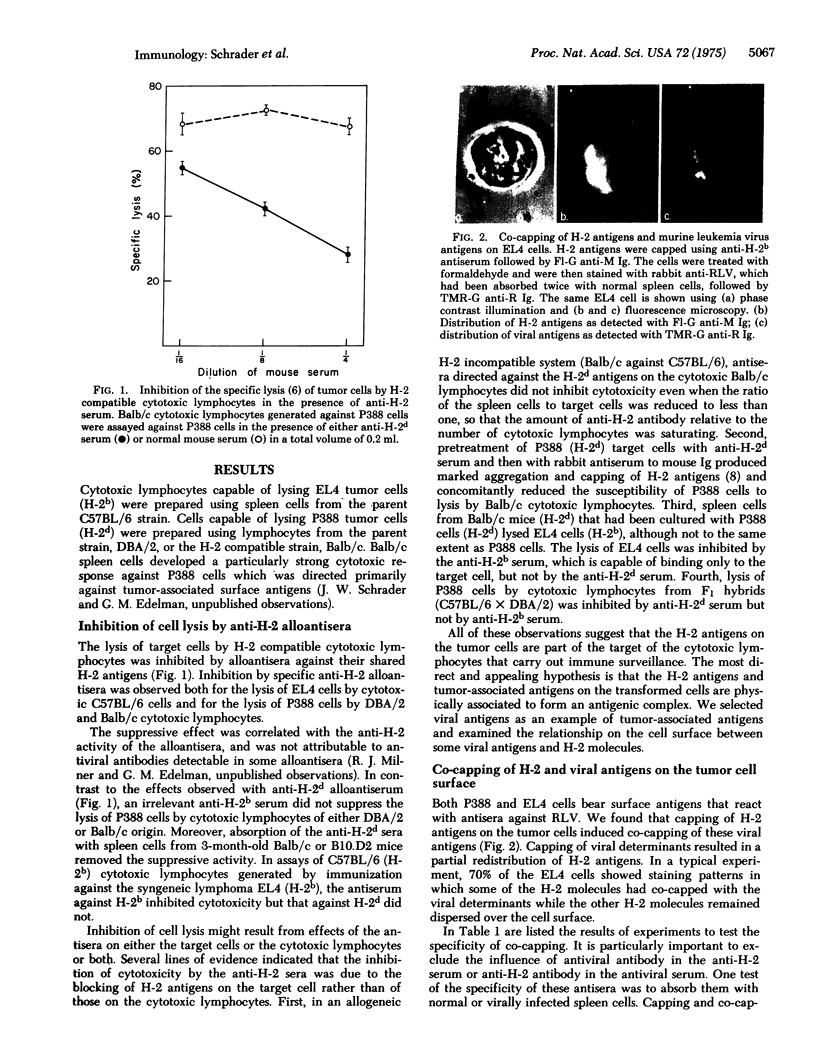
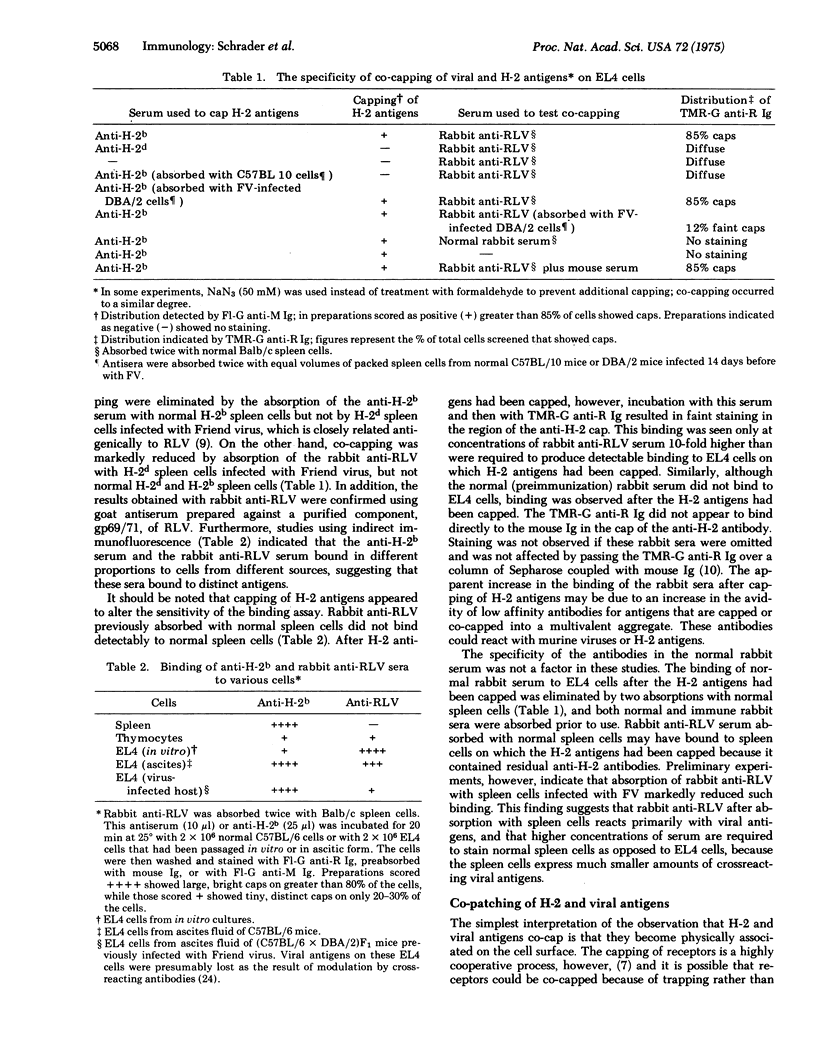
Abstract
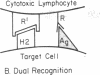
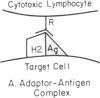
Several lines of evidence are presented to suggest that histocompatibility antigens can be physically associated on the cell surface with viral antigens and possibly other foreign antigens. The lysis of the murine tumor cells EL4 and P388 by syngeneic cytotoxic lymphocytes was inhibited by antisera directed against the H-2 antigens on the tumor cells, consistent with the hypothesis that H-2 antigens are part of the target of the cytotoxic lymphocytes. Moreover, it was found that patching and capping of the H-2 antigens on EL4 cells resulted in the co-patching and co-capping of viral antigens as detected by antisera against Rauscher leukemia virus. Capping of H-2 antigens also resulted in co-capping of determinants detected by an antiserum to the viral protein gp69/71. On the basis of these and other observations, we propose the hypothesis that the H-2 molecules serve as adaptors that combine with viral antigens on the cell surface to form hybrid antigens containing elements of self (H-2) and non-self (virus). The adaptor-antigen complex may then be recognized by a subclass of thymus-derived (T) lymphocytes that possesses a repertoire of receptors directed against hybirds of foreign and H-2 antigens. This raises the possibility that other products of the major histocompatibility complex may have analogous functions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter B. J., Schendel D. J., Bach M. L., Bach F. H., Klein J., Stimpfling J. H. Cell-mediated lympholysis. Importance of serologically defined H-2 regions. J Exp Med. 1973 May 1;137(5):1303–1309. doi: 10.1084/jem.137.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyse E. A., Stockert E., Old L. J. Modification of the antigenic structure of the cell membrane by thymus-leukemia (TL) antibody. Proc Natl Acad Sci U S A. 1967 Sep;58(3):954–957. doi: 10.1073/pnas.58.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny J., Essex M. Membrane antigens of murine leukaemia cells. Nature. 1974 Oct 25;251(5477):742–745. doi: 10.1038/251742a0. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. Enhanced immunological surveillance in mice heterozygous at the H-2 gene complex. Nature. 1975 Jul 3;256(5512):50–52. doi: 10.1038/256050a0. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19(0):89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Dunkley M., Miller R. G., Shortman K. A modified 51Cr release assay for cytotoxic lymphocytes. J Immunol Methods. 1974 Dec;6(1-2):39–51. doi: 10.1016/0022-1759(74)90088-x. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Yahara I., Wang J. L. Receptor mobility and receptor-cytoplasmic interactions in lymphocytes. Proc Natl Acad Sci U S A. 1973 May;70(5):1442–1446. doi: 10.1073/pnas.70.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb P., Feldmann M. The role of macrophages in the generation of T-helper cells. II. The genetic control of the macrophage-T-cell interaction for helper cell induction with soluble antigens. J Exp Med. 1975 Aug 1;142(2):460–472. doi: 10.1084/jem.142.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S., Chen C. H., Sabbadini E., Sehon A. H. Association of tumor and histocompatibility antigens in sera of lymphoma-bearing mice. J Immunol. 1973 Oct;111(4):1093–1100. [PubMed] [Google Scholar]
- Gooding L. R., Edidin M. Cell surface antigens of a mouse testicular teratoma. Identification of an antigen physically associated with H-2 antigens on tumor cells. J Exp Med. 1974 Jul 1;140(1):61–78. doi: 10.1084/jem.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht T. T., Summers D. F. Effect of vesicular stomatitis virus infection on the histocompatibility antigen of L cells. J Virol. 1972 Oct;10(4):578–585. doi: 10.1128/jvi.10.4.578-585.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernizzi G., Parmiani G. Tumour-associated transplantation antigens of chemically induced sarcomata cross reacting with allogeneic histocompatibility antigens. Nature. 1975 Apr 24;254(5502):713–714. doi: 10.1038/254713a0. [DOI] [PubMed] [Google Scholar]
- Koszinowski U., Ertl H. Lysis mediated by T cells and restricted by H-2 antigen of target cells infected with vaccinia virus. Nature. 1975 Jun 12;255(5509):552–554. doi: 10.1038/255552a0. [DOI] [PubMed] [Google Scholar]
- Lindahl K. F., Bach F. H. Human lymphocytes recognise mouse alloantigens. Nature. 1975 Apr 17;254(5501):607–609. doi: 10.1038/254607a0. [DOI] [PubMed] [Google Scholar]
- Nandi S. The histocompatability-2 locus and susceptibility to bittner virus borne by red blood cells in mice. Proc Natl Acad Sci U S A. 1967 Aug;58(2):485–492. doi: 10.1073/pnas.58.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse B. T., Röllinghoff M., Warner N. L. Anti-theta serum-induced supression of the cellular transfer of tumour-specific immunity to a syngeneic plasma cell tumour. Nat New Biol. 1972 Jul 26;238(82):116–117. doi: 10.1038/newbio238116a0. [DOI] [PubMed] [Google Scholar]
- Shearer G. M., Rehn T. G., Garbarino C. A. Cell-mediated lympholysis of trinitrophenyl-modified autologous lymphocytes. Effector cell specificity to modified cell surface components controlled by H-2K and H-2D serological regions of the murine major histocompatibility complex. J Exp Med. 1975 Jun 1;141(6):1348–1364. doi: 10.1084/jem.141.6.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Howard J. C., Nowell P. C. Some biological aspects of lymphocytes reactive to strong histocompatibility alloantigens. Transplant Rev. 1972;12:3–29. doi: 10.1111/j.1600-065x.1972.tb00051.x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974 Oct 11;251(5475):547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]