Abstract
Prepulse inhibition (PPI; also termed startle reduction or reflex modification, see [15]) provides an efficient and accurate method to assess both simple and complex acoustic discrimination in rodents [18]. Assessment of acoustic processing using PPI is less time consuming than operant conditioning paradigms, allows for the testing of many subjects simultaneously, and largely eliminates confounds due to motivation and attention [6]. Moreover, PPI procedures allow for data acquisition from the first day of testing, and can be used on rats as young as P14–15 [14, 31, 43]. For these and additional reasons, the PPI paradigm has more recently been adapted to the assessment of complex acoustic discrimination (tone sequences and FM sweeps), and applied to the study of normally developing as well as neuropathologically affected rodent populations.
The purpose of the current review is to provide a background on the PPI paradigm, and to summarize what has been learned more recently using modified versions of PPI with rodent models.
1. A Brief Review of Prepulse Inhibition
The acoustic startle reflex (ASR) is behaviorally manifest as a rapid contraction of muscles. This reflexive response is consistently evoked following the presentation of an unexpected intense stimulus (e.g., a loud noise burst). In rats, the ASR can be elicited by acoustic stimuli more than 80 dB above the auditory detection threshold [40]. In humans, ASR is typically indexed via eye-blink measures [21]. In rodents, however, ASR movements can be quantified and indexed by placing the animal on a load-cell platform that tranduces and transmits movement-induced pressure information (which is in turn recorded and analyzed [6; 28–32]).
The circuit mediating the ASR is composed of only a few synapses and is relatively simple, with extremely short latency. Data suggest that when a sufficiently intense startle eliciting auditory stimulus (SES) such as a noise burst is presented, the signal is transmitted from the auditory nerve to the ventral cochlear nucleus, the nuclei of the lateral lemniscus, the nucleus reticularis pontis caudalis (PnC), spinal interneurons, and finally to spinal motor neurons to elicit the characteristic “startle” response [9, 11, 26].
A reduction or attenuation of this ASR (startle reduction) can be induced through prepulse inhibition (PPI [18]). That is, if a non-startling stimulus or “prepulse” (i.e., auditory, visual or tactile) is presented 20 to 500 ms before the SES, the amplitude of the ASR is significantly reduced --- provided the subject is capable of processing the stimulus [11, 15, 42]. Moreover, the degree of ASR attenuation is directly related to the detectability of the stimulus preceding the SES (the prepulse [19, 25]). In its simplest form, PPI paradigms use a brief and moderately intense (65 – 75 dB) pure-tone, or a light flash, as the prepulse cue. However, more recent methods have also adapted this procedure to the use of silent gaps embedded in background white noise (see [16, 25]). In such paradigms, variable duration silent gaps (e.g., from 0 – 100 ms; 0 ms presented on uncued trials, and 2–100 ms gaps presented on cued trials) are embedded in background low-level (60–75 dB) broadband white noise. These variable duration gaps (including the 0 ms “no gap”) are presented in random order, usually 20 – 100 ms before the SES, on each trial. Using such paradigms, threshold gap detection can be reliably ascertained, since PPI will not be evident if the gap is too short to be detected [10, 14, 25].
The circuit mediating PPI appears to involve central nuclei including the cochlear nucleus, the inferior colliculus, the superior colliculus, and the pedunculopontine tegmental nucleus. Specifically, it is believed that when a non-startling acoustic stimulus (prepulse) is presented, the signal travels from the level of the cochlea to the inferior colliculus, that then sends collaterals to the superior colliculus (where somatosensory or visual prepulse information would enter the stream if present). From here, an excitatory input to the pedunculopontine tegmental nucleus appears to inhibit the PnC, a major nucleus involved in eliciting the startle reflex. Thus, when a SES occurs (20 to 500 ms after the presentation of a prepulse cue), there is an inhibition of the ASR via inhibition of the PnC [11, 22, 23, 26, 42]. With regard to the use of complex stimuli or stimulus changes to cue PPI, it also possible that higher acoustic structures may feed into this circuit (e.g., thalamus [46] and cortex [20]). However, it is unclear to what degree these structures are involved in stimulus processing per se versus general forebrain regulation of PPI. Overall, findings indicate that our understanding of the circuitry underlying PPI, particularly as concerns the use of variations in complex acoustic stimuli as prepulse cues, is not yet complete.
Numerous studies have been conducted to assess factors that influence PPI as a function of lesions or neurochemical manipulations to the PPI circuitry itself (see [42] for review). Accordingly, animal models of impaired PPI appear to translate to clinical models of impaired PPI in humans (e.g., schizophrenia [3]). However, in the current review, we discuss the use of PPI in a system where PPI circuitry is assumed to be largely intact (based on screening through simple tone and long silent gap PPI tasks), and the PPI paradigm can thus be used to assess individual and between-group variations in discrimination of the prepulse cue specifically as a function of spectral and temporal stimulus properties.
Our interest in the use of this paradigm is multi-faceted. First, evidence suggests that deficits in rapid auditory discrimination may be highly predictive of subsequent language difficulties in human populations [1, 2, 5]. As such, any acoustic discrimination paradigm that can easily be applied to animal models lends itself to the perceptual assessment of pathologies associated with language dysfunction in human populations (see [12] for further discussion). Second, the adapted PPI paradigm lends itself specifically to the study of neurodevelopmental models, because it can be used in very young animals (as young as P14 in rats [33, 45]). Third, PPI does not require associative learning or memory (although experience does lead to significant improvement [8, 10, 14, 44]), thus supporting experimental dissociation of cognitive processing (learning and memory) versus sensory discrimination thresholds. Finally, on a practical level, the paradigm can be automated and adapted to the assessment of large numbers of animals over relatively short periods of time.
2. Adapted Models of Prepulse Inhibition
As stated above, we have developed and implemented a modified version of the PPI paradigm, based loosely on classic neurophysiological mismatch negativity paradigms utilizing a background presentation of repeating tone-sequences interspersed by reversals or “oddball” tone pairs (e.g., [24]). This paradigm has been further adapted to the discrimination of 3-tone sequences and FM sweeps. The methods employed in these paradigms are detailed further below.
PPI (or Reflex Modification, Startle Reduction) Apparatus
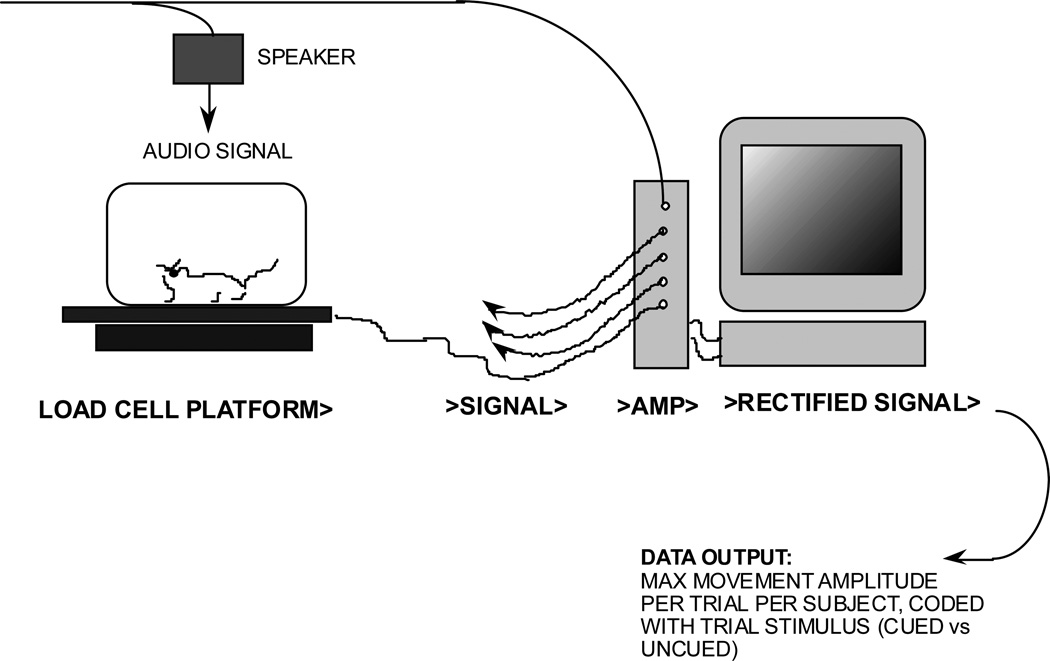
During testing each subject is placed on a Med Associates PHM-250 load cell platform (St. Albans, VT) in an opaque-walled, polypropylene cage located in a quiet testing room (Figure 1). The output voltages from the platforms are sent through a PHM-250–60 linear load cell amplifier, and passed into a Biopac MP100WS Acquisition system (Biopac Systems, CA) connected to a Power Macintosh 7200, where the signal is rectified on-line. This combined apparatus acts to record the amplitude of the subject’s whole-body acoustic startle reflex. The Biopac system acquires the transduced movement signals at a frequency of 1000 Hz throughout a session of testing. The epoch of interest is between 150- 200 ms in duration, beginning with the onset of the SES noise-burst. The peak amplitude of movement-induced pressure (measured in mV) is extracted via algorithm from this time-window, and serves as each subject's startle response measure for that trial. Auditory stimuli are generated on a Tucker-Davis sound system, and output through Cambridge Sound Works speakers positioned 50 cm above the platforms. The SES is a 50 ms “burst” of white noise with a 0-ms rise/fall time, presented at 105 dB.
Figure 1. Testing apparatus.
Importantly, all subjects in every study are always run on a simple single-tone detection procedure (using a 7 ms, 75dB pure-frequency tone as the pre-pulse cue) in order to establish baseline uncued startle and attenuated PPI (cued/uncued*100) values for each subject. These scores (along with subsequent scores on simple tasks such as long silent gap detection) provide a basis to ascertain comparability of PPI across groups. Assuming these scores do not differ, this equivalency supports a direct focus on spectrotemporal properties of the prepulse cue during further testing. For example, deficits in neuropathological groups may be seen in the detection of short but not long duration silent gaps using PPI, indicating group differences in auditory temporal acuity rather than PPI per se.
Silent Gap Detection Procedure
The gap detection test paradigm consists of repeated presentation of an SES with an inter-trial interval (ITI) of 24, 22, 18 or 16 sec [25]. The ITI is variable to prevent anticipation of the SES. A variable duration silent gap embedded in continuously presented broadband white background noise (75 dB) occurs 50 ms before the SES (gap duration on each trial randomly selected depending on task; long gap detection, 0–100 ms; short gap detection, 0–10 ms). A single trial consists of: 75 dB continuous background white noise; presentation of a silent gap; 50 ms of additional background white noise; and presentation of the SES (a 50 ms 105 dB white noise burst). This sequence is repeated for the next trial. Trials that do not contain gaps (uncued trials) are the same as above but the “gap” is 0 ms in duration. Thus, gap duration represents the independent variable. For the purpose of statistical comparison, the 0–gap condition is the “uncued” trial (baseline startle response), while the “cued” conditions include all other gap durations (which are compared individually to the 0-gap to ascertain threshold).
Oddball Two-Tone Sequence Detection Procedure
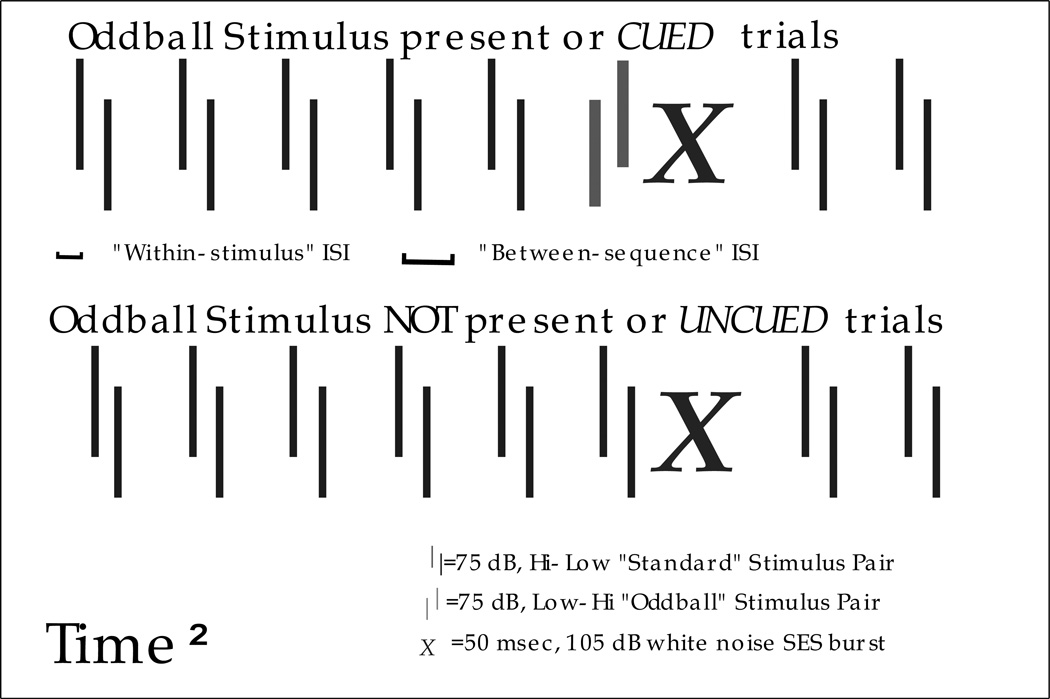
This auditory paradigm also consists of repeated presentation of the SES with a variable duration inter-trial interval (ITI) ranging from 16 to 24 sec [25]. However, the oddball presentation format involves the repeated presentation of a “standard” stimulus (75 dB), separated by an inter-stimulus interval. Stimuli presented are comprised of two or more 7 ms tones in a sequence (see Figure 2 for sample 2-tone stimulus trials). In half the trials, a standard stimulus is presented immediately before the SES (i.e., uncued trial). In the remaining trials, an “oddball” stimulus (also 75dB) is presented before the SES (i.e., cued trial). Presentation of cued and uncued trials is randomized. Subjects receive a variable number of trials per session and a variable number of days of testing (depending on test). Sessions typically progress from a long-duration within sequence inter-stimulus interval or ISI (e.g., 225 ms) to shorter ISIs (e.g., 125, 75, 50, 25, 10 ms), using only one ISI across a daily session. The between sequence ISI is typically 200 ms longer than the within sequence ISI, to maintain perceptual contiguity of the tone pair (although shorter between-sequence ISIs are sometimes used to increase difficulty of the task).
Figure 2. Sample two-tone sequence trials (cued and uncued).
Comparable results are obtained when the background stimulus is lo-hi, and the oddball is hi-lo (unpublished data); adapted from [6].
As an aside, this oddball startle reduction paradigm parallels electrophysiological procedures where a background (standard) stimulus is repeatedly presented, and an unexpected “oddball” (which is similar to the standard, but distinguished by specific spectral and/or temporal differences) unexpectedly replaces the repeating (standard) stimulus (see [24]). Whereas electrophysiologists look for differences in acoustic evoked potential signal as a function of oddball stimulus novelty (and thus discriminability), our paradigm uses the oddball stimulus to look for an attenuated behavioral startle response or PPI (which similarly indicates perception of the “oddball,” and thus discrimination of the distinguishing features of that stimulus).
Finally, we have adapted this oddball tone paradigm to the discrimination of 3- tone sequences, by adding a third 7 ms high tone to the standard (repeating background, 75 dB) and oddball pair. In this format, standard sequences are comprised of hi-lo-hi while the oddball triplet is lo-hi-hi. This stimulus format follows from backward masking paradigms, with the 3rd tone effectively representing a “masker” (because it is always high, and therefore irrelevant), and the critical discriminatory requirement representing tone order of the initial pair [38]. In this paradigm, the stimulus durations used include: 1) within ISI=60 ms, between ISI=260 ms; 2) within ISI=30 ms, between ISI=100 ms; and 3) within ISI=10 ms, between ISI=60 ms. We found that adult male sham rats require 5 days of testing at each of these conditions in order to elicit evidence of tone-triplet discrimination as measured by PPI [38].
Oddball FM Sweep Detection Procedure
As in the oddball tone-pair paradigm, repeating “down-sweeps” (or glides) are interspersed with a comparable “up-sweep” (same start/end frequencies and duration but spectrally reversed, 75 dB) on cued trials. Initial assessments use FM sweeps with start 2300 Hz/end 1100 Hz (linear, 0 ms ramp), presented at decreasing durations (225ms, 125 ms, 75 ms, 50 ms; one duration used per session). Findings show that these stimuli effectively differentiate groups with impaired rapid auditory processing [29, 30, 31, 32].
In closing, future applications should continue to apply the oddball presentation format to PPI in rodents to ascertain measurements of discrimination for other stimuli of interest, including for example the study of speech discrimination in rodents [13], as well as ethologically relevant stimuli such as ultrasonic vocalizations.
3. Findings on Complex Acoustic Discrimination Using Prepulse Inhibition (PPI)
a. PPI is reduced as prepulse cue complexity increases/duration decreases (i.e., as the cue becomes harder to distinguish from the background)
In one of our earliest reflex modification or PPI studies, Clark et al. [6] showed that as subjects were tested over a period of 13 days on the same task (a two-tone oddball discrimination), with incremental daily reductions in the between-tone ISI, shams evidenced a gradual shift in performance -- from around 75% attenuated scores at a 332 ms ISI (indicating cued responses averaged 75% of uncued responses), to around 95% attenuated scores at an ISI of 24 ms. In this context, higher scores indicate worse performance, with 100% indicating no difference between cued and uncued trials, and hence no detection of the cue. When subjects were returned to a longer stimulus ISI on Day 13, attenuation scores improved accordingly. Similar effects are seen when subjects are tested on a 2-tone oddball task using between-tone ISIs of 225, 75, 40 and 10 ms (with between-sequence ISIs always 200 ms greater to maintain perceptual contiguity of the tone pair). Moreover, when subjects tested on the above task were then tested on a “speeded-up” version, using within/between ISI ratios of 40/140 ms, 20/70 ms, and 10/60 ms (which produced a perceptual effect of “streamed” tones, rather than repeating pairs), subjects performed markedly worse as indicated by higher scores [38].
In practice, task difficulty effects are often masked or confounded by the prior experience required to move subjects smoothly from easier to more difficult tasks (and still elicit discrimination). Specifically, we have developed a standard battery of tasks that moves from use of a single tone (1 day), to 0–100 ms silent gap (4 days), to 0–10 ms silent gap (5 days), to 2-tone oddball (long to short ISI, minimum 5 days), to FM sweeps (long to short, minimum 5 days) . We have consistently found that if animals are “pushed ahead” to harder tasks too quickly, they perform at chance levels, and must be dropped back for further training on easier tasks (unpublished data). Conversely, the more training subjects receive on “easier” tasks, the better they perform when advanced to more difficult paradigms [44].
Finally, we have performed one study using a very difficult (3-tone sequence discrimination) version of our oddball task, and found that even adult sham rats with considerable prior experience perform at very marginal (albeit significant) levels on this task [38].
b. Increased age (up to adulthood) and increased experience improve performance
As might be expected, factors of age and experience are difficult to tease apart because young animals cannot perform difficult tasks, and older animals (at least in our studies) tend to have a great deal of prior experience. However, some studies have successfully dissociated these variables. For example, significant experience effects in PPI are seen across days for both young and adult rats, with several days age difference unlikely to be a major factor [8, 10, 14, 31, 41, 43].
In a more explicit assessment of experience effects, batches of animals with and without prior PPI experience were compared on the same task as adults. In all cases, prior experience led to significantly improved performance [44].
The interpretation of these findings is complicated by early assumptions that, because PPI can be assessed within a single trial, no “learning” per se is involved [15]. As such, alternate interpretations for experience effects could include improved sensory acuity for the prepulse cue, or increased attention (see [14, 41]). However, Crofton et al., [8] explicitly tested the putative contributions of associative learning between the prepulse cue and SES by presenting the stimuli in both a contingent and non-contingent format, and noted that PPI was seen only after contingent pairing, strongly supporting an associative learning component in the experience effects seen for PPI.
In addition, age effects can be assessed by looking at performance levels on comparable tasks using naïve subjects of different ages. For example, whereas rats tested for the first time at P15 on a silent gap detection task showed attenuated scores around 83% for trials cued by a 75 ms gap [14], littermates tested for the first time on this same task but at age P35 revealed attenuated scores around 73% for the same 75 ms gap [14]. Considered another way, minimum detectable gaps shifted from between 10–20 ms in P15 subjects to between 5–10 ms in P64 subjects ([14], see also [10]). Similar effects were seen on a 0–10 ms silent gap task, with animals tested for the first time at P23 performing around 97% on trials cued by a 6 ms gap (i.e., chance [37]), but animals tested for the first time at P50 performing at around 88% for the same 6 ms gap [37].
Thus it is apparent that both increasing age (up to adulthood), and increasing experience, both lead to improved performance in PPI paradigms using complex acoustic discrimination cues (although presumably these are asymptotic at some point). For example, Crofton et al. [8] reported asymptotic PPI performance in adult male rats measured after 5–6 sessions (using a 20 ms silent gap cue). Nevertheless, the parameters modulating the point at which asymptotic effects of experience are seen are likely to be unique to the stimulus properties and task demand for specific paradigms. For example, visually-cued PPI emerges later in development compared to acoustically-cued PPI [34], and requires a longer cue-burst interval [4; see below for discussion]. It follows that research may show that PPI cued by complex visual (versus acoustic) stimuli in rats requires a longer period of experience to reach asymptotic levels. In addition, evidence suggests that despite changes in baseline startle, PPI is not degraded with old age in mice [17] or humans [27]. Given that parallel literature suggests that acoustic discrimination thresholds plateau and even degrade in old age, future studies could investigate changes in complex acoustic processing thresholds over the entire lifespan in rodents using PPI.
c. Longer cue-burst intervals are required as prepulse cue (stimulus) complexity increases and/or age and experience decrease
Prior research has reported that prepulse cues presented at an optimal interval prior to the SES lead to maximum PPI, and this relationship appears to follow an inverted U-shaped function [25]. Specifically, at very short cue-burst intervals (<15 ms [41]), the prepulse cue may actually cause an increase in the startle response (termed “facilitation”). At longer cue-burst intervals (also called “stimulus onset asynchrony” (the time between prepulse start and SES start [20]) or “interstimulus interval” (the time between prepulse offset and SES onset [25])), PPI shows an optimal inhibition of startle, which then declines again with further increases in the cue-burst interval. In a key study utilizing psychophysical variation of both silent gap durations and also the interval between the prepulse and SES, Leitner et al. [25] demonstrated that optimal cue-burst intervals for silent gaps under 20 ms in duration was 50 ms, but for gaps above 25 ms, may approach closer to 20–30 ms (as measured by maximal PPI). These findings suggest an interaction between: 1) the duration of the prepulse, 2) the duration between the end of the prepulse and the start of the SES, and 3) the lead time from the start of the prepulse cue to the SES (which is the sum of 1 and 2). In effect, it appears that subjects require an adequate period of time to effectively process an incoming stimulus, in order for the prepulse cue to be relayed into the startle circuit in time to provide significant attenuation of the startle response.
This time requirement may explain why very short cue-burst intervals appear to produce facilitation of the startle response ([41], see also [26]). That is, when insufficient time for processing of the prepulse is provided, the prepulse may have the effect of perceptually “merging” with the SES, leading to enhanced arousal (and increased startle), rather than a classic PPI response.
Consistent with this view, we have frequently seen facilitation of the startle response in rats, even at longer duration cue-burst intervals (e.g., 50 ms), under the following conditions: 1) the initial day of testing with naïve subjects; 2) extremely young subjects (P14 – 16); and 3) very difficult discrimination tasks (e.g., human speech sounds; see [13]). In all of these cases, a requirement for longer stimulus processing time might be anticipated. Notably, Parisi and Ison [34] also reported startle facilitation for young rats (P17 – 35) when using a 25 ms noise burst prepulse presented only 4 ms before the SES, while PPI was seen for cue-burst intervals of 40 – 320 ms. These collective data support the view that adequate processing time for the prepulse cue is required for effective PPI, and that the criteria defining “adequate time” likely changes as a function of age, experience, and stimulus complexity.
Finally, prepulse cues presented in a non-auditory modality (e.g., a visual high frequency black-white checkerboard presented briefly against a “background” of equiluminant grey) appear to require much longer (around 250 ms) cue-burst intervals in order to elicit PPI as compared to the optimal range of 50 – 100 ms for tones and silent gaps [4]. In this visually-cued PPI paradigm, cue-burst intervals less than about 200 ms lead to startle facilitation in adult rats [4].
d. Deficits associated with neuropathology are most reliably seen on tasks that generate mid-level performance in shams
Multiple studies conducted over a period of years using different species (rats and mice), sexes, ages, and subjects with and without early induced neuropathologies, have revealed that in order to elicit performance differences between treatment groups on a given task, the task must neither be too difficult (>95% attenuated scores) nor too easy (<70% attenuated scores) for shams. Again, the task that will elicit deficits for a given group varies with age and experience. Thus while the 0–10 ms silent gap tasks elicit deficits in younger neurologically impaired rats and adult mice [7, 30, 31, 35, 37, 43], older rats (with some experience) typically only show deficits on short duration (< 75 ms) two-tone sequence or FM sweep oddball tasks [6, 31, 32, 36, 39]. Subjects with a great deal of prior testing may still be “pushed” to show deficits on very difficult tasks, such as a speeded version of the two-tone oddball, or a 3-tone oddball discrimination [28, 38].
4. Implications for the use of PPI in assessing sensory processing as a function of other variables
In this review, we have demonstrated the effective use of a modified PPI paradigm to assess the effects of early brain injury (and also concomitant treatment with neuroprotectants [30, 32]) on auditory discrimination of complex and short duration stimuli. Cumulative results indicate that this paradigm provides a sensitive assessment of acoustic discrimination thresholds, and can successfully differentiate groups that are otherwise unimpaired in PPI as a function of the spectral/temporal properties of stimuli used to cue PPI. Moreover, changes in these discrimination thresholds can be measured as a function of age and experience. The current findings have significant relevance in demonstrating that sensory processing indices can be quickly and easily obtained from large numbers of experimental subjects without the requirement of lengthy and time-consuming training typical of operant conditioning paradigms.
We suggest that in the future, the PPI paradigms described here could lend themselves to the study of acoustic (and potentially also visual) discrimination as a function of variables such as aging, pharmacological manipulations, environmental modifications, social context, and many additional as-yet unexplored variables known to influence sensory processing systems.
Acknowledgements
The authors wish to acknowledge the contributions of Matthew Clark to the initial adaptation and development of paradigms discussed here.
Footnotes
Conflict of Interest: None of the authors have any conflict of interest regarding the research performed here, the funding of said research, nor the publication of said research.
References
- 1.Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behavioral Brain Research. 2002;136:31–49. doi: 10.1016/s0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- 2.Benasich AA, Choudhury N, Friedman JT, Realpe-Bonilla T, Chojnowska C, Gou Z. The infant as a prelinguistic model for language learning impairments: Predicting from event-related potentials to behavior. Neuropsychologia. 2006;44:396–411. doi: 10.1016/j.neuropsychologia.2005.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, McClure MM, Fitch RH, Markus EJ. Cross modal sensory processing assessments in rodent models of early brain damage. Society for Neuroscience Abstracts. 2004:919.3. [Google Scholar]
- 5.Choudhury N, Leppanen PH, Leevers HJ, Benasich AA. Infant information processing and family history of specific language impairment: converging evidence for RAP deficits from two paradigms. Dev Sci. 2007;10:213–236. doi: 10.1111/j.1467-7687.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark M, Rosen G, Tallal P, Fitch RH. Impaired processing of complex auditory stimuli in rats with induced cerebrocortical microgyria. J Cog Neurosci. 2000;12:828–839. doi: 10.1162/089892900562435. [DOI] [PubMed] [Google Scholar]
- 7.Clark MG, Sherman GF, Bimonte HA, Fitch RH. Perceptual auditory gap detection deficits in ectopic male BXSB mice. NeuroReport. 2000;11:693–696. doi: 10.1097/00001756-200003200-00008. [DOI] [PubMed] [Google Scholar]
- 8.Crofton KM, Dean KF, Sheets LP. Evidence for involvement of associative conditioning in reflex modification of the acoustic startle response with gaps in background noise. Psychobiology. 1990;18:467–474. [Google Scholar]
- 9.Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: Lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean KF, Sheets LP, Crofton KM, Reiter LW. The effect of age and experience on inhibition of the acoustic startle response by gaps in background noise. Psychobiology. 1990;18:89–95. [Google Scholar]
- 11.Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology. 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- 12.Fitch RH, Tallal P, Brown C, Galaburda A, Rosen G. Induced microgyria and auditory temporal processing in rats: a model for language impairment? Cerebral Cortex. 1994;4:260–270. doi: 10.1093/cercor/4.3.260. [DOI] [PubMed] [Google Scholar]
- 13.Fitch RH, Tallal P. Neural mechanisms of language-based learning impairments: Insights from human populations and animal models. Behavioral & Cognitive Neurosciences Reviews. 2003;2:155–178. doi: 10.1177/1534582303258736. [DOI] [PubMed] [Google Scholar]
- 14.Friedman JT, Peiffer A, Clark M, Benasich A, Fitch RH. Age and experience related improvements in gap detection in the rat. Developmental Brain Research. 2004;152:83–91. doi: 10.1016/j.devbrainres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- 16.Ison JR. Temporal acuity in auditory function in the rat: reflex inhibition by brief gaps in noise. J Comp Physiol Psychol. 1982;96:945–954. [PubMed] [Google Scholar]
- 17.Ison JR, Bowen GP, Pak J, Gutierrez E. Changes in the strength of prepulse inhibition with variation in the startle baseline associated with individual differences and with old age in rats and mice. Psychobiology. 1997;25:266–274. [Google Scholar]
- 18.Ison JR, Hammond GR. Modification of the startle reflex in the rat by changes in the auditory and visual environments. J Comp Physiol Psychol. 1971;75:435–452. doi: 10.1037/h0030934. [DOI] [PubMed] [Google Scholar]
- 19.Ison JR, Hoffman HS. Reflex modification in the domain of startle: II. The anomalous history of a robust and ubiquitous phenomenon. Psychol Bull. 1983;94:3–17. [PubMed] [Google Scholar]
- 20.Ison JR, O’Connor K, Bowen GP, Bocirnea A. Temporal resolution of gaps in noise by the rat is lost with functional decortication. Behav Neurosci. 1991;105:33–40. doi: 10.1037//0735-7044.105.1.33. [DOI] [PubMed] [Google Scholar]
- 21.Ison JR, Pinckney LA. Reflex inhibition in humans: sensitivity to brief silent periods in white noise. Percept Psychophys. 1983;34:84–88. doi: 10.3758/bf03205900. [DOI] [PubMed] [Google Scholar]
- 22.Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 23.Koch M, Schnitzler HU. The acoustic startle response in rats - circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- 24.Kraus N, McGee T, Littman T, Nicol T, King C. Nonprimary auditory thalamic representation of acoustic change. J Neurophysiol. 1994;72:1270–1277. doi: 10.1152/jn.1994.72.3.1270. [DOI] [PubMed] [Google Scholar]
- 25.Leitner DS, Hammond GR, Springer CP, Ingham KM, Mekilo AM, Bodison PR, Aranda MT, Shawaryn MA. Parameters affecting gap detection in the rat. Percept Psychophys. 1993;54:395–405. doi: 10.3758/bf03205275. [DOI] [PubMed] [Google Scholar]
- 26.Leumann L, Sterchi D, Vollenweider F, Ludewig K, Fruh H. A neural network approach to the acoustic startle reflex and prepulse inhibition. Brain Research Bulletin. 2001;56:101–110. doi: 10.1016/s0361-9230(01)00607-4. [DOI] [PubMed] [Google Scholar]
- 27.Ludewig K, Ludewig S, Seitz A, Obrist M, Geyer MA, Vollenweider FX. The acoustic startle reflex and its modulation: effects of age and gender in humans. Biological Psychology. 2003;63:311–323. doi: 10.1016/s0301-0511(03)00074-7. [DOI] [PubMed] [Google Scholar]
- 28.McClure MM, Threlkeld SW, Rosen GD, Fitch RH. Auditory processing deficits in unilaterally and bilaterally injured hypoxic-ischemic rats. NeuroReport. 2005;16:1309–1312. doi: 10.1097/01.wnr.0000175613.16183.6c. [DOI] [PubMed] [Google Scholar]
- 29.McClure M, Peiffer AM, Rosen GD, Fitch RH. Auditory processing deficits in rats with neonatal hypoxic-ischemic injury. International Journal of Developmental Neuroscience. 2005;23:351–362. doi: 10.1016/j.ijdevneu.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 30.McClure M, Threlkeld S, Fitch RH. The effects of erythropoietin on auditory processing following neonatal hypoxic-ischemic injury. Brain Research. 2006;1087:190–195. doi: 10.1016/j.brainres.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 31.McClure M, Threlkeld S, Rosen G, Fitch RH. Rapid auditory processing and learning deficits in rats with P1 versus P7 neonatal hypoxic-ischemic injury. Behavioural Brain Research. 2006;172:114–121. doi: 10.1016/j.bbr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClure M, Threlkeld S, Fitch RH. Auditory processing and learning/memory following erythropoietin administration in neonatally HI injured rats. Brain Research. 2007;1132:203–209. doi: 10.1016/j.brainres.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Parisi T, Ison JR. Development of the acoustic startle response in the rat: Ontogenetic changes in the magnitude of inhibition by prepulse stimulation. Developmental Psychobiology. 1979;12:219–230. doi: 10.1002/dev.420120305. [DOI] [PubMed] [Google Scholar]
- 34.Parisi T, Ison JR. Ontogeny of control over the acoustic startle reflex by visual prestimulation in the rat. Developmental Psychobiology. 1981;14:311–316. doi: 10.1002/dev.420140403. [DOI] [PubMed] [Google Scholar]
- 35.Peiffer AM, Dunleavy CK, Frenkel M, Gabel LA, LoTurco JJ, Rosen GD, Fitch RH. Impaired detection of variable duration embedded tones in ectopic NZB/BINJ mice. NeuroReport. 2001;12:2875–2879. doi: 10.1097/00001756-200109170-00024. [DOI] [PubMed] [Google Scholar]
- 36.Peiffer AM, Rosen GD, Fitch RH. Rapid auditory processing and MGN morphology in rats reared in varied acoustic environments. Dev Brain Res. 2002;138:187–193. doi: 10.1016/s0165-3806(02)00472-8. [DOI] [PubMed] [Google Scholar]
- 37.Peiffer AM, Friedman JT, Rosen GD, Fitch RH. Impaired gap detection in juvenile microgyric rats. Developmental Brain Research. 2004;152:93–98. doi: 10.1016/j.devbrainres.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Peiffer AM, McClure MM, Threlkeld SW, Rosen GD, Fitch RH. Severity of focal microgyria and associated rapid auditory processing deficits. NeuroReport. 2004;15:1923–1926. doi: 10.1097/00001756-200408260-00018. [DOI] [PubMed] [Google Scholar]
- 39.Peiffer AM, Rosen GD, Fitch RH. Sex differences in auditory processing deficits in microgyric rats. Developmental Brain Research. 2004;148:53–57. doi: 10.1016/j.devbrainres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Pilz PK, Schnitzler HU, Menne D. Acoustic startle threshold of the albino rat (Rattus norvegicus) J Comp Psychol. 1987;101:67–72. [PubMed] [Google Scholar]
- 41.Reijmers LGJE, Peeters BWMM. Effects of acoustic prepulses on the startle reflex in rats; a parametric analysis. Brain Research. 1994;661:174–180. doi: 10.1016/0006-8993(94)91204-1. [DOI] [PubMed] [Google Scholar]
- 42.Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- 43.Threlkeld SW, McClure MM, Rosen GD, Fitch RH. Developmental timeframes for the induction of microgyria and rapid auditory processing deficits in the rat. Brain Research. 2006;1109:22–31. doi: 10.1016/j.brainres.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 44.Threlkeld SW, Hill CA, Rosen GD, Fitch RH. Effects of cortical developmental disruption and test experience on rapid auditory processing in rats. Society for Neuroscience Abstracts. 2007 doi: 10.1016/j.ijdevneu.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wecker J, Ison J, Foss J. Reflex modification as a test for sensory function. Neurobehav Toxicol Teratolol. 1985;7:733–738. [PubMed] [Google Scholar]
- 46.Zhang J, Engel JA, Ericson M, Svensson L. Involvement of the medial geniculate body in prepulse inhibition of acoustic startle. Psychopharmacology. 1999;141:189–196. doi: 10.1007/s002130050824. [DOI] [PubMed] [Google Scholar]