Abstract
A series of new coordination complexes of Cu(II), Co(II), Ni(II), Zn(II), Hg(II), Mn(II), and Fe(III) with the Schiff base 3-chloro-N′-[(1E)-(2-hydroxy phenyl)methylene]-6-methoxy-1-benzothiophene-2-carbohydrazide (HL) have been synthesized and characterized by elemental analysis, electrical conductivity measurements, IR spectra, 1H NMR, mass spectral data, electronic spectra, magnetic susceptibility, ESR spectra, TGA, and Powder XRD data. The Schiff base behaves as tridentate ONO donor ligand and forms the complexes of the type ML2 (metal-ligand) stoichiometry for Cu(II), Co(II), Ni(II), and Mn(II) complexes and ML stoichiometry for Zn(II), Hg(II), and Fe(III) complexes. All the complexes are colored and nonelectrolytes. It is found that Cu(II), Co(II), Ni(II), Mn(II) and Fe(III) complexes have exhibited octahedral geometry whereas Zn(II) and Hg(II) complexes exhibited tetrahedral geometry. The ligand and its metal complexes have been screened for their antibacterial activity against E. coli and S. aureus and antifungal activity against A. niger and A. flavus.
1. Introduction
Metal complexes with potentially tridentate and tetradentate ligands have evoked much interest in coordination chemistry [1]. Schiff base complexes of transition metals have played prominent role in the development of coordination chemistry [2]. Several Schiff base metal complexes have been studied because of their industrial and biological applications [3–5]. Schiff bases containing polyfunctional groups offer many practical advantages and unique structural environment for complexation [6]. Chen et al. [7] have reported Cu(II) complexes of thiophene-2,5-dicarboxylic acid with a view of constructing diverse low dimensional coordination polymers and the model coordination compounds. Literature survey reveals that many benzothiophene [8–10] derivatives are known to possess good biological activities like antimicrobial, anti-inflammatory, analgesic, diuretic, and antiviral activities.
In view of these findings and in continuation of our research work on coordination chemistry [11–16], we are reporting herewith the synthesis, characterization, and antimicrobial activity of Cu(II), Co(II), Ni(II), Zn(II), Hg(II), Mn(II), and Fe(III) complexes of 3-chloro-N′-[(1E)-(2-hydroxy phenyl)methylene]-6-methoxy-1-benzothiophene-2-carbohydrazide (HL) (Figure 9) in this communication (H refers to phenolate).
Figure 9.
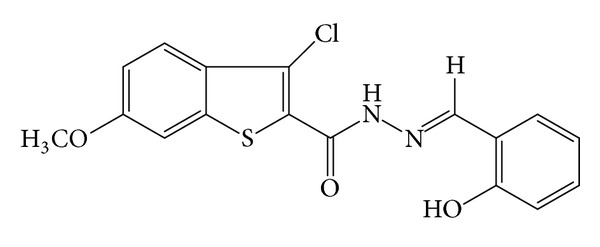
Schiff base HL.
2. Experimental
2.1. Material and Method
All the chemicals are of reagent grade. Solvents were dried and distilled before use according to standard procedure [17]. The precursor 3-chloro-6-methoxy benzothiophene-2-carbohydrazide was prepared by literature method [18]. The absorption of metal and chloride containt of the complexes were carried out by the standard procedure. [19]. The metal chlorides used were in their hydrated form.
2.2. Synthesis of the Ligand HL
An equimolar mixture of 3-chloro-6-methoxy-benzothiophene-2-carbohydrazide (0.001 mol) and salicylaldehyde (0.001 mol) in ethanol (30 mL) was refluxed in presence of catalytic amount of glacial acetic acid (1-2 drops) for about 6 h on water bath. The reaction mixture was cooled to room temperature, and the separated Schiff base (HL) (Figure 9) was collected by filtration, washed with ethanol, dried and recrystallized from absolute ethanol (Scheme 1).
Scheme 1.
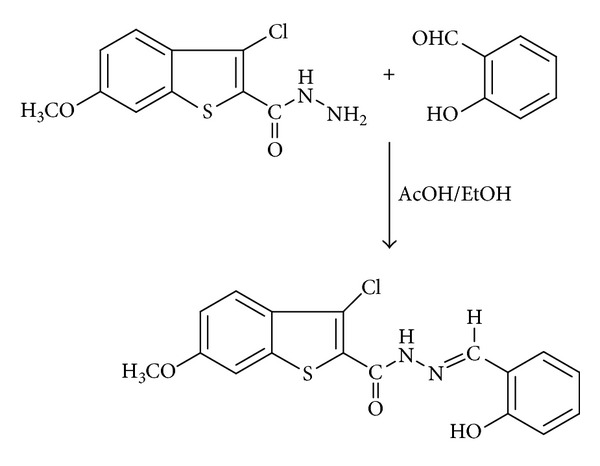
Synthesis of ligand HL.
2.3. Preparation of Cu(II), Co(II), Ni(II), Zn(II), Hg(II), Mn(II), and Fe(III) Complexes of Ligand HL
To the hot solution of 3-chloro-N′-[(IE)-(2-hydroxy phenyl)methylene]-6-methoxy-1-benzothiophene-2-carbohydrazide (HL) (Figure 10) (0.002 mol) in ethanol (30 mL) was added a hot ethanolic solution (10 mL) of respective metal chloride (0.002 mol); the reaction mixture was refluxed on a steam bath for 4 h, then sodium acetate (0.5 g) was added to it and refluxed for further 2 h. It was then poured into distilled water. The resulting solid complexes were collected by filtration, washed with sufficient quantity of distilled water, then with hot ethanol to apparent dryness, and dried in a vacuum over anhydrous calcium chloride in a desiccator (Table 1).
Figure 10.
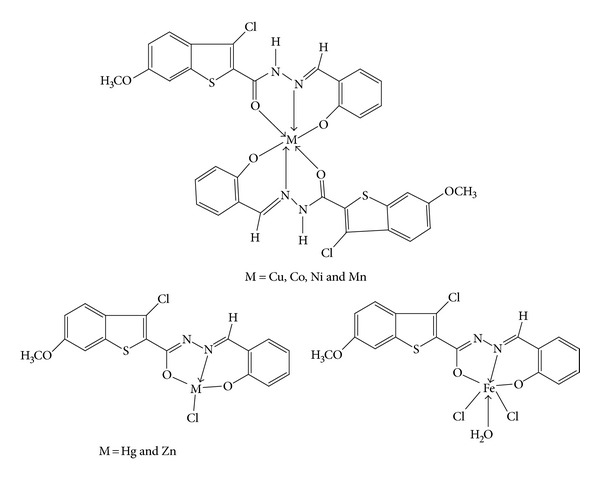
Proposed structure of the complexes of ligand HL.
Table 1.
Physical, analytical, and magnetic susceptibility and molar conductance data of ligand HL and its complexes.
| Compds | Molecular formula | Mol Wt. | M.P °C (yield in %) | Elemental analysis (%) Calcd (found) | Mag. moment µ eff (BM) |
Molar cond. (ƛ
M) ohm−1 cm−2 mol−1 |
Colour | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | C | H | N | Cl | |||||||
| HL | C17H13N2O3SCl | 360.81 | 266–268°C (65) | — | 56.59 (56.41) | 3.63 (3.53) | 7.76 (7.85) | 9.83 (9.60) | — | — | Colorless |
| Cu-complex | Cu(C34H24N4O6S2Cl2) | 783.15 | 308–310°C (70) | 8.11 (8.03) | 52.14 (52.31) | 3.09 (3.03) | 7.15 (7.21) | 9.05 (8.90) | 1.91 | 12 | Brown |
| Co-complex | Co(C34H24N4O6S2Cl2) | 778.55 | 275–278°C (67) | 7.32 (7.51) | 52.45 (52.21) | 3.11 (3.03) | 7.20 (7.25) | 9.11 (8.90) | 5.01 | 14 | Brown |
| Ni-complex | Ni(C34H24N4O6S2Cl2) | 778.55 | 300 above (77) | 7.54 (7.51) | 52.47 (52.29) | 3.11 (3.28) | 7.20 (7.08) | 9.11 (9.17) | 2.90 | 33 | Yellow |
| Zn-complex | Zn(C17H11N2O3SCl2) | 459.67 | 222–225°C (62) | 14.20 (14.10) | 44.32 (44.10) | 2.63 (2.41) | 6.08 (5.91) | 15.39 (15.29) | Diamagnetic | 37 | Yellow |
| Hg-complex | Hg(C17H11N2O3SCl2) | 594.85 | 280–283°C (72) | 33.66 (33.41) | 34.27 (34.05) | 2.03 (1.84) | 4.70 (4.79) | 11.90 (11.69) | Diamagnetic | 11 | Brown |
| Mn-complex | Mn(C34H24N4O6S2Cl2) | 774.55 | 300 above (74) | 7.09 (7.05) | 52.72 (52.66) | 3.12 (3.23) | 7.23 (7.15) | 9.15 (9.00) | 5.68 | 16 | Buff |
| Fe-complex | Fe(C17H13N2O4S1Cl3) | 504.57 | 294–296°C (70) | 11.07 (10.91) | 40.47 (40.27) | 2.80 (2.91) | 5.55 (5.39) | 21.08 (20.87) | 5.91 | 10 | Dark brown |
2.4. Physical Measurements
IR spectra of the synthesized ligand and its complexes were recorded as KBr pellets on Perkins-Elmer Spectrum One FT-IR spectrometer. 1H NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer in DMSO using TMS as an internal standard. UV-visible spectra of the complexes were recorded on Elico-SL 164 spectrometer in the range 200–1000 nm in DMF solution (10−3 M). Mass spectrum of ligand was acquired on MASPEC system. Elemental analysis was obtained from HERAEUS C, H, N–O rapid analyzer. ESR measurement was carried out on a Bruker BioSpin Gmbh spectrometer working at microwave frequency of 9.903 GHz. The experiment was carried out by using diphenylpicrylhydrazyl (DPPH) as reference with field set at 3200 gauss. Magnetic susceptibility was determined by the Faraday method using a model 300 Lewis coil force magnetometer of tesla field strength at room temperature and the instrument was calibrated with [Hg Co(SNC)4] [20].
2.5. Antimicrobial Activity
The in vitro biological screening effects of the investigated compounds were tested against the bacterial species E. coli and S. aureus, fungal species A. niger and A. flavus by the cup plate method at 1 mg/mL concentration.
The bacterial and fungal cultures were inoculated in nutrient broth (inoculation medium) and incubated overnight at 37°C. Inoculated medium containing 24 h grown culture was added aseptically to the nutrient medium and mixed thoroughly to get a uniform distribution. This solution was poured (25 mL in each dish) into Petri dishes and then allowed to attain room temperature. Wells (6 mm in diameter) were punched carefully using a sterile cork borer and were filled with test solution 25 μL. The plates were allowed to stand for an hour in order to facilitate the diffusion of the drug solutions, then the plates were incubated at 37°C for 24 h for bacteria and 48 h for fungi and the diameter of the zone of inhibition was measured [21]. The results were compared with those of standard drug streptomycin for bacterial and fluconazole for fungal activity of the same concentration as that of the test compounds under identical conditions.
2.6. Antioxidant Activity by DPPH Radical Scavenging Activity
The free radical-scavenging activity of the compounds was measured in terms of hydrogen donating or radical scavenging ability using the stable radical DPPH described by Blois method [22–24]. Briefly, stock solution of the sample (0.001 g/mL) was prepared by dissolving it in DMSO. 3 mL solutions of each with varying concentration (25–100 μg) were prepared from the stock solution in methanol. Solution of DPPH (0.01 mM) in methanol was prepared and 1 mL of this solution was added to the above test solutions. The mixture was shaken vigorously and incubated for 30 min, and then the absorbance was measured at 517 nm. All the tests were run in triplicate and expressed as the mean ± standard deviation (S.D). Vit-C and Vit-E were used as standard or positive control, parallel to the test compound and in the absence of the test compound/standard used as the negative control. The capability to scavenge the DPPH radical was calculated using the fallowing equation:
| (1) |
where A o corresponds to the absorbance of the negative control that is without sample; A e is the absorbance of sample with complex or ligand.
3. Results and Discussion
The physical and analytical data of the synthesized ligand HL and its Cu(II), Co(II), Ni(II), Zn(II), Hg(II), Mn(II), and Fe(III) complexes are given in Table 1. The molar conductance of the complexes was measured in DMF at 10−3 M concentration. Measured conductance values of these complexes are too low to account for their electrolytic behavior.
3.1. IR Spectrum of Ligand HL
In the IR spectrum of the ligand HL, a medium intensity band on 3351 cm−1 is assigned to ν NH vibrations and a sharp band of strong intensity at 1651 cm−1 due to ν C=O vibration, respectively [25]. A sharp intensity band observed at 1618 cm−1 is assigned to ν C=N vibrations. Presence of intramolecular hydrogen bonded (O–H … N) vibration of phenolic –OH with nitrogen of the azomethine group of the Schiff base [26] is evidenced by the appearance of a broad band at 2901 cm−1. Vibrations because C–O was observed at 1235 cm−1. The band due to C–S–C of thiophene ring was found to appear at 1518 cm−1 (Table 2).
Table 2.
The IR (in cm−1) data of ligand HL and its complexes.
| Compds | NH/NH | H/OH H2O |
C=O | C=N | Phenolic C–O | C–S–C | C–O–C | >C=N–N=C< | M–Cl | M–N | M–O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ligand HL | 3351 | 2901 | 1651 | 1618 | 1235 | 1518 | 1268 | — | — | — | — |
| Cu-complex | 3267 | — | 1618 | 1535 | 1302 | 1501 | 1268 | — | — | 448 | 564 |
| Co-complex | 3112 | — | 1635 | 1551 | 1317 | 1501 | 1268 | — | — | 445 | 545 |
| Ni-complex | 3351 | — | 1618 | 1560 | 1318 | 1518 | 1269 | — | — | 455 | 510 |
| Zn-complex | — | — | — | 1535 | 1318 | 1535 | 1268 | 1601 | 312 | 418 | 551 |
| Hg-complex | — | — | — | 1551 | 1318 | 1535 | 1268 | 1601 | 315 | 424 | 519 |
| Mn-complex | 3334 | — | 1618 | 1151 | 1314 | 1518 | 1270 | — | — | 415 | 505 |
| Fe-complex | 3317 | 3417 | 1585 | 1551 | 1302 | 1502 | 1268 | — | 314 | 427 | 516 |
3.2. IR Spectra of Cu(II), Co(II), Ni(II), Zn(II), Hg(II), Mn(II), and Fe(III) Complexes of Ligand HL
Appearance of band in the region 3112–3334 cm−1 in the IR spectra of Cu(II), Co(II), Ni(II), Mn(II), and Fe(III) complexes of ligand HL is assigned to ν NH of amide function [27]; further appearance of strong intensity bands in the region 1585–1635 cm−1 with a shift in the lower frequency side in these complexes by 16–66 cm−1 when compared to C=O of ligand which appear at 1651 cm−1 indicates the involvement of C=O oxygen atom in bonding with metal ions as such without undergoing enolization.
But in case of Zn(II) and Hg(II) complexes of ligand HL, disappearance of the band due to ν NH of amide function [28] and carbonyl function which were found to appear at 3351 and 1651 cm−1, respectively, in case of ligand clearly indicates the enolization of carbonyl function and subsequently co-ordination of enolized carbonyl oxygen to Zn(II) and Hg(II) ion via deprotonation [27]. The fact of enolization of amide function was further conformed by the appearance of a new band (enhanced intensity) in the region 1601 cm−1 in the case of these Zn(II) and Hg(II) complexes. Medium intensity band which appeared at 1618 cm−1 in case of ligand HL was found to be shifted to lower frequency side by 58–83 cm−1 and appeared in the region 1535–1560 cm−1 in all these complexes of ligand HL suggesting the coordination of the nitrogen atom of the azomethine function of ligand with metal atoms. In the IR spectra of all these complexes, the absence of band due to intramolecular hydrogen bonded phenolic OH vibration which appeared at 2901 cm−1 in ligand clearly indicates the deprotonation of hydrogen bonded phenolic OH group during complexation with metal ions. The band due to phenolic C–O function observed at 1235 cm−1 in case of ligand HL has been found to be shifted to higher frequency side by 83–67 cm−1 in the complexes of the ligand HL under present study and appeared in the region 1302–1318 cm−1, confirms the coordination of phenolic oxygen with the metal ions via deprotonation.
The thiophene ring vibration of the ligand which appeared at 1518 cm−1 in case of ligand remained unaffected in all the complexes and appeared at about the same region 1501–1535 cm−1 which rules out any possibility of coordination by ring sulphur atom to metal ions.
Assignments in the far IR region are purely tentative because of various skeletal vibrations associated with metal ligand vibrations. The bands of weak intensity 564–505 cm−1 in case of all the complexes of ligand HL are assigned to ν M−O vibrations. The absorption bands which appeared in the region 455–415 cm−1 in case of all the complexes are assigned to ν M−N vibrations. In Zn(II), Hg(II), and Fe(III) complexes ligand HL, new bands observed in the region 315–312 cm−1 are assigned to ν M−Cl vibrations. The broad band that appeared in the region 3417 cm−1 in case of Fe(III) complexes of ligand HL is assigned to ν OH vibration of the coordinate or lattice water molecules. The IR spectral data of the ligand HL and its complexes are tabulated in Table 2.
3.3. 1H NMR Spectrum of the Ligand HL
The 1H NMR spectrum (in ppm δ) of the ligand HL (Figure 1) which displayed a fine broad singlet observed at 11.21 (s, 1H, OH) is assigned to intramolecular hydrogen bonded proton of OH group with azomethine nitrogen. Another broad singlet observed at 11.20 (s, 1H, CONH) is assigned to proton of CONH function. A sharp singlet at 8.69 (s, 1H, N=CH) is due to azomethine proton. The three protons of methoxy group have resonated as a sharp singlet at 3.82 (s, 3H, OCH3). Seven aromatic protons have resonated as multiplet in the region of 6.85–7.74 (m, 7H, ArH).
Figure 1.
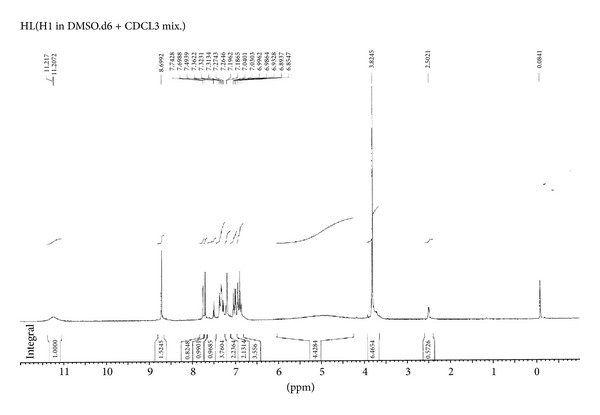
1H NMR spectrum of ligand HL.
3.4. Mass Spectrum of the Ligand HL
In the mass spectrum of the ligand HL (Figure 2), the molecular ion peak was observed at m/z 360, 362 (96%, 33%). The molecular ion underwent fragmentation to give fragment ions at m/z 241, 243 (92%, 32%) due to the expulsion of C7H5NO radical from the molecular ion. This fragment ion on further simultaneous loss of CS and HCNO species gave another fragment ion recorded at m/z 154, 156 (100%, 31%), which is also a base peak. This fragment ion further loses a chloride radical and gave a fragment ion recorded at m/z 119 (3%), (Scheme 2).
Figure 2.
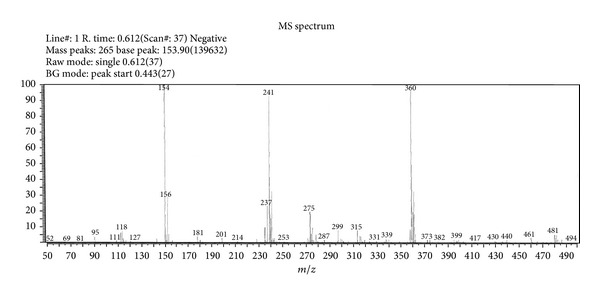
Mass spectrum of ligand HL.
Scheme 2.
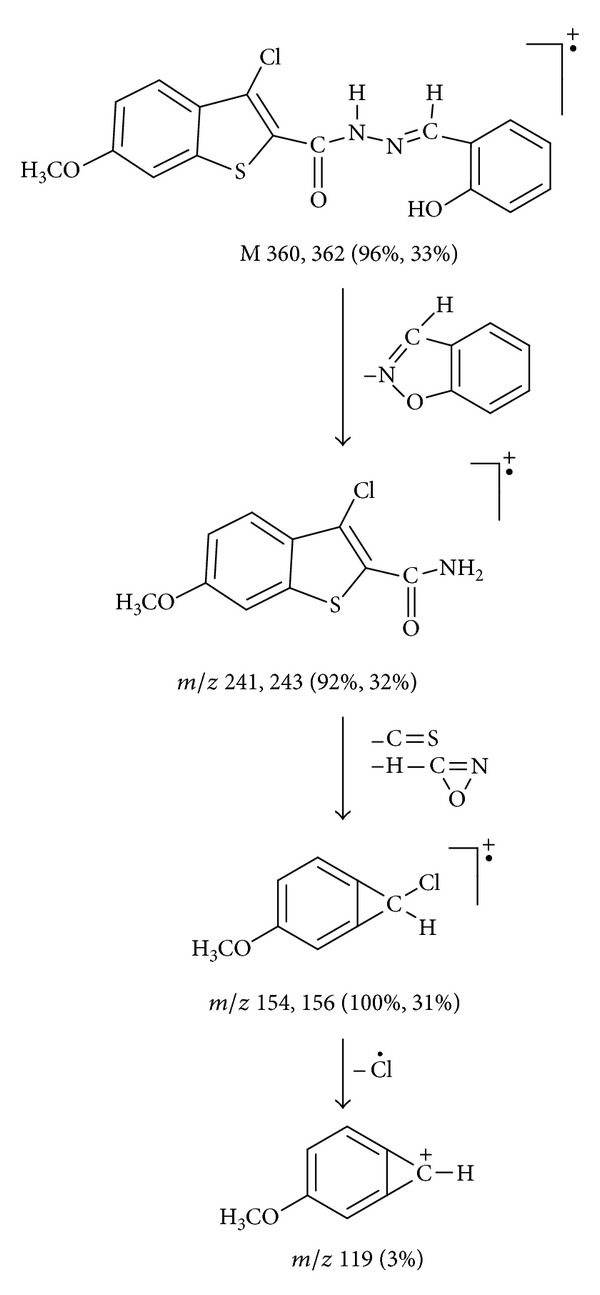
Mass fragmention pattern of ligand HL.
3.5. Electronic Spectra of Cu(II), Co(II), Ni(II), Mn(II), and Fe(III) Complexes of the Ligand HL
Electronic spectral data of the Cu(II), Co(II), Ni(II), Mn(II), and Fe(III) complexes of the ligand HL are given in Table 3. Electronic spectral studies of all these complexes were carried out in DMF at 10−3 M concentration.
Table 3.
Electronic and EPR spectral data of complexes of the ligand HL.
| Compds | Electronic spectral data (in cm−1) | ESR data | ||||||
|---|---|---|---|---|---|---|---|---|
| ν 1 | ν 2 | ν 3 | ν 4 | g ⊥ | g II | g av | G | |
| Cu-complex | 13485 | 18728 | — | 33870 | 2.07 | 2.36 | 2.171 | 5.14 |
| Co-complex | 10772 | 15961 | 19511 | — | — | — | — | — |
| Ni-complex | 10590 | 16598 | 25617 | — | — | — | — | — |
| Mn-complex | 17218 | 23435 | 25949 | 31434 | — | — | — | — |
| Fe-complex | 16429 | 20524 | 25635 | — | — | — | — | — |
3.5.1. Cu(II) Complex
The electronic spectrum of Cu(II) complex derived from the ligand HL showed three bands at 13485, 18728, and 33870 cm−1. Rao et al. [29] has suggested octahedral geometry for Cu(II) complex due to Schiff base ligand. These complexes displayed a band in the region 13000–19000 cm−1 due to d-d transitions. Shashidhara et al. [30] have observed a broad band 15174 cm−1 to Cu(II) complex which is assigned to 2Eg-2T2g and is a characteristic of distorted octahedral geometry. The observed broad band in the case of present Cu(II) complex of ligand HL can be assigned 2B1g → 2Eg, 2B2g, and 2Atg transition suggesting distorted octahedral geometry of Cu(II) complexes.
3.5.2. Co(II) Complex
Co(II) is d7 ion that exists both in octahedral and tetrahedral geometry. In octahedral Co(II) complexes three spin allowed transitions are expected corresponding to the transitions
4T1g(F)→4T2g(F) (ν 1) (~8000 cm−1)
4T1g(F)→4A2g(F) (ν 2) (~16000 cm−1)
4T1g(F)→4T2g(P) (ν 3) (~20000 cm−1).
Patel et al. [31] have reported three bands corresponding to ν 1, ν 2, and ν 3 transition around 9000 cm−1, 14500 cm−1, and 20620 cm−1, respectively, for octahedral Co(II) complex. The Co(II) complex of the ligand HL under present study has showed three bands at 10772 cm−1, 15961 cm−1, and 19511 cm−1 due to 4T1g(F) → 4T2g(F) (ν 1), 4T1g(F) → 4A2g(F) (ν 2), and 4T1g(F) → 4T2g(P) (ν 3) transition, respectively. These transitions suggest octahedral geometry for Co(II) complex.
3.5.3. Ni(II) Complex
The ground state of Ni(II) in octahedral coordination is 3A2g(t2g 6eg2). The Ni(II) complex shows three transitions in an octahedral field, namely,
-
3A2g(F) → 3T2g(F) (ν 1) (7000–13000 cm−1)
-
3A2g(F) → 3T1g(F) (ν 2) (11000–20000 cm−1)
-
3A2g(F) →3T1g(P) (ν 3) (20000–27000 cm−1).
The bands in the region ~10000, ~12000, and ~25000 cm−1 3A2g(F) →3T1g(P) (ν 3), 3A2g(F) → 3T1g(F) (ν 2), and 3A2g(F)→3T2g(F) (ν 1) for the transitions mentioned above are characteristics of octahedral geometry.
The electronic spectrum of Ni(II) complex of the ligand HL under present investigation exhibited three bands in the region 10590 cm−1, 16598 cm−1, and 25617 cm−1 which are assigned to 3A2g(F)→3T2g(F) (ν 1), 3A2g(F)→3T1g(F) (ν 2), and 3A2g(F)→3T1g(P) (ν 3) transition. All these observations favor the octahedral geometry for Ni(II) complex of the present study.
3.5.4. Mn(II) Complex
The ground term of Mn(II) ion is in the sextet. The only sextet term of the d5 configuration in octahedral stereochemistry is the 6A1g. The transitions of the spectrum are assigned as from the 6A1g ground term to the quartet excited term. Electronic absorption spectra of Mn(II) octahedral complex are expected to show four spin allowed transitions. The four narrow absorption bands approximately around 18000 cm−1, 24750 cm−1, 29500 cm−1, and 31900 cm−1 were assigned to 6A1g→4T1g(4G) (ν 1), 6A1g →4Eg(4G) (ν 2), 6A1g →4Eg(4D) (ν 3), and 6A1g→4T1g(4P) (ν 4) transitions, respectively, for octahedral Mn(II) complex [32]. The Mn(II) complex of the ligand HL under present study has exhibited four absorption bands at 17218, 23435, 25949, and 31434 cm−1, which corresponds to ν 1, ν 2, ν 3, and ν 4 transitions, respectively, suggesting octahedral geometry for the Mn(II) complex.
3.5.5. Fe(III) complex
The electronic spectrum of Fe(III) complex displays three bands at 16429, 20524, and 25635 cm−1, which corresponds to ν 1, ν 2, and ν 3. This may be assigned to 6A1g→4T1g, 6A1g→4T2g, and 6A1g→4T1g, 4Eg transitions, respectively, typical of an octahedral geometry. These observed values for Fe(III) complex in its visible spectrum are in agreement with the literature values [33] and thereby proved to have octahedral geometry for the Fe(III) complex of the ligand HL.
3.6. Magnetic Susceptibility Data
Magnetic susceptibility measurements of the complexes were performed at room temperature. The magnetic moment for Cu(II) complex of the ligand HL is 1.91 BM. The reported value for the mononuclear Cu(II) having no major spin interaction is 1.75–2.20 BM [34, 35]. Thus the present Cu(II) complex is devoid of any spin interaction with distorted octahedral geometry. In octahedral Co(II) complex, the ground state is 4T1g and a large orbital contribution to the singlet state lowers the magnetic moment values for the various Co(II) complexes which is in the range 4.70–5.20 BM. In the present investigation the observed magnetic moment value for Co(II) complex is 5.01 BM which indicates octahedral geometry for the Co(II) complex. For Ni(II) complex the observed magnetic moment value is 2.90 BM which is well within the expected range for Ni(II) complex with octahedral stereochemistry 2.83–4.00 BM [36, 37]. For Mn(II) and Fe(III) complexes the observed magnetic moment value is 5.68 BM and 5.91 BM, respectively, which are characteristic of octahedral geometry (Table 1).
3.7. ESR Spectral Studies of the Cu(II) Complex of the Ligand HL
The X-Band ESR spectrum of the powder Cu(II) complex was recorded at room temperature using DPPH as reference standard. One unpaired electron in Cu(II) complex with 2B1g as ground state lies in dx 2-y 2orbital and follows the trend g ||>g ⊥>g e (g e = 2.0036 free ion value).
The observed g || = 2.36, g ⊥ = 2.07 values of the Cu(II) complex under the present study followed the same trend g ||>g ⊥>g e which suggest the presence of unpaired electron in dx 2-y 2orbital giving octahedral geometry [35]. The observed G = 5.14 for the complexes under present study evidenced the monomeric nature of the complexes; this fact is further supported by the absence of a band corresponding to ΔMS = ± 2 transition in the observed ESR spectrum which is characteristic of monomeric complex (Table 3).
Consider
| (2) |
3.8. Thermogravimetric Analysis
Thermogravimetry (TG) is a technique in which the change in mass of the sample is determined as a function of temperature and/or time. Among all the methods, TG is the most widely used one. From TG curve, information related to the thermal stabilities, composition of the initial sample, intermediate compounds that are formed, and the final residue could be obtained (Figure 3).
Figure 3.
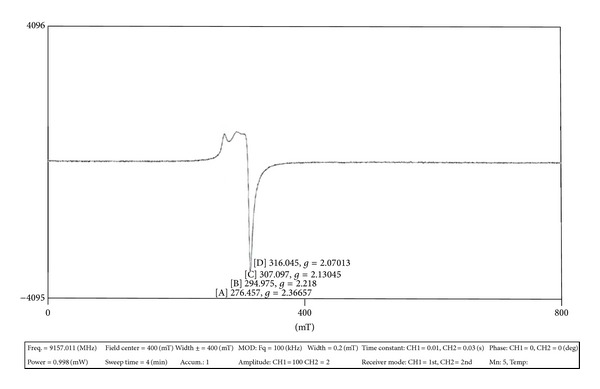
ESR spectrum of Cu(II) complex.
The TGA study on [Cu(HL)2] and [Co(HL)2] was carried out in the temperature range 38.69°C to 800°C.
3.8.1. Cu(II) Complex
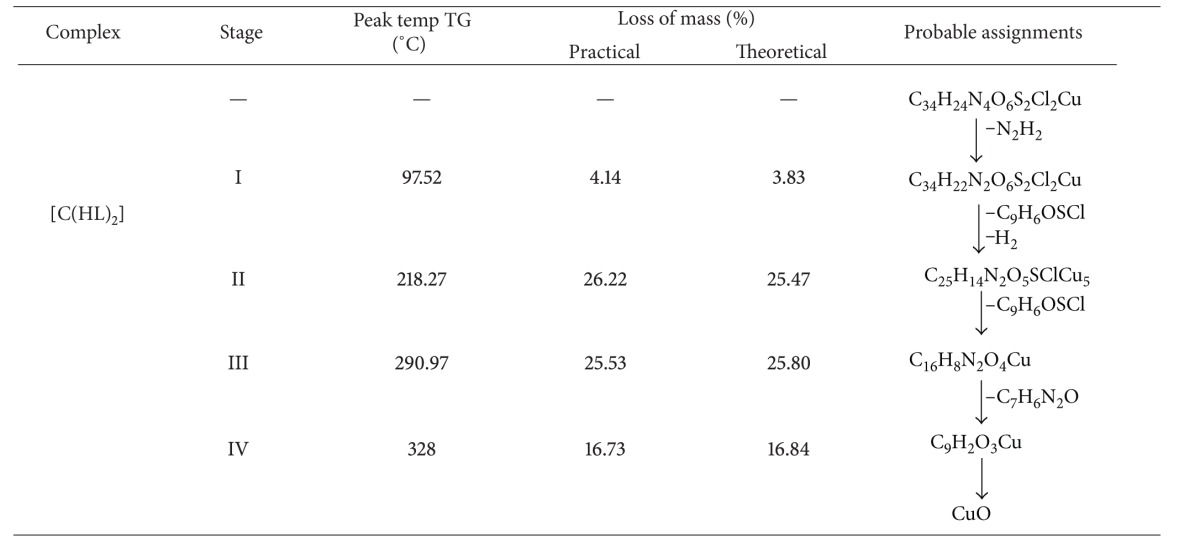
The decomposition studies of the compound Cu(II) complex [Cu(HL)2] have been carried out. In the thermogram of the [Cu(HL)2], (Figure 4) the first stage of the decomposition represents the weight loss of N2H2 at 97.52°C, with weight loss of 4.14%. The theoretical weight loss for this decomposition was 3.83% agreeing with observed value of 4.14%. The complex underwent further degradation and gave break at 218.27°C with a weight loss of 26.22%, which corresponds to the simultaneous decomposition of the C9H6OSCl and H2 species. This practical weight loss 26.22% is in accordance with theoretical weight loss of 25.47%. The third stage of decomposition occurs at 290.97°C, with weight loss of 25.53% which corresponds to the decomposition of C9H6OSCl species. This practical weight loss 25.53% is in accordance with theoretical weight loss of 25.80%. The fourth stage degradation at 328°C with weight loss of 16.73% corresponds to the decomposition of C7H6N2O species. This practical weight loss 16.73% is in accordance with theoretical weight loss 16.84%. Thereafter compound showed a gradual decomposition up to 800°C and onwards. The weight of the residue corresponds to copper oxide. The thermal decomposition of [Cu(HL)2] with probable assignments is given in Table 4.
Figure 4.
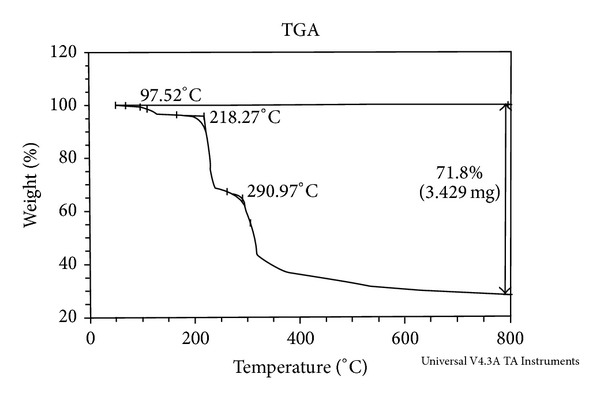
Thermogram of Cu(II) complex.
Table 4.
Thermal decomposition of Cu(II) complex of ligand HL.
|
3.8.2. Co(II) Complex
In the thermogram of the [Co(HL)2], (Figure 5) the loss of C26H18N2O4S2Cl2 was observed at 231.81°C, with weight loss of 71.67%. This practical weight loss 71.67% is in accordance with the theoretical weight loss 71.50%. The resultant intermediate complex underwent further degradation and gave another break at 392.99°C with a weight loss of 12.53%, which corresponds to the simultaneous decomposition of the C6H6O and H2 species from the above intermediate complexes. The theoretical weight loss for this decomposition corresponds to 12.07% agreeing well with the observed value 12.53%. Thereafter compound showed a gradual decomposition up to 800°C and onwards. The weight residue corresponds to cobalt oxide (Co3O4). The thermal decomposition of [Co(HL)2] with probable assignments is given in Table 5.
Figure 5.
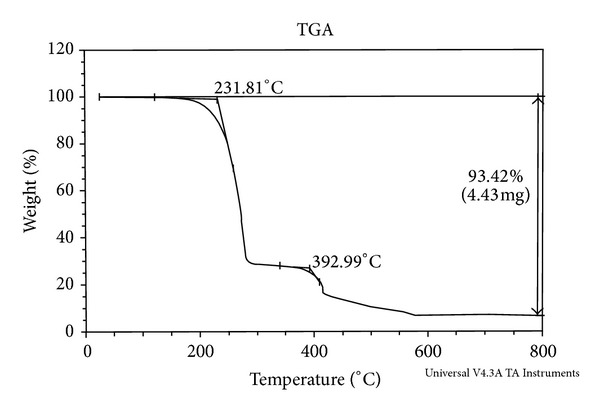
Thermogram of Co(II) complex.
Table 5.
Thermal decomposition of Co(II) complex of ligand HL.
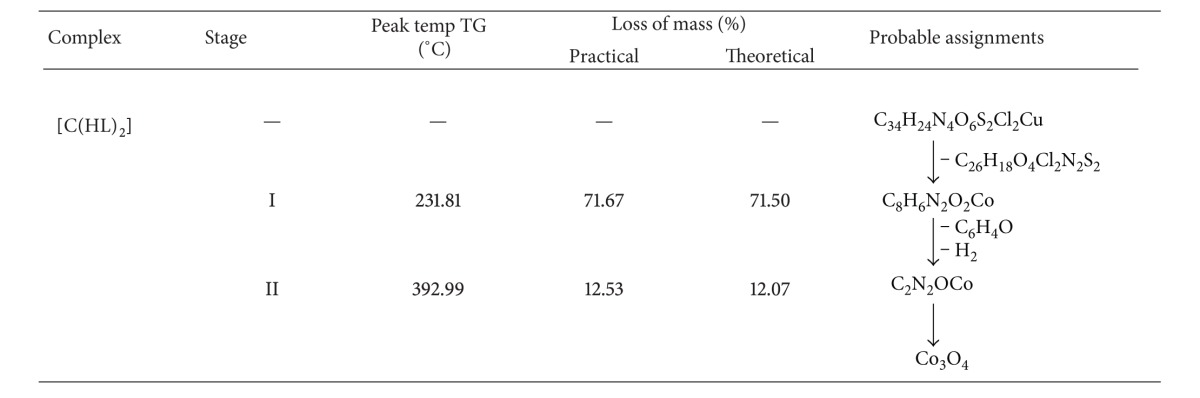
|
3.9. Powder X-Ray Diffraction
3.9.1. Cu(II) Complex
Powder X-ray diffraction pattern for Cu(II) complex of ligand HL has been depicted in Figure 6. The Copper complex of [Cu(HL)2] showed nine reflections in the range of 5–80° (2θ) arising from diffraction of X-ray by planes of complex. The interplanar spacing (d) has been calculated from the position of intense peak using Bragg's equation
| (3) |
where λ = wavelength of X-ray used (Cu K α = 1.54 Å).
Figure 6.
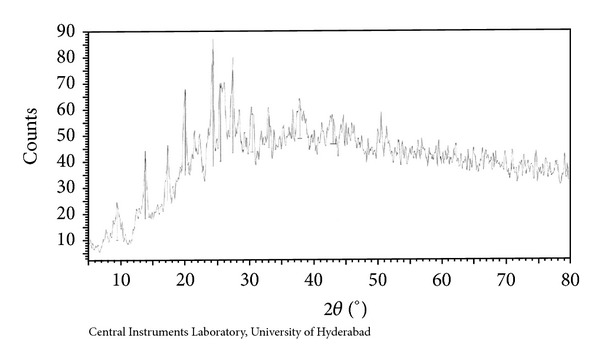
Powder X-ray diffraction of Cu(II) complex.
The calculated spacing together with relative intensities with respect to most intense peak has been recorded in Table 6.
Table 6.
Powder X-ray diffraction data of Cu(II) of the ligand HL.
| Peak | 2θ | θ | sinθ | sin2θ | hk l | d | h 2 + k 2 + l 2 | a in Å | |
|---|---|---|---|---|---|---|---|---|---|
| Calc. | Obser. | ||||||||
| 1 | 9.484 | 4.742 | 0.0826 | 0.00682 | 100 | 9.32567 | 9.32567 | 1 | 9.3277 |
| 2 | 13.899 | 6.9495 | 0.12099 | 0.01464 | 110 | 6.36664 | 6.36664 | 2 | 9.3277 |
| 3 | 17.399 | 8.6995 | 0.15125 | 0.02288 | 111 | 5.09289 | 5.09289 | 3 | 9.3277 |
| 4 | 20.154 | 10.077 | 0.17497 | 0.03061 | 200 | 4.40247 | 4.40247 | 4 | 9.3277 |
| 5 | 25.457 | 12.7285 | 0.22033 | 0.04855 | — | 3.49612 | 3.49612 | 7 | 9.3277 |
| 6 | 30.446 | 15.233 | 0.26257 | 0.06894 | 310 | 2.93369 | 2.93369 | 10 | 9.3277 |
| 7 | 33.056 | 16.528 | 0.28448 | 0.08093 | 222 | 2.70775 | 2.70775 | 12 | 9.3277 |
| 8 | 37.844 | 18.922 | 0.32428 | 0.10516 | — | 2.37542 | 2.37542 | 15 | 9.3277 |
| 9 | 42.952 | 21.476 | 0.36611 | 0.13404 | 420 | 2.10401 | 2.10401 | 20 | 9.3277 |
The 2θ value with maximum intensity of the peak for the compound was found to be 25.457 (2θ) which corresponds to d = 3.49612 Å. The 2θ values for the prominent peaks have been listed in Table 6. The entire important peaks have been indexed and unit cell calculations have been made for cubic symmetry of the complex. The observed values of interplanar distance have been compared with the calculated ones. It was observed that there is good agreement between the calculated and observed values. The experimental values of sin2 θ /common factor are recorded for each peak in Figure 6. The (h 2 + k 2 + l 2) values are 1, 2, 3, 4, 7, 10, 12, 15, and 20. The presence of forbidden no 7 and 15 indicates that the Cu(II) complex may belong to hexagonal or tetragonal system.
3.9.2. Co(II) Complex
Powder X-ray diffraction pattern for Co(II) complex of ligand HL has been depicted in Figure 7. The copper complex of [Co(HL)2] showed eleven reflections in the range of 5–80° (2θ) arising from diffraction of X-ray by planes of complex. The interplanar spacing (d) has been calculated from the position of intense peak using Bragg's equation
| (4) |
Figure 7.
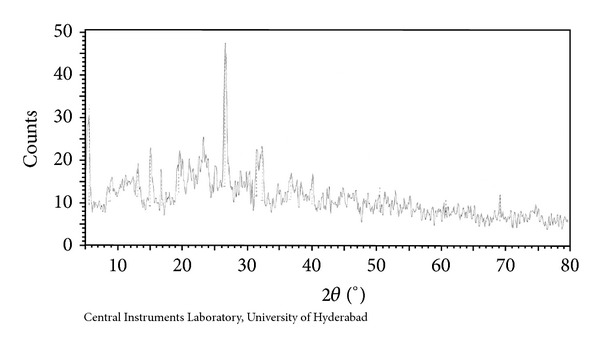
Powder X-ray diffraction of Co(II) complex.
The calculated spacing together with relative intensities with respect to most intense peak has been recorded in Table 7.
Table 7.
Powder X-ray diffraction data of Co(II) complex of the ligand HL.
| Peak | 2θ | θ | sinθ | sin2θ | hk l | d | h 2 + k 2 + l 2 | a in Å | |
|---|---|---|---|---|---|---|---|---|---|
| Calc. | Obser. | ||||||||
| 1 | 8.8805 | 4.44025 | 0.07742 | 0.00599 | 100 | 9.94963 | 9.94939 | 1 | 9.9518 |
| 2 | 13.0916 | 6.5458 | 0.1139 | 0.01299 | 110 | 6.75761 | 6.75698 | 2 | 9.9518 |
| 3 | 15.0632 | 7.5316 | 0.1310 | 0.01718 | 111 | 5.87701 | 5.87671 | 3 | 9.9518 |
| 4 | 19.5309 | 9.7654 | 0.1696 | 0.02877 | 210 | 4.54133 | 4.54133 | 5 | 9.9518 |
| 5 | 26.5511 | 13.2755 | 0.2296 | 0.05273 | 300 | 3.35453 | 3.35438 | 9 | 9.9518 |
| 6 | 30.7545 | 15.3772 | 0.2651 | 0.07032 | 222 | 2.90493 | 2.90481 | 12 | 9.9518 |
| 7 | 36.8253 | 18.4126 | 0.3158 | 0.09977 | 410 | 2.43874 | 2.43869 | 17 | 9.9518 |
| 8 | 40.0795 | 20.0397 | 0.3426 | 0.11742 | 420 | 2.24794 | 2.24787 | 20 | 9.9518 |
| 9 | 50.5420 | 25.2710 | 0.4269 | 0.18224 | 521 | 1.8044 | 1.80436 | 30 | 9.9518 |
| 10 | 60.7614 | 30.3807 | 0.5057 | 0.25577 | 533 | 1.52311 | 1.52306 | 43 | 9.9518 |
| 11 | 69.1800 | 34.5900 | 0.5677 | 0.32228 | 721 | 1.35688 | 1.35684 | 54 | 9.9518 |
The 2θ value with maximum intensity of the peak for the compound was found to be 26.5511 (2θ) which corresponds to d = 3.35453 Å. The 2θ values for the prominent peaks have been listed in Table 7. The entire important peaks have been indexed and unit cell calculations have been made for cubic symmetry of the complex. The observed values of interplanar distance have been compared with the calculated ones. It was observed that there is good agreement between the calculated and observed values. The experimental values of sin2 θ/common factor are recorded for each peak in Figure 7. The (h 2 + k 2 + l 2) are 1, 2, 3, 5, 9, 12, 17, 20, 30, 43, and 54. The absence of forbidden no 7, 15, and 23 conforms the cubic symmetry. The experimental values are in good agreement with (h 2 + k 2 + l 2) values of primitive type cubic cell, with lattice parameter equal to a = b = c = 9.9518.
3.10. Antimicrobial Activity
Antimicrobial activity was carried out by the cup-plate method [33]. The ligand HL and its Cu(II), Co(II), Ni(II), Zn(II), Hg(II), Mn(II), and Fe(III) complexes have been tested for their antibacterial and antifungal activity at 1 mg/mL concentration. The results of the antimicrobial activity have been presented in Table 8. The results of the antibacterial activity testing showed that the complexes of Hg(II) and Mn(II) showed good activity, complexes of Cu(II), Co(II), Ni(II), and Fe(III) exhibited moderate activity, and ligand HLand complex of Zn(II) showed less activity against E. coli when compared with that of standard drug Streptomycin. The complexes of Hg(II) and Mn(II) showed good activity, and complexes of Cu(II), Co(II), Ni(II), and Fe(III) exhibited moderate activity against S. aureus when compared with that of standard drug Streptomycin.
Table 8.
Antimicrobial activity screening data of the ligand HL and its complexes.
| Ligand/complexes | Antimicrobial activity (zone of inhibition in mm)* | |||
|---|---|---|---|---|
| Antibacterial activity | Antifungal activity | |||
| E. coli | S. aureus | A. niger | A. flavus | |
| HL | 12 | 13 | 11 | 10 |
| Cu-complex | 16 | 16 | 11 | 13 |
| Co-complex | 17 | 16 | 14 | 12 |
| Ni-complex | 17 | 17 | 15 | 14 |
| Zn-complex | 14 | 15 | 12 | 14 |
| Hg-complex | 21 | 20 | 18 | 17 |
| Mn-complex | 18 | 18 | 14 | 15 |
| Fe-complex | 17 | 16 | 12 | 11 |
| Streptomycin | 22 | 21 | — | — |
| Fluconazole | — | — | 19 | 20 |
| DMF (control) | 0 | 0 | 0 | 0 |
*Bore size-6 mm.
The results of the antifungal activity testing of the ligand HL and its Cu(II), Co(II), Ni(II), Zn(II), Hg(II), Mn(II), and Fe(III) complexes showed that the complex of Hg(II) showed good activity and complexes of Co(II), Ni(II), and Mn(II) exhibited moderate activity against A. niger when compared with standard drug Fluconazole at the same concentration as that of the test compound. Ligand HL and complexes of Cu(II), Zn(II), and Fe(III) showed less activity. The complex of Hg(II) showed good activity, complexes of Ni(II), Zn(II), and Mn(II) exhibited moderate activity, and ligand HL and complexes of Cu(II), Co(II), and Fe(III) showed less activity against A. flavus when compared with standard drug Fluconazole.
3.11. Antioxidant Activity by DPPH Radical Scavenging Activity
DPPH scavenging activity of ligand and its complexes against vitamin-E as standard were analyzed at 50–100 μg in DMF solution at T = 30 min. This investigation indicates that there is a great possibility of finding potent antioxidants. The Cu(II), Ni(II) Zn(II), and Fe(III) complexes have exhibited very good free radical scavenging activity, and Co(II) showed moderate activity. Ligand HL and its Hg(II) and Mn(II) complexes showed less activity compared with Vit-E. The bar graph representation of percentage of free radical scavenging activities is shown in Figure 8.
Figure 8.
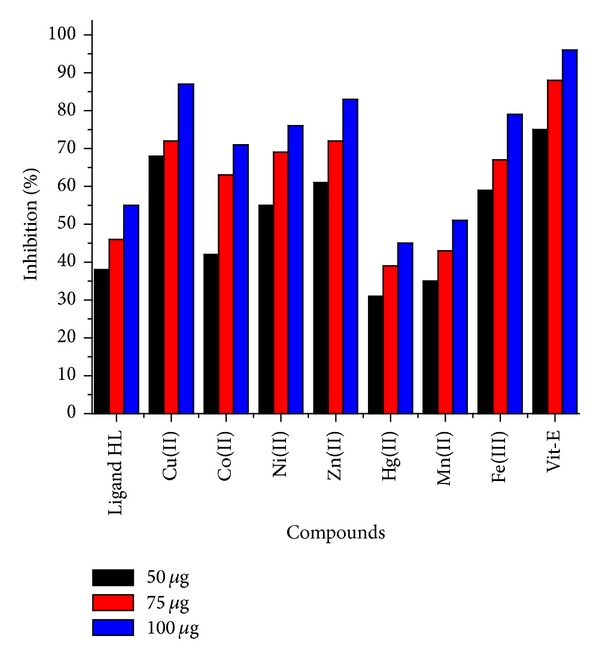
Antioxidant activities of ligand HL and its complexes.
4. Conclusion
In the light of above discussion we have proposed that Cu(II), Co(II), Ni(II), Mn(II), and Fe(III) complexes have exhibited octahedral geometry whereas Zn(II) and Hg(II) complexes exhibited tetrahedral geometry. The ligand behaves as ONO tridentate chelating agent coordinating through the deprotonation of hydroxyl group, carbonyl group, and azomethine nitrogen. The elemental analysis, electrical conductivity measurements, IR spectra, 1H NMR, mass spectral data, electronic spectra, magnetic susceptibility, ESR spectra, and TGA revealed mononuclear nature of the complexes. The ligand HL was found to be less active against the bacteria as well as fungi whereas its complexes were found to be highly active against the bacteria as well as fungi and some of the complexes showed good antioxidant activity. On the basis of spectral evidence following structures have been assigned for synthesized complexes.
Acknowledgments
The authors are grateful to Chairman, Department of Chemistry, Gulbarga, University, Gulbarga, for providing the laboratory facilities. They also thank the directors of IISc, Bangalore, STIC, Kochi, and CDRI, Lucknow, for 1H NMR, ESR, FAB-Mass, TGA, and elemental data.
References
- 1.Deshpande UG, Shah JR. Coordination Polymers. IV. physicochemical studies on chelate polymers of Cr(III), Mn(II), Fe(II), Co(II), Ni(II), and Cu(II) with a schiff base of 4,4′-(4,4′-Biphenylylene Bisazo)di-(salicylaldehyde) with m-Toluidine. Journal of Macromolecular Science: Part A. 1983;20(3):355–363. [Google Scholar]
- 2.Cotton FA. Progress in Inorganic Chemistry. Vol. 7. New York, USA: Wiley; 1966. [Google Scholar]
- 3.Torayama H, Nishide T, Asada H, Fujiwara M, Matsushita T. Preparation and characterization of novel cyclic tetranuclear manganese (III) complexes: Mn4 III (X-salmphen)6(X-salmphenH2 = N,N′-di-substituted-salicylidene-1,3-diaminobenzene (X = H, 5-Br)) Polyhedron. 1997;16(21):3787–3794. [Google Scholar]
- 4.Punniyamurthy T, Kalra SJS, Iqbal J. Cobalt(II) catalyzed biomimetic oxidation of hydrocarbons in the presence of dioxygen and 2-methylpropanal. Tetrahedron Letters. 1995;36(46):8497–8500. [Google Scholar]
- 5.Chavan SS, Hankare PP. X-ray diffraction studies of Ni(II), Cu(II) and Zn (II) complexes with 2-[2′-hydroxysalicylidene-5′-(2″-thiazolylazo)]phenol. Journal of the Indian Chemical Society. 2005;82(6):494–496. [Google Scholar]
- 6.Chohan ZH, Praveen M, Ghaffar A. Synthesis, characterisation and biological role of anions (Nitrate, Sulphate, Oxalate and Acetate) in Co(II), Cu(II) and Ni(II) metal chelates of some Schiff base derived Amino Acids. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 1998;28(10):1673–1687. [Google Scholar]
- 7.Chen B-L, Mok K-F, Ng S-C, Drew MGB. Syntheses, structures and properties of copper(II) complexes with thiophene-2,5-dicarboxylic acid (H2Tda) and nitrogen-containing ligands. Polyhedron. 1999;18(8-9):1211–1220. [Google Scholar]
- 8.Abele E, Lukevics E. Synthesis of sterically hindered heteroaromatic ketones under phase-transfer and metal-complex catalysis conditions. Chemistry of Heterocyclic Compounds. 2001;37(1):5–14. [Google Scholar]
- 9.Farghaly TA, Abdallah ZA. Synthesis, azo-hydrazone tautomerism and antitumor screening of N-(3-ethoxycarbonyl-4,5,6,7-tetrahydro-benzo[b]thien-2-yl)-2-arylhydrazono-3- oxobutanamide derivatives. Arkivoc. 2008;2008(17):295–305. [Google Scholar]
- 10.Vasoya SL, Paghdar DJ, Chovatia PT, Joshi HS. Synthesis of some new thiosemicarbazide and 1, 3, 4-thiadiazole hetrocycles bearing benzo[b]thiophene nucleus as a potent antitubercular and antimicrobial agents. Journal of Sciences Islamic Republic of Iran. 2005;16(1):33–36. [Google Scholar]
- 11.Jadegoud Y, Ijare OB, Mallikarjuna NN, Angadi SD, Mruthyunjayaswamy BHM. Synthesis and antimicrobial activity of copper, cobalt and nickel(II) complexes with Schiff bases. Journal of the Indian Chemical Society. 2002;79(12):921–924. [Google Scholar]
- 12.Mruthyunjayaswamy BHM, Jadegoud Y, Ijare OB, Patil SG, Kudari SM. Synthesis, characterization and antimicrobial activity of macrocylic phenoxo-bridged di and tetra-nuclear complexes from N,N-bis[2,6-diiminomethyl-4-methyl-1-hydroxyphenyl]succinoyl/sebacoyldicarboxamides. Transition Metal Chemistry. 2005;30(2):234–242. [Google Scholar]
- 13.Mruthyunjayaswamy BHM, Ijare OB, Jadegoud Y. Synthesis, characterization and biological activity of symmetric dinuclear complexes derived from a novel macrocyclic compartmental ligand. Journal of the Brazilian Chemical Society. 2005;16(4):783–789. [Google Scholar]
- 14.Jadegoud Y, Ijare OB, Somashekar BS, Gowda GAN, Mruthyunjayaswamy BHM. Synthesis, characterization and antimicrobial activity of homodinuclear complexes derived from 2,6-bis[3′-methyl-2′-carboxamidyliminomethyl(6′,7′)benzindole]-4-methylphenol, an end-off compartmental ligand. Journal of Coordination Chemistry. 2008;61(4):508–527. [Google Scholar]
- 15.Rahaman F, Hiremath B, Basavarajaiah SM, Jayakumarswamy BHM, Mruthyunjayaswamy BHM. Synthetic, spectral, thermal and antimicrobial activity studies of some transition metal complexes derived from 2-hydroxy-methylbenzaldehyde N-(4′-phenyl-1′,3′-thiazol-2′-yl)semicarbazone. Journal of the Indian Chemical Society. 2008;85(4):381–386. [Google Scholar]
- 16.Rahaman F, Ijare OB, Jadegoud Y, Mruthyunjayaswamy BHM. Phenoxo-bridged symmetrical homobinuclear complexes derived from an “end-off” compartmental ligand, 2,6-bis[5′-chloro-3′-phenyl-1H-indole-2′-carboxamidyliminomethyl]-4-methylphenol. Journal of Coordination Chemistry. 2009;62(9):1457–1467. [Google Scholar]
- 17.Vogel AI. A Text Book Quantitative Organic Analysised. 3rd edition 1962. [Google Scholar]
- 18.Hiremath SP, Shivaramayya K, Purohit MG. Synthesis of substituted 2,5-bis(Oxadiazolyl/Thiazolidino/Pyrazolyl/Pyrimidinediono) indoles and Oxadiazolyl/Thiadiazolyl/Triazolyl/Thiazolidinone analogs of Benzothiophene and their antibacterial activity. Indian Journal of Heterocyclic Chemistry. 1992;1:177–184. [Google Scholar]
- 19.Vogel AI. A Text Book of Quantitative Inorganic Analysis. 3rd edition. London, UK: Longman ELBS; 1968. [Google Scholar]
- 20.O’Connor CJ, Sinn E, Cukauskas EJ, Deaver BS., Jr. Low temperature magnetic properties and antiferromagnetic interactions of the magnetic susceptibility calibrant HgCo(NCS)4 . Inorganica Chimica Acta. 1979;32(C):29–32. [Google Scholar]
- 21.Barry AL. The Antimicrobial Susceptibility Test, Principles and Practices. 4th edition. E.L.B.S; 1976. [Google Scholar]
- 22.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199–1200. [Google Scholar]
- 23.Molyneux P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. The Songklanakarin Journal of Science and Technology. 2004;26:211–219. [Google Scholar]
- 24.Topçua G, Ertas A, Kolak U, Öztürk M, Ulubelen A. Antioxidant activity tests on novel triterpenoids from Salvia macrochlamys . Arkivoc. 2007;7:195–208. [Google Scholar]
- 25.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual. 2nd edition. Cold Spring Harbor, New York, NY, USA: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 26.Abu-Hussen AAA. Synthesis and spectroscopic studies on ternary bis-Schiff-base complexes having oxygen and/or nitrogen donors. Journal of Coordination Chemistry. 2006;59(2):157–176. [Google Scholar]
- 27.Singh NK, Singh SB, Shrivastav A, Singh SM. Spectral, magnetic and biological studies of l,4-dibenzoyl-3-thiosemicarbazide complexes with some first row transition metal ions. Journal of Chemical Sciences. 2001;113(4):257–273. [Google Scholar]
- 28.Singh NK, Singh SB. Synthesis, characterization and biological properties of manganese(II), cobalt(II), nickel(II), copper(II), zinc(II), chromium(III) and iron(III) complexes with a new thiosemicarbazide derivative. Indian Journal of Chemistry: Section A. 2001;40(10):1070–1075. [Google Scholar]
- 29.Rao CNR. Chemical Applications of Infrared Spectroscopy. New York, NY, USA: Academic Press; 1963. [Google Scholar]
- 30.Shashidhara GM, Goudar TR. Synthesis of Uranium(IV) complexes of Schiff bases. Journal of the Indian Chemical Society. 2001;78(7):360–361. [Google Scholar]
- 31.Patel MK, Patel NH, Patel KN, Patel MN. Synthesis, structural characterization and biocidal studies of Cu(II), Ni(II), Zn(II), Co(II), Fe(II), Mn(II) and Cd(II) complexes derived from Schiff bases (ONO) and bidentate (NN) heterocycle. Journal of Indian Council of Chemists. 2000;17(1):19–24. [Google Scholar]
- 32.Chandra S, Gupta K. Synthesis and spectral studies on chromium(III), manganese(II), iron(III), cobalt(II), nickel(II) and copper(II) complexes of fourteen-membered and sixteen membered tetradentate macrocyclic ligands. Indian Journal of Chemistry: Section A. 2001;40(7):775–779. [Google Scholar]
- 33.Nicolas D, Bailar JC, Jr., Emelens HJ, Nyloms RS. Comprehnsive Inorganic Chemistry. 5th edition 1973. [Google Scholar]
- 34.Tian JL, Gao EQ, Li YT, Liu SX. Synthesis and characterization of Glyoxalic Acid Thiosemicarbazone complexes of some Bivalent metal Ions. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 1995;25(3):417–427. [Google Scholar]
- 35.Mishra LK, Jha Y, Sinha BK, Kant R, Singh R. Studies of chelates of cobalt-, nickel-, copper-, zinc- and cadmium(II) with 4-amino-3,5-dioxo-6-methyl-2,3,4,5-tetrahydro-1,2,4-triazine. Journal of the Indian Chemical Society. 1999;76(2):65–66. [Google Scholar]
- 36.Melník M, Potočňak I, Macášková L, Mikloš D, Holloway CE. Spectral study of copper(II) flufenamates: crystal and molecular structure of biis(Flufenamato)di(N,N-diethylnicotinamide)di(Aqua)copper(II) Polyhedron. 1996;15(13):2159–2164. [Google Scholar]
- 37.Hathaway BJ, Tomlinson AAG. Copper(II) ammonia complexes. Coordination Chemistry Reviews. 1970;5(1):1–43. [Google Scholar]


