Abstract
Rationale
Developing models to efficiently explore the mechanisms by which stress can mediate reinstatement of drug-seeking behavior is crucial to the development of new pharmacotherapies for alcohol use disorders.
Objectives
We examined the effects of multiple reinstatement sessions using the pharmacological stressor, yohimbine, in ethanol- and sucrose-seeking rats in order to develop a more efficient model of stress-induced reinstatement.
Methods
Long–Evans rats were trained to self-administer 10% ethanol with a sucrose-fading procedure, 20% ethanol without a sucrose-fading procedure, or 5% sucrose in 30-min operant self-administration sessions, followed by extinction training. After reaching extinction criteria, the animals were tested once per week with yohimbine vehicle and yohimbine (2 mg/kg), respectively, 30 min prior to the reinstatement sessions or blood collection. Levels of reinstatement and plasma corticosterone (CORT) were determined each week for four consecutive weeks.
Results
Yohimbine induced reinstatement of ethanol- and sucrose-seeking in each of the 4 weeks. Interestingly, the magnitude of the reinstatement decreased for the 10% ethanol group after the first reinstatement session but remained stable for the 20% ethanol group trained without sucrose. Plasma CORT levels in response to injection of both vehicle and yohimbine were significantly higher in the ethanol-trained animals compared to sucrose controls.
Conclusions
The stable reinstatement in the 20% ethanol group supports the use of this training procedure in studies using within-subject designs with multiple yohimbine reinstatement test sessions. Additionally, these results indicate that the hormonal response to stressors can be altered following extinction from self-administration of relatively modest amounts of ethanol.
Keywords: Ethanol, Self-administration, Corticosterone, Stress, Alcohol, Animal model, Behavior, Relapse, Extinction, Glucocorticoid
Introduction
A critical need in the treatment of alcohol use disorders (AUDs) is the development of medications to prevent relapse to alcohol seeking. The high rate of recidivism is a major problem in treating the disease. Stress has long been established to play a critical role in mediating pathological ethanol intake and relapse in both humans and laboratory animals (Brown et al. 1995; Cooper et al. 1992; Sinha 2001). Chronic ethanol intake has been proposed to lead to dysregulation of stress hormone systems inducing neuro-adaptations that increase susceptibility to the development of AUDs [for reviews, see Koob (2008) and Shaham and Hope (2005)]. The major stress hormones that are altered by chronic ethanol exposure are the glucocorticoids [cortisol in humans and corticosterone (CORT) in rodents]. In humans, cortisol has been shown to be elevated following chronic ethanol exposure and in early withdrawal, and although levels normalize during protracted abstinence, the hormonal response to a stress challenge is reduced (Adinoff et al. 1998; Heinz et al. 1999; Lovallo et al. 2000; Prendergast and Little 2007). Similarly, in rodents, it has been shown that circulating CORT levels are elevated in early withdrawal from long-term forced ethanol exposure and quickly returns to baseline; however, brain CORT levels remain elevated in the hypothalamus, hippocampus, midbrain, prefrontal cortex, cortex, and striatum for at least 2 months following removal of the ethanol, and this elevation has been postulated to increase vulnerability to relapse (Little et al. 2008).
The protocol developed for studying reinstatement of drug seeking in animals has been shown to have validity for the study of relapse to drug addiction in humans (Epstein et al. 2006; Katz and Higgins 2003; Shaham et al. 2003; Spanagel 2003). Stress and re-exposure to cues or to the context previously associated with drug availability are common reasons for relapse to drug seeking in humans and induce reinstatement of drug seeking in rodents (Liu and Weiss 2003; Shaham et al. 2000; Zironi et al. 2006). A “stress response” is generally believed to involve the corticotropin releasing factor (CRF) system and activation of the hypothalamic–pituitary–adrenal (HPA) axis (for review see (Koob 1999). Footshock has been the most commonly used method of stress-induced reinstatement in rodents and has been shown to effectively reinstate heroin (Shaham et al. 1997), cocaine (Erb et al. 1998), nicotine (Buczek et al. 1999), and ethanol seeking (Le et al. 1998). However, it has recently been shown that the pharmacological stressor yohimbine is a viable alternative, not only in its ability to reinstate drug seeking but also in its effects on CRF production and activation of the same circuitry as footshock, including the nucleus accumbens, the basolateral and central amygdalar nuclei, and the dorsal bed of the stria terminalis (Funk et al. 2006). Yohimbine is an alkaloid that acts as an α-2 adrenoceptor antagonist, leading to an increase in circulating noradrenaline, which stimulates the sympathetic nervous system and CORT release and has been shown to produce anxiety-like states in both humans (Charney et al. 1983; Holmberg and Gershon 1961) and laboratory animals (Davis et al. 1979; Lang and Gershon 1963; Stine et al. 2002). This stress response has been shown to induce reinstatement of methamphetamine (Shepard et al. 2004), cocaine (Lee et al. 2004), heroin (Banna et al. 2010), palatable food (Ghitza et al. 2006), and ethanol seeking (Le et al. 2005; Simms et al. 2010). Studies exploring the effects of yohimbine on ethanol-seeking behavior have shown that several receptor systems can modulate this behavior including CRF (Marinelli et al. 2007), orexin (Richards et al. 2008), serotonin (Le et al. 2009a,b), neuropeptide Y (Cippitelli et al. 2010), peroxisome proliferator-activated receptor-gamma (Stopponi et al. 2011), adrenoreceptors (Le et al. 2011), and delta opioid receptors (Nielsen et al. 2011). Clinical research has shown that yohimbine increases anxiety and cortisol levels in patients recovering from AUDs (Krystal et al. 1994; Krystal et al. 1996). However, the CORT response to multiple yohimbine challenges in rats with a history of ethanol self-administration has yet to be measured against sucrose controls. The aim of the present study is to examine the behavioral and hormonal effects of yohimbine given across multiple reinstatement sessions.
Materials and methods
Subjects
Male, Long–Evans rats, weighing 150–180 g upon arrival (Harlan Indianapolis, IN, USA), were individually housed in ventilated Plexiglas cages with a single bottle grommet at the front end of the cage. Rats were housed in a climate controlled room on a 12 h light–dark cycle (lights on at 0700 hours). Operant conditioning sessions occurred Monday through Friday. Food and water were available ad libitum, except for short periods during initial training, as outlined below. All procedures were pre-approved by the Gallo Center Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health (NIH) guidelines for the Humane Care and Use of Laboratory Animals.
Drugs
Yohimbine (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in distilled water and administered intraperito-neally at a dose of 2 mg/kg in a volume of 0.5 ml/kg. Sucrose solutions, 5% or 10% (w/v) (Fisher Scientific, New Jersey, USA), were prepared using filtered tap water. The 10% ethanol (v/v) solution was prepared using 95% ethyl alcohol (Gold Shield Chemical Co., Hayward, CA, USA, DSP-CA-151) and filtered tap water. In the sucrose fade experiments, 10%, 5%, 3%, and 1.5% sucrose, respectively, were dissolved in 10% ethanol (w/v).
Operant self-administration apparatus
Self-administration testing was conducted in standard operant conditioning chambers (Coulbourn Instruments, Allentown, PA, USA). Details regarding the apparatus have been described extensively elsewhere (Richards et al. 2008; Steensland et al. 2007). Importantly, the Coulbourn chambers are equipped with lickometers that provide information about whether the animals are drinking the solution they are responding for, allowing for more accurate estimates of ethanol and sucrose intake (g/kg).
Ten percent ethanol self-administration with sucrose fade
Two groups of Long–Evans rats (n=12–14 per group) were initially exposed to 10% ethanol as the only liquid source in their home cages for 4 days. This forced ethanol exposure has been postulated to facilitate the initiation of ethanol self-administration by reducing the influence of neophobia for ethanol during operant training (Nadal et al. 2002). Following the fourth day of forced ethanol exposure, rats were placed in the operant conditioning chambers for a 14-h overnight session on a fixed ratio (FR) 1 schedule of reinforcement (0.1 ml reinforcer after a single lever press with a 30-s timeout period between reinforcer deliveries). The start of the training session was signaled by the illumination of the house light and extension of the active lever. During this phase, only the active lever was available for the rat to press to establish lever pressing behavior. Rats were trained to respond for 10% sucrose in overnight sessions (one to three nights) and continued on 10% sucrose until they reached the FR3 stage of training. Initial daily training consisted of 45-min FR1 sessions and 1-h daily water access, with water access immediately following the training sessions. Furthermore, the timeout period was reduced from 30 to 10 s between reinforcer deliveries for this stage of the training. Responding on the lever during the timeout period was recorded but did not count toward the next reinforcer delivery. Once responding was established (2–4 days), rats were given free access to water in the home cage and continued on a 45-min FR1 schedule for an additional 3–4 days. Subsequently, training sessions were reduced to 30 min, and the work ratio was increased to an FR3 schedule of reinforcement (three active lever presses required for 0.1 ml reinforcer with a 10-s timeout period between reinforcer deliveries). A second, inactive lever was also introduced at this time. Upon pressing the inactive lever, no reinforcer, visual (light), or auditory stimuli were presented, and the event was merely recorded as a measure of nonspecific behavioral activity. Again, responding on the active and inactive levers during the timeout period was recorded but did not count toward the next reinforcer delivery. Following three sessions of FR3 training with 10% sucrose as the reinforcer, a modified sucrose fade technique (Samson 1986) was initiated. Ten percent ethanol was added to the 10% sucrose solution, and over the next 12 sessions, the sucrose concentration was gradually decreased (10%, 5%, 3%, and 1.5%, respectively) until rats responded on an FR3 schedule for 10% ethanol alone. Rats continued on the FR3 protocol with 10% ethanol as the reinforcer for a minimum of 20 sessions. Any animals not reaching 0.3 g/kg ethanol intake per session were excluded from further study.
20% ethanol self-administration
Using a previously described method (Simms et al. 2010), daily 20% ethanol self-administration was initiated in two separate groups of Long–Evans rats (n=12–13 per group). Importantly, food and water were available ad libitum at all times in the home cage throughout the training. On the first day of training, animals were placed in the operant conditioning chambers for a 14-h overnight session on an FR1 schedule of reinforcement (0.1 ml after a single lever press) with 20% ethanol solution as the reinforcer. These FR1 overnight sessions were performed 5 days per week for a total of 12 sessions. During this phase, only the active lever was available for the rat to press to establish lever pressing behavior. Following the completion of these sessions, rats were then exposed to 45-min FR1 sessions for a total of six sessions. Subsequently, training sessions were reduced to 30 min, and the work ratio was increased to an FR3 schedule of reinforcement (three active lever presses required for 0.1 ml reinforcer). The second (inactive) lever was also introduced at this time. Upon pressing the inactive lever, no reinforcer, cue light, or auditory stimuli were presented, and the event was merely recorded as a measure of nonspecific behavior. Rats continued on the FR3 protocol with 20% ethanol as the reinforcer for a minimum of 20 sessions. Any animals not reaching 0.3 g/kg ethanol intake per session were excluded from further study.
Five percent sucrose self-administration
Two groups of Long–Evans rats (n=12–16 per group) were trained to self-administer sucrose using the protocol described above for 10% ethanol with 5% sucrose substituted as the reinforcer at each stage of the training. The training included four nights forced sucrose exposure in the home cage, one to three overnight sessions, two to four 45-min FR1 sessions with 1 h water access, three to four 45-min FR1 sessions with full water, and a minimum of 20 30-min FR3 sessions.
Extinction
In order to extinguish lever pressing, rats continued with daily operant conditioning sessions under FR3 conditions; however, active lever pressing did not result in reinforcer delivery despite light and tone cues still being presented. Ethanol (10% and 20%) and 5% sucrose were not available throughout extinction training. Extinction training continued until the rats responded with <10% of their baseline pressing on the active lever for two consecutive sessions, which took 22 sessions for the 10% ethanol and 5% sucrose groups and 16 sessions for the 20% ethanol group. At this time, yohimbine vehicle tests and yohimbine-stress-induced reinstatement tests were performed once per week with regular extinction sessions on non-reinstatement days.
Multiple yohimbine-induced reinstatements
Following extinction, one group of 10% ethanol-trained animals (n=15), one group of 20% ethanol-trained animals (n=13), and one group of sucrose-trained animals (n=16) were tested with the pharmacological stressor, yohimbine (2 mg/kg, i.p.), and its vehicle (distilled water) each week for four consecutive weeks. Thirty minutes following yohimbine or vehicle administration, a standard FR3 session was run with active and inactive levers available, accompanied by light and tone cues on successful FR3 responding. No reinforcer deliveries were presented during the reinstatement sessions. Vehicle injections were given on the day prior to yohimbine administration to allow for acclimatization to the injection procedure and to determine if the animals were sensitive to the vehicle itself. Yohimbine dose was determined in accordance with previous studies (Le et al. 2005; Simms et al. 2010). All rats received vehicle (distilled water) injections on Wednesday and yohimbine injections on Thursday of each reinstatement test week with regular extinction sessions run on each of the other days (i.e., Monday, Tuesday, and Friday).
Corticosterone measurements following administration of yohimbine
In order to obtain blood CORT measurements following yohimbine or vehicle treatment in extinction, one group of 10% ethanol-trained animals (n=12), one group of 20% ethanol-trained animals (n=12), and one group of sucrose-trained animals (n=12) each received either an i.p. yohimbine or vehicle injection once weekly for four consecutive weeks, each test 7 days apart. Due to high variability in the CORT measures for the 10% ethanol group in week 1, another group of 10% ethanol animals (n=12, divided into vehicle and yohimbine groups) was tested for CORT responses for this week only. Blood samples were collected on Thursday of each week with regular extinction sessions run on each of the other days (i.e., Monday, Tuesday, Wednesday, and Friday). Thirty minutes after the injection, the animals were anesthetized with isoflurane (Baxter Health Care, Deerfield, IL, USA), and blood was collected from the lateral tail vein. The samples were centrifuged at 4°C for 13 min at 8,000 rpm then frozen at −80°C until analysis. Plasma CORT concentrations were determined by a commercially available ELISA kit according to the manufacturer’s instructions (Assay Designs Inc., Ann Arbor, MI, USA).
Data analysis
Statistical analyses were performed using SigmaStat version 3.5 (Systat Software, San Jose, CA, USA). The numbers of active and inactive lever presses during the last three ethanol or sucrose self-administration sessions prior to extinction were averaged to obtain a baseline value for self administration. Likewise, the numbers of active and inactive lever presses during the first and last three extinction sessions prior to the first reinstatement test were averaged to obtain a value for extinction. Lever presses for the multiple reinstatements were analyzed by repeated measures two-way analysis of variance (ANOVA), followed by Newman–Keuls post hoc test where statistical significance was p<0.05. CORT was analyzed by repeated measures two-way ANOVA and non-repeated measures two-way ANOVA, followed by Newman–Keuls post hoc test where statistical significance was p<0.05. All data are presented as mean±SEM.
Results
Acquisition and extinction of ethanol and sucrose operant self-administration
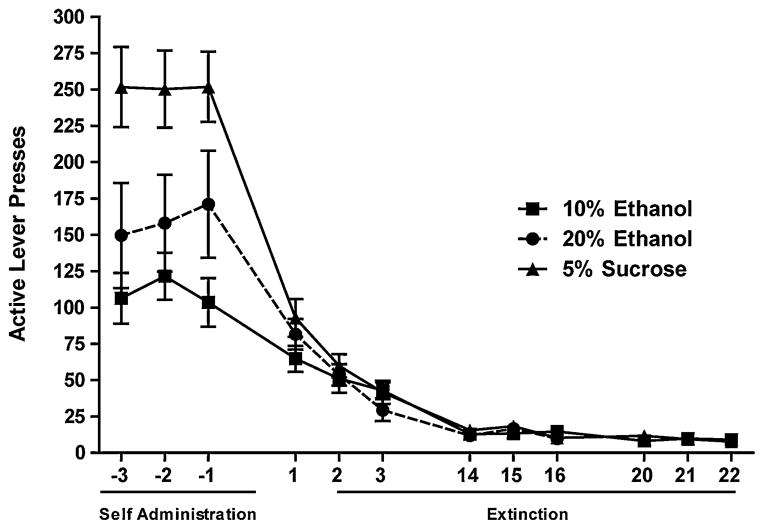
A total of 87 male Long Evans rats were trained to respond for either 10% ethanol (n=38), 20% ethanol (n=22), or 5% sucrose (n=28), respectively, and met acquisition criteria (Fig. 1). During the last three self-administration sessions, rats exhibited total active lever presses of 118±12 (10% ethanol group), 142±17 (20% ethanol group), and 244±22 (sucrose group), respectively. This equated to an average consumption of 0.59±0.05 and 1.50±0.17 g/kg ethanol per session for the 10% and 20% ethanol self-administering groups, respectively, and 0.66±0.05 g/kg sucrose per session for the sucrose self-administering animals. Although we did not calculate blood ethanol levels in the present study, we have previously shown that animals self-administering similar amounts of 10% and 20% ethanol reach mean blood ethanol concentrations of 20 and 60 mg%, respectively, with several 20% ethanol trained animals near 100 mg% (Simms et al. 2010). During the first three extinction sessions, the rats averaged 58±9 (10% ethanol group), 56±6 (20% ethanol group), and 65±8 (sucrose group) active lever presses. Before the first reinstatement test, the lever pressing had decreased to 12±1, 11±2, and 8±1, respectively. A repeated measures two-way ANOVA comparing extinction responding between the groups revealed that responding was significantly different between the 10% ethanol and 5% sucrose groups only in the first session and between the 20% ethanol and 5% sucrose groups only in the first, third, and eighth extinction sessions (data not shown). The responding for the two ethanol groups was only significantly different in the third session (data not shown). This indicates that although the 20% ethanol began reinstatement testing six sessions earlier than the other groups, there were no significant differences in the extinction rate following the eighth extinction session. One animal from the 10% ethanol-trained CORT collection group, two animals from the 20% ethanol reinstatement group, and one animal from the 20% ethanol-trained CORT were excluded due to failure to meet acquisition criteria (0.3 g/kg/30 min session). Additionally, one animal from the 20% ethanol reinstatement group was excluded due to lack of reinstatement in any of the four test weeks.
Fig. 1.
Operant training and extinction of responding for 10% ethanol, 20% ethanol and 5% sucrose. Mean±SEM number of active and inactive lever presses during the last 3 days of operant training, first 3 days of extinction training, and the last 3 days of extinction training are shown
Multiple yohimbine-induced reinstatements
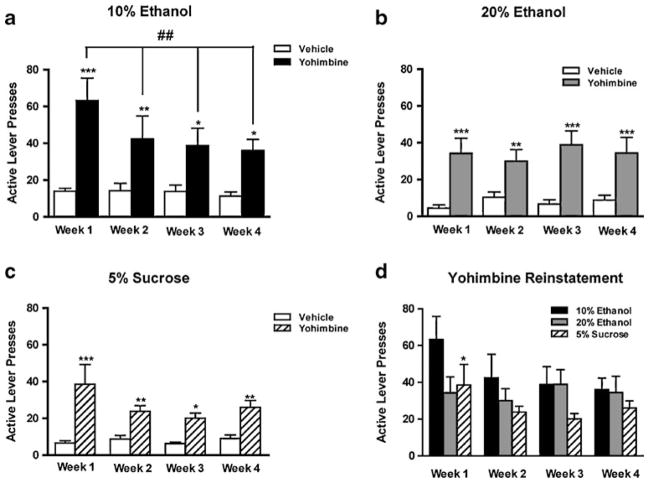
Yohimbine administration, in both of the ethanol groups and in the sucrose group, induced significant reinstatement in each of the four consecutive weeks of testing. Statistical analysis of active lever pressing using a repeated measures two-way ANOVA for the 10% ethanol group revealed an overall effect of treatment (vehicle or yohimbine) [F(1,111)=18.94, p< 0.001], an overall effect of week (1, 2, 3, or 4) [F(3,111)= 3.34, p<0.05], and an overall interaction between treat-ment×week [F(3,111)=2.97, p<0.05]. Post hoc analysis of active lever presses revealed a significant effect of yohimbine compared to vehicle for all weeks (Fig. 2a, week 1, p<0.001; week 2, p<0.01; week 3, p<0.05; week 4, p<0.05) and a significantly higher yohimbine-induced reinstatement when comparing week 1 with weeks 2, 3, and 4 (Fig. 2a, p<0.01). Statistical analysis of inactive lever pressing using a repeated measures two-way ANOVA revealed an overall effect of treatment (vehicle or yohimbine) [F(1,111)=10.22, p<0.01] but no effect of week or interaction between treatment× week. Post hoc analysis of inactive lever presses revealed a significant effect of yohimbine compared to vehicle for week 3 (Table 1, p<0.01).
Fig. 2.
Yohimbine challenge induces reinstatement of ethanol- and sucrose-seeking behavior each week for four consecutive weeks. Yohimbine (2 mg/kg i.p.) was administered once weekly for four consecutive weeks and induced a significant increase in active lever pressing in a 10% ethanol-seeking animals (***p<0.001; **p<.01; *p<.05 compared to vehicle: ##p<.01 compared to week 1 yohimbine reinstatement), b 20% ethanol-seeking animals (***p<0.001; **p< 0.01 compared to vehicle), and c 5% sucrose-seeking animals (***p< 0.001; **p<.01; *p<.05 compared to vehicle). Injection of vehicle had no effect on active or inactive lever responding (n.s., compared to extinction baseline, data not shown) in ethanol and sucrose-seeking animals. d 10% ethanol-trained animals had a significantly higher level of reinstatement in the first week of testing when compared to sucrose animals (*p<.05 compared to the sucrose reinstatement for the corresponding week). Data are presented as mean±SEM, (n=14 for 10% ethanol group, n=10 for 20% ethanol group, and n=16 for sucrose groups). Statistical analysis was performed by repeated-measures ANOVA with Newman–Keuls post hoc testing
Table 1.
Inactive lever presses following vehicle and yohim-bine administration for each of the reinstatement weeks
| Solution | Week 1 | Week 2 | Week 3 | Week 4 | |
|---|---|---|---|---|---|
| 10% ethanol | Vehicle | 0.8±0.4 | 0.7±0.3 | 0.7±0.3 | 1.3±0.5 |
| Yohimbine | 1.6±0.5 | 2.1±1.1 | 3.1±0.9** | 2.0±0.8 | |
| 20% ethanol | Vehicle | 0.3±0.2 | 0.2±0.1 | 0.6±0.5 | 0.5±0.5 |
| Yohimbine | 1.0±0.5* | 0.5±0.2 | 0.8±0.4 | 1.4±0.8 | |
| 5% sucrose | Vehicle | 0.3±0.2 | 0.8±0.4 | 0.9±0.5 | 1.3±0.9 |
| Yohimbine | 1.3±0.6 | 2.1±0.6 | 0.7±0.3 | 1.5±0.8 |
p<0.05,
p<0.01 compared to vehicle for the corresponding week and solution
Statistical analysis, using a repeated measures two-way ANOVA for the 20% ethanol group, revealed an overall effect of treatment (vehicle or yohimbine) [F(1,79)=24.60, p<0.001]. There was no overall effect of week, and no interaction between treatment×week. Post hoc analysis of active lever presses revealed a significant effect of yohimbine compared to vehicle for all weeks (Fig. 2b, week 1, p<0.001; week 2, p<0.01; week 3, p<0.001; week 4, p<0.001). Statistical analysis of inactive lever pressing using a repeated measures two-way ANOVA revealed an overall effect of treatment (vehicle or yohimbine) [F(1,79)=16.58, p<0.01] but no effect of week or interaction between treatment× week. Post hoc analysis of inactive lever presses revealed a significant effect of yohimbine compared to vehicle for week 1 (Table 1, p<0.05). Yohimbine-induced increases in inactive lever presses have been reported in the literature (Le et al. 2011; Marinelli et al. 2007); however, these effects are inconsistent as our group and others have failed to find such an effect (Le et al. 2005; Nielsen et al. 2011; Richards et al. 2008). More evidence of this inconsistency is offered in the present study where we show that inactive lever responding is increased only in one of the weeks for the animals trained to respond for 10% ethanol and 20% ethanol and not at all for 5% sucrose.
Statistical analysis of active lever pressing using a repeated measures two-way ANOVA for the 5% sucrose group revealed an overall effect of treatment (vehicle or yohimbine) [F (1,127)=46.39, p<0.001]. There was no overall effect of week and no interaction between treatment×week. Post hoc analysis of active lever presses revealed a significant effect of yohimbine compared to vehicle for all weeks (Fig. 2c, week 1, p<0.001; week 2, p<0.01; week 3, p<0.05; week 4, p<0.01). There was no effect of yohimbine on inactive lever responding compared to vehicle (Table 1).
A repeated measures two-way ANOVA comparing the active lever responding following yohimbine treatment for the 10% ethanol and 5% sucrose groups revealed an overall effect of solution (10% ethanol or 5% sucrose) [F(1,119)= 4.22, p<0.05] and an overall effect of week (1, 2, 3, or 4) [F(3,119)=5.35, p<0.01] but no interaction between solu-tion×week. Post hoc analysis revealed significantly greater reinstatement in the 10% ethanol group in week 1 (Fig. 2d). Repeated measures ANOVAs comparing active lever responding for the 20% ethanol group with the 5% sucrose group and the 10% ethanol group with the 20% ethanol group failed to reveal any significant differences.
Corticosterone measurements following multiple yohimbine challenges
Yohimbine administration, in ethanol- and sucrose-trained animals, induced a significant increase in plasma CORT levels. Statistical analysis of plasma CORT, using a two-way ANOVA, for 10% ethanol-seeking animals revealed overall effects of treatment (vehicle or yohimbine) [F(1,55)=11.22, p<0.01] but no overall effect of week or interaction between treatment× week. Post hoc analysis revealed a significant effect of yohimbine compared to vehicle for week 1 (p<0.001) and week 2 (p<0.05) but no significant difference in the two final weeks (Fig. 3a). Statistical analysis of plasma CORT, using a repeated measures two-way ANOVA for 20% ethanol-seeking animals, revealed an overall effect of treatment (vehicle or yohimbine) [F(1,47)=79.14, p<0.001] and an overall effect of week (1, 2, 3, or 4) [F(3,47)=3.47, p< 0.05], but no interaction between treatment×week. Post hoc analysis revealed a significant effect of yohimbine compared to vehicle for all weeks (Fig. 3b, week 1, p< 0.05; week 2, p<0.001; week 3, p<0.001; week 4, p< 0.01) and significant increases in yohimbine-induced CORT release when comparing week 1 with weeks 2 and 3 (Fig. 3b, p<0.05). Statistical analysis, using a two-way repeated measures ANOVA for sucrose-seeking animals, revealed an overall effect of treatment (vehicle or yohimbine) [F(1,47)=10.36, p<0.01] but no overall effect of week and no interaction between treatment×week. Post hoc analysis revealed a significant effect of yohimbine compared to vehicle for weeks 1, 2, and 3 (Fig. 3c, week 1, p<0.01; week 2, p<0.01; week 3, p<0.05).
Fig. 3.
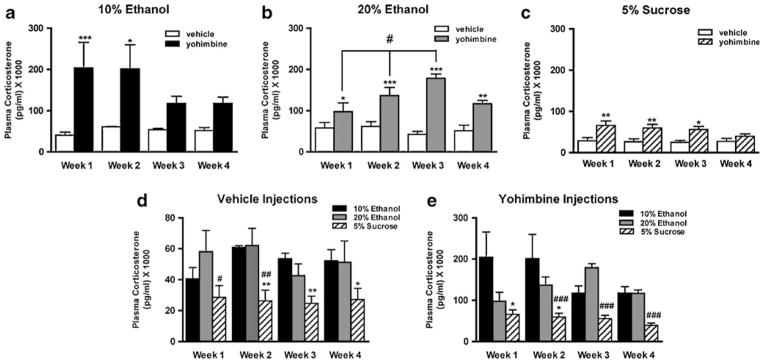
Yohimbine administration induces elevation of CORT in ethanol- and sucrose-trained animals. CORT levels following both vehicle and yohimbine administration are significantly higher in ethanol-seeking animals than in sucrose-seeking animals. Yohimbine (2 mg/kg i.p.) or vehicle (distilled water) were administered once weekly for four consecutive weeks and induced a significant increase in CORT in a 10% ethanol-trained animals for the first 2 weeks (***p< 0.001; *p<0.05 compared to vehicle), in b 20% ethanol-trained animals for all 4 weeks (***p<0.001; **p<0.01; *p<0.05 compared to vehicle: #p<0.05 compared to week 1 yohimbine plasma CORT), and in c sucrose-seeking animals for the first 3 weeks (**p<.01; *p<0.05 compared to vehicle). d Ethanol-trained animals had a significantly higher CORT response to vehicle injection than sucrose controls (**p< 0.01; *p<.05 comparing 10% ethanol CORT to 5% sucrose CORT for the corresponding week: ##p<0.01; #p<.05 comparing 20% ethanol CORT to 5% sucrose CORT for the corresponding week). e Ethanol-trained animals had a significantly higher CORT response to yohimbine injection than sucrose controls (*p<.05 comparing 10% ethanol CORT to 5% sucrose CORT for the corresponding week: ###p<0.001 comparing 20% ethanol CORT to 5% sucrose CORT for the corresponding week). Data are presented as mean±SEM, (n=11 for the 10% ethanol, n=11 for the 20% ethanol group, and n=12 for sucrose group). Statistical analysis was performed by ANOVA with Newman–Keuls post hoc testing
Statistical tests comparing CORT levels during treatment with either vehicle or yohimbine between both of the ethanol- and the sucrose-trained group revealed significant differences in response to mild stress (vehicle injection) and pharmacological stress (yohimbine injection). A two-way ANOVA comparing the CORT levels between the 10% ethanol- and 5% sucrose-trained groups following yohimbine vehicle administration revealed overall effects of solution (10% ethanol or 5% sucrose) [F(1,49)=24.80, p< 0.01] but no effect of week or interaction between solution×week. Post hoc analysis revealed that the 10% ethanol-trained animals had significantly higher CORT levels than sucrose-trained animals when administered vehicle in weeks 2, 3, and 4 (Fig. 2d, week 2, p<0.01; week 3, p<0.01; week 4, p<0.05). A repeated measures two-way ANOVA comparing the CORT levels between the 20% ethanol- and 5% sucrose-trained groups following yohimbine vehicle administration revealed overall effects of solution (20% ethanol or 5% sucrose) [F(1,43)=13.38, p< 0.01] but no effect of week or interaction between solution×week. Post hoc analysis revealed that the 20% ethanol-trained animals had significantly higher CORT levels than sucrose-trained animals when administered vehicle in weeks 1 and 2, with a strong trend in week 4 (Fig. 2d, week 1, p<0.05; week 2, p<0.01; week 4, p= 0.07, n.s.). A two-way ANOVA comparing the CORT levels between the 10% and 20% ethanol-trained groups following yohimbine vehicle administration revealed no significant differences.
A two-way ANOVA comparing the CORT levels between the 10% ethanol- and 5% sucrose-trained groups following yohimbine administration revealed an overall effect of solution (10% ethanol or 5% sucrose) [F(1,53)= 10.26, p<0.01] but no overall effect of week and no interaction between solution×week. Post hoc analysis revealed that 10% ethanol-trained animals had significantly higher CORT levels than 5% sucrose-trained animals when challenged with yohimbine in weeks 1 and 2 (Fig. 2e: p< 0.05). A repeated measures two-way ANOVA comparing the CORT levels between the 20% ethanol- and 5% sucrose-trained groups following yohimbine administration revealed an overall effect of solution (20% ethanol or 5% sucrose) [F(1,47)=84.92, p<0.001], an overall effect of week (1, 2, 3, or 4) [F(3,47)=3.68, p<0.05] and an interaction between solution×week [F(3,47)=3.75, p< 0.05]. Post hoc analysis revealed that 20% ethanol-trained animals had significantly higher CORT levels than 5% sucrose-trained animals when challenged with yohimbine in weeks 2, 3, and 4 (Fig. 2e, p<0.001). A two-way ANOVA comparing the CORT levels between the 10% and 20% ethanol-trained groups following yohimbine administration revealed no significant differences.
Discussion
In the present study, we show that the pharmacological stressor, yohimbine, significantly reinstates ethanol- and sucrose-seeking over four consecutive weeks. Although the magnitude of the reinstatement decreased for the 10% ethanol with sucrose-fade group following the first test week, the reinstatement for the 20% ethanol group was sustained at a stable level throughout the experiment. In the first week of testing, the reinstatement levels for the 10% ethanol group were significantly higher than those of the 5% sucrose group and showed a trend toward higher levels in comparison to 20% ethanol-trained animals. However, this higher reinstatement level was transient, and levels were similar to both the 5% sucrose and the 20% ethanol groups in each of the subsequent weeks. It is unclear whether the higher levels of reinstatement behavior in the animals in the 10% ethanol group are due to the sucrose-fading procedure or some other variable (i.e., ethanol concentration), but we have reported similar trends in our previous work (Simms et al. 2010). The use of sucrose to initiate ethanol self-administration is ubiquitous in the preclinical alcohol research field. However, several recent studies have shown that sucrose may be addictive in rodents (Avena et al. 2008; Colantuoni et al. 2002). Sucrose exposure has been shown to cause several stages of addiction in rats, including bingeing, withdrawal, craving, and sensitization [for review, see Avena et al. (2008)]. It has also been shown that withdrawal symptoms can be induced by administration of an opioid antagonist, suggesting the formation of dependence on the endogenous opioid release caused by excessive sugar intake (Colantuoni et al. 2002). Others researchers have found that sucrose may result in similar brain activation and have greater reward salience to rodents than drugs that are commonly abused by humans, such as opioids (Spangler et al. 2004) and cocaine (Lenoir et al. 2007). Therefore, the addition of these sweetened solutions may introduce a confound to studies exploring ethanol-reinforced behaviors. Furthermore, the use of sucrose fading in reinstatement models may complicate interpretation of results because of prior sucrose experience (i.e., questions may arise as to whether the animals are reinstating for ethanol or sucrose or the combination of the two solutions). The removal of these sweetened solutions from the 20% ethanol training regimen described here allows for unambiguous interpretation of our results; the animals are unequivocally reinstating for ethanol.
Animal models that explore drugs of abuse can also be used to explore maladaptive intake of natural reinforcers, including high fat, high calorie foods, and sweets. This is largely due to the fact that the mechanisms that underlie drug reinforcement overlap with those of natural reward (Colantuoni et al. 2002; Ghitza et al. 2006). Indeed, yohimbine has previously been shown to reinstate palatable food seeking (Ghitza et al. 2006) and sucrose seeking (Richards et al. 2008). Such studies have relevance in today’s society because of rampant obesity and type 2 diabetes. Developing models to more thoroughly understand the link between stress and maladaptive eating will become critical to public health in the future. The repeated yohimbine-induced reinstatement of sucrose-seeking described in the present study may represent a more efficient model for exploring these behaviors. The reinstatement levels for the sucrose group are quite stable across the 4 weeks of testing. The model presented here may allow researchers to develop within-subjects, Latin-square designs to study the reinstatement behavior. In addition, the findings may extend to palatable food seeking, although studies are needed to determine the stability of this reinstatement behavior over time.
The other major finding of the present study is that the hormonal response to stressors is elevated in animals with a history of ethanol self-administration compared to sucrose controls. Our data indicate that ethanol self-administration dysregulates HPA axis function, as demonstrated by an increase in plasma CORT in response to both mild stressors (vehicle injections) and pharmacological stressors (yohimbine challenges) in comparison to sucrose controls. Importantly, these changes were observed 5 weeks after the last reinforced self-administration sessions. This suggests that relatively modest levels of ethanol intake (~0.7–1.5 g/kg 5 days per week) can cause long-lasting disruptions in the functioning of stress hormone systems. Interestingly, the CORT response to yohimbine administration sensitizes over time in the 20% ethanol group, while the levels decrease for the 10% ethanol and 5% sucrose groups. This may be one factor that helps to maintain the stability of the reinstatement behavior in the animals trained to respond for 20% ethanol. CORT function has been shown to be disrupted in animals with a history of ethanol self-administration, and these changes may persist several months into abstinence and/or extinction and may affect susceptibility to reinstatement (Little et al. 2008; Prendergast and Little 2007; Sinha 2001). Similarly, normal cortisol release has been shown to be compromised in humans following chronic alcohol exposure and in early withdrawal, and this dysregulation may increase vulnerability to relapse (Adinoff et al. 1998; Brown et al. 1995; Cooper et al. 1992; Heinz et al. 1999; Koob 2008; Lovallo et al. 2000; Prendergast and Little 2007; Sinha 2001). It has been postulated that chronic alcohol exposure can create a state of allostasis, or a deviation from normal, homeostatic functioning of the brain. This increase in “allostatic load” leads to the creation of a new dysregulated state, which drives dependence (Koob 2009). This new allostatic set point, which may result in a hypo- or hyper-responsive HPA axis, may alter the dependent individuals’ response to psychosocial stress and increases the likelihood of relapse to AUDs (Adinoff et al. 1998; Brown et al. 1995; Lovallo et al. 2000; Sinha 2001). In agreement with the present study, the response to yohimbine has also been shown to be altered in recovering alcoholics (Krystal et al. 1994; Krystal et al. 1996). Krystal and colleagues found that while baseline cortisol levels are suppressed in patients recovering from AUDs, the cortisol response to yohimbine is elevated compared to healthy controls. This mirrors the increased sensitivity to yohimbine challenge in the ethanol-trained animals in the present study. However, it is important to note that the animals described here are consuming only moderate amounts of ethanol and are therefore not dependent. Future studies exploring the behavioral and hormonal effects of yohimbine in ethanol-dependent vs. non-dependent animals could potentially uncover other important changes that would be of interest to the preclinical alcohol research field.
Traditionally, reinstatement studies are designed to examine differences between subjects and focus only on the first reinstatement session for data collection. This experimental design can limit months of work (training, extinction, etc.) to one data point and significantly increase the number of animals needed to complete studies. The sustained and stable yohimbine-induced reinstatement observed in the 20% ethanol group indicates that yohimbine may be useful in the study of multiple reinstatements using within-subject designs.
Acknowledgments
We would like to thank Rui Li, Haley Pierson, and Tiffany Ho for excellent technical assistance. This work was supported by funding from the State of California for Medical Research through UCSF to S.E.B., Department of Defense Grant W81XWH-06-0240 and W81XWH-10-1-0247 to S.E.B. The experiments contained herein comply with the current laws of the USA. All procedures were pre-approved by the Gallo Center Institutional Animal Care and Use Committee and were in accordance with NIH guidelines for the Humane Care and Use of Laboratory Animals. This work was supported by funding the NIH, 1RO1AA017924-01 to S.E.B.
References
- Adinoff B, Iranmanesh A, Veldhuis J, Fisher L. Disturbances of the stress response: the role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health Res World. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banna KM, Back SE, Do P, See RE. Yohimbine stress potentiates conditioned cue-induced reinstatement of heroin-seeking in rats. Behav Brain Res. 2010;208:144–148. doi: 10.1016/j.bbr.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. J Stud Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- Buczek Y, Le AD, Wang A, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Charney DS, Heninger GR, Redmond DE., Jr Yohimbine induced anxiety and increased noradrenergic function in humans: effects of diazepam and clonidine. Life Sci. 1983;33:19–29. doi: 10.1016/0024-3205(83)90707-5. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, Thorsell A, Heilig M. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacology (Berl) 2010;208:417–426. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Rada P, McCarthy J, Patten C, Avena NM, Chadeayne A, Hoebel BG. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res. 2002;10:478–488. doi: 10.1038/oby.2002.66. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Russell M, Skinner JB, Frone MR, Mudar P. Stress and alcohol use: moderating effects of gender, coping, and alcohol expectancies. J Abnorm Psychol. 1992;101:139–152. doi: 10.1037//0021-843x.101.1.139. [DOI] [PubMed] [Google Scholar]
- Davis M, Redmond DE, Jr, Baraban JM. Noradrenergic agonists and antagonists: effects on conditioned fear as measured by the potentiated startle paradigm. Psychopharmacology (Berl) 1979;65:111–118. doi: 10.1007/BF00433036. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138:235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Weingartner H, George D, Hommer D, Wolkowitz OM, Linnoila M. Severity of depression in abstinent alcoholics is associated with monoamine metabolites and dehydroepiandrosterone-sulfate concentrations. Psychiatry Res. 1999;89:97–106. doi: 10.1016/s0165-1781(99)00099-2. [DOI] [PubMed] [Google Scholar]
- Holmberg G, Gershon S. Autonomic and psychic effects of yohimbine hydrochloride. Psychopharmacologia. 1961;2:93–106. doi: 10.1007/BF00592678. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Koob GF. Stress, corticotropin-releasing factor, and drug addiction. Ann N YAcad Sci. 1999;897:27–45. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Cooney N, Kranzler HR, Charney DS. Specificity of ethanollike effects elicited by seroto-nergic and noradrenergic mechanisms. Arch Gen Psychiatry. 1994;51:898–911. doi: 10.1001/archpsyc.1994.03950110058008. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Cooney NL, Kranzler HR, Southwick SW, Heninger GR, Charney DS. Serotonergic and noradrenergic dysregulation in alcoholism: m-chlorophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatry. 1996;153:83–92. doi: 10.1176/ajp.153.1.83. [DOI] [PubMed] [Google Scholar]
- Lang WJ, Gershon S. Effects of psychoactive drugs on yohimbine induced responses in conscious dogs. A proposed screening procedure for anti-anxiety agents. Arch Int Pharmacodyn Ther. 1963;142:457–472. [PubMed] [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ. The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology (Berl) 2009;204:477–488. doi: 10.1007/s00213-009-1481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Juzytsch W, Coen K, Navarre BM, Cifani C, Shaham Y. Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2178-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsycho-pharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little HJ, Croft AP, O’Callaghan MJ, Brooks SP, Wang G, Shaw SG. Selective increases in regional brain glucocorticoid: a novel effect of chronic alcohol. Neuroscience. 2008;156:1017–1027. doi: 10.1016/j.neuroscience.2008.08.029. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Stimulus conditioned to foot-shock stress reinstates alcohol-seeking behavior in an animal model of relapse. Psychopharmacology (Berl) 2003;168:184–191. doi: 10.1007/s00213-002-1267-z. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–658. [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162:333–338. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- Nielsen CK, Simms JA, Bito-Onon JJ, Li R, Ananthan S, Bartlett SE. The delta opioid receptor antagonist, SoRI-9409, decreases yohimbine stress-induced reinstatement of ethanol-seeking. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2010.00295.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast MA, Little HJ. Adolescence, glucocorticoids and alcohol. Pharmacol Biochem Behav. 2007;86:234–245. doi: 10.1016/j.pbb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35:1453–1463. doi: 10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcohol addiction research: from animal models to clinics. Best Pract Res Clin Gastroenterol. 2003;17:507–518. doi: 10.1016/s1521-6918(03)00031-3. [DOI] [PubMed] [Google Scholar]
- Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–142. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine SM, Southwick SM, Petrakis IL, Kosten TR, Charney DS, Krystal JH. Yohimbine-induced withdrawal and anxiety symptoms in opioid-dependent patients. Biol Psychiatry. 2002;51:642–651. doi: 10.1016/s0006-3223(01)01292-6. [DOI] [PubMed] [Google Scholar]
- Stopponi S, Somaini L, Cippitelli A, Cannella N, Braconi S, Kallupi M, Ruggeri B, Heilig M, Demopulos G, Gaitanaris G, Massi M, Ciccocioppo R. Activation of nuclear PPARgamma receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol Seeking. Biol Psychiatry. 2011;69:642–649. doi: 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Zironi I, Burattini C, Aicardi G, Janak PH. Context is a trigger for relapse to alcohol. Behav Brain Res. 2006;167:150–155. doi: 10.1016/j.bbr.2005.09.007. [DOI] [PubMed] [Google Scholar]