Abstract
Age-related changes in the concentration of factors like TGF-1β, DHEA-S and IGF-1 may increase the risk of disease and illnesses in advanced life. A better understanding of these changes would aid in the development of more appropriate treatments and/or preventative care for many conditions associated with age. Due to their similar immune system and vulnerability to pathogens, baboons are an ideal model for humans. However, little research has been done examining the general effects of age in baboons. Therefore, we wanted to further examine the effects of aging in baboons by determining the age-dependent changes in serum TGF-1β, DHEA-S and IGF-1 concentrations. Blood samples were collected during routine health checks in 113-118 captive baboons. In addition, longitudinal samples from 23-27 adult individuals were collected an average of 10.7 years apart. Both age and gender influenced the concentrations of serum TGF-1β and IGF-1. When both genders were analyzed together, TGF-1β increased 16.1% as adults, compared to younger and older animals, but male and female baboons showed a slightly different temporal pattern of change. IGF-1 decreased with increasing age and males had a 30% greater concentration of IGF-1 than did females. While there was no effect of gender among our population, serum DHEA-S was negatively correlated with age, decreasing by 51.6% in the oldest animals. There were no effects of age or gender on serum IGFBP-3. In longitudinal samples collected from the same individuals, the concentrations of TGF-1β, DHEA-S and IGF-1 were reduced with age. The results presented herein provide additional knowledge of the aging process in baboons and further validate the use of this species as an appropriate model for aging in humans.
Keywords: Non-human primates, aging, TGF-1β, DHEA-S, IGF-1, baboon
1. Introduction
Transforming growth factor-1β (TGF-1β), insulin like growth factor-1 (IGF-1), and the adrenal steroid hormone, dehydroepiandrosterone-sulfate (DHEA-S) are important factors that are essential for normal physiological processes. In the aging human, there is evidence that changes in the concentration of factors like TGF-1β, DHEA-S and IGF-1 may increase the risk of disease and pathological conditions seen among the elderly. In order to develop treatments and preventatives for chronic conditions that occur in aged individuals, a better understanding of changes that occur during the aging process and its impact on biological systems is required.
Transforming growth factor-1β is an anti-inflammatory cytokine member of the prototypic TGF-β superfamily. Although originally found to induce anchorage-independent growth in fibroblasts [41], it is now known to be a multifunctional protein that plays an important role in many physiological systems [28]. Mice null for TGF-1β show impairment in the development and regulation of the immune system as well as in the maintenance of immunological homeostasis. Furthermore, specific endocrine, paracrine and autocrine actions were discovered for TGF-1β [27]. Circulating concentrations of TGF-1β have been found to both decrease and increase with age in humans. Okamoto et al., found that serum concentration TGF-1β were decreased in adults 21-67 years of age compared to children 1-14 years of age [37]. Similarly, in adults 40-79 years of age, serum concentrations declined in an age-dependent manner [31]. However, in centenarians, serum TGF-1β concentration was increased when compared to younger adults [8], perhaps suggesting that high concentrations of TGF-1β are beneficial during extreme old age. Research with human diseases has shown that a greater concentration of TGF-1β is associated with a higher risk of diseases such as heart failure, lung cancer and metastatic prostate cancer in adults [13, 17, 43]. It can also exhibit differing roles in normal compared to diseased tissues. In normal breast tissue it is a potent inhibitor of proliferation, but TGF-1β appears to promote proliferation during the development of breast cancer [34].
Dehydroepiandrosterone and its more stable sulfate ester, DHEA-S, are mainly produced by the zona reticularis in the adrenal cortex. They are an important precursor of sex steroids, and consequently, adrenal production increases during adrenarche [14, 25, 32, 42]. In people, maximal serum DHEA-S concentrations are reached in the thirties then begin to decline considerably, such that by 70 years of age, DHEA-S concentrations are only approximately 20% of their peak values [14, 25, 32, 42]. In addition to its role as a sex steroid precursor, DHEA-S also functions as an important regulator of the immune system. For example, studies in vitro and in vivo found that treatment with DHEA-S increased mitogenic responses by T and B lymphocytes and cytotoxicity of natural killer cells [21, 45]. High doses of DHEA can have both immune stimulatory and anti-glucocorticoid effects in vitro and in vivo [6, 14]. Exogenous DHEA-S can also exhibit either anti- or pro-oxidant effects, depending on the dose and on tissue specificity. In the cardiovascular system, for instance, its main effects appear to be anti-oxidant [42].
The growth hormone–IGF-1 axis has been known to be essential for body growth and maintenance for decades. It is necessary for both the transition of fetus to neonate and for continued normal development after birth. Insulin-like growth factor-1 knockout mice are infertile and have considerably impaired growth [36, 40]. In people, the concentration of serum IGF-1 peaks around the peri-pubertal period and then begins to continuously decline after adulthood [2, 10, 46]. Like TGF-1β and DHEA-S, IGF-1 also affects immune function and the aging process. In vitro, treatment with IGF-1 increased the production of immunoglobulin-E (Ig) and IgG4, and enhanced expression of type II IgE receptors on human B cells [22, 23]. Its major binding protein in blood, IGFBP-3, aids in the transport and modulation of circulating IGFs and is itself a negative acute phase protein. In rodents, the administration of lipopolysaccharide endotoxin decreases both IGFBP-3 in serum and its synthesis in the liver [39]. There is also evidence that IGFBP-3 has potent anti-proliferative and pro-apoptotic effects independent of IGFs. These effects may be important in tumor development and progression [3, 7].
Although aging may be studied in humans using clinical subjects, animal models for humans are highly beneficial by providing a greater supply of subjects and ease of manipulation. To date, the majority of general aging studies using animal models have been done in invertebrates and rodent species. However, large animal models offer distinct advantages over rodents and invertebrates due to a closer similarity in genetics and physiology to more complex mammals. Captive non-human primates (NHP) provide an excellent model for humans. In particular, baboons are an ideal model, as they share greater similarity in immune system than other old world monkeys, with four IgG antibody subclasses and they demonstrate similar vulnerability to pathogens as people [1, 20, 35, 44, 51]. However, beyond their use for specific disease models, little research has been done examining the general effects of age using the baboon model.
A previous study by our laboratory found that aged baboons showed age-associated inflammatory changes similar to what had been found in people and rodents [33]. In that study, we found that the serum aging biomarkers, C-reactive protein, IL-6, and IL-6:IL-10 ratio increased with age in captive baboons. Furthermore, cytokine response of peripheral blood mononuclear cells and in whole blood increased in an age-dependent manner in baboons [33]. Therefore in the present study, we wanted to further examine the effects of aging in baboons by determining the age dependent changes in serum concentration of the aging biomarkers TGF-1β, DHEA-S and IGF-1.
2. Materials and Methods
2.1 Animals for population study and sample collection
All captive baboons were held at the Baboon Research Resources, Department of Comparative Medicine, University of Oklahoma Health Sciences Center (BRR-OUHSC). Animals were housed outdoors in hierarchical troops of approximately 60 to 80 animals per troop and were fed a commercial diet of monkey chow and fresh fruits and vegetables. Water was provided ad libitum. Saphenous venous blood was collected during routine annual health checks from 113-118 captive baboons. Coagulated blood was centrifuged at 500 g for 15 minutes and serum was collected and stored on ice until transported to the laboratory. Samples were then frozen at −80° C until analysis. Ninety three to 96 females and 20 to 22 males ranging in age from 2 to 26.7 years old were included in the study. The mean age of the study groups was 12 years. Table 1A provides a summary of the demographic information for the animals included in the population study. All procedures with animals were approved by the University of Oklahoma's Animal Care and Use Committee.
Table 1.
Summary of the demographic information for the population (A) and longitudinal (B) study animals.
| A. Population Study | Total Animal Number | Total Female Number | Total Male Number | Range of Animal Ages | Mean Animal Age |
| n = 113-118 | n = 93-96 | n = 20-22 | 2-26.7 yrs1 | 12 yrs1 | |
| Age Group Distributions | All Animals | Less than 9 yrs1 | 9-17 yrs1 | Greater than 17 yrs1 | |
| n = 43 | n = 40 | n = 35 | |||
| Number of Females* | n = 31 | n = 34 | n = 31 | ||
| Number of Males* | n = 12 | n = 6 | n = 4 | ||
| B. Longitudinal Study | Total Animal Number | Range of Animal Ages | Mean Age at 1st Collection | Mean Age at 2nd Collection | Mean Difference Between Collections |
| n = 23-27 | 4.6 to 26.7 yrs1 | 9.3 ± 2.9 yrs1 | 20.3 ± 3.5 yrs1 | 10.71 ± 2.9 yrs1 |
Yrs = years
Male and female baboons showed a different response to age in the concentration of TGF-1β; therefore, serum TGF-1β concentration was analyzed separately by gender in the age group analysis.
2.2 Longitudinal Study in Adult Baboons
To determine the effects of age on TGF-1β, DHEA-S and IGF-1 within the same animal, multiple samples were collected overtime from the same individuals. Samples were collected at two different ages in 23-27 animals. All animal were considered adults and ranged in age from 4.6 to 26.7 years old when samples were collected. Baboons averaged 9.3 ± 2.9 years of age at first sample collection and 20.3 ± 3.5 years old at second sample collection, with a mean difference of 10.71 ± 2.9 years between collections. Archived and fresh samples were frozen and held at −80°C until analysis. A summary of the demographic information for the longitudinal study animals is included in Table 1B.
2.3 Radioimmunoassays and enzyme-immunoassays
DHEA-S was measured in serum using a commercial single-antibody radioimmunoassay (RIA) for human DHEA-S (Siemen's Coat-a-Count DHEA-Sulphate RIA kit, Siemens Medical Solutions USA, Inc., Los Angeles, CA). Assay sensitivity is 9 ng/ml and cross-reactivity of the antibody with DHEA, androsterone, androstenedione, estrone, progesterone, testosterone and 17β-estradiol is 100% 20%, 6%, 0.4%, 0.2%, < 0.1% and < 0.1%, respectively. TGF-1β was measured by a commercially available double-antibody sandwich enzyme-immunoassay (EIA) for human TGF-1β (R&D Systems, Minneapolis, MN). The assay sensitivity is 31.2 pg/mL and cross-reactivity of the antibody is 57% for TGF-β1.2, 0.96% for TGF-β3, and 0.15% for TGF-β2. Both the DHEA-S RIA and TGF-1β EIA were validated previously for baboons in our laboratory by demonstrating linearity of diluted baboon serum samples to the standard curve and by determining the degree of relatedness of measured concentration to the true concentration in samples spiked with a known amount of hormone [33].
Serum IGF-1 was measured using a commercial double-antibody sandwich immunoradiometric assay for human IGF-1 (Diagnostic Systems Laboratories, Inc., Webster, TX). In addition, IGFBP-3 was measured using Diagnostic Systems Laboratories’ double-antibody sandwich EIA for human IGFBP-3 in a subset of samples (n=54), as the assay was discontinued while in the midst of the study. Due to low serum volumes, we could not repeat measurement of IGFBP-3 with a different assay. Assay sensitivity is 2.06 ng/ml and 0.04 ng/ml for the IGF-1 and IGFBP-3 assays, respectively. No cross-reactivity of similar peptides was detected for either antibody. Diagnostic Systems Laboratory's assay kits for human IGF-1 and IGFBP-3 have been validated for and used previously in numerous non-human primate species, including baboons [29, 47-50]. In addition, our laboratory ensured the validation of both assays for baboons by demonstrating parallelism of diluted baboon samples to the standard curve.
2.4 Statistical Analyses
Data were tested for normality prior to analyses and were log-transformed (DHEA-S) or rank-transformed (age), as needed. To determine the effects of age and gender on serum TGF-1β, DHEA-S, IGF-1 and IGFBP3, backward pairwise regression was performed followed by linear regression with the significant factors. Differences between mean concentrations were analyzed by ANOVA after being tested for both normality and homogeneity of variance. Tukey's HSD was used for multiple comparisons and Student's t tests were used for pairs of means. Welch ANOVA followed by Kruskal-Wallis rank sum test was used for non-parametric data, when no transformation was found suitable. Longitudinal data were compared by paired t tests. Values were considered significant at P ≤ 0.05. All statistical analyses were performed using JMP 7 Statistical Discovery (SAS, Cary, NC).
3. Results
3.1 The effects of age and gender on serum TGF-1β and DHEA-S concentration in baboons
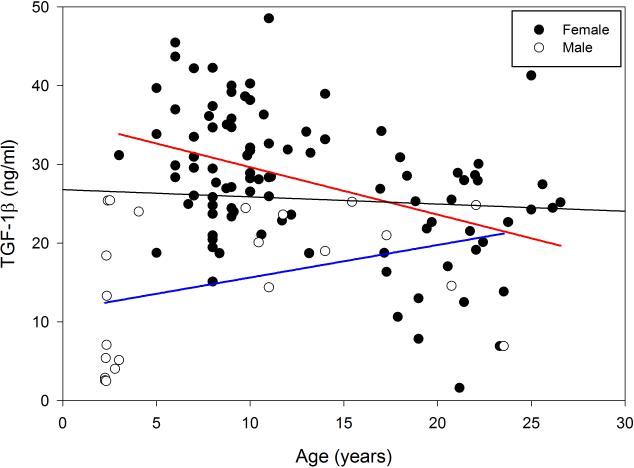
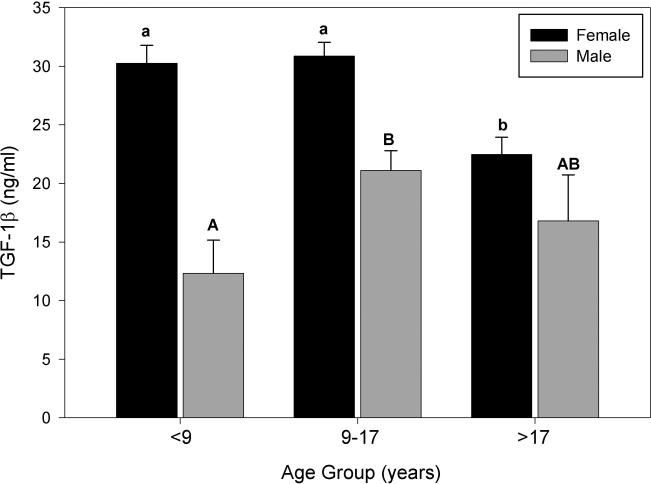
Both age and gender influenced serum TGF-1β concentration in baboons (Figure 1). When mean concentration of TGF-1β was examined by both gender and age, male and female baboons showed a different response to age (Figure 2). Therefore, an analysis of TGF-1β concentration from all animals together by age groups was not included. In female baboons, although the intermediate age group showed the highest mean concentration of TGF-1β it was not significantly different than the youngest group (means = 30.24 ng/ml for females less than 9 years old and 31.26 ng/ml for ages 9-17). However, a reduction of up to 25.9% was observed in serum TGF-1β in the over 17 age group, compared to the younger age groups (Figure 2). In contrast to what was observed in females, TGF-1β concentration in male baboons was greatest in the intermediate age group (9-17 years old) peaking 46.4% above that observed in the youngest animals. However, by the time males had reached 17 years old, the concentrations began to return back to what was observed in young males (means = 11.32 ng/ml for males less than 9 years old and 16.80 ng/ml for males over 17 years of age; Figure 2). Due to the considerably fewer number of males than females per age group, a comparison between males and females at each age was not performed.
Figure 1.
The effects of age and gender on serum TGF-1β concentration (ng/ml) in baboons. Distribution of serum TGF-1β in individual baboons of different ages (n = 118). Both age (p < 0.05) and gender (p < 0.01) were correlated to serum TGF-1β concentration. Female baboons are depicted in solid circles and male baboons are depicted in open circles. The black line represents the linear regression results for all individuals, while the blue line represents males and the red line represents females.
Figure 2.
Comparison of the mean serum TGF-1β concentration (ng/ml) in baboons by gender and age group (<9 yrs, n = 31 females, 12 males; 9-17 yrs, n = 34 females, 6 males; >17 yrs, n = 31 females, 4 males). Different lower case letters represent differences between female age groups and different upper case letters represent differences between male age groups. Due to the lower number of males in each age group, a comparison of the mean levels of serum TGF-1β between females and males within the same age group was not included. Data are expressed as means ± SEM. Means with no common letters differ significantly, P ≤ 0.05.
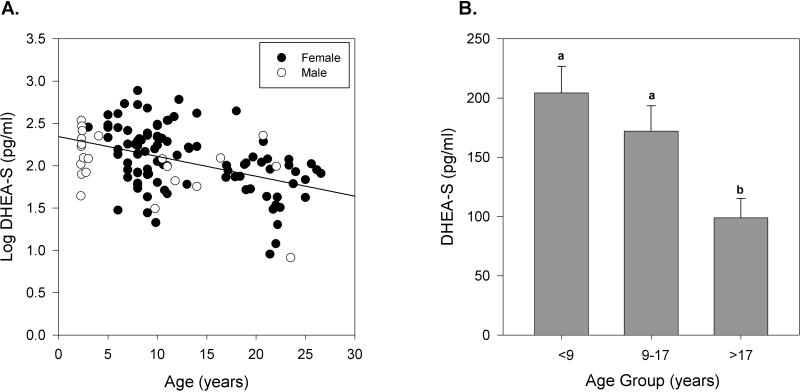
Although serum DHEA-S was negatively correlated with age (r = −0.4, P < 0.01), there was no effect of gender (Figure 3A). Furthermore when mean concentration was examined by age group, a steady decrease in serum DHEA-S was observed. In baboons greater than 17 years old, mean serum DHEA-S concentration decreased by as much as 2.1-fold (51.6%), when compared to younger animals (Figure 3B).
Figure 3.
The effects of age and gender on serum DHEA-S concentration (pg/ml) in baboons. A. Distribution of serum DHEA-S (log-transformed) in individual baboons of different ages (n = 118). Age (P < 0.01) but not gender was significantly correlated to serum DHEA-S concentration within this population of baboons. Female baboons are depicted in solid circles and male baboons in open circles. The black line represents the linear regression results for all individuals. B. Comparison of mean serum DHEA-S (pg/ml) in all baboons by age group (<9 yrs, n = 43; 9-17 yrs, n = 40; >17 yrs, n = 35). Data are expressed as means ± SEM of the non-transformed values. Means with no common letters differ significantly, P ≤ 0.05.
3.2 The effects of age and gender on serum IGF-1 and IGFBP-3 concentration in baboons
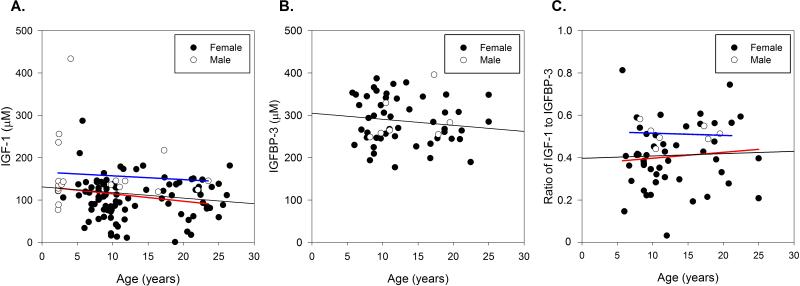
Both age and gender were significant factors in predicting serum IGF-1 concentration, with decreased IGF-1 observed with increasing age (r = −0.2, P < 0.05) and a greater concentration of IGF-1 in males compared to females (Figure 4A). In the whole population, serum IGF-1 concentration in females was reduced by 30% compared to males. However, serum IGFBP-3, measured in a subset of animals (mean age: 12 yrs, ranged 5.7 to 25 yrs), was not correlated with age or gender (Figure 4B). To evaluate the effect of age and gender on biologically available or “free” IGF-1, the ratio of serum IGF-1 to IGFBP-3 was assessed. Similar to what was found with IGF-1, the ratio of IGF-1 to IGFBP-3 was 22% lower in females than in male baboons (Figure 4C), however no effect of age on IGF-1 to IGFBP-3 ratio with this animal subset.
Figure 4.
The effect of age and gender on serum IGF-1 concentration (μM) in all baboons (Panel A, n = 113 total) and on serum IGFBP-3 concentration (μM) and ratio of IGF-1 to IGFBP-3 (n=54) in a subset of baboons (Panels B and C, n = 54). A. Serum IGF-1 concentration decreased with age (P < 0.05), with a greater concentration in male baboons (P < 0.05). B. Serum IGFBP-3 was not correlated with age or gender within this population. C. The ratio of IGF-1 to IGFBP-3 was only correlated with gender in the subset, with males having an increased ratio (P = 0.05). Female baboons are depicted in solid circles and male baboons in open circles. The black line represents the linear regression results for all individuals, while the blue line represents males and the red line represents females.
3.3 Longitudinal changes in serum TGF-1β, DHEA-S and IGF-1 in aging adult baboons
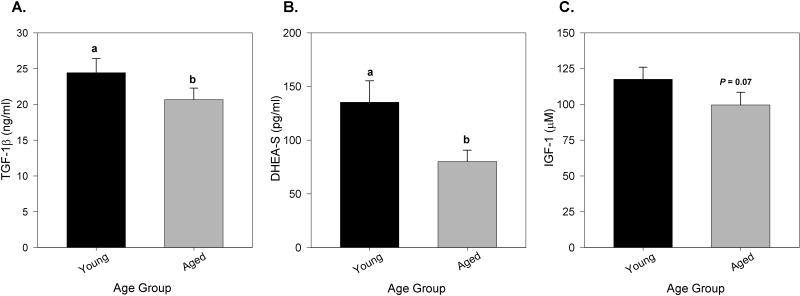
To determine the age-related changes in individual animals, serum concentration of TGF-1β, DHEA-S and IGF-1 were measured in paired samples collected, on average, 10.71 ± 2.9 years apart in the same individuals. Mean serum concentration of TGF-1β and DHEA-S were 15.4% and 46.1% lower, respectively, with age (Figure 5A and B). Although serum IGF-1 concentration also decreased with age in paired samples from the same individuals this failed to reach significance (P = 0.07; Figure 5C).
Figure 5.
Serum TGF-1β (ng/ml), DHEA-S (pg/ml) and IGF-1 (μM) concentration over time in paired samples from individual adult baboons (n = 23-27). Panels A and B. Serum TGF-1β and DHEA-S decreased by 15.4% and 46.1%, respectively, with increasing age when paired samples from individual animals were assessed (paired t-test, P ≤ 0.05). C. A trend toward decreased serum IGF-1 was observed with age in paired samples P = 0.07). Black bars represent baboons when young (mean age =9.3 ± 2.9 yrs) and gray bars represent the same baboons when aged (mean age= 20.3 ± 3.5 yrs). Different lower case letters represent differences between the young- and aged-adult groups. Data are expressed as means ± SEM.
4. Discussion
Little is known about the aging process in baboons and the effect aging has on factors that impact risk of age-associated diseases in this species. The development of baboons as an animal model for human aging has the potential to be highly beneficial in understanding this complex process more in depth. Baboons have a shorter life span than humans and can be more easily studied throughout their life, their environment can be more easily controlled, and they offer easier manipulation of research parameters. Although baboons have been used very little in aging research, they have been used as models for many specific human diseases and pathologies, and for the development of other research methodologies, like xenotransplantation. Thus, there is abundant knowledge about this species and there are good husbandry practices for maintaining healthy baboon colonies. Therefore, our first step towards developing this model is to determine the normal effects of aging on this species.
In the baboon population study, we found that serum TGF-1β changed with both gender and age. Different temporal patterns of change were found when data were analyzed by gender. In females only, TGF-1β was reduced in the aged animals compared to younger animals; while in males, the concentration was highest in adulthood (9-17 years) and then began to decline again with age (Figure 2). Moreover, when longitudinal samples collected from both female and male adults were examined, a significant decrease in TGF-1β was appreciated (Figure 5A).
The results of our study are comparable to those reported in aging humans. Although there is not a single study that examines age as a continuum, when the studies are considered together, previous research suggests that serum concentration of TGF-1β declines with age until individuals reach an extreme old age of approximately 100 years [8, 31, 37]. One study examined the concentration of TGF-1β in a broader age group of 25-80 years old in Chinese women [53]. The authors found a steady decrease in TGF-1β and when the data were analyzed relative to menopause age, post-menopausal women had lower concentrations of TGF-1β than did pre- and peri-menopausal women [53]. There is also other evidence that reproductive hormones help to regulate TGF-1β. Increases in mRNA and protein for TGF-1β were found in bone samples obtained from women who had been on estrogen hormone replacement therapy for 15 years when compared to controls [5]. Furthermore, ovarian hormones help modulate the effects of TGF-1β in breast tissue [12] while androgens can inhibit TGF-1β in normal tissues [11, 24]. Consequently, it may be appropriate that there are gender differences in the concentration of and/or in the temporal patterns of change in this factor. However, little research has be done examining the changes in circulating concentration as a function of and by gender in humans and thus, more research will be needed to examine the effects of gender more closely in both people and baboons.
Human studies also indicate that TGF-1β increases again when extreme old age is reached [8]; unfortunately, there were only a limited number of extremely geriatric animals in the colony so we were unable to determine if this occurs in baboons. Previous research examining human diseases associated with age has shown that a greater concentration of TGF-1β is associated with a higher risk of disease. Higher plasma TGF-1β concentration was associated with an increased risk of heart failure among older people [13], while increased concentrations were also found in lung cancer patients and in metastatic prostate cancer patients [17, 43]. The complex actions of TGF-1β on the development and progression of disease in the elderly are still being studied.
Similar to what has been widely reported in people, we found the adrenal steroid DHEA-S decreased in baboons with age independent of gender in both the population cross-sectional study and the longitudinal study (Figures 3A, 3B and 5B). In contrast to what we observed in baboons, men were shown to have a higher concentration of DHEA-S in serum than women [25, 42]. It is important to consider that although we did not find gender differences in the baboon, our population was biased towards females and the small sample size of males could obscure a difference. For example, Young et al., found gender differences in the correlation between serum DHEA-S concentration and age using 412 men and 395 women [52]. A power analysis based on our results indicated that at least 35 male baboons would be needed for an 80% likelihood of determining a gender difference in the concentration of serum DHEA-S. Thus, the addition of more male baboons would allow for the direct comparison between genders and more definitively determine the gender effects for this hormone in baboons.
A decrease in DHEA/DHEA-S concentration is thought to play a major role in the age related decline in immune system function. It also has utility as a biomarker of aging. A recent longitudinal study with both men and women found that low serum DHEA-S concentration correlated to increased frailty among individuals 10 years later [4]. In addition, low DHEA-S has been associated with many age-related conditions such as muscle loss, cardiovascular disease, poor cognitive performance and an increased overall risk of death. In women specifically, DHEA and DHEA-S are the main sources of sex steroid hormones after menopause, and it is thought that low concentrations in women may be associated with post-menopause diseases like osteoporosis, muscle loss and fat accumulation, hot flashes, type 2 diabetes, sexual dysfunction, memory and cognition loss and, potentially, even Alzheimer's disease [25]. As further data is accumulated, it will be interesting to examine the utility of this hormone in predicting disease in aged baboons as well.
Similar to DHEA-S, IGF-1 declines with age in people reaching its nadir at approximately 60 years of age [18]. Likewise, we also found that serum IGF-1 was negatively correlated with age in the baboon population and within the same individual (Figures 4A and 5C). The biological effects of IGF-1 are strictly controlled by its association with binding proteins. Some gender differences have been reported for both IGF-1 and its main binding protein in blood, IGFBP-3, though the results are inconsistent. Kaklamani et al. found that the concentration of IGF-1 was higher, while IGFBP-3 was lower in men compared to women, suggesting that men have higher free IGF-1 [19]. Landin-Wilhelmsen et al. observed higher concentrations of IGF-1 in young women compared to men but men showed higher concentrations than women when aged [26]. In contrast to both of these reports, another study indicated that there was no difference in IGF-I values between men and women. Furthermore, these authors found that IGFBP-3 was not correlated with age but that women had greater overall IGFBP-3, suggesting, again, that men have more free IGF-1 [30]. We found that male baboons had a greater concentration of IGF-1 (Figures 4A), while no effects of age or gender were observed in serum IGFBP-3 (Figures 4B). While these results suggest that male baboons have more free IGF-1 compared to females (Figure 4C), results should be interpreted cautiously until a larger sample size of male baboons is available for IGFBP-3 assessment.
The reasons for a gender difference in the concentration of serum IGF-1 or in free IGF-1 is not yet well understood. However, differences in IGF-1 concentrations may be due to differences in the pattern of growth hormone secretion between genders. Growth hormone is a major regulator of serum IGF-1. Studies examining growth hormone in people and in rodents found that females show a more disorderly pattern of growth hormone secretion than do males [16, 38]. The increasing disorder of growth hormone secretion in women has been associated with lower concentrations of serum IGF-I [16]. As stated previously, IGF-1 has essential roles in promoting growth and mitogenesis, and mediates the effects of growth hormone through feedback in the growth hormone/IGF-1 axis. Insulin-like growth factor-1 can also have multiple effects on immune cell types and is a neurotrophic hormone, contributing to information processing. There is substantial evidence that IGF-1 deficiency is a contributing factor for reduced cognitive abilities in both adults and aged humans, and in rodent models of aging [10]. It has implications in age-related diseases like Alzheimer's and atherosclerosis [10, 15, 40]. Furthermore, low concentrations of serum IGF-1 are associated with reduced bone remodeling and preservation of bone strength during aging [9].
The results presented herein further validate the use of baboons as an appropriate model for human aging. However, future studies are needed to more closely determine the effects of gender, the temporal patterns of change during extreme old age, and the influence of these factors on age-related diseases in baboons in order to clarify the roles of these factors in the aging process. We then can begin to examine more in depth how these factors (as well as others) may contribute to diseases and pathologies associated with age in an in vivo system.
Acknowledgements
The authors wish to thank the animal veterinarians, technicians and animal care workers at the University of Oklahoma Health Sciences Center's Baboon Research Resources who assisted in the organization and collection of samples. The authors also thank K. A. McDaniel and K. Hill for their assistance with enzyme- and radioimmunoassays. This study was supported by the National Institutes of Health, under award number K01RR023946 (McFarlane). The University of Oklahoma Health Sciences Center's Baboon Resources were supported by the Office of the Director, National Institutes of Health, under award numbers P40OD010431 for Specific Pathogen Free animals and P40OD010988 for Conventional animals (Wolf and White).
Abbreviations
- TGF-1β
Transforming growth factor-1beta
- DHEA
Dehydroepiandrosterone
- DHEA-S
Dehydroepiandrosterone sulfate
- IGF-1
Insulin like growth factor-1
- IGFBP-3
Insulin like growth factor binding protein-3
- IL
Interleukin
- Ig
Immunoglobulin
References
- 1.Attanasio R, Jayashankar L, Engleman CN, Scinicariello F. Baboon immunoglobulin constant region heavy chains: identification of four IGHG genes. Immunogenetics. 2002;54:556–561. doi: 10.1007/s00251-002-0505-1. [DOI] [PubMed] [Google Scholar]
- 2.Bartke A. Growth hormone and aging: a challenging controversy. Clinical interventions in aging. 2008;3:659–665. doi: 10.2147/cia.s3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter RC, Butt AJ, Schedlich LJ, Martin JL. Antiproliferative and pro apoptotic activities of insulin-like growth factor-binding protein-3. Growth Horm IGF Res. 2000;10(Suppl A):S10–11. doi: 10.1016/s1096-6374(00)90004-2. [DOI] [PubMed] [Google Scholar]
- 4.Baylis D, Bartlett DB, Syddall HE, Ntani G, Gale CR, Cooper C, et al. Immune-endocrine biomarkers as predictors of frailty and mortality: a 10-year longitudinal study in community-dwelling older people. Age (Dordrecht, Netherlands) 2013;35:963–971. doi: 10.1007/s11357-012-9396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bord S, Beavan S, Ireland D, Horner A, Compston JE. Mechanisms by which high-dose estrogen therapy produces anabolic skeletal effects in postmenopausal women: role of locally produced growth factors. Bone. 2001;29:216–222. doi: 10.1016/s8756-3282(01)00501-4. [DOI] [PubMed] [Google Scholar]
- 6.Buford TW, Willoughby DS. Impact of DHEA(S) and cortisol on immune function in aging: a brief review. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2008;33:429–433. doi: 10.1139/H08-013. [DOI] [PubMed] [Google Scholar]
- 7.Butt AJ, Williams AC. IGFBP-3 and apoptosis--a license to kill? Apoptosis. 2001;6:199–205. doi: 10.1023/a:1011388710719. [DOI] [PubMed] [Google Scholar]
- 8.Carrieri G, Marzi E, Olivieri F, Marchegiani F, Cavallone L, Cardelli M, et al. The G/C915 polymorphism of transforming growth factor beta1 is associated with human longevity: a study in Italian centenarians. Aging cell. 2004;3:443–448. doi: 10.1111/j.1474-9728.2004.00129.x. [DOI] [PubMed] [Google Scholar]
- 9.Courtland HW, Kennedy OD, Wu Y, Gao Y, Sun H, Schaffler MB, et al. Low levels of plasma IGF-1 inhibit intracortical bone remodeling during aging. Age (Dordrecht, Netherlands) 2012 doi: 10.1007/s11357-012-9469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deak F, Sonntag WE. Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1. The journals of gerontology. 2012;67:611–625. doi: 10.1093/gerona/gls118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. The Prostate. 1996;28:251–265. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Ewan KB, Shyamala G, Ravani SA, Tang Y, Akhurst R, Wakefield L, et al. Latent transforming growth factor-beta activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. The American journal of pathology. 2002;160:2081–2093. doi: 10.1016/s0002-9440(10)61158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glazer NL, Macy EM, Lumley T, Smith NL, Reiner AP, Psaty BM, et al. Transforming growth factor beta-1 and incidence of heart failure in older adults: the Cardiovascular Health Study. Cytokine. 2012;60:341–345. doi: 10.1016/j.cyto.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazeldine J, Arlt W, Lord JM. Dehydroepiandrosterone as a regulator of immune cell function. The Journal of steroid biochemistry and molecular biology. 2010;120:127–136. doi: 10.1016/j.jsbmb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. The journals of gerontology. 2012;67:626–639. doi: 10.1093/gerona/gls102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hindmarsh PC, Dennison E, Pincus SM, Cooper C, Fall CH, Matthews DR, et al. A sexually dimorphic pattern of growth hormone secretion in the elderly. The Journal of clinical endocrinology and metabolism. 1999;84:2679–2685. doi: 10.1210/jcem.84.8.5915. [DOI] [PubMed] [Google Scholar]
- 17.Hou YL, Chen H, Dong ZH, Xue CJ, Wu YF, Luo HX, et al. Clinical significance of serum transforming growth factor-beta1 in lung cancer. Cancer epidemiology. 2013 doi: 10.1016/j.canep.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Junnila RK, List EO, Berryman DE, Murrey JW, Kopchick JJ. The GH/IGF-1 axis in ageing and longevity. Nature reviews. 2013;9:366–376. doi: 10.1038/nrendo.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaklamani VG, Linos A, Kaklamani E, Markaki I, Mantzoros C. Age, sex, and smoking are predictors of circulating insulin-like growth factor 1 and insulin-like growth factor-binding protein 3. J Clin Oncol. 1999;17:813–817. doi: 10.1200/JCO.1999.17.3.813. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy RC, Shearer MH, Hildebrand W. Nonhuman primate models to evaluate vaccine safety and immunogenicity. Vaccine. 1997;15:903–908. doi: 10.1016/s0264-410x(96)00277-0. [DOI] [PubMed] [Google Scholar]
- 21.Khorram O, Vu L, Yen SS. Activation of immune function by dehydroepiandrosterone (DHEA) in age-advanced men. The journals of gerontology. 1997;52:M1–7. doi: 10.1093/gerona/52a.1.m1. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Park HH, Lee CE. IGF-1 potentiation of IL-4-induced CD23/Fc(epsilon)RII expression in human B cells. Molecules and cells. 2003;15:307–312. [PubMed] [Google Scholar]
- 23.Kimata H, Fujimoto M. Growth hormone and insulin-like growth factor I induce immunoglobulin (Ig)E and IgG4 production by human B cells. The Journal of experimental medicine. 1994;180:727–732. doi: 10.1084/jem.180.2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyprianou N, Isaacs JT. Expression of transforming growth factor-beta in the rat ventral prostate during castration-induced programmed cell death. Molecular endocrinology (Baltimore, Md. 1989;3:1515–1522. doi: 10.1210/mend-3-10-1515. [DOI] [PubMed] [Google Scholar]
- 25.Labrie F. DHEA, important source of sex steroids in men and even more in women. Progress in brain research. 2010;182:97–148. doi: 10.1016/S0079-6123(10)82004-7. [DOI] [PubMed] [Google Scholar]
- 26.Landin-Wilhelmsen K, Lundberg PA, Lappas G, Wilhelmsen L. Insulin-like growth factor I levels in healthy adults. Hormone research. 2004;62(Suppl 1):8–16. doi: 10.1159/000080753. [DOI] [PubMed] [Google Scholar]
- 27.Letterio JJ, Roberts AB. Transforming growth factor-beta1-deficient mice: identification of isoform-specific activities in vivo. Journal of leukocyte biology. 1996;59:769–774. doi: 10.1002/jlb.59.6.769. [DOI] [PubMed] [Google Scholar]
- 28.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annual review of immunology. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Levitz M, Hubbard GB, Jenkins SL, Han V, Ferry RJ, Jr., et al. The IGF axis in baboon pregnancy: placental and systemic responses to feeding 70% global ad libitum diet. Placenta. 2007;28:1200–1210. doi: 10.1016/j.placenta.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin CM, Huang YL, Lin ZY. Influence of gender on serum growth hormone, insulin-like growth factor-I and its binding protein-3 during aging. Yonsei medical journal. 2009;50:407–413. doi: 10.3349/ymj.2009.50.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Y, Nakachi K, Ito Y, Kikuchi S, Tamakoshi A, Yagyu K, et al. Variations in serum transforming growth factor-beta1 levels with gender, age and lifestyle factors of healthy Japanese adults. Disease markers. 2009;27:23–28. doi: 10.3233/DMA-2009-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maninger N, Wolkowitz OM, Reus VI, Epel ES, Mellon SH. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Frontiers in neuroendocrinology. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McFarlane D, Wolf RF, McDaniel KA, White GL. Age-associated alteration in innate immune response in captive baboons. The journals of gerontology. 2011;66:1309–1317. doi: 10.1093/gerona/glr146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moses H, Barcellos-Hoff MH. TGF-beta biology in mammary development and breast cancer. Cold Spring Harbor perspectives in biology. 2011;3:a003277. doi: 10.1101/cshperspect.a003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murthy KK, Salas MT, Carey KD, Patterson JL. Baboon as a nonhuman primate model for vaccine studies. Vaccine. 2006;24:4622–4624. doi: 10.1016/j.vaccine.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 36.Oberbauer AM. The Regulation of IGF-1 Gene Transcription and Splicing during Development and Aging. Frontiers in endocrinology. 2013;4:39. doi: 10.3389/fendo.2013.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamoto Y, Gotoh Y, Uemura O, Tanaka S, Ando T, Nishida M. Age-dependent decrease in serum transforming growth factor (TGF)-beta 1 in healthy Japanese individuals; population study of serum TGF-beta 1 level in Japanese. Disease markers. 2005;21:71–74. doi: 10.1155/2005/381215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pincus SM, Gevers EF, Robinson IC, van den Berg G, Roelfsema F, Hartman ML, et al. Females secrete growth hormone with more process irregularity than males in both humans and rats. The American journal of physiology. 1996;270:E107–115. doi: 10.1152/ajpendo.1996.270.1.E107. [DOI] [PubMed] [Google Scholar]
- 39.Priego T, Ibanez de Caceres I, Martin AI, Villanua MA, Lopez-Calderon A. Endotoxin decreases serum IGFBP-3 and liver IGFBP-3 mRNA: comparison between Lewis and Wistar rats. Molecular and cellular endocrinology. 2003;199:23–28. doi: 10.1016/s0303-7207(02)00356-8. [DOI] [PubMed] [Google Scholar]
- 40.Puche JE, Castilla-Cortazar I. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. Journal of translational medicine. 2012;10:224. doi: 10.1186/1479-5876-10-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts AB, Anzano MA, Lamb LC, Smith JM, Sporn MB. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proceedings of the National Academy of Sciences of the United States of America. 1981;78:5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savineau JP, Marthan R, Dumas de la Roque E. Role of DHEA in cardiovascular diseases. Biochemical pharmacology. 2013;85:718–726. doi: 10.1016/j.bcp.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Shariat SF, Shalev M, Menesses-Diaz A, Kim IY, Kattan MW, Wheeler TM, et al. Preoperative plasma levels of transforming growth factor beta(1) (TGF-beta(1)) strongly predict progression in patients undergoing radical prostatectomy. J Clin Oncol. 2001;19:2856–2864. doi: 10.1200/JCO.2001.19.11.2856. [DOI] [PubMed] [Google Scholar]
- 44.Shearer MH, Dark RD, Chodosh J, Kennedy RC. Comparison and characterization of immunoglobulin G subclasses among primate species. Clinical and diagnostic laboratory immunology. 1999;6:953–958. doi: 10.1128/cdli.6.6.953-958.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solerte SB, Fioravanti M, Vignati G, Giustina A, Cravello L, Ferrari E. Dehydroepiandrosterone sulfate enhances natural killer cell cytotoxicity in humans via locally generated immunoreactive insulin-like growth factor I. The Journal of clinical endocrinology and metabolism. 1999;84:3260–3267. doi: 10.1210/jcem.84.9.6003. [DOI] [PubMed] [Google Scholar]
- 46.Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing research reviews. 2005;4:195–212. doi: 10.1016/j.arr.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Tarantal AF, Castillo A, Ekert JE, Bischofberger N, Martin RB. Fetal and maternal outcome after administration of tenofovir to gravid rhesus monkeys (Macaca mulatta). Journal of acquired immune deficiency syndromes (1999) 2002;29:207–220. doi: 10.1097/00042560-200203010-00001. [DOI] [PubMed] [Google Scholar]
- 48.Ulijaszek SJ. Serum insulin-like growth factor-I, insulin-like growth factor binding protein-3, and the pubertal growth spurt in the female rhesus monkey. American journal of physical anthropology. 2002;118:77–85. doi: 10.1002/ajpa.10072. [DOI] [PubMed] [Google Scholar]
- 49.Wang XL, Wang J, Rainwater DL. Acute effects of insulin-like growth factor-1 and recombinant growth hormone on liporotein(a) levels in baboons. Metabolism: clinical and experimental. 2002;51:508–513. doi: 10.1053/meta.2002.31328. [DOI] [PubMed] [Google Scholar]
- 50.Wilson ME. Insulin-like growth factor I (IGF-I) replacement during growth hormone receptor antagonism normalizes serum IGF-binding protein-3 and markers of bone formation in ovariectomized rhesus monkeys. The Journal of clinical endocrinology and metabolism. 2000;85:1557–1562. doi: 10.1210/jcem.85.4.6522. [DOI] [PubMed] [Google Scholar]
- 51.Wolf RF, Papin JF, Hines-Boykin R, Chavez-Suarez M, White GL, Sakalian M, et al. Baboon model for West Nile virus infection and vaccine evaluation. Virology. 2006;355:44–51. doi: 10.1016/j.virol.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 52.Young DG, Skibinski G, Mason JI, James K. The influence of age and gender on serum dehydroepiandrosterone sulphate (DHEA-S), IL-6, IL-6 soluble receptor (IL-6 sR) and transforming growth factor beta 1 (TGF-beta1) levels in normal healthy blood donors. Clinical and experimental immunology. 1999;117:476–481. doi: 10.1046/j.1365-2249.1999.01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang N, Wu XY, Wu XP, Fu XH, Du XY, Xie H, et al. Relationship between age-related serum concentrations of TGF-beta1 and TGF-beta2 and those of osteoprotegerin and leptin in native Chinese women. Clinica chimica acta; international journal of clinical chemistry. 2009;403:63–69. doi: 10.1016/j.cca.2009.01.021. [DOI] [PubMed] [Google Scholar]