Abstract
The identification of the optimal administration schedule for an effective medical countermeasure is critical for the effective treatment of individuals exposed to potentially lethal doses of radiation. The efficacy of filgrastim (Neupogen®), a potential medical countermeasure, to improve survival when initiated at 48 hours following total body irradiation in a nonhuman primate model of the hematopoietic syndrome of the acute radiation syndrome was investigated. Animals were exposed to total body irradiation, antero-posterior exposure, total midline tissue dose of 7.5 Gray, (target lethal dose 50/60) delivered at 0.80 Gray minute-1, using linear accelerator-derived 6 Megavolt photons. All animals were administered medical management. Following irradiation on day 0, filgrastim (10 μg kg day-1) or the control (5% dextrose in water) was administered subcutaneously, daily through effect (absolute neutrophil count ≥ 1,000 cells μL-1 for 3 consecutive days). The study (n = 80) was powered to demonstrate a 25% improvement in survival following the administration of filgrastim or control beginning at 48 ± 4 hours post-irradiation. Survival analysis was conducted on the intention-to-treat population using a two-tailed null hypothesis at a 5% significance level. Filgrastim, initiated 48 hours after irradiation, did not improve survival (2.5% increase, P = 0.8230). These data demonstrate that efficacy of a countermeasure to mitigate lethality in the hematopoietic syndrome of the acute radiation syndrome can be dependent on the interval between irradiation and administration of the medical countermeasure.
Keywords: blood, laboratory animals, radiation, whole body irradiation
Introduction
The development of medical countermeasure (MCM) requires reliable and validated animal models that allow translation of treatment to the human condition. In this case, the efficacy of MCM must be determined within the context of use and in adherence to the criteria of the FDA “animal rule” (AR) (Crawford 2002; Food and Drug Administration 2009). The predictive validity of a model requires that the performance of the model in subsequent tests is reliable and reproducible so that it can be used for meaningful and accurate measurement of a desired bioeffect. The established dose response relationship (DRR) must permit the reliable choice of a dose and effect for additional studies, e.g., the lethal dose (LD) 50/60. The reproducible bioeffect permits further analysis of MCM efficacy relative to a desired lethal effect within the established DRR, in this case the LD50/60. Current models of the hematopoietic syndrome of the acute radiation syndrome (H-ARS) in nonhuman primate (NHP) employ total-body irradiation (TBI) or partial-body irradiation with 5% bone marrow sparing (PBI/BM5) (Neelis et al. 1997; Bertho et al. 2005b; Bertho et al. 2005a; MacVittie et al. 2012; Farese et al. 2012). Both models can be used to assess MCM efficacy with a degree of predictive validity that is dependent on the context of use. Herein, we determined the efficacy of filgrastim to mitigate mortality in a NHP model when administered 48 hours (hr) post-TBI. We had previously developed a NHP model of the H-ARS induced by TBI and subsequently demonstrated the significant efficacy of filgrastim when administered at 24 hr post-TBI (Farese et al. 2013; Farese et al. 2012). The requirement to assess the efficacy of candidate MCM with increasing intervals between exposure and treatment reflects the knowledge required for optimal clinical utility of MCM and supportive care in the context of use in a nuclear terrorist scenario.
Methods
Test and Control Articles
The test article, Neupogen® (filgrastim) (Amgen Inc., Thousand Oaks, CA), and control article, dextrose 5% in water (D5W) (Baxter Healthcare Corporation, 1 Baxter Pkwy, Deerfield, IL 60015), was stored from 2° C to 8° C, and from 20° C to 30° C, upon receipt, respectively.
Animals
A total of 80 male, rhesus macaques, Macaca mulatta, Chinese substrain, mean ± 2 standard errors (SE) kilogram (kg) body weight was 5.8 kg ± 0.2 kg (range 5.0 kg-8.0 kg). Animals were housed in individual stainless steel cages at the AAALAC accredited Testing Facility (Farese et al. 2012). All animals were acclimated to a supine restraint device prior to irradiation.
Radiation Exposure
Restrained and sedated NHP were exposed antero-posteriorly to 6 Megavolt [MV] LINAC-derived photons (approximately 2 MV average energy, Varian Model EX or Model HET SN 6) (Varian Medical Systems, Inc., 3100 Hansen Way, Palo Alto, CA 94304-1038) at a dose rate of 0.80 Gy min-1 to a total midline tissue dose of 7.50 Gy as described previously (Farese et al. 2012).
A cylindrical, saline-filled lucite phantom that approximated the mean diameter of the NHP was used to calibrate the LINAC source for midline exposure doses. Depth dose measurements were made at the center of the phantom with paired 0.5 cc ion chambers, specifically an A-150 plastic tissue-equivalent chamber with methane-based, tissue-equivalent gas in a magnesium chamber with argon gas. Real time exposure dose was confirmed with dosimeters (Landauer® nanoDot™ system, 2 Science Rd, Glenwood, IL 60425). Two dosimeters were placed on each animal at and just above the xiphoid process at the time of antero-irradiation, then removed and replaced with unexposed dosimeters in the same locations on the animal prior to posterior-exposure.
TBI: Rationale for the dose
The midline tissue target dose for 6 MV photon irradiation of 7.50 Gy was the estimated (LD)50/60 for rhesus macaques administered medical management (Farese et al. 2012).
Animal cage-side observations
Cage-side observations of the animals were performed twice daily by individuals blinded to the treatment assignment. Activity, posture, stool consistency, vomit, hemorrhage, alopecia, respiratory and seizure activity were observed, scored and recorded.
Animal clinical observations
NHP were anesthetized and clinical parameters such as a complete blood count, body weight, body temperature, dehydration status, presence of mouth ulcers, and observation of blood in the stool were assessed as described previously (Farese et al. 2013).
Medical Management
Medical management was provided to all NHP as indicated by specified criteria. This included hydration fluids, antibiotics, analgesics, anti-diarrheals, antipyretics, anti-emetics, anti-ulceratives, nutritional support and blood transfusions. Specific details as to initiation, dosages and duration of treatments was described previously (Farese et al. 2013). Note that Invanz® (ertapenem sodium) (Merck & Co. Inc., One Merck Drive, Whitehouse Station, NJ 08889-0100) was administered at 15 ± 1 mg kg-1, intramuscularly (IM), twice daily, in place of Primaxin® when microbial resistance was demonstrated to enrofloxacin, gentamicin and ceftriaxone.
Euthanasia
The study director, who was blinded to the filgrastim or the control D5W randomization assignments, adhered to a specific set of criteria as described previously when determining if euthanasia was the appropriate action prior to the end of the study (d60) (Farese et al. 2013). Expiration was confirmed by a minimum of 2 methods (e.g. lack of pulse and respiration).
Experimental Design
Randomization of Treatment Groups and Study Blinding
The plan for the full study was set up with 14 blocks of 8 animals and 1 block of 6 animals to encompass the maximum sample size of the study, 118 animals. For each block, animals were assigned to treatment type [test article (filgrastim) or control (D5W)] using computer generated random numbers and the next open slots in the next block of the design. Random numbers were generated for each cohort independently. Animal randomizations were performed using SAS® version 9.3.
All study personnel were blinded to the treatment assignments with the exception of a select group of laboratory personnel who were responsible for preparing syringes for the injection of either the control article or the filgrastim.
Dose and Route of Administration
Filgrastim (10 μg kg d-1) or D5W were administered subcutaneously (SC), at 48 ± 4 hr following TBI and then daily until the ANC ≥ 1,000 cells μL-1 for three (3) consecutive days or if at any time the ANC was ≥ 10,000 cells μL-1. At any point following discontinuation of dosing, if the ANC was < 500 cells μL-1, daily injections were re-initiated the same day and continued until the ANC was ≥ 1,000 cells μL-1 for three (3) consecutive days.
Specimen Collection
Blood was collected for complete blood counts (CBC) and aseptically for microbial analysis as described previously (Farese et al. 2013; Farese et al. 2012).
CBC Analysis
The CBC was analyzed using an automated cell counter Beckman Coulter Ac·T diff™ or Ac·T diff™ 2 (Beckman Coulter, Inc., 11800 SW 147th Avenue, Miami, FL). The manufacturer’s published white blood cell (WBC) linearity range of the parameter for the instrument was 0 to 99,900 cells μL-1 ± the greater of 300 cells μL-1 or 5.0%. Additionally, A WBC differential, which included verification of all electronically generated WBC < 1,000 cells μL-1 was performed by trained personnel using light microscopy and a blood-film stained (Hema- Tek II™, Bayer Corp., Diagnostic Division, 511 Benedict Ave., Tarrytown, NY 10591-5097) with Wright-Giemsa Stain Pack (Fisher Scientific, 2000 Park Lane Dr., Pittsburgh, PA, 15275) on all CBC samples.
Determination of Endogenous Platelet Recovery
Only animals who demonstrate a platelet count ≥ 20,000 platelets μL-1 prior to death or end of the study, according to the criteria previously described are included when determining the mean ± SE duration of thrombocytopenia and time to recovery to a platelet count ≥ 20,000 platelets μL-1 (Farese et al. 2013).
Necropsy, Microbiology and Histopathology
A necropsy was performed on all animals as previously described (Farese et al. 2013).
Statistical Design
The primary endpoint of the statistical analysis was to determine and analyze all-cause survival measured at 60 days post-TBI. An interim analyses for efficacy or futility to determine if the study may be terminated prior to completion was included in the protocol design and would be conducted after the cohort associated with at least 68% of the monkeys were 60 days past irradiation according to the following statistical analyses. The primary analysis was conducted on the intention to treat population using a chi square test of a two-tailed null hypothesis using a 5% significance level.
All animal randomizations and statistical analyses were performed using Statistical Analysis Software SAS® version 9.3. An interim analysis for efficacy and futility was conducted based on the Lan-DeMets version of the O’Brien-Fleming boundary to provide an overall two-sided P = 0.05 test (Lan and DeMets 1983; O’Brien and Fleming 1979). Futility was assessed informally based on conditional power using stochastic curtailment (Davis and Hardy 1994). Secondary endpoints (e.g. first day, duration and recovery from neutropenia, and thrombocytopenia, ANC and platelet nadir) were analyzed as follows: Continuous data were summarized descriptively by mean, median, standard deviation, standard error and range. Two-sample t-tests or Mann-Whitney-U tests were done to compare continuous variables between treatment treatments; Categorical data was presented as enumerations and percentages. Chi-squared or Fisher’s Exact tests were done to compare categorical data between treatment.
Results
Survival, the primary endpoint
Administration of neupogen (filgrastim) at 48 hr post-TBI of animals exposed to an estimated LD50/60 of 7.50 Gy resulted in mortality of 47.5% (19/40 survivors/total) relative to the control cohort of 50.0% (20/40, survivors/total). The 2.5% difference in survival was not significant (P = 0.82) (Figure 1); therefore, the study was halted for futility following the interim analysis.
Figure 1.
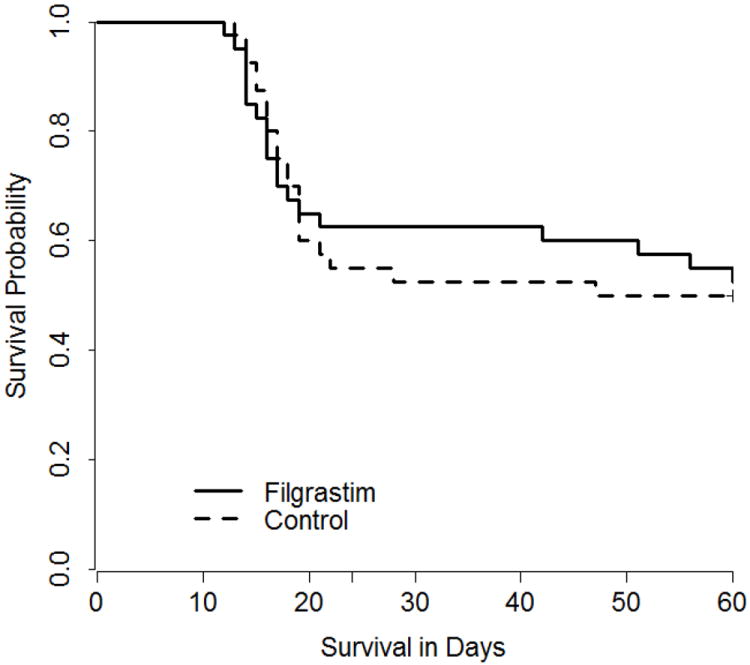
Kaplan Meier survival curve in rhesus macaques following total-body irradiation. Rhesus macaques were exposed to 7.50Gy TBI with 6MV LINAC photons (2MV average energy) at a dose rate of 0.80Gy/minute. The TBI was delivered as 50% in the anterior (AP), then 50% in the posterior (PA) directions. NHP (n=80) were observed for 60d post-TBI for cage side and clinical observations and protocol euthanasia criteria for all-cause mortality. All animals were administered medical management and either filgrastim at 10μg/kg/d or control article at 164μL/kg/d, by subcutaneous injection, starting day 2 post-TBI and continued daily until the ANC > 1,000 cells/μL for three (3) consecutive days. Lethality in the filgrastim treated-cohort was 47.5% (19/40) and occurred on d12 (n=1), d13 (n=1), d14 (n=4), d15 (n=1), d16 (n=3), d17 (n=2), d18 (n=1), d19 (n=1), d21 (n=1), d45 (n=1), d51 (n=1), d56 (n=1), d60 (n=1). Lethality in the control cohort was 50.0% (20/40) and occurred on d13 (n=1), d14 (n=2), d15 (n=2), d16 (n=3), d17 (n=2), d18 (n=2), d19 (n=4), d21 (n=1), d22 (n=1), d28 (n=1), d47 (n=1).
Survival time of decedents
Administration of filgrastim increased the mean survival time of the decedents from 19.2 for the control cohort to 23.4 days. The median ST of decedents was 17.5 and 16.0 days for control and filgrastim-treated animals, respectively.
Hematologic parameters, secondary endpoints
Neutrophil-related parameters
TBI at 7.50 Gy reduced the ANC in control and filgrastim-treated cohorts to < 500 cells μL-1 within 5 days (P > 0.05) and to values < 100 cells μL-1 within 7.8 (±0.3) and 6.5 (±0.1) (P = 0.0002) days respectively (Figure 2). The mean duration of neutropenia (ANC < 500 cells μL-1) was 16.4 (± 0.5) and 13.1 (± 0.4) days for control and filgrastim-treated cohorts, respectively) (P < 0.0001). The mean time to recovery to an ANC ≥ 1,000 cells μL-1 was 23.5 and 18.9 days, respectively (P < 0.0001) (Table 2). The first day of febrile neutropenia (FN) (ANC < 500 cells μL-1 and body temperature ≥ 103.0° F) occurred on day 11.8 (± 0.5) and day 9.8 (± 0.5) for control and G-CSF-treated cohorts, respectively. FN occurred in 85% of the filgrastim-treated animals and 95% of the controls (P = 0.2633). Positive blood cultures were noted in 67.5% of the animals. Although the administration of filgrastim diminished the duration of neutropenia and time to recovery of neutrophils by several days it did not mitigate the mortality associated with the 7.50 Gy (LD50/60) dose of TBI.
Figure 2.
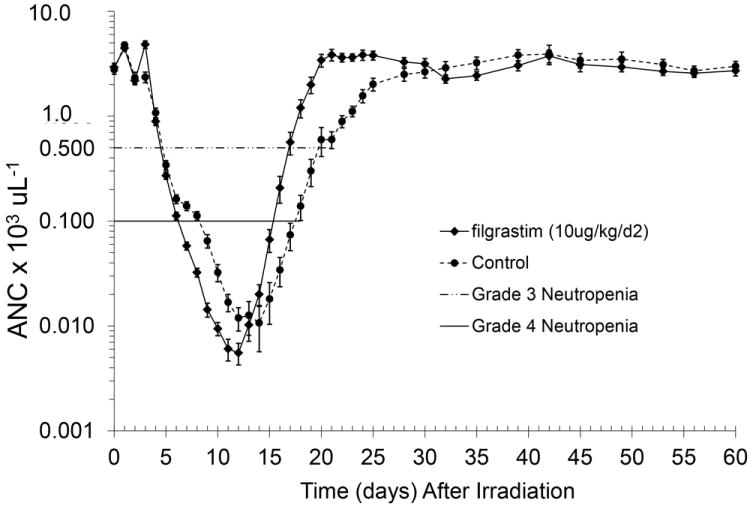
Mean absolute neutrophil counts in rhesus macaques following total-body irradiation and administration of filgrastim or control. Animals (n=80) were exposed to 7.50 Gy total body irradiation (TBI) with 6MV LINAC-derived photons at a dose rate of 0.80 Gy/min and administered medical management and either filgrastim (n=40) or control (5% dextrose in water) (n=40) by subcutaneous injection beginning on d2 post-TBI. Shown is the decrease in the absolute neutrophil count (ANC) in the peripheral blood of rhesus macaques as a function of time post-TBI. Mean values (± standard error) are displayed for all animals that survived at each data point.
Table 2.
Neutrophil-related parameters for rhesus macaques following exposure to 7.50 Gy TBI. Animals were total body-irradiated by 6 MV LINAC-derived photons at a dose rate of 0.80 Gy min-1 and administered either filgrastim (n=40) at 10 μg kg d-1 or control (5% dextrose in water) (n=40) at 164μL kg d-1, by subcutaneous injection, starting day 2 post TBI and continued daily until the ANC > 1,000 cells μL-1 for three (3) consecutive days. Each time point is determined for each animal and the mean, one standard error (SE), median and range (where applicable) are reported. The absolute neutrophil count (ANC) nadir, duration and the first and final day that grade 3 or 4 neutropenia [ANC < 500 cells μL-1 or < 100 cells μL-1, respectively] for filgrastim and control cohorts, and ANC recovery ≥ 1000 cells μL-1 occurred are shown. The durations last day of grade 3 or 4 neutropenia do not include data from decedent animals unless recovery occurred to that level, e.g., ANC ≥ 500 cells μL-1 prior to death. The duration of neutropenia was estimated as the number of days that a subject has an observed or an extrapolated ANC below 500 cells μL-1. If an animal’s ANC was ≥ 500 cells μL-1 for a single day but < 500 cells μL-1 on the day preceding and following this day, it was considered a day of severe neutropenia when determining the animal’s duration of ANC < 500 cells μL-1. The time to recovery was estimated as the number of days from study day 1 until the first 2 consecutive observed or extrapolated ANC after the nadir was ≥ 1,000 cells μL-1. The nadir was the lowest observed or extrapolated ANC μL-1 observed at least 2d after irradiation.
| Treatment | First day (d) of Neutropenia | Duration (d) of Neutropenia | Recovery to ANC ≥ 1000 μL-1 ≥ 1000 μL-1 | Nadir (ANC μL-1) | |||
|---|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | ||||
| Control | Mean ± SE | 5.0±0.1a | 7.8±0.3a | 16.4±0.5b | 10.4±0.6 b | 23.5±0.7c | 2.6±1.0a |
| Median | 5a | 7a | 16.5b | 9b | 23.0c | 0a | |
| Range | d4-7 | d5-11 | d13-21 | d6-17 | d19-33 | 0-36 | |
|
| |||||||
| Filgrastim | Mean ± SE | 4.9±0.1a | 6.5±0.1a | 13.1±0.4d | 9.9±0.3d | 18.9±0.3d | 1.9±0.5a |
| P | 0.4802 | 0.0002 | <0.0001 | 0.5504 | <0.0001 | 0.9032 | |
| Median | 5a | 6a | 13.0d | 10d | 19.0d | 0a | |
| Range | d3-6 | d5-8 | d11-18 | d8-15 | d16-22f | 0-12 | |
All animals (n=40 per treatment group) are included in analysis for first day of Grade 3 (ANC < 500 μL-1) and Grade 4 (ANC <100 μL-1) neutropenia and ANC nadir. Survivors and the non-survivors that recovered to respective levels are included in duration of neutropenia and recovery to ANC ≥ 1000 μL-1 analysis. P values are a result of the Wilcoxon rank-sum analysis for nadir and a two-sample t-test for all other analyses.
n=40;
n=22;
n=21;
n=25
Platelet-related parameters
Platelet-related parameters were also severely diminished following exposure to 7.50 Gy of TBI. The mean duration of thrombocytopenia (platelet count < 20,000 platelets μL-1) was 13.6 day (d) (± 0.9) and 11.4 d (± 0.6) (P = 0.0437) (Table 3). The platelet nadir was essentially the same in both treatment groups (1,700 platelets μL-1 controls, 1,775 platelets μL-1 figrastim). The average number of transfusions per animals was 2.5 and 1.9 for control and filgrastim-treated cohorts, respectively (P = 0.0010).
Table 3.
Platelet-related parameters for rhesus macaques following exposure to 7.50 Gy TBI. Animals were total body-irradiated by 6 MV LINAC-derived photons at a dose rate of 0.80Gy min-1 and administered either filgrastim (n=40) at 10 μg kg d-1 or control (5% dextrose in water) (n=40) at 164μL kg d-1, by subcutaneous injection, starting day 2 post TBI and continued daily until the ANC > 1,000 cells μL-1 for three (3) consecutive days. Data at each time point is determined for each animal and the mean, one standard error (SE), median and range (where applicable) are reported for each parameter. The first day of thrombocytopenia (platelet count < 20,000 platelets μL-1) in each treatment cohort, duration of thrombocytopenia, platelet nadir, day recovery from thrombocytopenia occurs, number of whole blood transfusions administered (54 mL) and first day a transfusion is administered are displayed.
| Treatment | First day Thrombocytopenia | Duration (d) Thrombocytopenia | Nadira (Platelet μL-1) | Day (d) of Recovery from Thrombocytopenia | # Transfusionsa (54mL) | First day (d) of transfusiona | |
|---|---|---|---|---|---|---|---|
| Control | Mean ± SE | 9.4a | 13.6±0.9b | 1,700±140a | 23.0±1.0b | 2.5±0.2a | 11.5a |
| Median | 9a | 12.5b | 1500a | 21.5b | 2a | 11a | |
| Range | d7-12 | d8-24 | 1000-4000 | d16-32 | 1-6 | d9-17 | |
|
| |||||||
| Filgra-stim | Mean ± SE | 9.1a | 11.4±0.6c | 1775±233a | 20.4±0.5c | 1.9±0.1a | 10.9d |
| P | 0.1657 | 0.0437 | 0.9794 | 0.0289 | 0.0010 | 0.819 | |
| Median | 9a | 11.0c | 2000a | 20.0c | 2a | 11d | |
| Range | d8-10 | d6-17 | 0-9000 | d16-26 | 0-3 | d11-13 | |
All animals (n=40 per treatment group) are included in analysis for first day of thrombocytopenia (platelet count < 20000 μL-1) and platelet nadir. Survivors and the non-survivors that recovered to platelet counts ≥ 20,000 μL-1 are included in duration of and recovery from thrombocytopenia analysis. P values are a result of the Wilcoxon rank-sum analysis for nadir and a two-sample t-test for all other analyses.
n=40;
n=22;
n=25;
n=38;
Red Blood Cell-related Parameters
The pre-irradiation hemoglobin (Hgb) values were similar [12.0 grams (g) deciliter (dL)-1 (± 0.3 g dL-1) and 12.5 g dL-1 (± 0.1 g dL-1) in the control and filgrastim cohorts respectively. The maximum decrease in Hgb ranged between 26% [8.9 g dL-1 (± 0.9 g dL-1)] in the controls to 28% [9.0 g dL-1 (± 0.3 g dL-1)] in the filgrastim-treated cohort which occurred within 3 weeks post-TBI. Similarly, both groups demonstrated a gradual recovery of Hgb following their nadirs to near baseline levels reached by d60 [86% [10.4 g dL-1 (± 0.7 g dL-1)] in the controls to 85% [10.7 g dL-1 (± 0.2 g dL-1) in the filgrastim-treated cohort].
Predictive validity of the H-ARS model for an LD50/60 dose of TBI
The initial study to determine the dose response relationship (DRR) for the H-ARS in the rhesus macaque exposed to 6 MV LINAC-derived photons estimated that the LD50/60 was 7.53 Gy [6.50, 7.88] (Farese et al. 2012). Herein, animals (n=40) of similar age and body weight were exposed to the same radiation dose of 7.50 Gy and dose rate, 0.80 Gy min-1 as previously reported and only received medical management following TBI. The resulting mortality in this study was 50% (20 deaths/40 total) over the 60 d in-life phase of the study and in this case confirmed the predictive ability of the DRR for this model as performed herein (Farese et al. 2012).
Discussion
The efficacy of filgrastim to enhance survival in NHP when administered within 24 hr post-TBI has been established. Filgrastim, when administration was initiated within 24 hr post-TBI, significantly increased survival to 78.3% relative to 40% for the control-treated cohort in a standard NHP model of the H-ARS (Farese et al. 2013; Farese et al. 2012). The efficacy of filgrastim when administered within 24 hr post-TBI is consistent and substantial when assessed across three species, e.g., mouse, canine and NHP, different radiation sources and varied doses of high sub-lethal and lethal radiation (Patchen et al. 1990; Patchen et al. 1993; Tanikawa et al. 1990; MacVittie et al. 2005; Schuening et al. 1989; Schuening et al. 1993; MacVittie et al. 1996; Neelis et al. 1996; Storb et al. 1993).
The delayed administration of filgrastim has been assessed in a number of preclinical studies; however, the limited results do not provide a consensus on efficacy when the interval between TBI and initiation of filgrastim administration is increased. However, studies in the mouse do provide a more consistent assessment. Tanikawa et al., and Patchen et al., suggested that the earlier and more frequently filgrastim was administered, the earlier the hematopoietic recovery was observed to occur (Patchen et al. 1990; Tanikawa et al. 1990). The survival of mice was diminished but not completely lost when administration of G-CSF was delayed. In contrast, a canine study used to evaluate the delayed administration of G-CSF did not show efficacy (Schuening et al. 1989). The high, lethal dose of radiation as well as the length of the interval, 7 days, between exposure and treatment used in the canine study may have been critical factors in the lack of efficacy. Schuening et al., exposed canines to an LD99/30 and could not demonstrate efficacy when G-CSF was initiated at 7 days post-TBI and continued with daily administration, relative to that observed when G-CSF administration was initiated within 2 hr post exposure. In contrast with results from the radiation studies, Meisenberg et al., showed efficacy of G-CSF when administered in a delayed schedule in a NHP model of chemotherapy-induced myelosuppression (Meisenberg et al. 1992). It is likely that the differential radiation dose-effect on the hematopoietic system between chemotherapy-induced, sublethal myelosuppression, and high-dose, lethal irradiation contributed to the variable response in the large animal models. The marginal data base suggested that the more severe the insult the more critical was the early administration of G-CSF and that G-CSF efficacy was diminished with longer delay in administration.
The questions remains: What cellular parameters and associated mechanisms of action noted for lethal doses of TBI and G-CSF can account for the observed lack of treatment efficacy on survival, the primary clinically relevant endpoint for this study? All animals received an equivalent dose of lethal TBI. The LD50/60 of 7.5 Gy reduced the number of hematopoietic progenitor cells (HPC) in a dose-dependent manner for all animals. The dose-dependent reduction of HPC is a defining consequence for the H-ARS. A recent article, focused on modeling treatment strategies for G-CSF, identified the influence of intensive cytotoxic chemotherapy on marrow-derived hematopoietic progenitor cells and emphasized a key variable noted as the “acute marrow capacity” (AMC) (Shochat and Rom-Kedar 2008). It was suggested that the AMC dominates neutrophil kinetics and determines the grade of neutropenia (nadir) and the maximal neutrophil flux that can be induced by administration of G-CSF and supportive care after intensive chemotherapy. There is no doubt that the AMC is a critical parameter consequent to dose-dependent, high-dose lethal radiation. The early administration of G-CSF in the lethal TBI models is necessary for optimal survival. The efficacy of early administration of G-CSF is linked to enhanced viability of responsive HPC, stimulation of HPC proliferation and differentiation, enhanced mitotic activity within the immature neutrophil lineage, a reduced transit time through the marrow to the peripheral pool, enhanced viability and amplification of the post-mitotic pool entering the peripheral blood. These events culminate in marked amplification of the marrow-derived neutrophil compartment (Hess et al. 2002; Lord et al. 1989; Lord et al. 2001; Engel et al. 2004: Mackey et al. 2003; Chatta et al. 1994; Zhuge et al. 2012). The consensus from modeling efforts using clinical and preclinical data from chemotherapy-induced myelosuppression suggests that the timing of G-CSF administration after intensive chemotherapy is crucial for a positive outcome. In effect, the earlier the administration of G-CSF post cytotoxic insult, the better result in enhancing neutrophil dynamics. The delayed administration of G-CSF should only exacerbate the recovery time of the marrow-derived HPC. Farese et al and others have demonstrated the efficacy of early administration of G-CSF in enhancing survival of lethally irradiated large animals based on the aforementioned mechanism (Schuening et al. 1989; MacVittie et al. 2005: Farese et al. 2013).
The lack of efficacy characterized by equivalent survival between control and G-CSF-treated cohorts following an additional 24 hr delay in administration of G-CSF convolutes the proposed mechanism and suggests a diminished effect at all mechanistic levels including apoptotic effects of TBI, normal programmed apoptosis during neutrophil maturation and transit through the marrow, diminished stimulation of marrow–derived progenitor cells and less amplification of functional neutrophils. Another possibility is that the pool of G-CSF-target cells has been depleted by 48 hr post TBI and therefore the ability to regenerate neutrophils is significantly diminished.
We did not measure circulating or tissue neutrophil half-life or neutrophil function as assessed by microbicidal activity or chemotaxis into relevant tissues. We note that there was no difference in positive blood cultures between the control and the 48 hr G-CSF-treated cohorts, both had 67.5% positive blood cultures. The comparative parameter for the recent study using G-CSF at 24 hr post-TBI was 86% and 58% positive blood cultures for the control and 24 hr G-CSF-treated cohorts, respectively (Farese et al. 2013). Delayed administration of G-CSF to 48 hr post-TBI did not reduce the percentage of positive blood cultures and thus more animals are at risk for sepsis-induced morbidity and mortality.
The administration of MCM in the context of the aftermath of a nuclear terrorist event requires knowledge of their efficacy when utilized in a less than optimal treatment protocol, e.g., with increasing interval between the irradiation and initial administration. Furthermore, the exposure doses will range across the dose-dependent, lethal H-ARS. As noted above the data base in this regard is minimal relative to assessing efficacy in large animal models of lethal exposure. The data presented herein suggested that G-CSF administration delayed until 48 hr could not mitigate the 50% lethality induced by the 7.50 Gy of TBI in the presence of medical management. The lack of efficacy occurred despite the comparable recovery of neutrophils relative to the control cohort. We hypothesized that a 24 hr further delay in administration of G-CSF to 48 hr post-TBI would result is a less efficacy than noted previously with the 24 hr administration of G-CSF but would certainly mitigate lethality and increase survival to a lesser degree. The authors understand that a model of TBI may not be the appropriate model to assess efficacy of a MCM within the context of the post-nuclear radiation exposure environment. TBI to an expected uniform, midline tissue delivery of dose is a laboratory model that may be used to estimate early efficacy of selected MCM but caution must be exercised when extending use of a TBI model to assess efficacy relevant to “context of use” in a reality environment post a nuclear event where dose inhomogeneity and non-uniformity may prevail.
The human anecdotal experience with accidental irradiation and use of growth factors (GF) such as G-CSF, GM-CSF and/or IL-3 is noteworthy since the majority of exposed personnel were administered cytokines in a delayed, less than optimal schedule (Butturini et al. 1988; Rafael-Hurtado et al.; Baranov et al. 1994; Liu et al. 2008; Gourmelon et al. 2010; Hirama et al. 2003; IAEA 2000; IAEA 2002). All the accidental radiation exposures are anecdotal with regard to radiation dose, treatment and biological response. The majority of cases noted a definitive leucocyte response to the GF administration but efficacy of the treatment was difficult to ascertain due to the delayed administration and likely spontaneous recovery of hematopoiesis as a consequence of the non-uniform nature of the radiation exposure. With heterogeneous and non-uniform exposure, bone marrow was spared and responsive hematopoietic progenitor cells were likely in a spontaneous recovery phase when GFs were administered.
To more realistically model a likely human exposure scenario, an animal model, with some degree of bone marrow sparing is required to assess the efficacy of delayed administration of MCM (MacVittie et al. 2012). A small fraction of active BM spared from lethal doses of radiation can prevent lethality from the acute H-ARS (Bertho et al. 2005a; Bertho et al. 2005b; Drouet et al. 2004; Monroy et al. 1988; Baltschukat and Nothdurft 1990; Nothdurft et al. 1997) and has been shown to shift the dose response relationship and LD50/60 in NHP from 7.50 Gy after TBI to 11.0 Gy with 5% BM sparing (MacVittie et al. 2012). An animal model that incorporates a minimal percentage of marrow sparing may be more relevant to what has been observed in human accidental radiation exposures. This type of model may be better suited to assess MCM efficacy when the MCM is administered with increasing time interval between exposure and initiation of treatment. The data presented herein are significant in demonstrating the lack of efficacy when filgrastim is administered with an increased interval (48 hr) between TBI and treatment. These results underscore the importance of radiation dose and exposure geometry, as well as the timing of MCM intervention during a radiological nuclear event on survival. Current studies are underway to assess the efficacy of delayed administration of G-CSF in a model of partial-body irradiation with 5% marrow sparing (MacVittie et al. 2012). We hypothesize that in this model of heterogeneous exposure, G-CSF will mitigate lethality and/or severe myelosuppression when administered with an interval of several days between exposure and treatment.
Table 1.
Percent lethality and mean survival time of decedents following total-body irradiation in rhesus macaques.
Rhesus macaques (n=80) were exposed to target radiation dose of 7.5 Gy, total-body irradiation (TBI) using 6 MV LINAC-derived photons at a dose rate of 0.80Gy min-1 in a blinded and randomized study. The TBI was delivered as 50% in the anterior (AP), then 50% in the posterior (PA) directions. Following TBI, animals received medical management and either filgrastim (n=40) or control (n=40) beginning on study day 2 by subcutaneous injection. Animals were observed for 60d post TBI for cage side observations by study personnel and euthanasia under protocol criteria for all-cause mortality by the study director who were “blinded” to the treatment assignments for each animal. The percent lethality, number of decedents versus the total number, mean survival time (days) of decedents ± standard error (SE), median survival time (days) of decedents are reported for each radiation cohort. All animals received IACUC-approved supportive care as per defined signs of morbidity.
| Treatment | Control | Filgrastim |
|---|---|---|
| % Lethality | 50.0% | 47.5% |
| Decedents/total | 20/40 | 19/40 |
| Mean survival time of decedents (days) | 19.2±1.6 | 23.4±3.6 |
| Median survival time of decedents (days) | 17.5 | 16.0 |
Acknowledgments
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272201000046C. Authors gratefully acknowledge the support of our NIAID colleagues, Bert Maidment, Ph.D. and David Cassatt, Ph.D. for their guidance and helpful insight during the conduct of the study and review of this manuscript; all the members of our Preclincial Radiobiology Laboratory for their dedication to the health and well-being of our animals.
Footnotes
The authors declare no conflict of interest.
Reference List
- Baltschukat K, Nothdurft W. Hematological effects of unilateral and bilateral exposures of dogs to 300-kVp X rays. Radiat Res. 1990;123:7–16. [PubMed] [Google Scholar]
- Baranov AE, Selidovkin GD, Butturini A, Gale RP. Hematopoietic Recovery After 10-Gy Acute Total Body Radiation. Blood. 1994;83:596–599. [PubMed] [Google Scholar]
- Bertho JM, Frick J, Prat M, Demarquay C, Dudoignon N, Trompier F, Gorin C, Thierry D, Gourmelon P. Comparison of Autologous Cell Therapy and Granulocyte colony-stimulating Factor (G-CSF) Injection vs G-CSF Alone for the Treatment of Acute Radiation Syndrome in a Nonhuman Primate Model. Int J Radiat Oncol Biol Phys. 2005a;63:911–920. doi: 10.1016/j.ijrobp.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Bertho JM, Prat M, Frick J, Demarquay C, Gaugler MH, Dudoignon N, Clairand I, Chapel A, Gorin NC, Thierry D. Application of autologous hematopoietic cell therapy to a nonhuman primate model of heterogeneous high-dose irradiation. Radiat Res. 2005b;163:557–570. doi: 10.1667/rr3352. [DOI] [PubMed] [Google Scholar]
- Butturini A, Gale RP, Lopes DM, Cunha CB, Ho WG, Sanpai JM, de Souza PC, Cordiero JM, Neto C, DeSouza CEP, Tabak DG. Use of recombinant granulocyte-macrophage colony stimulating factor in the Brazil radiation accident. The Lancet. 1988:471–474. doi: 10.1016/s0140-6736(88)90121-3. [DOI] [PubMed] [Google Scholar]
- Crawford LM. New drug and biological drug products; Evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Federal Register. 2002;67(105):37988–37998. 21 CFR parts 314 and 601, FDA, HHS; ACTION: Final Rule. [PubMed] [Google Scholar]
- Davis BR, Hardy RJ. Data monitoring in clinical trials: the case for stochastic curtailment. J Clin Epidemiol. 1994;47:1033–1042. doi: 10.1016/0895-4356(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Drouet M, Mourcin F, Grenier N, Leroux V, Denis J, Mayol J-F, Thullier P, Lataillade J-J, Herodin F. Single administration of stem cell factor, FLT-3 ligand, megakaryocyte growth and development factor, and interleukin-3 in combination soon after irradiation prevents nonhuman primates from myelosupression: long-term follow-up of hematopoiesis. Blood. 2004;103(3):878–885. doi: 10.1182/blood-2003-05-1400. [DOI] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Jackson W, III, Cohen DM, MacVittie TJ. A nonhuman primate model of the hematopoietic acute radiation syndrome plus medical management. Health Phys. 2012;103(4):367–382. doi: 10.1097/HP.0b013e31825f75a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, MacVittie TJ. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res. 2013;179:89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. Office of Communications, Division of Drug Information. Center for Drug Evaluation and Research (CDER) Center for Biologics Evalaution and Research (CBER) Silver Spring, MD: FDA; 2009. Draft Guidance for Industry: Animal Models-Essential Elements to Address Efficacy Under the Animal Rule; pp. 1–19. 21 CFR Part 314.600 for drugs; 21 CFR 601.90 for biological products. [Google Scholar]
- Gourmelon P, Benderitter M, Bertho J-M, Gorin NC, De Revel P. European Consensus on the Medical Management of Acute Radiation Syndrome and Analysis of the Radiation Accidents in Belgium and Senegal. Health Physics. 2010;98:825–832. doi: 10.1097/HP.0b013e3181ce64d4. [DOI] [PubMed] [Google Scholar]
- Hirama T, Tanosaki S, Kandatsu S, Kuroiwa N, Kamada T, Tsuji H, Tamada S, Katoh H, Yamamoto N, Tsuji H, Suzuki G, Akashi M. Initial Medical Management of Patients Severely Irradiated in the Tokai-mura Criticality Accident. Brit J of Radiol. 2003;76:246–253. doi: 10.1259/bjr/82373369. [DOI] [PubMed] [Google Scholar]
- IAEA. The Radiological Accident in Istanbul, IAEA 2000. Vienna, Austria: International Atomic Energy Agency; 2000. pp. 1–75. [Google Scholar]
- IAEA. The Radiological Accident in Samut Prakarn, IAEA 2002. Vienna Austria: International Atomic Energy Agency; 2002. pp. 1–52. [Google Scholar]
- Lan KKG, DeMets DL. Discrete Sequential Boudaries for Clinical Trials. Biometrika. 1983;70:659–663. [Google Scholar]
- Liu Q, Jiang B, Jiang L-P, Wu Y, Wang X-G, Zhao F-L, Fu B-H, Turai I, Jiang E. Clinical report of three cases of acute radiation sickness from a 60Co radiation accident in Henan province in China. Journal of Radiation Research. 2008;49:63–69. doi: 10.1269/jrr.07071. [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Bennett A, Booth C, Garofalo M, Tudor G, Ward A, Shea-Donohue T, Gelfond D, Mcfarland E, Jackson IW, Lu W, Farese AM. The prolonged gastrointestinal syndrome in rhesus macaques: The relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation. Health Phys. 2012;103(4):427–453. doi: 10.1097/HP.0b013e318266eb4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Herodin F, Grab LB, Baum CM, McKearn JP. Combination therapy of radiation-induced bone marrow aplasia in nonhuman primates using synthokine SC-55494 and recombinant human granulocyte colony-stimulating factor. Blood. 1996;87:4129–4135. [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Jackson WI. Defining the Full Therapeutic Potential of Recombinant Growth Factors in the Post Radiation-Accident Environment: The effect of supportive care plus administration of G-CSF. Health Physics. 2005;89:546–555. doi: 10.1097/01.hp.0000173143.69659.5b. [DOI] [PubMed] [Google Scholar]
- Meisenberg BR, Davis TA, Melaragno AJ, Stead R, Monroy RL. A comparison of therapeutic schedules for administering granulocyte colony-stimulating factor to nonhuman primates after high-dose chemotherapy. Blood. 1992;79:2267–2272. [PubMed] [Google Scholar]
- Monroy RL, Skelly RR, Taylor P, Dubois A, Donahue RE, MacVittie TJ. Recovery from severe hemopoietic suppression using recombinant human granulocyte-macrophage colony stimulating factor. Experimental Hematology. 1988;16:334–338. [PubMed] [Google Scholar]
- Neelis KJ, Dubbelman YD, Qingliang L, Thomas GR, Eaton DL, Wagemaker G. Simultaneous administration of TPO and G-CSF after cytoreductive treatment of rhesus monkeys prevents thrombocytopenia, accelerates platelet and red cell reconstitution, alleviates neutropenia, and promotes the recovery of immature bone marrow cells. Experimental Hematology. 1997;25:1084–1093. [PubMed] [Google Scholar]
- Neelis KJ, Hartong SCC, Thomas GR, Eaton DL, Wagemaker G. Distinct hematopoietic response patterns to Tpo/GM-CSF and Tpo/G-CSF treatment in myelosuppressed rhesus monkeys. Blood. 1996;88(Suppl 1):352a. [Google Scholar]
- Nothdurft W, Kreja L, Selig C. Acceleration of hematopoietic recovery in dogs after extended-field partial-body irradiation by treatment with colony-stimulating factors: rhG-CSF and rhGM-CSF. Int J Radiat Oncol Biol Phys. 1997;37:1145–1154. doi: 10.1016/s0360-3016(97)00112-0. [DOI] [PubMed] [Google Scholar]
- O’Brien PC, Fleming TR. A multiple testing procedure for clincial trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- Patchen ML, Fischer R, MacVittie TJ. Effects of combined administration of IL-6 and G-CSF on recovery from radiation-induced hemopoietic aplasia. Experimental Hematology. 1993;21:338–344. [PubMed] [Google Scholar]
- Patchen ML, MacVittie TJ, Solberg BD, Souza LM. Therapeutic administration of recombinant human granulocyte colony stimulating factor accelerated hemopoietic regeneration and enhances survival in a murine model of radiation-induced myelosuppression. International Journal of Cell Cloning. 1990;8:107–122. doi: 10.1002/stem.5530080204. [DOI] [PubMed] [Google Scholar]
- Rafael-Hurtado M, Alanis A, Raul-Alvarez T, Frankel D, Pasquel C, Pasquetti CA, Juan-Ortega C, Guadalajara BJ, Cesin R, Masse S, Jesus-Simon, Mutchinick O, Valenzuela J, Garcia-Velasco J, Madrazo M, Ibarrola JL, Souto MC, Maisterrena J, Armendariz C, Ricks R, Berger ME, Littlefield G, Joiner G, Clouthier R, Carranza M, Echegoyen A, Gomez-Fuentes G. Recombinant Human Granulocyte/Macrophage Colony-Stimulating Factor for the Treatment of Bone Marrow Aplasia in Accidentally Irradiated (60Co) Patients: Report of Three New Cases. In: MacVittie TJ, Weiss JF, Browne D, editors. Avances in the Treatment of Radiation Injuries: Advances in the Biosciences. Vol. 94. Tarrytown, NY: Pergamon; Elsevier Science Ltd; 1996b. pp. 295–301. [Google Scholar]
- Schuening FG, Appelbaum FR, Deeg HJ, Sullivan-Pepe M, Grahm R, Hackman R, Zsebo KM, Storb R. Effects of recombinant canine stem cell factor, a c-kit ligand and recombinant granulocyte colony stimulating factor on hematopoietic recovery after otherwise lethal total body irradiation. Blood. 1993;81:20–26. [PubMed] [Google Scholar]
- Schuening FG, Storb R, Goehle S, Graham TC, Appelbaum FR, Hackman R, Souza LM. Effect of recombinant human granulocyte colony-stimulating factor on hematopoiesis of normal dogs and on hematopoietic recovery after otherwise lethal total body irradiation. Blood. 1989;74:1308–1313. [PubMed] [Google Scholar]
- Storb R, Raff RF, Graham T, et al. Marrow toxicity of fractionated versus single dose total body irradiation is identical in a canine model. Int J Radiat Oncol Biol Phys. 1993;26:275–283. doi: 10.1016/0360-3016(93)90207-c. [DOI] [PubMed] [Google Scholar]
- Tanikawa S, Nose M, Yoshiro A, Tsuneoka K, Shikita M, Nara N. Effects of recombinant human granuloctye colony-stimulating factor on the hematologic recovery and survival of irradiated mice. Blood. 1990;76:445–449. [PubMed] [Google Scholar]


