Abstract
Spilanthes spp. are popular, over-the-counter remedies; they are sold over the internet under various names and are widely used in traditional medicine in various cultures. This review will summarize the important reports on the ethnopharmacology, botany, phytochemistry, and pharmacological properties as described in the literature from recent years (1920 to 2013). Spilanthes spp. are used for more than 60 types of disorders. They are reported to contain a number of biologically active phytochemicals, although a large number of ethnopharmacological uses have been documented; only a few of these species have been investigated for their chemical and biological activities. The studies are carried out mainly on Spilanthes extracts and a few metabolites substantiate the uses of these plants in traditional medicine. Well-conducted pharmacological studies are still needed for several traditional indications, and the mechanisms of action by which the plant extracts and the active compounds exert their pharmacological effects remain to be studied. They are predominantly used as extracts in personal care products, traditional medicines, and the pharmaceutical and culinary areas. Suggestions are made regarding some of the possible mechanisms of action as to how the known compounds may exert their biological activity.
1. Introduction
Several species in the genus Spilanthes Jacq. are tropical plants and are used extensively in traditional medicine and in flavoring foodstuffs. Most people find the spilanthol-induced tingling of the tongue unpleasant, but when cooked, the plants lose their strong flavor and may be used as a green leafy vegetable. For culinary purposes, a small amount of shredded fresh leaves adds unique flavors to salads. In addition, both fresh and cooked leaves are used in dishes such as stews and soups. There have been significant advances in all aspects of Spilanthes research, and an increasing number of commercial Spilanthes products have appeared in the market place as personal care products, health care products, and for culinary use. Commercial Spilanthes plantations have been established to address the need for sustainable supplies of standardized, high quality raw material. The extensive use of this genus in traditional medicine around the globe has been described in many ethnopharmacological reports. Alongside its traditional applications, the importance of this genus lies in the type of disorders for which preparations of its aerial parts and roots are used. Various plants in the genus are used for anti-inflammatory, hepatoprotective, and diuretic properties and in a wide range of disorders like toothache, diuretic, gastritis, gastric ulcers, mucous membrane inflammation, burns, and wounds [1, 2]. For these purposes, infusions and decoctions are prepared from the aerial parts or roots and administered either orally or topically as compresses or baths. Moreover, many biologically active compounds have been isolated from this genus. In recent decades there is a growing research literature on this genus, mainly for the validation of ethnopharmacological usage.
Spilanthes spp. have recently been the object of many claims concerning its medicinal properties. A number of publications have shown that these plant extracts, formulations, and bioactive components have a wide range of potential applications in pharmaceutical and cosmetic industries [3]. The patents on Spilanthes products and its formulations are increasing. For instance, in the United States alone, some 30 patents have been registered by the US Patent and Trademark Office since 1976 [4]. Spilanthes extracts have found applications in pharmaceuticals as an antitoothache formulation, for pain relief, swelling, and gum infections, periodontosis, and in mouthwashes. For instance, A. Vogel Herbal Remedies in the United Kingdom sells organically grown S. oleracea L. plant and leaf extracts in alcohol (67% v/v) as a botanical food supplement [5]. A dermal health compound, oral health tonic, and fungus fighter compound marketed by HerbPharm, USA, contains organically grown S. acmella Murray and is recommended for skin care, oral health, and antifungal uses [6]. A. Vogel Australia Pty. Ltd. sells formulations which contain S. oleracea extract in the Dentaforce herbal mouth spray which is indicated to assist the treatment of moderate cases of periodontal disease and gingivitis. Dentaforce herbal mouthwash and an aftershave cream are also available. Commercial interest in Spilanthes has increased tremendously, as indicated by the number of personal care products in the market in which S. acmella flower extract is present. For example, in Gatuline from Gattefosse and Antiwrinkle firming light cream from Laboratories SVR S. acmella flower extract is added for for its antiaging properties [7, 8]. Nevertheless, despite the real market opportunities in the medicinal, personal care, and food industries, there has been little scientific research to review the potential uses of this genus. Furthermore, the phytochemical compounds responsible for their alleged properties have not yet been reviewed. As a result, the optimization of scientific technologies for their quality control has been neglected. In addition, there is also a need to identify the lacunas where further research is needed to fill in the knowledge gaps.
2. Botanical Aspects
The genus Spilanthes Jacq. belongs to the family Asteraceae (formerly Compositae) and has more than 300 species, generally distributed in the tropics [9, 10]. The validity of the different species names of plants in the genus Spilanthes was searched in international databases, namely, the International Plant Names Index (IPNI), which generated 343 records, and these were verified. This family is known as the aster, daisy, or sunflower family and is the largest family of flowering plants, in terms of number of species. The name “Asteraceae” is derived from the type genus aster, while “Compositae” an older but still valid name referring to the characteristic inflorescence. These herbs originated in tropical Africa and South America and are widely distributed in the tropics and subtropics, including tropical America, North Australia, Africa, Malaya, Borneo, India, and Sri Lanka [11, 12]. These plants have been popularly called toothache plant because of their traditional use. Other common folkloric names include eyeball plant, spot plant, para cress (after the Brazilian province), Brazil cress, alphabet plant, and Australian cress.
This genus is often confused taxonomically with the genus Acmella Rich. ex Pers. and sometimes with the genus Salmea DC. Comparative morphological and chromosomal studies suggest that these genera are different. The confusion among these species and the misuse of names are very common among traditional and complementary medicine practitioners, herbal users, and particularly among anonymous information sources on the internet [13]. The similar morphological features and ingredient spectra of these species and even genera were and are the cause for this confusion. The anomalies present various problems, such as a large number of overlapping botanical names, synchronous with the formation of frequent and diverse taxonomic revisions, and reclassifications of individual plants expressed in other taxa. Furthermore the traditional uses of these plants and the properties and effects thereof have produced a lot of confusion among earlier researchers. Subsequent genetic, anatomical, morphological, and phytochemical studies showed that although there are indeed close family ties between Spilanthes and Acmella, they can be distinguished by at least eight morphological characters and by distinctive chromosomes. The main morphological difference between the genera Spilanthes and Acmella is that the latter has rayed heads and lacks pappus [14]. Comparative morphological studies showed that Spilanthes spp. has discoid heads and Acmella spp. have rayed heads. The nature of the head is a good taxonomic character for the identification of species in this taxon. Thus the rayed and nonrayed heads seem to be a very reliable characteristic for the broad classification of the genus Spilanthes. In chromosome studies, Spilanthes has a chromosome number of 16, whereas Acmella has 12 or 13 [15]. Jansen recircumscribed the genus and restored the generic status of Acmella, which had long been subsumed to a section under Spilanthes by earlier taxonomists. Studies of other genera within the Heliantheae suggest that both Spilanthes and Acmella are allied to Salmea in the Verbesininae.
The genus Spilanthes is represented by six species in India; they are Spilanthes calva DC., S. paniculata DC., S. radicans Jacq., S. ciliata Kunth, S. uliginosa Sw., and S. oleracea [16]. Typically, these plants are annual herbs or short lived perennials, approximately a half-meter tall with prostrate or ascending cylindrical hairy stems and simple ovate opposite leaves with stipules. They belong to the family Asteraceae, the tribe Heliantheae, and the subtribe Ecliptinae and have characteristic of flower heads, which distinguish individual species [17]. The flower heads are either solitary or occur in compact or spreading inflorescences. Aerial parts are usually hairy or woolly, and the plants occur as herbs or shrublets that are sometimes dwarfed and are aromatic; the roots are hairy. The key identification characteristics of the genera Spilanthes are head solitary, pappus of stiff awns; achenes monomorphic, rhombic, stramineous, cork like margin at maturity, leaves sessile; heads discoid and corollas white to purplish white [18, 19]. The flowers and leaves have a pungent taste, accompanied with tingling and numbness [20, 21].
3. Traditional Medicinal Uses
For this review, the ethnopharmacology and ethnobotanical reports were selected with quantitative data and with special attention to the frequency of citation which prioritization for further possible studies [22]. In the tropics and subtropics, these plants are widely used in traditional medicine. The major use in all these systems of medicine is for toothache where the fresh flower head and/or leaves is chewed or placed in tooth cavities to relieve pain [20, 23–27]. Confusion among various species and the misapplication of names are common place among folk herbalists. Other major traditional uses include the following: in India juice of inflorescence of S. acmella is used to treat mouth ulcers, and the dried fruits of S. calva are powdered and mixed with coconut oil and then used on boils and wounds [28]. Ethiopian traditional healers use the crushed aerial parts in a paste dressing for external injuries [29]. In Nigeria and Sri Lanka, S. acmella and S. uliginosa are used as a sialagogue [11, 30]. The aerial parts of S. africana DC. are crushed and given orally to induce labour during childbirth in western Uganda [31]. In China, S. callimorpha A. H. Moore is used as a fertility regulating agent and for amenorrhea [32]. S. filicaulis (Schumach.&Thonn.) C. D. Adams and S. acmella are used in the treatment of snake bite and rheumatic fever [33, 34]. Plants of this genus are also used to treat parasitic diseases in different traditional systems of medicine [35–37]. For centuries they have been widely cultivated for horticultural, medicinal, insecticidal, and culinary purposes [15], and the application for this purpose is still widespread in different parts of the world.
The uses of the genus Spilanthes in traditional medicine can be generally summarized as follows: (a) oral and throat-related conditions like toothache, tooth decay, tooth infections, sore throats, mouth ulcers, paralysis of the tongue, bleeding from gums, throat complaints, stomatitis, gingivitis, and as a sialagogue; (b) other painful conditions like headache, muscle pain, and rheumatism, rubbed on the skin as a local anesthetic; (c) common cold, fever, and cough; (d) gastrointestinal disorders such as stomach ache, dysentery, gastritis, intestinal diseases, diarrhea, and constipation, as an emetic, for liver trouble, and as a tonic during jaundice; (e) others like diuretic activity and the ability to dissolve urinary calculi as an aphrodisiac, amenorrhea, leucorrhoea, anemia, and fertility regulating agent, on boils and wounds and cuts; (f) as an anti-infective, used as an antibacterial, antifungal, and antiviral, and in tuberculosis and pneumonia; (g) antiparasitic activity like malaria, antitrypanocidal, and other worm infections, for the treatment of head infections accompanied by itchiness and as an insecticidal agent; and (h) as a soup, as a fortifier for infants and to get rid of unpleasant symptoms of an alcoholic hangover. Selected, well-conducted ethnopharmacological surveys are summarized in Table 1.
Table 1.
Reported uses of genus Spilanthes in ethnopharmacological surveys.
| Name of the plant | Type of use | Population or geographic zone | Part used and method | References |
|---|---|---|---|---|
| Spilanthes acmella L. | Toothache and throat complaints | India | Flowers and leaves | [23] |
| Spilanthes acmella Murr. | Toothache, insecticidal, colic, gastrointestinal disorders. Dried leaves are strewn around the home to ward off insect pests; a combination of leaf and flower juice is taken for colic |
Bangladesh | Flowers and leaves | [92] |
| Pain which includes headache, toothache, and muscle pain | Bangladesh | [93] | ||
| Cough | Haryana, India | Whole plant | [94] | |
| Head infections accompanied by itchiness | Jamalpur District, Bangladesh | [92] | ||
| Toothache | Hasanur Hills, Erode, Tamil Nadu, India | Flowers | [95] | |
| Spilanthes acmella (L.) Murray | Anticancer agent | Indonesia | Entire plant Indonesia | [96] |
|
| ||||
| Spilanthes clava L. | Toothache and throat complaints | Apatani tribe of Arunachal Pradesh, India | Leaf | [24] |
| Teeth were brushed with flowers as remedy for toothache | Bangladesh | Flowers | [92] | |
|
| ||||
| Spilanthes calva DC. | Toothache and throat complaints | Theni District, Tamil Nadu, India | Flower | [25] |
| Dry cough | Tribals of Nandurbar District, Maharashtra, India | Two to three inflorescences crushed and mixed in a spoon with honey taken twice a day for 2-3 days | [97] | |
| Tuberculosis | Chakma tribe in Hill Tracts Districts of Bangladesh | Root juice | [98] | |
| Pain | Palani Hills of Tamil Nadu, India | Crushed head inflorescence | [90] | |
|
| ||||
| Spilanthes uliginosa | Sore throats and gums and in paralysis of the tongue | India | Flower | [20] |
|
| ||||
| Spilanthes acmella Murr. | Placed in tooth cavities to relieve pain | Kelantan, Malaysia | Pounded flowers | [26] |
| Decotion of roots and leaves is used as gargle for tooth pain | Philippines | Roots and leaves | [99] | |
| Toothache and dysentery | Saurashtra region, Gujarat, India | Flower | [100] | |
| Leucorrhoea, toothache, anti-inflammatory, astringent, stop bleeding from gums, dysentery, antibacterial, and anemia | Vhabaniganj village, Bogra District, Bangladesh | Leaves and flowers | [101] | |
|
| ||||
| Spilanthes acmella L. | Ulcer in mouth | Karnataka, India | Juice of inflorescence | [102] |
|
| ||||
| Spilanthes calva DC. | Toothache and on boils and wounds | Nagarcoil, India | Fruit dried, powdered, and mixed with coconut oil | [28] |
| Dental caries | Nandurbar District, Maharashtr, India | Root and flower head | [103] | |
|
| ||||
| Spilanthes oleracea | Curing stammering, toothache, stomatitis, and throat complaints | India | Leaves and flowers decoction | [104] |
|
| ||||
| Spilanthes acmella | Sialagogue | Sri Lanka | Flowers tincture | [11] |
|
| ||||
| Spilanthes uliginosa | Sialagogue | Nigeria and Cameroon | Flower | [30] |
|
| ||||
| Spilanthes acmella | Diuretic activity and the ability to dissolve urinary calculi | Uva Province, Sri Lanka | Cold infusion flowers | [11] |
|
| ||||
| Spilanthes leiocarpa DC. | Diuretic activity | Andean people of Canta, Lima, Peru | Leaves and flowers infusion | [105] |
|
| ||||
| Spilanthes calva | Cuts and mud infection | Bhaudaha, Morang, Nepal | Leaves | [106] |
| Cough, cold, and gingivitis | Nawalparasi District, Nepal | Flowers | [107] | |
|
| ||||
| Spilanthes uliginosa | External injury, crushed paste dressing | Ethiopia | Aerial parts | [29] |
|
| ||||
| Spilanthes callimorpha A. H. Moore | Dermatitis | Yunnan Province, China | Aerial part | [108] |
|
| ||||
| Spilanthes africana DC. | Induce labor during childbirth | Western Uganda | Aerial part | [31] |
|
| ||||
| Spilanthes callimorpha A. H. Moore | Amenorrhea | Hong Kong | Whole plant | [32] |
|
| ||||
| Spilanthes mauritiana DC. | Aphrodisiac, treatment of convulsions in children with malaria fever | Mwanga District, Tanzania | Fresh plant | [109] |
|
| ||||
| Spilanthes filicaulis | Snakebite | Ghana | Aerial parts | [33] |
| Emetic | Benin City, Nigeria | Leaves | [110] | |
|
| ||||
| Spilanthes acmella L. | Snakebite and rheumatic fever | — | Entire plant | [34] |
|
| ||||
| Spilanthes caulirhiza (Delile) DC. | Common cold | Masango, Gabon | Leaves are chewed | [111] |
|
| ||||
| Spilanthes acmella Murr. | Fortifier for infants | Madagascar | Leaves soup | [112] |
|
| ||||
| Spilanthes africana DC. | Hypertension | Bafia region, Cameroon | Paste mixed with other plants | [113] |
|
| ||||
| Spilanthes mauritiana DC. | Convulsions in children malaria, pneumonia, and tonsillitis | — | Leaves | [114] |
| Rwanda | Whole plant | [115] | ||
|
| ||||
| Spilanthes filicaulis Jacq. | Peptic ulcer and treatment of tooth decay | Cameroon | Aerial parts | [27] |
| Genital infections | Baham, Cameroon | Whole plant | [116] | |
|
| ||||
| Spilanthes acmella (L.) Murray | Used as an anticancer agent | Indonesia | Entire plant | [117] |
|
| ||||
| Spilanthes filicaulis | Intestinal diseases and diarrhoea | Mbalmayo, Cameroon | Leaves | [118] |
|
| ||||
| Spilanthes paniculata DC. | Constipation | Apatani tribe of Arunachal Pradesh, India | Leaves | [24] |
| Constipation, liver trouble, toothache, worm infection, and as tonic during jaundice | Assam, India | Young stem and leaf | ||
| For the treatment of intestinal worms, constipation, and toothache | Apatani tribe of Arunachal Pradesh, India | Leaves | [119] | |
|
| ||||
| Spilanthes paniculata Wall. DC. | Skin disease | Mt.Yinggelin, Hainan Island, China | Flowers | [120] |
|
| ||||
| Spilanthes paniculata L. | Toothache, cough, and fever | The Nocte, the Nyishi and the Adi in the Eastern Himalayan region of Arunachal Pradesh, India | Leaf, water decoction | [121] |
|
| ||||
| Spilanthes paniculata | Toothache | Tripuri, India | Fresh whole plant | [122] |
| Toothache, tooth infections Flowers are chewed followed by closing of the mouth for 5 minutes followed by gargling with water | Barisal District, Bangladesh | Flowers | [123, 124] | |
| Worm infection | Assam, India | Leaf and flower extracts | [123, 124] | |
| Decoction of plant is used in dysentery and rheumatism and tincture of flowers relieves toothache | Noakhali District, Bangladesh | Whole plant flowers | [125] | |
| Cuts | Amazonian Ecuador | Leaves | [126] | |
|
| ||||
| Spilanthes oleracea Linn. | Malaria | Mali | Flower decoction | [35] |
|
| ||||
| Spilanthes oleracea Jacq. | Antitrypanocidal | Mali | Flower decoction | [36] |
|
| ||||
| Spilanthes filicaulis | Chest pain, eczema, guinea worm, stomach problems, headache, cough, and toothache; an enema for side pain; used to coagulate blood; rubbed on skin as a local anaesthesia | Cameroon | Entire plant | [37] |
| Toothache, stomach ache, gastritis, and malaria | Babungo, Northwest Region, Cameroon | Whole plant | [127] | |
|
| ||||
| Spilanthes callimorpha A. H. Moore | Fertility regulating agent | China | Aerial part | [32] |
|
| ||||
| Spilanthes acmella Murr. | Soup and as a fortifier for infants | Betsimisaraka and Tanala people of Madagascar | Leaves | [112] |
|
| ||||
| Spilanthes acmella (L.) Murray | Get rid of unpleasant symptoms of the alcoholic hangover | Brazil | Leaves | [128] |
|
| ||||
| Spilanthes americana (Mutis) Hieron | Cough | San Jose Succotz in Belize, Argentina | Prepare a tea from the leaves and drink | [129] |
4. Phytochemistry
In this genus, the major phytochemicals present are saturated and unsaturated alkyl ketones, alkamides, hydrocarbons, acetylenes, lactones, alkaloids, terpenoids, flavonoids, and coumarins. They are the main constituents considered responsible for the pharmacological activity. Reported chemical constituents from Spilanthes are summarized in Table 2 and the compound structures are given in Figure 1. Alkylamides are predominantly found in this genus and have been shown to possess varied biological activities. For instance, they act on cannabinoid type 2 receptor dependent and independent and also have been found to possess useful immunomodulatory effects as chemotaxonomic markers [38]. The principal pungent and bioactive N-isobutylamide compound spilanthol 1 is known to modulate chemosensory receptors and ligands associated with these receptors. Other N-isobutylamides such as undeca-2E,7Z,9E-trienoic acid isobutylamide 2 and undeca-2E-en-8,10-diyonic acid isobutylamide 3 [38, 39]; 2E-N-(2-methylbutyl)-2-undecene-8,10-diynamide; 2E,7Z-N-isobutyl-2,7-tridecadiene-10,12-diynamide; and 7Z-N-isobutyl-7-tridecene-10,12-diynamide from S. acmella have been reported [23]. N-Isobutyl-2E,4E,8E,l0Z-dodeca-2,4,8,10-tetraenamide was isolated from aerial parts of S. mauritiana [40]. N-Isobutylamides have also been reported in other species, such as S. alba [41] and S. oleracea [42].
Table 2.
Reported phytochemicals from genus Spilanthes.
| Name of the plant | Type of nucleus | Name of the compound | Part used and method | References |
|---|---|---|---|---|
| Spilanthes acmella | Alkamide | Spilanthol 1, undeca-2E,7Z,9E-trienoic acid isobutylamide 2, and undeca-2E-en-8,10-diynoic acid isobutylamide 3, and | Hexane extract of dried flower buds | [39] |
| Spilanthes acmella | Alkamide | Spilanthol 1, N-2-methylbutyldeca-2E, GZ, 8E-trienamide Q α and β-amyrm esters and sitosterol-O-β-D-glucoslde | Whole plant | [43] |
| Spilanthes acmella | Aliphatic compounds | Lauric, myristic, palmitic linoleic, and linolenic acids as their methyl esters | Whole plant | [130] |
| Spilanthes acmella | Sterols, coumarin | Vanillic acid 18, trans-ferulic acid 21, scopoletin 20, 3-acetylaleuritolic acid 17, β-sitostenone 19, and mixture of stigmasteryl-and β-sitosteryl-3-O-β-D-glucopyranosides | Aerial parts | [2] |
| Spilanthes acmella | Triterpenoidal saponin | Olean-12-en-3-O-beta-D-galactopyranosyl (1→4)-O-alpha-L rhamnopyranoside | Root | [131] |
| Spilanthes acmella | Long chain 2-ketol ester | Acmellonate N-isobutyl-dodeca-2E,4E,8Z,l 0 E-tetraenamide 3 | Ethyl acetate extract Whole plant | [132] |
| Spilanthes oppositifolia | Alkamide | Spilanthol 1 | Aerial parts | [133] |
| Spilanthes alba | Unsaturated amides | Acetylemc amides | Aerial parts | [134] |
| Spilanthes mauritiana | Alkamide | N-isobutyl-2E,4E,8E.lOZdodeca-2,4,8,1O-tetraenamide | Aerial parts | [40] |
| Spilanthes leiocarpa | Terpeniods | β Isocomene, lupeyl acetate, caryophyllen-l,l0-epoxide, and alantolactone, eudesmanolide 13 | Aerial parts | [45] |
| Spilanthes ocymifolia | Amides Terpenoid | N-2-Phenylethylcinnamide, stigmasterol Taraxasterol acetate lupeyl acetate | Leaves | [42] |
| Spilanthes callimorpha | Alkamides | 8,11-dihydroxy-dodeca-2E,4E, 9E-triensaureisobutylamide and 7-hydroxy-trideca-2E, 8E-dien-10, 12-diynoic acid isobutylamide | Whole plant | [135] |
| Spilanthes oleracea L. | Alkamides | Z-Non-2-en-6,8-diynoic acid isobutylamide 3 and (Z)-dec-2-en-6,8-diynoic acid isobutylamide | Whole plant | [41] |
Figure 1.
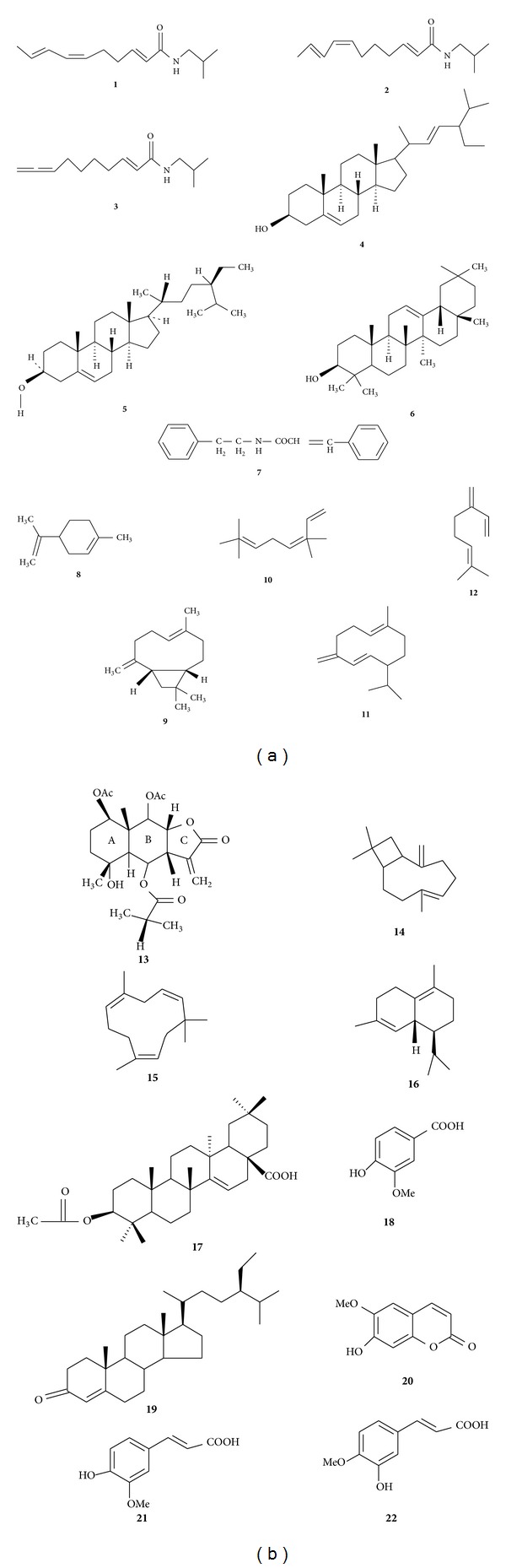
The aromatic amide, N-2-phenylethylcinnamamide 4, which is rarely found in plants was isolated from the leaves of S. ocymifolia [42]. Acetylenes and related compounds were found in S. alba L'Herit, S. americana Hieron, S. mauritiana DC., S. ocymifolia, S. oleracea, S. oleracea, and S. stolonifera DC. Myricyl alcohol, pentacyclic triterpenes α- and β-amyrins, plant sterols β-sitosterol 5, and stigmasterol 6 were isolated from the air-dried whole plant of S. acmella [43, 44]. From the aerial parts of S. leiocarpa, a sesquiterpene β-isocomene, lupeol acetate, an epoxide caryophyllene-l, l0-epoxide, alantolactone, onosenlolide, and the sesquiterpene lactone eudesmanolide were isolated [45, 46]. From the leaves of S. ocymifolia, stigmasterol, the triterpenoid taraxasterol 7, and lupeyl acetate were isolated [42]. A new triterpenoidal saponin olean-12-en-3-O-β-D-galactopyranosyl (1→4)-O-α-L-rhamnopyranoside was isolated from the roots of S. acmella. A steiractinolide derivative was isolated from S. leiocarpa [47], and eudesmanolide was isolated from the aerial parts of S. leiocarpa [46]. Bioassay-guided isolation from S. acmella resulted in the isolation of phenolics, vanillic acid 8, trans-ferulic acid 9, trans-isoferulic acid 10, coumarin, scopoletin 11, triterpenoid 3-acetylaleuritolic acid 12, β-sitostenone 13, stigmasterol, and stigmasteryl-3-O-β-D-glucopyranoside, in addition to a mixture of stigmasterol- and β-sitosteryl-3-O-β-D-glucopyranosides [2]. S. acmella flower heads and S. paniculata have been reported to contain amino acids [48, 49].
4.1. Essential Oil
Spilanthes is one of the oil-rich genera belonging to the family Asteraceae, although only a few species have been explored for their essential oils. Most of the investigations studied the essential oils from plants of this genus by conventional methods of analysis, namely, gas chromatography, in most cases coupled with mass spectrometry. The composition of the essential oil is very variable, suggesting the existence of a high number of chemotypes. From the flower heads of S. acmella volatile constituents were characterized [50]. In the same plant, the presence of a mixture of C22 to C35 hydrocarbons was also reported [51]. Simultaneous distillation extraction (SDE) and supercritical (CO2) extraction from the flowers, leaves, and stems of S. americana resulted in the isolation of volatile metabolites, including sesquiterpenes (α- and β-bisabolenes, β-caryophyllene, α-caryophyllene, and an isomeric hydrocarbon cadinene, nitrogenated alkamides (N-(isobutyl)-2E,6Z,8E-decatrienamide; N-(2-methylbutyl)-2E,6Z,8E-decatrienamide; decatrienamide; N-(isobutyl)-6Z,8E-decadienamide; and N-(2-phenylethyl)-2E,6Z,8E-decatrienamide), and oxygenated compounds have been isolated by simultaneous distillation solvent extraction (SDE). Supercritical fluid extraction (SFE) extracts from the stems were found to be rich (>40%) in sesquiterpenes, while those from leaves and flowers were abundant in nitrogenated (43 and 27%) and oxygenated (36 and 23%) compounds [52]. Seven components from the essential oil have been identified, including the sesquiterpene caryophyllene oxide, caryophyllene, limonene, and myrcene as significantly dominating compounds of the essential oil from the inflorescences of S. calva DC [53].
5. Pharmacological Activities
Spilanthes products are sold as over-the-counter herbal medicines and are commonly recommended by traditional healers and used by patients in many countries, derived from raw plant tissue or plant extracts. There are many websites which sell these herbal supplements for their analgesic, antibacterial, and antifungal properties [13]. S. acmella is one of the most studied species in this genus from a biological perspective, and biological evaluations are generally based on the traditional uses. Because of the chemical variability of the plants, the main identified compounds are cited when available.
5.1. Analgesic and Anti-Inflammatory Activities
Different Spilanthes species are used for toothache and sore throat and to relieve pain from boils, cut wounds, and other types of wounds in traditional medicine (Table 1). The analgesic effects were studied using different extracts and animal models. A 100 mg/kg p.o. dose of the ethanol extract of the fresh leaves of S. acmella and standard pethidine at 5 mg/kg was used in the tail flick response assay in albino rats to determine central analgesic activity, and significant protection (62.95%) was obtained [54]. The analgesic activity of an aqueous extract of S. acmella was tested in the acetic acid-induced writhing response assay in albino mice and the tail flick method in albino rats at doses of 100, 200, and 400 mg/kg. At the end of three hours, the protection from writhing was 46.9%, 51.0%, and 65.6%, respectively [55]. When the isolated compound N-isobutyl-4,5-decadienamide was tested for analgesic activity using the tail flick method in albino rats by applying a thermal method (Hotwire), it showed significant activity in a dose-dependent manner [56]. The aqueous extract of fresh flowers of S. acmella showed significant analgesic activity at doses of 111, 335, and 671 mg/kg when administered to male rats. The activity was in a dose-dependent manner, had a rapid onset and a short duration of action, and was not blocked by naloxone, an opioid receptor antagonist. Consequently, it is assumed that analgesic activity is mediated supraspinally accompanied with sedation [57]. Persistent pain attenuation and hyperalgesia by a cold water extract of S. acmella flowers were evaluated in rats at doses of 500, 1000, and 1500 mg/kg given orally in the formalin test of nociception and carrageenan-induced thermal hyperalgesia test. The extract showed significant activity in a dose-dependent manner [58].
Spilanthes spp. are an important source of anti-inflammatory compounds, and there are numerous studies that validate their use in ethnomedicine for stomatitis, rheumatism, and other painful conditions. A dose of 100 mg/kg p.o. and 500 mg/kg p.o. of an ethanol extract of the fresh leaves of S. acmella exhibited anti-inflammatory activity similar to that produced by 100 mg/kg of acetylsalicylic acid (P < 0.01). An increase in the tail flick reaction time in albino mice as compared to the control, a peak analgesic effect of the extract, was observed after 90 min of administration [54]. An ethanol extract of the fresh leaves of S. acmella was reported to possess potent anti-inflammatory effects in acute (carrageenan-induced), subacute (granuloma pouch), and chronic inflammation (adjutant induced arthritis) models, and in both central (tail flick) and peripheral (glacial acetic acid induced writhing) analgesic assays [54]. An aqueous extract of S. acmella was evaluated for anti-inflammatory activity by the carrageenan-induced rat paw edema assay in albino rats, administering the extract at doses of 100, 200, and 400 mg/kg, which showed 52.6%, 54.4%, and 56.1% inhibition of paw edema, respectively. Aspirin at 100 mg/kg p.o. was used as a standard [55]. Extracts of S. acmella were obtained by extraction with 85% ethanol, followed by liquid partition against hexane, chloroform, ethyl acetate, and butanol. These fractions were tested for anti-inflammatory activity in the lipopolysaccharide-activated murine macrophage model RAW 264.7. The chloroform extract significantly inhibited nitric oxide production (P < 0.01) and was selected for further fractionation to yield the bioactive compound, spilanthol. The diminished levels of LPS-induced inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX-2) mRNA and protein expression support the postulation that spilanthol inhibits proinflammatory mediator production at the transcriptional and translational levels [59].
5.2. Antipyretic Activity
In the antipyretic model, an aqueous extract of S. acmella at doses of 100, 200, and 400 mg/kg reduced the temperature of pyretic rats significantly from the first hour to the third hour, respectively (P < 0.05–0.01). However, the reduction in pyrexia at the fourth hour was not found to be significant, as compared to aspirin [60, 61]. Ethanol and aqueous leaf extracts of S. acmella showed significant antipyretic activity in the TAB vaccine-induced pyrexia model, and the extracts exhibited antipyretic activity at a higher dose level 200 mg/kg/p.o. [60].
5.3. Antioxidant Activity
The ethyl acetate extract of S. acmella showed the most potent antioxidant effect by the DPPH assay. At 200 μg/mL, the ethyl acetate and methanol extracts displayed comparable activity and the highest radical scavenging activity (47.90 and 47.76%) with IC50 values of 216 and 223 μg/mL, respectively 116. The ethyl acetate extract of S. acmella exhibits a stronger free radical scavenging capacity than other fractions, as determined by DPPH and ABTS radical scavenging assays. The chloroform extract significantly inhibited nitric oxide production (P < 0.01) and for further fractionation yielded the active compound, spilanthol [59]. In vitro antioxidant activity of the methanol extracts of the leaves of S. calva was studied using various models, including scavenging of ABTS, DPPH, hydroxyl radical, hydrogen peroxide, lipid peroxidation, nitric oxide, and superoxide radical. The methanol extract was found to have significant antioxidant activity [62].
5.4. Antiulcer Activity
Spilanthes plants are used to treat various types of ulcers, and studies with S. filicaulis aqueous extract showed complete mucosal cytoprotection at doses of 500, 1000, and 1500 mg/kg, respectively, in HCl/EtOH-induced gastric lesions in male Wistar rats [63].
5.5. Local Anesthetic Activity
The local anesthetic activity of an aqueous extract of S. acmella was tested by intracutaneous wheal method in guinea pigs and the plexus method anesthesia in frogs. The test drug, in concentrations of 10% and 20%, produced 70.36% and 87.02% anesthesia, respectively, compared to 97.22% anesthetic effect produced by 2% xylocaine, the standard drug (P < 0.001). In the plexus anesthesia model, the time taken by the animals failing to withdraw their feet was recorded as the “onset of local anesthetic action.” A 20% aqueous extract of S. acmella and 2% xylocaine were used as standard; the extract showed significant activity (P < 0.001). The mean onset of anesthesia with the test drug was faster compared to the standard drug. The onset of local anesthetic activity in the test and standard groups was significantly different from the control group. The anesthetic action of the standard and test drugs continued for 30 minutes. Thus S. acmella has a potent local anesthetic effect [61]. Local anesthetic activity is attributed to the presence of the bioactive alkamides in these extracts.
5.6. Vasorelaxant Activity
Methanol, chloroform, ethyl acetate, and hexane extracts of S. acmella were tested for vasorelaxation on phenylephrine-induced contraction of rat thoracic aorta. Among these, the chloroform extract showed maximum activity; the R max of the chloroform extract was 96.6% (ED50 4.28 × 10−7) and the ethyl acetate extract exerts immediate vasorelaxation (ED50 76.1 ng/mL). All the tested extracts elicited maximal vasorelaxations in a dose-related manner, although such vasorelaxations are less than those produced by acetylcholine. The vasorelaxation effects of the extracts are completely abolished on the removal of endothelial cells [64].
5.7. Diuretic Activity
Based on traditional use, S. acmella flower heads were screened for diuretic activity. Oral administration of a cold water extract of 1500 mg/kg in hydrated rats exhibited strong diuretic action and significantly increased in a dose dependent manner. In addition, the extract caused a marked increase in urinary Na+ and K+ levels and a reduction in the osmolarity of urine, suggesting that it is mainly acting as a loop diuretic, which validates the traditional use of this plant [58]. Recent studies on an ethanolic extract of leaves of S. acmella at 500 mg/kg body weight p.o. showed significant antidiuretic activity. The extract showed increase in total urine volume and electrolyte excretion of sodium, potassium, and chloride ions. The diuretic activity of the extract may be attributed to its alkaloids, flavonoids, and the presence of mono or divalent salts [65].
5.8. Hepatoprotective Activity
A significant hepatoprotective effect has been reported for an ethanolic extract of S. ciliata whole plant at different doses, namely, 100, 200, and 400 mg/kg in Wistar rats against paracetamol-induced hepatic damage. This was evident from decreased levels of serum enzymes and an almost normal histological architecture of the liver, following treatment with the plant extract prior to paracetamol treatment. Furthermore, the extract was also effective in increasing the choleretic activity of anesthetized normal rats, and it also shortened hexobarbitone-induced sleeping time in mice, which was increased by carbon tetrachloride treatment, besides showing significant antilipid preoxidant effects in vitro [66].
Oral administration of an S. ciliata ethanol extract to rats prior to aflatoxin B1 treatment was found to provide significant protection against toxin-induced liver damage, determined [40] hours after the aflatoxin B1 challenge (1.5 mg/kg, i.p.) as evidenced by a significant lowering of the activity of the serum enzymes, and enhanced hepatic reduced glutathione status. Pathological examination of the liver tissues supported the biochemical findings. The plant extracts also showed a significant antilipid peroxidant effect in vitro [67].
5.9. Antiobesity Properties
Aqueous ethanol (70%) extracts of S. acmella flower buds showed pancreatic lipase inhibitory activities in a concentration-(0.75–2.0 mg/mL) dependent manner under in vitro conditions. The extract also inhibited lipase, and this plant has potential as a candidate for weight reduction and obesity control [68].
5.10. Immunomodulatory Studies
An ethanol extract of the leaves of S. acmella was studied for immune stimulatory activity by modulation of macrophage function, carbon clearance assay through Indian ink dispersion in mice (0.5 mL/100 g b.w.i.v.), and for immune prophylactic effects using E. coli in mice (0.5 mL/100 g b.w.i.p.). The extract showed significant (P < 0.01) peritoneal macrophage stimulation and 25–50% mortality as compared to control mice, indicating its prominent immune stimulant activity [69]. Immunomodulatory activity may be due to the presence of alkamides and polysaccharides in these extracts.
5.11. Antimutagenic Studies
A chloroform extract of S. calva flower buds produced dose-dependent inhibition of mutagenicity. S. calva extract showed more significant inhibition (86.4%) of mutagenesis when evaluated using the Ames salmonella/microsome assay [70]. This activity may be due to the presence of abundant flavonoids and alkamides in these extracts.
5.12. Anticancer Activity
S. spirulina was tested for anticancer activity (10 μg/mL–5 mg/mL) by tumoricidal effects in an immortal neuroblastoma of spontaneous malignant origin. The findings indicated no pattern of tumoricidal effects with anticancer screen category 5 and hence are considered weak [71].
5.13. Metabolic Studies
Ethanol extracts from fresh S. acmella were examined with regard to their ability to inhibit cytochrome P4502E1-mediated oxidation of 4-nitrophenol in vitro. The alkylamides present in S. acmella showed significant inhibition at concentrations as low as 25 μM [72].
5.14. Antibacterial and Antifungal Activities
Spilanthes spp. are used to treat infections in traditional medicine specifically in the treatment of mouth wounds, boils, cuts, and respiratory tract infections, but only a few species have been established to have good anti-infective properties. Extracts of several species (Table 3) have been subjected to antibacterial testing using a group of randomly selected bacteria, including the Gram-positive bacteria Bacillus cereus, B. pumilus, B. subtilis, B. cereus, Staphylococcus aureus, Enterobacter faecalis, Pseudomonas aeruginosa, and Corynebacterium diphtheriae and the Gram-negative bacteria E. coli by different methods. Among these, the methanol extract of S. calva was reported to have significant activity [73]. Agar dilution method assays against strains of microorganisms showed that fractions from the chloroform and methanol extracts weakly inhibited the growth of many tested organisms, for example, C. diphtheria NCTC 10356 with minimum inhibitory concentration (MIC) of 64–256 μg/mL and B. subtilis ATCC 6633 with MIC of 128–256 μg/mL 2. Aqueous, ethanol, and hexane extracts of S. americana were tested against S. aureus, B. cereus, Streptococcus hemolyticus, E. coli, P. aeruginosa, and the fungus Candida albicans. Ethanol extracts were active against all the organisms except P. aeruginosa. Hexane extracts were only active against S. hemolyticus and E. coli, while the aqueous extract was inactive against all the above organisms. None of the three extracts showed antifungal activity [74].
Table 3.
Antibacterial and antifungal activities of genus Spilanthes.
| Classification | Species | Tested material | MIC | Active/inactive | References |
|---|---|---|---|---|---|
| Gram + | Staphylococcus aureus | Spilanthes calva methanol extract | 8 μg/mL | Active | [73] |
| Gram + | Bacillus subtilis | Spilanthes calva methanol extract | 8–9.9 μg/mL | Active | [73] |
| Gram + | Bacillus subtilis ATCC 6633 | Fraction of chloroform extract Spilanthes acmella Murr. | 128 μg/mL | Active | [2] |
| Gram + | Bacillus subtilis ATCC 6633 | Fraction of chloroform extract Spilanthes acmella Murr. | 128 μg/mL | Active | [2] |
| Gram + | Bacillus subtilis | Spilanthes calva Methanol extract | 8.0–9.9 μg/mL | Active | [73] |
| Gram + | Bacillus cereus | Fraction of chloroform extract Spilanthes acmella Murr. | 256 μg/mL | Active | [2] |
| Gram + | Corynebacterium diphtheriae NCTC 10356 | Fraction of chloroform extract Spilanthes acmella Murr. | 64 μg/mL | Active | [2] |
| Gram + | Staphylococcus epidermidis ATCC 12228 | Fraction of chloroform extract Spilanthes acmella Murr. | 128 μg/mL | Active | [2] |
| Gram + | Enterobacter faecalis | Spilanthes clava methanol extract | — | Inactive | [73] |
| Gram + | Mycobacter phlei | Spilanthes clava methanol extract | — | Inactive | [73] |
| Gram + | Micrococcus luteus ATCC 10240 | Fraction of chloroform extract Spilanthes acmella Murr. | 128 μg/mL | Active | [2] |
| Gram + | Staphylococcus epidermidis ATCC 12228 | Fraction of chloroform extract Spilanthes acmella Murr. | 128 μg/mL | Active | [2] |
| Gram + | Streptococcus pyogenes II | Fraction of chloroform extract Spilanthes acmella Murr. | 256 μg/mL | Active | [2] |
| Gram + | Micrococcus luteus ATCC 10240 | Fraction of chloroform extract Spilanthes acmella Murr. | 128 μg/mL | Active | [2] |
| Gram + | Streptococcus mutans | Chloroform extract of S. acmella | 250 μg/mL disk (7.5 mm zone) | Active | [136] |
| Gram − | Escherichia coli | Spilanthes calva methanol extract | — | Inactive | [73] |
| Fungi | Candida albicans | Spilanthes calva methanol extract | — | Inactive | [73] |
| Saccharomyces cerevisiae ATCC 2601 | Fraction of chloroform extract Spilanthes acmella Murr. | 256 μg/mL | Active | [2] | |
| Saccharomyces cerevisiae ATCC 2601 | Hexane extract Spilanthes acmella Murr. | 256 μg/mL | Active | [2] |
S. mauritania roots and flowers were tested against 105 strains of bacteria belonging to seven genera, namely, Staphylococcus, Enterococcus, Pseudomonas, Escherichia, Klebsiella, Salmonella, and Mycobacterium and exhibited an MIC and MBC value >8 mg/mL, but mycobacteria were not inhibited at extract concentrations of 0.5–2 mg/mL [75]. S. acmella aqueous alcoholic (90–10%) extracts were found to be inactive against E. coli ATCC 25922, P. aeruginosa ATCC 15442, B. subtilis ATCC 6623, S. aureus ATCC 25923, and C. albicans, C. krusei, C. parapsilosis, and C. tropicalis [76]. S. calva methanol extracts were also inactive [73, 77]. S. mauritiana and S. calva methanol extracts were inactive against mycobacteria, and it was concluded that the aqueous extracts of this genus are usually microbially inactive [74, 76, 77]. Antimycobacterial activity against Mycobacterium tuberculosis H37Ra of the chloroform, methanol, and water extracts of S. acmella was studied and found to be less effective, with the MIC being 0.12–1000 μg/mL [78]. A 95% aqueous ethanol extract of S. acmella and spilanthol at concentrations of 1 mg, 5 mg, and 10 mg was tested for antibacterial activity against S. aureus by the agar diffusion method. All samples were also tested in combination with 5 μg/mL berberine. A 30 μg kanamycin disk was used as a positive control. Spilanthol and the Spilanthes extract showed no zone of inhibition and hence no antibacterial activity at that concentration against S. aureus. The same samples were also tested with 5 mg/mL berberine and showed no additional inhibition beyond the berberine control [79]. In vivo studies showed that spilanthol did not have any antimicrobial activity, whereas the Spilanthes extract showed antimicrobial activity, indicating that other metabolites should be examined for this activity. The aqueous, ethanol, and hexane extracts of S. americana and the methanol extracts of S. calva did not show activity against C. albicans and the chloroform, methanol, and water extracts of S. acmella whole plants did not show significant activity against Entamoeba histolytica at 1,000 μg/mL [73, 74, 77]. Hexane and chloroform extracts of S. acmella completely inhibited the growth of S. cerevisiae with a MIC 256 μg/mL 2. Petroleum ether extracts of S. acmella flower heads were evaluated for antifungal activity against Fusarium oxysporium, Fusarium moniliforme, Aspergillus niger, and Aspergillus parasiticus and good inhibition zones were observed against F. oxysporium [80]. Extracts from S. mauritiana roots and flowers exhibited no activity against Candida spp. [81]. S. iabadicensis whole plant dichloromethane extracts were reported to have antifungal activity against both Cladosporium cucumerinum and C. albicans [82].
5.15. Antiviral Activity
There are only limited reports regarding the antiviral activity of this genus. Leaves of S. mauritiana were explored for antiviral activity with moderate activity shown against HSV, herpes simplex virus, Cox, Coxsackie B2 virus and significant activity against measles, measles edmonston A, polio, poliomyelitis virus type 1 strain 1A/S3,SF, Semliki Forest virus A7 and VSV, and vesicular stomatitis virus T2 [83].
5.16. Antiparasitic and Insecticidal Activities
Antiparasitic activity has been widely studied for Spilanthes because of its extensive range of use in traditional medicine. This work is summarized in Tables 4 and 5. In sub-Saharan countries these plants are used to treat many parasitic diseases, including malaria and sleeping sickness (Trypanosomiasis), and the plant is also used against various insects. Dichloromethane and methanol extracts of S. mauritiana were highly active against Plasmodium falciparum strain D [84]. A methanol extract of the flowers of S. oleracea showed significant activity against Trypanosoma brucei [36, 85]. S. acmella extract was investigated for potential larvicide activity [86] and was highly toxic against larvae of Culex quinquefasciatus with LC50 value 61.43 ppm [87]. Dichloromethane (1 and 5%) and methanol (1 and 5%) extracts of S. stolonifera aerial parts were found to be toxic against Sitophilus oryzae. S. acmella showed acute toxicity against adult Periplaneta americana, and electrophysiological studies indicated immediate hyperexcitation followed by complete inhibition of the cockroach cercal nerve activity [88]. S. acmella stimulated cell growth and differentiation of Herpetomonas samuelpessoai, a nonpathogenic trypanosomatid, used as biological model because of its similar antigens to Trypanosoma cruzi. Crude extracts (1000 μg/mL) or essential oil (250 μg/mL) was added in a defined medium [89]. Cell growth [90] was estimated by counting in Neubauer's chamber, and cell differentiation was examined by light microscopy. S. acmella stimulated cell differentiation, which indicates that this plant contains inhibitors against the parasite [76].
Table 4.
Antiparasitic activities of extracts of Spilanthes.
| Classification | Species | Tested material | MIC (μg/mL) | Other results | References |
|---|---|---|---|---|---|
| Trypanosoma | Trypanosoma brucei | Dichloromethane extract Flowers of S. oleracea Jacq. | 100 | Active | [85] |
| Trypanosoma | Trypanosoma brucei STlB 345 | Flowers of Spilanthes oleracea methanol extract | 10 | Active | [36] |
| Plasmodium | Plasmodium falciparum strain D10 | Cold DCM extract of stems of S. mauritiana (Pers.) DC. | 38 | Active | [84] |
| Dichloromethane and methanol Spilanthes mauritiana (Pers.) DC. | 5.3 | Active |
Table 5.
Insecticidal activities of extracts of Spilanthes.
| Classification | Species | Tested material | Results of the test | References |
|---|---|---|---|---|
| Arthropoda | Sitophilus Oryzae | Dichloromethane (1 and 5%) and methanol (1 and 5%) extract of S. stolonifera DC. aerial part | Toxic | [89] |
| Arthropoda | Larvae of Culex quinquefasciatus | S. acmella | LC50 value 61.43 ppm |
[87] |
| Glyprhelrnins | Physa occidentalis | S. oleracea | 50 (LC100) ppm | [137] |
| Arthropoda | Periplaneta americana adult | S. acmella Murr. | Acute toxic | [88] |
| Cypriniformes | Danio rerio embryos | Spilanthes acmella (Linn.) Murr. 20% aquous extract | No lethel effect | [138] |
6. Clinical Trials
A double-blind clinical trial was carried out in Thammasat University Hospital, Thailand, to examine the effects of S. acmella in reducing postoperative sore throat soreness after endotracheal intubation. The study used a dose of 180 mg S. acmella spray (spilanthol) extract with a sample size of 120 patients. There were no differences in incidence of throat soreness or hoarseness in both recovery (0–2 hr) and postoperative period (24–48 hr), but severity of the sore throat was significantly reduced in the S. acmella extract treated group at 0–2 hr after the operation [91].
7. Food Preservation
Excellent growth restriction by S. acmella extract on the red halophilic cocci isolated from salt cured fish and solar salt was observed. The total viable bacterial count (TVBC) was reported as 4.7, and the total halophile bacterial count was 3.5 (THBC) [139].
8. System for Biocontrol of Parasitic Diseases
Extracts of three Spilanthes species were used to develop a biocontrol system against the late third and early fourth instar larvae of A. stephensi Liston, A. culicifacies, C. quinquefasciatus, S. acmella L. var. oleracea Clarke, S. calva, and S. paniculata; hexane extracts obtained from the flower heads were used. The extracts were potent, with LC50 and LC90 values being 4.57 and 7.83 (A. stephensi), 0.87 and 1.92 (A. culicifacies), and 3.11 and 8.89 ppm (C. quinquefasciatus), respectively. Among the three plant species, S. acmella extract proved to be the most effective in inducing complete lethality at minimum dose [140]. Spilanthol, at 7.5 ppm concentration, caused 100% motility of eggs, larvae, and pupae of Anopheles, Culex, and Aedes mosquitoes at lower doses; it is also effective against eggs and pupae [141]. The extracts were potent, with LC50 and LC90 values being 4.57 and 7.83 (A. stephensi), 0.87 and 1.92 (A. culicifacies), and 3.11 and 8.89 ppm (C. quinquefasciatus), respectively. Among the three plant species, S. acmella extract proved to be the most effective in inducing complete lethality at a minimum dose [140]. Spilanthol found in this genus, at 7.5 ppm concentration, causes 100% motility of eggs, larvae, and pupae of Anopheles, Culex, and Aedes mosquito at lower doses; it is effective against eggs and pupae as well [141].
9. Toxicological Considerations
Reports on the toxicity of this genus are very limited. Acute oral toxicity of the essential oil of the flower heads of S. urens Jacq. was found to be 2000 mg/kg. Irritability to the eyes, skin, and oral and rectal mucosa was carried out using OECD methods, and it was concluded not to be a potential danger in rats [142]. A quasiexperimental double-blind study conducted in Swiss albino mice studied S. americana extract applied topically for a period of 30 days and did not produce any acute toxicity in the tissues of these mice [143]. No adverse effects or mortality were detected in albino rats up to 3 g/kg, p.o. of an aqueous extract of S. acmella during the 24 h observation period [61]. The hexane extract of S. acmella in male Wistar rats was injected i.p. at 50 to 150 mg/kg b.w. of the extract, and EEG and behaviour were observed for periods up to 2 hours. The lower doses (50 and 75 mg/kg) only elicited minor behavioural changes, such as grooming and wet dog shakes. Higher doses (100 to 150 mg/kg) induced full tonic clonic convulsions in a dose-dependent manner, which were accompanied by typical electrographic seizures in the EEG [144]. The USFDA has added S. acmella to the FDA poisonous plant database and listed it as an Indian fish poisons [145]. In Tripura, India, S. paniculata leaf paste is added to stagnant water pools for the intoxication of fish in order to capture them easily, and the Miri tribe of Arunachal Pradesh uses coarsely powdered S. oleracea whole plant in small ponds and streams and waits for 10–15 minutes before collection of fish [146, 147].
10. Biological Activities of Isolated Compounds
Mainly alkylamides are isolated from S. acmella, S. oppositifolia, S. oleracae, S. mauritiana, S. callimorpha, S. oleracea, and S. ocymifolia [39, 40, 43, 130, 133–135, 146, 148]. Generally, these alkyl amides are responsible for sialagogue, local anesthetic, analgesic, antibacterial, and antifungal activities. Among these alkyl amides, spilanthol ((E,E,Z)-2,6,8-decatrienoic acid N-isobutylamide) is considered to be one of the most potent alkylamides found in Spilanthes spp. Many derivatives of spilanthol have been synthesized and evaluated for their trigeminal sensory properties like burning, pungency, tingling, scratching, numbing, warming, mouthwatering, and cooling effects. Spilanthol was found to be the most active tingling and mouthwatering compound among the natural alkamides present in this genus [148].
Alkyl amides isolated from Heliopsis longipes S. F. Blake (Asteraceae) showed good analgesic activity [149], so the ethnopharmacological reports regarding Spilanthes analgesic activity may be due to the alkylamides. The mosquitocidal activity was exhibited by dodeca-2E,4E,8E,10Z-tetraenoic acid isobutylamide, an alkamide isolated from S. mauritiana [40]. Further studies should be aimed at the validation of traditional uses, including for the treatment of hypertension, peptic ulcer, constipation, infertility, amenorrhea, and alcohol abuse. Other reported compounds are vanillic acid, trans-ferulic acid, scopoletin, 3-acetylaleuritolic acid, β-sitostenone, and a mixture of stigmasteryl- and β-sitosteryl-3-O-β-D-glucopyranosides 2. The above compounds lower the support for the folkloric use. The phytochemistry of this genus needs to be explored in more detail to examine the traditional claims. Pharmacological activities of this genus show inconsistent results because the secondary metabolites vary in extraction method and from time to time. For example, the alkylamides obtained from the different parts of Echinacea and Spilanthes plants require a variety of extraction methods. Studies have shown that the concentrations of the alkylamides differ among various parts of Echinacea plant; the roots usually contain higher levels than the aerial parts [150]. An HPLC/ES I-MS validated method for the analysis of the alkylamides in S. acmella was developed to determine the alkylamide content in the flower heads, leaves, and roots [151].
11. Discussion and Outlook
Spilanthes spp. extracts and formulations have been used for centuries in traditional medicine in several parts of the world. Different studies, some of them with controversial methodologies, showed that this genus has diverse pharmacological activities and contains several bioactive compounds, although most of them have not been quantified. Several phytochemicals from these plants with supposed pharmacological activities have been identified as possible candidates; these include alkamides, sterols, coumarins, flavonoids, saponins, terpenoids, and polysaccharides. These plants have been reported to contain greater than 0.5% alkylamides, responsible for the local anesthetic, analgesic, antiseptic, sialogogue, and insecticidal properties of this genus which were likely exploited by traditional medicine practitioners [41].
Alkylamides are fatty acid derivatives whose general structure derives from the condensation of an unsaturated or saturated fatty acid and an amine [152]. Alkylamides are known to modulate plant physiology. N-Acylethanolamines (NAEs) are common in both plants and animals. Animal physiology is regulated by low concentrations of long chain polyunsaturated NAEs, and because of their potential role in cell signaling, NAEs and their analogues appear to have diverse activities, with a number of receptor systems, including CB2 and PPARγ [153]. In vitro studies of alkylamides have shown various modes of anticancer activity for complex extracts [71]. Another in vitro research has shown inhibition of the angiogenesis induced by lung and kidney cancers by an alkylamide containing extract [154]. Structure-activity relationship studies of alkylamides have suggested that they promote differentiation of leukemia to a benign state. Alkylamides at a dose level of 12 mg/kg/day had a significant influence on the phagocytic activity of alveolar macrophages. In addition, the alkylamides caused a dose-dependent increase in NO release from the alveolar macrophages on stimulation with LPS [155]. Recent reports show that these amides demonstrate good bioavailability in humans. Alkylamides also function as cannabinoid receptor 2 (CB2) ligands and have the PPAR receptor and antiviral activity of fatty acid derivatives [156–158]. Alkylamides are abundant in ethanolic extracts that comprise a significant portion of the Spilanthes spp. products purchased by patients. These plant products are prepared by maceration or percolation of the starting plant material in a variable ratio of ethanol and water depending on the plant species, plant part, and manufacturing process. Unfortunately, storage conditions are commonly not assessed, which is frequently unreported factor in research investigating medicinal plant efficacy, leaving a gap in the assessment of the research on medicinal plants, and one possible explanation for the inconsistencies in outcomes in such popular herbal remedies such as Spilanthes spp. [159]. The traditional healers check the quality of Spilanthes spp. extract by the amount of tingling produced by a few drops when placed on their tongue.
Spilanthol, a branched chain, unsaturated aliphatic amide isolated from members of this genus, is a good example of a compound from a traditional medicine which has acted as a drug “lead” in the discovery of many sialagogue compounds, such as N-alkyl-carboxamide derivatives. The presence of flavonoids in this genus [160, 161] justifies its use in traditional medicine for digestive, urinary, analgesic, anti-inflammatory, and skin conditions. The antioxidant effect of these plants may be due to the presence of flavonoids as they act by different mechanisms. For example, some flavonoids inhibit the metabolic activating process of dietary carcinogens, such as aflatoxin Bl. Flavonoids have also been shown to inhibit the cytochrome P450-monooxygenase system, which is involved in the oxidative activation of mutagens. Flavonoids may confer protection through activation of transporters that mediate the extrusion of mutagens from the cell like P glycoprotein and the multidrug resistant protein, MDRP, and detoxifying enzymes like ADPH, quinone reductase, glutathione-S transferase, and so forth. They also block reactive oxygen, nitrogen, and so forth. Metabolites that are involved in mutagenesis, particularly in the context of chronic inflammation, such as flavonoids, inhibit inducible nitric oxide synthase, the main source of NO, and subsequent nitrogen reactive substances in inflammation, and are capable of scavenging these reactive molecules [162–164]. Furthermore, flavonoids have been reported to exhibit a wide range of biological effects, including antibacterial, antiviral, anti-inflammatory, spasmolytic, vasodilator, and hepatoprotective activities. Studies have corroborated that different flavonoid-containing extracts are able to exert an antidiarrheal effect. Thus the antidiarrheal activity of S. filicaulis leaf decoction used in traditional medicine and the antibacterial and anti-adhesive properties of these plants may be due to the presence of flavonoids [64, 118, 165–168]. The above pharmacological activities exerted by flavonoids are established in vitro, but bioavailability studies did not demonstrate that these compounds reach the particular site to initiate a pharmacological effect, and there is a lack of evidence regarding their bioavailability, in vivo enzyme inhibition, pharmacokinetics, and metabolism. Though flavonoids are an important class of compounds, isolation of flavonoids has not been carried out for all Spilanthes species, which may be an important area of research, as this will help in understanding the possible mechanism of action of many therapeutic benefits bestowed by these species.
The antipyretic activity of S. acmella may be because of the flavonoids present in these plants. Phytochemical studies showed the presence of flavonoids in S. acmella. Some flavonoids are predominant inhibitors of either cyclooxygenase or lipoxygenase, so the antipyretic activity may be due to the presence of flavonoids, and again this is proven only in vitro and not in vivo. Several metabolites in these plants are responsible for the diuretic action, although the contribution level of each one to the total diuretic activity is not yet clear. The main active metabolites that can intervene in diuretic activity are flavonoids, saponins, and monovalent and bivalent cations, but some of these substances could be more active on the glomerular level than on the tubule, provoking an increase in renal circulation and, in this way, glomerular filtration rate and primary urine formation. However, the salts could induce a diuretic effect as a result of an osmotic process. These plants may also act as aquaretics, agents that increase water excretion without affecting renal handling of electrolytes and are unlikely to affect edema or hypertension [169, 170]. Thus, these plants may have potential for the treatment of excessive weight, hypertension, congestive heart failure, kidney stones, and premenstrual syndrome.
The five constituent groups currently believed to be the source of activity in the genus Spilanthes spp. are alkamides, coumarins, flavonoids, terpenoids, and polysaccharides. Synergy is often cited as a potential basis of action for many medicinal plant species [171]. Generally, Spilanthes spp. extracts are used more than isolated compounds for commercial use for traditional, cosmetic, pharmaceutical, or medicinal purposes. This raises the question of why Spilanthes spp., as an extract, is superior to isolated compounds. Considering the number of compounds found in a medicinal plant extract, it seems that either pharmacokinetic potentiation or pharmacodynamic enhancement is highly likely. The presence of plant secondary metabolites in an extract may not be sufficient to exert a pharmacological effect; it depends on the bioavailability of the molecules which reach the target sites to produce a required pharmacological effect. Potential synergies also play a major role in enhancing this effect. The complexity of studying these extracts requires models that can assess multiple pathways simultaneously, since ingestion of a medicinal plant product likely activates multiple pathways. The polyphytochemical nature of these extracts adds to the potential of complex signal transduction, which is difficult to assess in current in vitro and in vivo models. However, recent technological breakthroughs such as high throughput screening, gene microarrays, and the “omics” platforms in combination with new constructs, such as network pharmacology, offer more realistic biological maps to study these polyphytochemical extracts and to deduce the activity [172]. As traditional medicine continues to increase in popularity, it has become vital to educate the medical and scientific establishments that there are some features which are unique to phytotherapy, and which contribute to both efficacy and safety.
One of these is the concept of synergy, in that a plant extract is more than the sum of its parts, which will substantiate the perception that natural medicines have something special to offer, at least a scientifically based explanation for the clinical bioequivalence of many plant extracts with synthetic drugs for the same therapeutic indications [173]. Market interest in Spilanthes spp. products in the area of pharmaceutical, cosmetic, and food industries has a good future, while more studies are needed to identify the potential applications and properties which may explain their mechanisms of action. In order to determine the real potential of these products and to develop new technologies, greater understanding is needed to produce enhanced patient compliance.
12. Conclusions
In conclusion, the genus Spilanthes offers a wide range of research possibilities. From a botanical point of view, the large numbers of species are confused with other genera and species and require accurate studies to clarify the controversial aspects in the botanical classification of this genus. Without the correct understanding of the genus and species, all of the phytochemical and pharmacological studies will be controversial. The unique morphological diversity of the genus results in a challenging taxonomy, hence accurate botanical identification is important to achieve authentic biological and phytochemical outcomes. Regarding the pharmacological activity of this genus, the studies that have been performed have justified, to some extent, the traditional uses for these plants and also helped to uncover new pharmacological actions. More pharmacological validations are required to support some of the traditional claims. For example, the common name of this species is toothache plant. Therefore, clinical trials to determine its efficacy in tooth decay and tooth infections are essential. Also, pharmacological studies directed at oral infections are desired to validate this popular claim. Microbiological validation against specific mouth organisms Streptococcus mutans or P. gingivalis is required. The phytochemistry of this genus is complex. So far, the research has been targeted in isolating the alkamides, while more studies are needed to isolate other biologically active compounds. The phytochemistry and biological activity of S. acmella have been studied extensively, whilst other species are less studied, and there are no reports on many of the plants in this genus, which is a lacuna in the research on this genus. It is quite often that the flowers are investigated for their biological, chemical, and traditional uses, while the other parts are unexplored. In this genus, biological activity determinations have only targeted alkamides, mainly spilanthol and related amides. Other metabolites should be studied to validate the wide range of traditional uses of this genus. The genus has been found to be rich in coumarins, flavonoids, terpenoids, and polysaccharides, apart from the alkamides. All these groups have significant biological activity which should be studied, validated, and established. Long-term toxicity studies of alkamides are also needed to weigh the activity and toxicity benefits. The studies carried out with the extracts and purified compounds from these plants support many of their reported uses in traditional medicine as an antimicrobial, antifungal, antiviral, and analgesic for the treatment of genitourinary disorders and as an antinociceptive agent. However, well-controlled, double-blind clinical trials are lacking. In many countries, Spilanthes spp. products are sold as over-the-counter medicine. Thus a safety evaluation needs to be done on these plants immediately. Well-conducted pharmacological and chemical studies are still necessary for several indications of this species. Its use as an analgesic deserves clinical investigation. It is also astonishing to note that a plant so widely used and also available as over-the-counter medicine has very few or only an isolated clinical trial report. Well-directed clinical trials are required to substantiate the traditional use in order to increase the acceptance and patient compliance.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgments
The authors thank Dr. A. G. Pandurangan, Head of Plant Systematics and Evolutionary Science Tropical Botanical Garden and Research Institute (TBGRI) Palode, Kerala, India, for his help with enquiries on name changes and taxonomy of this genus. The authors also thank Dr. John McEwan, Hurstville, NSW, Australia who corrected the English in the paper.
References
- 1.Christophe W. Ethnopharmacology of Medicinal Plants. Asia and the Pacific. Totowa, NJ, USA: Humana Press; 2006. [Google Scholar]
- 2.Prachayasittikul S, Suphapong S, Worachartcheewan A, Lawung R, Ruchirawat S, Prachayasittikul V. Bioactive metabolites from Spilanthes acmella Murr. Molecules. 2009;14(2):850–867. doi: 10.3390/molecules14020850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James ES. Market Assessment of Selected Colombian Natural Products for Putumayo. PHASE I. 2002, http://pdf.usaid.gov/pdf_docs/PNADS460.pdf.
- 4.USPTO. Patent Full-Text and Image Database. Patents (Spilanthes) 2013, http://patft.uspto.gov/netahtml/PTO/search-bool.html.
- 5.A. Vogel herbal-remedies. 2013, http://www.avogel.co.uk/herbal-remedies/spilanthes/
- 6.Herb Pharm. 2013, http://www.herb-pharm.com/
- 7.Gattefosse. 2013, http://www.gattefosse.com/node.php?articleid=42?
- 8.Laboratoires SVR. 2013, http://www.labo-svr.com/
- 9.Anonymous. 2013, http://data.gbif.org/species/browse/taxon/13219744/
- 10.Harold RA, Powell M, King RM, et al. Compositae, XII: Heliantheae. Washington, DC, USA: Smithsonian Institution Press; 1981. Chromosome numbers. [Google Scholar]
- 11.Jayaweer DMA. Medicinal Plants. Part III. Columbo, Sri Lanka: National Science Council of Sri Lanka; 1981. [Google Scholar]
- 12.Altaffer P. Herbs and Botanicals from South America. Ramsey, NJ, USA: Nutraceut World; 2006. [Google Scholar]
- 13.Hind N, Biggs N. Acmella oleracea compositae. Curtis's Botanical Magazine. 2003;20(1):31–39. [Google Scholar]
- 14.Cassini H. Dictionnaire des Sciences Naturelles. Vol. 24. Paris, France: Le Normant; 1822. Spilanthes; pp. 328–331. [Google Scholar]
- 15.Jansen RK. Systematics of Spilanthes (Compositae-Heliantheae) Systematic Botany. 1981;6:231–232. [Google Scholar]
- 16.Sivarajan VV, Remesan C. The genus Spilanthes Jacq. (Composite-Heliantheae) in India. Journal of Economic & Taxonomic Botany. 1987;10:1–3. [Google Scholar]
- 17.Raju CP, Raju RR. Some rare and interesting Asteraceous taxa from the forests of Andhra Pradesh, India . Journal of Economic & Taxonomic Botany. 1996;20:261–263. [Google Scholar]
- 18.Sundara Rajan S. Embryological studies in compositae. IV. A contribution to the life history of Spilanthes acmilla, Murr. (S. calva, Wt. Ic.) Proceedings of the Indian Academy of Science. 1974;79(6):267–282. [Google Scholar]
- 19.Chandra S, Sharma HP, Chandra R, Jha S. Effect of 2,4-D and BAP on Callusing response of Spilanthus Paniculata (D.C) Jansen. Int. J. Mendel. 2006;23(3-4):129–130. [Google Scholar]
- 20.Chopra RN, Nayara SL, Chopra IC. Glossary of Indian Medicinal Plants. New Delhi, India: Council of Scientific and Industrial Research; 1956. [Google Scholar]
- 21.Anonymous. The Wealth of India: A Dictionary of Indian Raw Materials and Industrial Products. Vol. 10. New Delhi, India: Council of Scientific & Industrial Research; 1989. [Google Scholar]
- 22.Verpoorte R, Houghton PJ, Heinrich M, et al. Editorial. Journal of Ethnopharmacology. 2006;103(3):309–310. [Google Scholar]
- 23.Nakatani N, Nagashima M. Pungent alkamides from Spilanthes acmella var. oleracea Clarke. Bioscience, Biotechnology, and Biochemistry. 1992;56(5):759–762. doi: 10.1271/bbb.56.759. [DOI] [PubMed] [Google Scholar]
- 24.Kala CP. Ethnomedicinal botany of the Apatani in the Eastern Himalayan region of India. Journal of Ethnobiology and Ethnomedicine. 2005;1, article no. 11:1–8. doi: 10.1186/1746-4269-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ignacimuthu S, Ayyanar M, Sankarasivaraman K. Ethnobotanical study of medicinal plants used by Paliyar tribals in Theni district of Tamil Nadu, India. Fitoterapia. 2008;79(7-8):562–568. doi: 10.1016/j.fitote.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Ong HC, Nordiana M. Malay ethno-medico botany in Machang, Kelantan, Malaysia. Fitoterapia. 1999;70(5):502–513. [Google Scholar]
- 27.Noumi E, Dibakto TW. Medicinal plants used for peptic ulcer in the Bangangte region, western Cameroon. Fitoterapia. 2000;71(4):406–412. doi: 10.1016/s0367-326x(00)00144-1. [DOI] [PubMed] [Google Scholar]
- 28.Pushpangadan P, Atal CK. Ethnomedical and ethnobotanical investigations among some scheduled caste communities of travancore, kerala, india. Journal of Ethnopharmacology. 1986;16(2-3):175–190. doi: 10.1016/0378-8741(86)90088-7. [DOI] [PubMed] [Google Scholar]
- 29.Teklehaymanot T, Giday M, Medhin G, Mekonnen Y. Knowledge and use of medicinal plants by people around Debre Libanos monastery in Ethiopia. Journal of Ethnopharmacology. 2007;111(2):271–283. doi: 10.1016/j.jep.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Dalziel JM. The Useful Plants of West Tropical Africa. London, UK: Academic Press, Crown Agents for the Colonies; 1937. [Google Scholar]
- 31.Kamatenesi-Mugisha M, Oryem-Origa H. Medicinal plants used to induce labour during childbirth in western Uganda. Journal of Ethnopharmacology. 2007;109(1):1–9. doi: 10.1016/j.jep.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Kong YC, Xie J-X, But PP-H. Fertility regulating agents from traditional Chinese medicines. Journal of Ethnopharmacology. 1986;15(1):1–44. doi: 10.1016/0378-8741(86)90102-9. [DOI] [PubMed] [Google Scholar]
- 33.Abbiw D. Useful Plants of Ghana. London, UK: Intermediate Technology; 1990. [Google Scholar]
- 34.Santesson CG. Einige Drogen aus dem Kamerungebiet und ihreeinheimische verwendung. Arkiv för Botanik. 1926;20:1–34. [Google Scholar]
- 35.Ahua KM, Ioset J-R, Ioset KN, Diallo D, Mauël J, Hostettmann K. Antileishmanial activities associated with plants used in the Malian traditional medicine. Journal of Ethnopharmacology. 2007;110(1):99–104. doi: 10.1016/j.jep.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 36.Bizimana N, Tietjen U, Zessin K-H, et al. Evaluation of medicinal plants from Mali for their in vitro and in vivo trypanocidal activity. Journal of Ethnopharmacology. 2006;103(3):350–356. doi: 10.1016/j.jep.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 37.Ndenecho EN. Herbalism and resources for the development of ethnopharmacology in Mount Cameroon region. African Journal of Pharmacy and Pharmacology. 2009;3(3):078–086. [Google Scholar]
- 38.Raduner S, Majewska A, Chen J-Z, et al. Alkylamides from Echinacea are a new class of cannabinomimetics: cannabinoid type 2 receptor-dependent and -independent immunomodulatory effects. Journal of Biological Chemistry. 2006;281(20):14192–14206. doi: 10.1074/jbc.M601074200. [DOI] [PubMed] [Google Scholar]
- 39.Ramsewak RS, Erickson AJ, Nair MG. Bioactive N-isobutylamides from the flower buds of Spilanthes acmella . Phytochemistry. 1999;51(6):729–732. doi: 10.1016/s0031-9422(99)00101-6. [DOI] [PubMed] [Google Scholar]
- 40.Jondiko IJO. A mosquito larvicide in Spilanthes mauritiana . Phytochemistry. 1986;25(10):2289–2290. [Google Scholar]
- 41.Greger H, Hofer O, Werner A. New amides from Spilanthes oleracea—short communication. Monatshefte für Chemie. 1984;116(2):273–277. [Google Scholar]
- 42.Borges-Del-Castillo J, Vazquez-Bueno P, Secundino-Lucas M, Martinez-Martir AI, Joseph-Nathan P. The N-2-phenylethylcinnamamide from Spilanthes ocymifolia . Phytochemistry. 1984;23(11):2671–2672. [Google Scholar]
- 43.Krishnaswamy NR, Prasanna S, Seshandri TR, Vedantham TNC. α- and β-Amyrin esters and sitosterol glucoside from Spilanthes acmella . Phytochemistry. 1975;14(7):1666–1667. [Google Scholar]
- 44.Tiwari HP, Kakkar A. Phytochemical examination of Spilanthus acemella (Murr.) Journal of the Indian Chemical Society. 1990;67(9):784–785. [Google Scholar]
- 45.Marshall JA, Cohen N. The structure of alantolactone. Journal of Organic Chemistry. 1964;29(12):3727–3729. [Google Scholar]
- 46.Bohlmann F, Ludwig GW, Jakupovic J, et al. Spirosesquiterpenlactone, Germacranolide und Eudesmanolide aus Wunderlichia mirabilis. Liebigs Annalen der Chemie. 1984:228–239. [Google Scholar]
- 47.Bohlmann F, Jakupovic J, Hartono L, King RM, Robinson H. A further steiractinolide derivative from Spilanthes leiocarpa . Phytochemistry. 1985;24(5):1100–1101. [Google Scholar]
- 48.Mondal AK, Parui S, Mandal S. Analysis of the free amino acid content in pollen of nine Asteraceae species of known allergenic activity. Annals of Agricultural and Environmental Medicine. 1998;5(1):17–20. [PubMed] [Google Scholar]
- 49.Dinda B, Guha S. Amino acids from Spilanthes paniculata . Journal of the Indian Chemical Society. 1987;64(6):376–377. [Google Scholar]
- 50.Baruah RN, Leclercq PA. Characterization of the essential oil from flower heads of Spilanthes acmella . Journal of Essential Oil Research. 1993;5(6):693–695. [Google Scholar]
- 51.Baruah RN, Pathak MG. Hydrocarbons from the flower heads of Spilanthes acmella . Journal of Medicinal and Aromatic Plant Sciences. 1999;3:p. 675. [Google Scholar]
- 52.Stashenko EE, Puertas MA, Combariza MY. Volatile secondary metabolites from Spilanthes americana obtained by simultaneous steam distillation-solvent extraction and supercritical fluid extraction. Journal of Chromatography A. 1996;752(1-2):223–232. [Google Scholar]
- 53.Begum J, Bhuiyan MNI, Chowdhury JU. Essential oil from inflorescence of Spilanthes calva D.C. Bangladesh Journal of Botany. 2008;37(2):217–218. [Google Scholar]
- 54.Barman S, Sahu N, Deka S, Dutta S, Das S. Anti-inflammatory and analgesic activity of leaves of Spilanthes acmella (ELSA) in experimental animal models. Pharmacologyonline. 2009;1:1027–1034. [Google Scholar]
- 55.Chakraborty A, Devi RKB, Rita S, Sharatchandra K, Singh TI. Preliminary studies on antiinflammatory and analgesic activities of Spilanthes acmella in experimental animal models. Indian Journal of Pharmacology. 2004;36(3):148–150. [Google Scholar]
- 56.Ansari AH, Mukharya DK, Saxena VK. Analgesic study of N-isobutyl-4,5-decadienamide isolated from the flowers of Spilanthes acmella (Murr) Indian Journal of Pharmaceutical Sciences. 1988;50(2):p. 106. [Google Scholar]
- 57.Peiris KPP, Silva GKJ, Ratnasooriya WD. Analgesic activity of water extract of Spilanthes acmella flowers on rats. Journal of Tropical Medicinal Plants. 2001;2(2):201–204. [Google Scholar]
- 58.Ratnasooriya WD, Pieris KPP, Samaratunga U, Jayakody JRAC. Diuretic activity of Spilanthes acmella flowers in rats. Journal of Ethnopharmacology. 2004;91(2-3):317–320. doi: 10.1016/j.jep.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 59.Wu L-C, Fan N-C, Lin M-H, et al. Anti-inflammatory effect of spilanthol from Spilanthes acmella on murine macrophage by down-regulating LPS-induced inflammatory mediators. Journal of Agricultural and Food Chemistry. 2008;56(7):2341–2349. doi: 10.1021/jf073057e. [DOI] [PubMed] [Google Scholar]
- 60.Vijeyaanandhi M, Vasuki R, Anbu J, Jayakumari S, Sujatha R, Shanmugasundaram P. Antinociceptive and antipyretic activity of aqueous and ethanolic extracts of leaves of Spilanthes acmella . Biomedicine. 2007;27(3):109–112. [Google Scholar]
- 61.Chakraborty A, Devi BRK, Sanjebam R, Khumbong S, Thokchom IS. Preliminary studies on local anesthetic and antipyretic activities of Spilanthes acmella Murr. in experimental animal models. Indian Journal of Pharmacology. 2010;42(5):277–279. doi: 10.4103/0253-7613.70106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Badami S, Channabasavaraj KP. In vitro antioxidant activity of thirteen medicinal plants of India’s Western Ghats. Pharmaceutical Biology. 2007;45(5):392–396. [Google Scholar]
- 63.Tan PV, Njimi CK, Ayafor JF. Screening of some African medicinal plants for antiulcerogenic activity. Phytotherapy Research. 1997;11(1):45–47. [Google Scholar]
- 64.Wongsawatkul O, Prachayasittikul S, Isarankura-Na-Ayudhya C, Satayavivad J, Ruchirawat S, Prachayasittikul V. Vasorelaxant and antioxidant activities of Spilanthes acmella Murr. International Journal of Molecular Sciences. 2008;9(12):2724–2744. doi: 10.3390/ijms9122724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kumar BNS, Swamy BMV, Swamy A, Murali A. A review on natural diuretics. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2010;1(4):615–634. [Google Scholar]
- 66.Suja SR, Latha PG, Rajasekharan S, Pushpangadan P. Antihepatotoxic activity of Spilanthes ciliata . Pharmaceutical Biology. 2003;41(7):536–541. [Google Scholar]
- 67.Shyamal L, Latha PG, Suja SR, et al. Hepatoprotective effect of three herbal extracts on aflatoxin B1-intoxicated rat liver. Singapore Medical Journal. 2010;51(4):326–331. [PubMed] [Google Scholar]
- 68.Ekanem AP, Wang M, Simon JE, Moreno DA. Antiobesity properties of two African plants (Afromomum meleguetta and Spilanthes acmella) by pancreatic lipase inhibition. Phytotherapy Research. 2007;21(12):1253–1255. doi: 10.1002/ptr.2239. [DOI] [PubMed] [Google Scholar]
- 69.Savadi RV, Yadav R, Yadav N. Study on immunomodulatory activity of ethanolic extract of Spilanthes acmella Murr. leaves. Indian Journal of Natural Products and Resources. 2010;1(2):204–207. [Google Scholar]
- 70.Sukumaran K, Kuttan R. Inhibition of tobacco-induced mutagenesis by eugenol and plant extracts. Mutation Research. 1995;343(1):25–30. doi: 10.1016/0165-1218(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 71.Mazzio EA, Soliman KFA. In vitro screening for the tumoricidal properties of international medicinal herbs. Phytotherapy Research. 2009;23(3):385–398. doi: 10.1002/ptr.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raner GM, Cornelious S, Moulick K, Wang Y, Mortenson A, Cech NB. Effects of herbal products and their constituents on human cytochrome P4502E1 activity. Food and Chemical Toxicology. 2007;45(12):2359–2365. doi: 10.1016/j.fct.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rajakaruna N, Harris CS, Towers GHN. Antimicrobial activity of plants collected from serpentine outcrops in Sri Lanka. Pharmaceutical Biology. 2002;40(3):235–244. [Google Scholar]
- 74.Rojas JJ, Ochoa VJ, Ocampo SA, Muñoz JF. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: a possible alternative in the treatment of non-nosocomial infections. BMC Complementary and Alternative Medicine. 2006;6, article no. 2 doi: 10.1186/1472-6882-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fabry W, Okemo PO, Ansorg R. Antibacterial activity of East African medicinal plants. Journal of Ethnopharmacology. 1998;60(1):79–84. doi: 10.1016/s0378-8741(97)00128-1. [DOI] [PubMed] [Google Scholar]
- 76.Holetz FB, Pessini GL, Sanches NR, Cortez DAG, Nakamura CV, Dias Filho BP. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Memorias do Instituto Oswaldo Cruz. 2002;97(7):1027–1031. doi: 10.1590/s0074-02762002000700017. [DOI] [PubMed] [Google Scholar]
- 77.Sawangjaroen N, Phongpaichit S, Subhadhirasakul S, Visutthi M, Srisuwan N, Thammapalerd N. The anti-amoebic activity of some medicinal plants used by AIDS patients in southern Thailand. Parasitology Research. 2006;98(6):588–592. doi: 10.1007/s00436-005-0119-2. [DOI] [PubMed] [Google Scholar]
- 78.Phongpaichit S, Vuddhakul V, Subhadhirasakul S, Wattanapiromsakul C. Evaluation of the antimycobacterial activity of extracts from plants used as self-medication by AIDS patients in Thailand. Pharmaceutical Biology. 2006;44(1):71–75. [Google Scholar]
- 79.Stacy B. A HPLC/ESI-MS method developed and validated to evaluate the quantity, identity, and stability of the alkylamides in ethanolic extracts of Spilanthes acmella [M.S. thesis] Greensboro, NC, USA: University of North Carolina; 2007. [Google Scholar]
- 80.Sabitha AR, Murthy US. Antifungal potential of flower head extract of Spilanthes acmella Linn. African Journal of Biomedical Research. 2006,;9:67–69. [Google Scholar]
- 81.Fabry W, Okemo P, Ansorg R. Fungistatic and fungicidal activity of East African medicinal plants. Mycoses. 1996;39(1-2):67–70. doi: 10.1111/j.1439-0507.1996.tb00087.x. [DOI] [PubMed] [Google Scholar]
- 82.Cavin A, Dyatmyko W, Hostettmann K. Screening of Indonesian plants for antifungal and free radical scavenging activities. Pharmaceutical Biology. 1999;37(4):260–268. [Google Scholar]
- 83.Cos P, Hermans N, De Bruyne T, et al. Further evaluation of Rwandan medicinal plant extracts for their antimicrobial and antiviral activities. Journal of Ethnopharmacology. 2002;79(2):155–163. doi: 10.1016/s0378-8741(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 84.Clarkson C, Maharaj VJ, Crouch NR, et al. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. Journal of Ethnopharmacology. 2004;92(2-3):177–191. doi: 10.1016/j.jep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 85.Aderbauer B, Clausen P-H, Kershaw O, Melzig MF. In vitro and in vivo trypanocidal effect of lipophilic extracts of medicinal plants from Mali and Burkina Faso. Journal of Ethnopharmacology. 2008;119(2):225–231. doi: 10.1016/j.jep.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 86.Pendse GS, Bhide BV, Phalnikar NK. Investigation of new plant larvicides with special reference to Spilanthes acmella . Journal of the Malaria institute of India. 1946;6(3):p. 321. [PubMed] [Google Scholar]
- 87.Pitasawat B, Choochote W, Kanjanapothi D, Panthong A, Jitpakdi A, Chaithong U. Screening for larvicidal activity of ten carminative plants. Southeast Asian Journal of Tropical Medicine and Public Health. 1998;29(3):660–662. [PubMed] [Google Scholar]
- 88.Kadir HA, Zakaria MB, Kechil AA, Azirun MS. Toxicity and electrophysiological effects of Spilanthes acmella Murr. extracts on Periplaneta americana L. Pesticide Science. 1989;25(4):329–335. [Google Scholar]
- 89.Broussalis AM, Ferraro GE, Martino VS, Pinzón R, Coussio JD, Alvarez JC. Argentine plants as potential source of insecticidal compounds. Journal of Ethnopharmacology. 1999;67(2):219–223. doi: 10.1016/s0378-8741(98)00216-5. [DOI] [PubMed] [Google Scholar]
- 90.Ganesan S, Suresh N, Kesaven L. Ethnomedicinal survey of lower Plani hills of Tamilnadu. Indian Journal of Traditional Knowledge. 2004;3:299–304. [Google Scholar]
- 91.Manuwong S, Prapaitrakool S, Nandhasri P, et al. The effect of Spilanthes acmella for reduction of postoperative sore throat after endotracheal intubation. Thai Journal of Anesthesiology. 2006;32(4):247–254. [Google Scholar]
- 92.Rahmatullah M, Mollik MAH, Harun-or-Rashid M, et al. A comparative analysis of medicinal plants used by folk medicinal healers in villages adjoining the Ghaghot, Bangali and Padma rivers of Bangladesh. American-Eurasian Journal of Sustainable Agriculture. 2010;4(1):70–85. [Google Scholar]
- 93.Mollik MAH, Hossan MSH, Paul AK, Taufiq-Ur-Rahman M, Jahan R, Rahmatullah M. A comparative analysis of medicinal plants used by folk medicinal healers in three districts of Bangladesh and inquiry as to mode of selection of medicinal plants. Ethnobotany Research and Applications. 2010;8:195–218. [Google Scholar]
- 94.Panghal M, Arya V, Yadav S, Kumar S, Yadav JP. Indigenous knowledge of medicinal plants used by Saperas community of Khetawas, Jhajjar District, Haryana, India. Journal of Ethnobiology and Ethnomedicine. 2010;6, article no. 4 doi: 10.1186/1746-4269-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Revathi P, Parimelazhagan T. Traditional knowledge on medicinal plants used by the Irula tribe of Hasanur Hills erode district, Tamilnadu India. Ethnobotanical Leaflets. 2010;14:136–160. [Google Scholar]
- 96.Hsu YT. Study on the Chinese drugs used as cancer remedy. Journal of South Asian Researches. 1967;3:p. 63. [Google Scholar]
- 97.Patil HM, Bhaskar VV. Medicinal knowledge system of tribals of Nandurbar District, Maharashtra. Indian Journal of Traditional Knowledge. 2006;5(3):327–330. [Google Scholar]
- 98.Atiqur RM, Uddin SB, Wilcock CC. Medicinal plants used by Chakma tribe in hill tracts districts of Bangladesh. Indian Journal of Traditional Knowledge. 2007;(3):508–517. [Google Scholar]
- 99.Balangcod TD, Balangcod AKD. Ethnomedical knowledge of plants and healthcare practices among the Kalanguya tribe in Tinoc, Ifugao, Luzon, Philippines. Indian Journal of Traditional Knowledge. 2011;10(2):227–238. [Google Scholar]
- 100.Jadeja BA, Nakar RN. Study on ethno-medico botany of weeds from Saurashtra region, Gujarat, India. Plant Archives. 2010;10(2):761–765. [Google Scholar]
- 101.Hossan MS, Hanif A, Agarwala B, et al. Traditional use of medicinal plants in Bangladesh to treat urinary tract infections and sexually transmitted diseases. Ethnobotany Research and Applications. 2010;8:61–74. [Google Scholar]
- 102.Harsha VH, Hebbar SS, Hegde GR, Shripathi V. Ethnomedical knowledge of plants used by Kunabi Tribe of Karnataka in India. Fitoterapia. 2002;73(4):281–287. doi: 10.1016/s0367-326x(02)00078-3. [DOI] [PubMed] [Google Scholar]
- 103.Badgujar SB, Mahajan RT, Kosalge SB. Traditional practice for oral health care in Nandurbar District of Maharashtra, India. Ethnobotanical Leaflets. 2008;12:1137–1144. [Google Scholar]
- 104.Chadha ML. Indigenous vegetables of India with a potential for improving livelihoods. International Symposium on Underutilized Plants for Food Security, Nutrition, Income and Sustainable Development; 2008; http://www.actahort.org/books/806/806_72.htm. [Google Scholar]
- 105.De-la-Cruz H, Vilcapoma G, Zevallos PA. Ethnobotanical study of medicinal plants used by the Andean people of Canta, Lima, Peru. Journal of Ethnopharmacology. 2007;111(2):284–294. doi: 10.1016/j.jep.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 106.Acharya E, Pokhrel B. Ethno-medicinal plants used by Bantar of Bhaudaha, Morang, Nepal. Our Nature. 2006;4(1):96–103. [Google Scholar]
- 107.Bhattarai S, Chaudhary RP, Taylor SL. Ethnomedicinal plants used by the people of Nawalparasi District, Central Nepal. Our Nature. 2009;7:82–99. [Google Scholar]
- 108.Lee S, Xiao C, Pei S. Ethnobotanical survey of medicinal plants at periodic markets of Honghe Prefecture in Yunnan Province, SW China. Journal of Ethnopharmacology. 2008;117(2):362–377. doi: 10.1016/j.jep.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 109.Chhabra SC, Mahunnah RLA, Mshiu EN. Plants used in traditional medicine in Eastern Tanzania. II. Angiosperms (capparidaceae to ebenaceae) Journal of Ethnopharmacology. 1989;25(3):339–359. doi: 10.1016/0378-8741(89)90038-x. [DOI] [PubMed] [Google Scholar]
- 110.Gill LS. Ethnomedicinal Uses of Plants in Nigeria. Benin City, Nigeria: Uniben Press; 1992. [Google Scholar]
- 111.Akendengué B, Louis AM. Medicinal plants used by the Masango people in Gabon. Journal of Ethnopharmacology. 1994;41(3):193–200. doi: 10.1016/0378-8741(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 112.Novy JW. Medicinal plants of the eastern region of Madagascar. Journal of Ethnopharmacology. 1997;55(2):119–126. doi: 10.1016/s0378-8741(96)01489-4. [DOI] [PubMed] [Google Scholar]
- 113.Noumi E, Houngue F, Lontsi D. Traditional medicines in primary health care: plants used for the treatment of hypertension in Bafia, Cameroon. Fitoterapia. 1999;70(2):134–139. [Google Scholar]
- 114.Raza M, Chaudhary MI. Medicinal plants with anticonvulsant activities. Studies in Natural Products Chemistry. 2000;22, part C:507–553. [Google Scholar]
- 115.Hatil HK. Medicinal plants in East and Central Africa: challenges and constraint. Ethnobotanical Leaflets. 2009;13:364–369. [Google Scholar]
- 116.Telefo PB, Lienou LL, Yemele MD, et al. Ethnopharmacological survey of plants used for the treatment of female infertility in Baham, Cameroon. Journal of Ethnopharmacology. 2011;136(1):178–187. doi: 10.1016/j.jep.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 117.Graham JG, Quinn ML, Fabricant DS, Farnsworth NR. Plants used against cancer—an extension of the work of Jonathan Hartwell. Journal of Ethnopharmacology. 2000;73(3):347–377. doi: 10.1016/s0378-8741(00)00341-x. [DOI] [PubMed] [Google Scholar]
- 118.Noumi E, Yomi A. Medicinal plants used for intestinal diseases in Mbalmayo Region, Central Province, Cameroon. Fitoterapia. 2001;72(3):246–254. doi: 10.1016/s0367-326x(00)00288-4. [DOI] [PubMed] [Google Scholar]
- 119.Srivastava RC, Singh RK, Mukherjee TK. Indigenous biodiversity of Apatani plateau: Learning on biocultural knowledge of Apatani tribe of Arunachal Pradesh for sustainable livelihoods. Indian Journal of Traditional Knowledge. 2010;9(3):432–442. [Google Scholar]
- 120.Zheng X-L, Xing F-W. Ethnobotanical study on medicinal plants around Mt.Yinggeling, Hainan Island, China. Journal of Ethnopharmacology. 2009;124(2):197–210. doi: 10.1016/j.jep.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 121.Tangjang S, Namsa ND, Aran C, Litin A. An ethnobotanical survey of medicinal plants in the Eastern Himalayan zone of Arunachal Pradesh, India. Journal of Ethnopharmacology. 2011;134(1):18–25. doi: 10.1016/j.jep.2010.11.053. [DOI] [PubMed] [Google Scholar]
- 122.Majumdar K, Datta BK. A study on ethnomedicinal usage of plants among the folklore herbalists and Tripuri medical practitioners: part-II. Natural Product Radiance. 2007;6(1):66–73. [Google Scholar]
- 123.Chowdhury AR, Jahan FI, Seraj S, et al. A survey of medicinal plants used by Kavirajes of Barisal town in Barisal District, Bangladesh. American-Eurasian Journal of Sustainable Agriculture. 2010;4(2):237–246. [Google Scholar]
- 124.Tamuli P, Sharma P. Ethno-medico-botany of the Dimasa Kachari of North Cachar hills district of Assam. Indian Journal of Traditional Knowledge. 2010;9(4):718–720. [Google Scholar]
- 125.Rahmatullah M, Abdullah-Al-Mahmud A-A, Rahman MA, et al. An ethnomedicinal survey conducted amongst folk medicinal practitioners in the two southern districts of Noakhali and Feni, Bangladesh. American-Eurasian Journal of Sustainable Agriculture. 2011;5(1):115–131. [Google Scholar]
- 126.Friedman J, Bolotin JD, Rios M, et al. A novel method for identification and domestication of indigenous useful plants in Amazonian Ecuador. In: Janick J, Simon JE, editors. New Cropseds. New York, NY, USA: Wiley; 1993. pp. 167–174. [Google Scholar]
- 127.Simbo DJ. An ethnobotanical survey of medicinal plants in Babungo, Northwest Region, Cameroon. Journal of Ethnobiology and Ethnomedicine. 2010;6, article no. 8 doi: 10.1186/1746-4269-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Elisaldo AC, Eliana R, Fulvio RM, et al. Treatment of drug dependence with Brazilian herbal medicines. Brazilian Journal of Pharmacognsosy. 2006;16:690–695. [Google Scholar]
- 129.Arnason T, Uck F, Lambert J, Hebda R. Maya medicinal plants of San Jose Succotz, Belize. Journal of Ethnopharmacology. 1980;2(4):345–364. doi: 10.1016/s0378-8741(80)81016-6. [DOI] [PubMed] [Google Scholar]
- 130.Molinatorres J, Salgado-Garciglia R, Ramirez-Chavez E, Del Rio RE. Purely olefinic alkamides in Heliopsis longipes and acmella (Spilanthes) oppositifolia . Biochemical Systematics and Ecology. 1996;24(1):43–47. [Google Scholar]
- 131.Mukharya DK, Ansari AH. Olean-12-en-3-O-beta-D-galactopyranosyl (1 → 4)-O-alpha-L-rhamnopyranoside: a new triterpenoidal saponin from the roots of Spilanthes acmella (Murr.) Indian Journal of Chemistry. 1987;26(4):86–87. [Google Scholar]
- 132.Ley JP, Blings M, Krammer G, Reinders G, Schmidt C-O, Bertram H-J. Isolation and synthesis of acmellonate, a new unsaturated long chain 2-ketol ester from Spilanthes acmella . Natural Product Research. 2006;20(9):798–804. doi: 10.1080/14786410500246733. [DOI] [PubMed] [Google Scholar]
- 133.Standley PC, Calderon S. Lista Preliminar de las Plantas de El Savador. 1944. [Google Scholar]
- 134.Bohlmann F, Ziesche J, Robinson H, King RM. Neue amide aus Spilanthes alba . Phytochemistry. 1980;19(7):1535–1537. [Google Scholar]
- 135.Li G-P, Shen B-C, Zhao J-F, Yang X-D, Li L. Two new alkamides from Spilanthes callimorpha . Journal of Integrative Plant Biology. 2007;49(11):1608–1610. [Google Scholar]
- 136.Voravuthikunchai SP, Phongpaichit S, Subhadhirasakul S. Evaluation of antibacterial activities of medicinal plants widely used among AIDS patients in Thailand. Pharmaceutical Biology. 2005;43(8):701–706. [Google Scholar]
- 137.Johns T, Graham K, Towers GHN. Molluscicidal activity of affinin and other isobutylamides from the asteraceae. Phytochemistry. 1982;21(11):2737–2738. [Google Scholar]
- 138.Aranya P, Nopadon P, Wanwipa S, Anong B. Toxicity test of Kameng (Eclipta prostrata Linn.) and Kradhuawean (Spilanthes acmella (Linn.) Murr.) to early life stage of zebrafish (Danio rerio) Thai Journal of Veterinary Medicine. 2011;4:523–527. [Google Scholar]
- 139.Prasad MM, Seenayya G. Effect of spices on the growth of red halophilic cocci isolated from salt cured fish and solar salt. Food Research International. 2000;33(9):793–798. [Google Scholar]
- 140.Pandey V, Agrawal V, Raghavendra K, Dash AP. Strong larvicidal activity of three species of Spilanthes (Akarkara) against malaria (Anopheles stephensi Liston, Anopheles culicifacies, species C) and filaria vector (Culex quinquefasciatus Say) Parasitology Research. 2007;102(1):171–174. doi: 10.1007/s00436-007-0763-9. [DOI] [PubMed] [Google Scholar]
- 141.Saraf DK, Dixit VK. Spilanthes acmella Murr.: study on its extract spilanthol as larvicidal compound. Asian Journal of Experimental Sciences. 2002;16(1-2):9–19. [Google Scholar]
- 142.Gaston GSL, Lic AM, Pilot A, et al. Pharmacotoxicological study of the essential oil obtained from the flower of Spilanthes urens Jacq. Anuario de Toxicología. 2001;1:120–125. [Google Scholar]
- 143.Cardona RD, Zuluaga O, Ramirez N. Acute Toxicity of Spilanthes americana in Swiss Albino Mice. 2003. http://iadr.confex.com/iadr/2006Brisb/preliminaryprogram/abstract_76207.htm. [Google Scholar]
- 144.Moreira VM, Maia JG, de Souza JM, Bortolotto ZA, Cavalheiro EA. Characterization of convulsions induced by a hexanic extract of Spilanthes acmella var. oleracea in rats. Brazilian Journal of Medical and Biological Research. 1989;22(1):65–67. [PubMed] [Google Scholar]
- 145.Hooper D. FDA poisonous plant database USFDA #:F25534. Druggists Bulletin. 1989;4:368–369. [Google Scholar]
- 146.Shil S, Dutta M. Indigenous knowledge on healthcare practices by the Reang tribe of Dhalai District of Tripura, North East India. Ethnobotanical Leaflets. 2009;13:775–790. [Google Scholar]
- 147.Hui T, Dass AK, Pallabi K. Plants used by the Miri hill tribe of Arunachel Pradesh in ethanofisheries. Indian Journal of Traditional Knowledge. 2005;4:57–64. [Google Scholar]
- 148.Jakob PL, Gerhard K, Jan L, Gerald R, Heinz JB. Structure-activity relationships of trigeminal effects for artificial and naturally occurring alkamides related to spilanthol. In: Bredie WLP, Petersen MA, editors. Flavour Science: Recent Advances and Trends. Amsterdam, The Netherlands: Elsevier B.V.; 2006. [Google Scholar]
- 149.Rios MY, Aguilar-Guadarrama AB, Gutiérrez MDC. Analgesic activity of affinin, an alkamide from Heliopsis longipes (Compositae) Journal of Ethnopharmacology. 2007;110(2):364–367. doi: 10.1016/j.jep.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 150.Hall C., III echinacea as a functional food ingredient. Advances in Food and Nutrition Research. 2003;47:113–173. doi: 10.1016/s1043-4526(03)47003-4. [DOI] [PubMed] [Google Scholar]
- 151.Stinson SL, Craven BM, Cech NB. HPLC/ESI-MS method validated for the analysis of alkylamides in Spilanthes acmella . Proceedings of the 58th SE Regional Meeting (SERMACS '06); 2006; Augusta, GA, USA. [Google Scholar]
- 152.Gertsch J. Immunomodulatory lipids in plants: Plant fatty acid amides and the human endocannabinoid system. Planta Medica. 2008;74(6):638–650. doi: 10.1055/s-2008-1034302. [DOI] [PubMed] [Google Scholar]
- 153.Gertsch J, Raduner S, Altmann K-H. New natural noncannabinoid ligands for cannabinoid type-2 (CB2) receptors. Journal of Receptors and Signal Transduction. 2006;26(5-6):709–730. doi: 10.1080/10799890600942674. [DOI] [PubMed] [Google Scholar]
- 154.Rogala E, Skopińska-Rózewska E, Wasiutyński A, Siwicki AK, Sommer E, Pastewka K. Echinacea purpurea diminishes neovascular reaction induced in mice skin by human cancer cells and stimulates non-specific cellular immunity in humans. Central-European Journal of Immunology. 2008;33(3):127–130. [Google Scholar]
- 155.Goel V, Chang C, Slama JV, et al. Alkylamides of Echinacea purpurea stimulate alveolar macrophage function in normal rats. International Immunopharmacology. 2002;2(2-3):381–387. doi: 10.1016/s1567-5769(01)00163-1. [DOI] [PubMed] [Google Scholar]
- 156.Karin W, Reginald F, Hartmut D, Veronika B, Rudolf B. Solute and relative bioavailabilities of dodeca-2E,4E,8E,10E/Z-tetraenoic acid isobutylamides after intravenous and oral single doses in rats. BMC Pharmacology. 2009;9:p. 36. [Google Scholar]
- 157.Vimalanathan S, Kang L, Amiguet VT, Livesey J, Arnason JT, Hudson J. Echinacea purpurea aerial parts contain multiple antiviral compounds. Pharmaceutical Biology. 2005;43(9):740–745. [Google Scholar]
- 158.Christensen KB, Petersen RK, Petersen S, Kristiansen K, Christensen LP. Activation of PPARγ by metabolites from the flowers of purple coneflower (Echinacea purpurea) Journal of Natural Products. 2009;72(5):933–937. doi: 10.1021/np900003a. [DOI] [PubMed] [Google Scholar]
- 159.Sun L, Rezaei KA, Temelli F, Ooraikul B. Supercritical fluid extraction of alkylamides from Echinacea angustifolia. Journal of Agricultural and Food Chemistry. 2002;50(14):3947–3953. doi: 10.1021/jf0200265. [DOI] [PubMed] [Google Scholar]
- 160.Jayaraj P, Govindarajan R, Alex R. Isolation of a Flavonoid and Its spectral Analysis from Spilanthes calva DC. Lincoln, Neb, USA: University of Nebraska; 2010. (Periyar Maniammi University Poster Presentation). [Google Scholar]
- 161.Alam M, Azim U, Rahman S, et al. Evaluation of antimicrobial and cytotoxic properties of Leucas aspera and Spilanthes paniculata . International Journal of Biosciences. 2001;50(14):3947–3953. [Google Scholar]
- 162.Moon YJ, Wang X, Morris ME. Dietary flavonoids: Effects on xenobiotic and carcinogen metabolism. Toxicology In Vitro. 2006;20(2):187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 163.Capdevila JH, Falck JR, Harris RC. Cytochrome P450 and arachidonic acid bioactivation: molecular and functional properties of the arachidonate monooxygenase. Journal of Lipid Research. 2000;41(2):163–181. [PubMed] [Google Scholar]
- 164.Yao LH, Jiang YM, Shi J, et al. Flavonoids in food and their health benefits. Plant Foods for Human Nutrition. 2004;59(3):113–122. doi: 10.1007/s11130-004-0049-7. [DOI] [PubMed] [Google Scholar]
- 165.Borgi W, Recio M-C, Ríos JL, Chouchane N. Anti-inflammatory and analgesic activities of flavonoid and saponin fractions from Zizyphus lotus (L.) Lam. South African Journal of Botany. 2008;74(2):320–324. [Google Scholar]
- 166.Bamidele VO, Stephen OO, Kemi D, et al. Analgesic, antiinflammatory and antipyretic activities from flavonoid fractions of Chromolaena odorata . Journal of Medicinal Plants Research. 2008;9(2):219–225. [Google Scholar]
- 167.Wu Y, Wang F, Zheng Q, et al. Hepatoprotective effect of total flavonoids from Laggera alata against carbon tetrachloride-induced injury in primary cultured neonatal rat hepatocytes and in rats with hepatic damage. Journal of Biomedical Science. 2006;13(4):569–578. doi: 10.1007/s11373-006-9081-y. [DOI] [PubMed] [Google Scholar]
- 168.Cushnie TPT, Lamb AJ. Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents. 2005;26(5):343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kerry B. Phytotherapy for recurrent kidney stones. Phytotherapy review and commentary. Townsend Letter for Doctors and Patients. 2005:51–53. [Google Scholar]
- 170.Yarnell E. Botanical medicines for the urinary tract. World journal of urology. 2002;20(5):285–293. doi: 10.1007/s00345-002-0293-0. [DOI] [PubMed] [Google Scholar]
- 171.Kirakosyan A, Kaufman PB, Boik J, et al. Types of interactions of plant metabolites at target sites-synergistic modes of interactions. In: Kaufman PB, editor. Recent Advances in Plant Biotechnology. New Delhi, India: I.K International; 2009. pp. 213–230. [Google Scholar]
- 172.Gertsch J. Botanical drugs, synergy, and network pharmacology: Forth and back to intelligent mixtures. Planta Medica. 2011;77(11):1086–1098. doi: 10.1055/s-0030-1270904. [DOI] [PubMed] [Google Scholar]
- 173.Williamson EM. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8(5):401–409. doi: 10.1078/0944-7113-00060. [DOI] [PubMed] [Google Scholar]


