Abstract
Acute kidney injury (AKI) continues to have an exceedingly high mortality rate, despite advances in dialysis technology. Current dialysis therapies replace only the filtration function of the kidney, not the critical transport, metabolic, and endocrine functions of renal tubule cells. Replacement of these additional functions would provide more complete AKI therapy and thereby change the natural history of this disease process. A renal tubule assist device (RAD) containing living renal proximal tubule cells has been successfully engineered and has demonstrated differentiated absorptive, metabolic, and endocrine functions of normal kidney in vitro and ex vivo in animal experiments. The addition of the RAD containing human cells to conventional continuous renal replacement therapy has been shown in preclinical and clinical studies to have the potential to advance AKI treatment, from enhancing renal clearance to providing more complete renal replacement therapy. This “bioartificial kidney” demonstrates metabolic activity with systemic effects and improvement of survival in patients with AKI and multiorgan failure. It also appears to influence systemic leukocyte activation and the balance of inflammatory cytokines, suggesting that cell therapy by use of the RAD may improve morbidity and mortality by altering the proinflammatory state of patients with renal failure. In addition to providing cellular metabolic function, technologies directed toward disrupting systemic inflammatory response may well enhance the clinical outcome of critically ill patients in the future. Innovative approaches to intensive renal care such as the RAD may break the mold of current institutional dialysis therapies and provide numerous opportunities to develop lifesaving technologies.
Keywords: Acute kidney injury, systemic inflammatory response syndrome, bioartificial kidney, cell therapy, renal replacement therapy
INTRODUCTION
Acute kidney injury (AKI), also called acute renal failure (ARF), affects 5–7% of hospitalized patients and up to 30% of patients in intensive care units (ICUs). The development of AKI in a hospitalized patient results in a 5- to 8-fold higher risk of death [1, 2]. Improved treatment regimens in patients with AKI in the ICU have not shared the same quantum leaps of progress that have been seen in other illnesses, such as myocardial infarction, despite an improved understanding of the pathophysiology of AKI and techniques for renal replacement therapy [1, 2].
Currently, the main treatment modalities for AKI are the dialysis therapies based on diffusion, convection, or absorption. Although these therapies are undoubtedly life-sustaining in patients with renal failure, morbidity and mortality rates are still unacceptably high because dialysis replaces only the filtration function of the kidney, not the lost homeostatic, regulatory, metabolic, and endocrine functions of the defected tubular elements of the kidney [3]. Novel therapeutic approaches must be formulated to replace fully all functions of the kidney and change the dismal prognosis for patients with AKI. The present review will focus on the use of renal cell therapy in the treatment of AKI as an extension to the current renal substitution processes of dialysis.
KIDNEY AS A FILTRATIVE, ENDOCRINE, METABOLIC, AND IMMUNOREGULATORY ORGAN
The kidney plays a critical role in maintaining body water and electrolyte homeostasis through excretory and regulatory functions of glomerulus and tubule. Current dialysis therapies, including hemodialysis (HD), hemofiltration, and peritoneal dialysis (PD), replace these functions by removing waste products and excess electrolytes from blood using artificial or natural semipermeable membranes. In this regard, the kidney is unique in that it is the first organ for which long-term ex vivo substitutive therapy has been available and lifesaving.
The kidney, however, has many other roles. It is regarded as an important endocrine organ, responsible for the secretion of hormones that are critical in maintaining hemodynamics (renin, angiotension II, prostaglandins, nitric oxide, endothelin, and bradykinin), red blood cell production (erythropoietin), and bone metabolism (1,25-(OH)2-vitD3 or calcitriol) [4]. It synthesizes glutathione and free-radical scavenging enzymes and provides gluconeogenic and ammoniagenic capabilities [5, 6]. Catabolism of low-molecular-weight proteins, including peptide hormones, cytokines, and growth factors, is also accomplished by the kidney [7].
The kidney also has an immunoregulatory function, which has been less recognized. The mammalian renal proximal tubule cells are immunologically active. They are antigen-presenting cells [8] that have co-stimulatory molecules [9] that synthesize and process a variety of inflammatory cytokines [9, 10]. The roles of the renal tubule cells in glutathione metabolism [5], regulation of vitamin D, and production and catabolism of multiple cytokines [4] are critical to immunoregulation to maintain tissue integrity and host defense under stress conditions [11]. A growing body of evidence indicates that inflammation plays a major role in the pathophysiology of AKI, so that this disorder is, to some extent, an inflammatory disease [12].
AKI AS SYSTEMIC INFLAMMATORY RESPONSE SYNDROME
The cause of death in AKI patients is usually the development of a systemic inflammatory response syndrome (SIRS) [13]. The exceptionally high mortality associated with SIRS is caused in part by the development of the multiple system organ failure (MSOF) syndrome in a subset of patients and is not ameliorated by conventional renal replacement therapy (CRRT), which treats volume overload, uremia, acidosis, and electrolyte derangements [13]. The propensity of patients with AKI to develop SIRS and sepsis suggests that renal function, specifically renal tubule cell function, plays a critical immunomodulatory role in individuals under stress states [11].
Inflammatory cascades initiated by endothelial dysfunction in SIRS can be augmented dramatically by the generation of several potent mediators by the ischemic proximal tubule. These are evidenced by recent human studies demonstrating that the levels of the proinflammatory cytokines interleukin (IL)-6 and IL-8 in the plasma predict mortality in patients with AKI [14]. Strategies that modulate the inflammatory response provide significant beneficial effects in experimental AKI [15]. A more promising direction to improve the outcome of AKI is to better understand and interrupt the pathophysiologic processes that are activated in AKI, resulting in distant multi-organ dysfunction and eventually death. Technologies directed to disrupting multi-organ dysfunction may well be the next major improvement to enhance the clinical outcome of these critically ill patients. Renal cell therapy may alter the natural history of this disorder if the renal tubular epithelium has an immunoregulatory role in whole-body homeostasis.
CURRENT RENAL REPLACEMENT THERAPIES IN AKI
Current renal replacement therapy for AKI is predominantly supportive in nature. The therapeutic goals are the maintenance of fluid and electrolyte balance, adequate nutrition, and treatment of infection and uremia when they are present. Uremia is treated with either intermittent hemodialysis or continuous hemofiltration. This approach has had substantial impact on this disease process over the past 40 years, but patients with acute tubular necrosis (ATN) still have an exceedingly high mortality of greater than 50% [14, 16]. Although intensivists and nephrologists have attempted to improve outcomes of AKI by increasing the dose of hemodialysis or hemodiafiltration, the results have been disappointing. The improvements in renal small-solute clearance alone have had minimal impact on clinical outcomes [17–19].
During the maintenance phase of AKI, a patient undergoing hemodialysis or hemofiltration may die from multi-organ failure while in exquisite electrolyte and fluid balance. This high mortality is caused by the propensity of these patients to develop SIRS, most commonly secondary to bacterial sepsis, with resulting multiorgan damage from cardiovascular collapse and ischemia [20]. Since AKI secondary to ischemic and/or nephrotoxic insults predominantly arises from the necrosis of renal proximal tubule cells, replacement of the functions of these cells during the episode of AKI would provide almost full renal replacement therapy in conjunction with hemofiltration. The addition of metabolic activity such as ammoniagenesis and glutathione reclamation, endocrine activity such as vitamin D3 activation, and cytokine homeostasis may provide additional physiologic replacement activities to change the current natural history of this disease process [11]. Still lacking are methods to down-regulate the inflammatory response, which plays a major role in the pathophysiology of AKI.
RENAL TUBULE ASSIST DEVICE (RAD) FOR CELL THERAPY
Cell therapy harnesses the natural ability of cells to recognize various biological signals and respond with a complex array of cell products to replace physiologic functions deranged or lost in acute or chronic disease processes [21]. One extension of cell therapy is the developing field of tissue engineering in which techniques from the biological and engineering sciences are combined to create structures and devices to replace lost tissue or organ functions [22, 23]. The majority of current applications involve the seeding of animal or human cells within artificial constructs such as hollow-fiber bioreactors or encapsulating membranes. Given the success of renal replacement therapy with hemodialysis and hemofiltration during the last four decades, a natural application of tissue engineering is the treatment of acute and chronic renal failure [16].
Our laboratory developed an extracorporeal bioartificial renal tubule assist device (RAD) utilizing a standard hemofiltration cartridge containing more than 109 renal tubule cells grown in confluent monolayers along the inner surface of the fibers [3, 24, 25]. The nonbiodegradability and the pore size of the hollow fibers allow the membranes to act as both scaffolds for the cells and as an immunoprotective barrier. In vitro and ex vivo studies of the RAD have demonstrated that the cells retain vectorial fluid transport properties as a result of Na, K-ATPase; other differentiated active transport properties, including active glucose and bicarbonate transport; differentiated metabolic activities, including intra-luminal glutathione breakdown, constituent amino acid uptake and ammonia production; and the important endocrinologic conversion of 25-OH-vitD3 to 1,25-(OH)2-vitD3 (Table 1). These characteristics were observed in RADs that maintained viability and functionality ex vivo when connected in series to a hemofiltration cartridge within an extra-corporeal perfusion circuit in an acutely uremic animal [26]. The RADs reabsorbed 40–50% of ultrafiltrate (UF) volume presented to the devices and achieved active transport of potassium, bicarbonate, and glucose, as well as ammonia excretion, glutathione metabolism, and 1,25-(OH)2-vitD3 production.
Table 1.
In vitro and Ex Vivo Properties of Renal Tubule Cell Assist Device
| In vitro | Ex Vivo | |
|---|---|---|
|
| ||
| Transport | Sodium—ouabain inhibitable | Sodium |
| Bicarbonate—acetazolamide inhibitable | Potassium | |
| Glucose—phlorizin inhibitable | Bicarbonate | |
| PAH—probenecid inhibitable | Glucose | |
|
| ||
| Metabolic | Ammoniagenesis—pH sensitive | Ammonia excretion |
| Glutathione synthesis—acivicin inhibitable | Glutathione reclamation | |
|
| ||
| Endocrinologic | 1-hydroxylation of vitamin D3-PTH - Pi sensitive | 1-hydroxylation of 25-OH vit D3 |
|
| ||
| Immunomodulatory | ↑ IL-10, ↓ IL-6, ↓ TNF-γ in uremic animal; ↓ IL-6 / IL-10 ratio in human | |
BIOARTIFICIAL KIDNEY FOR FULL RENAL REPLACEMENT THERAPY
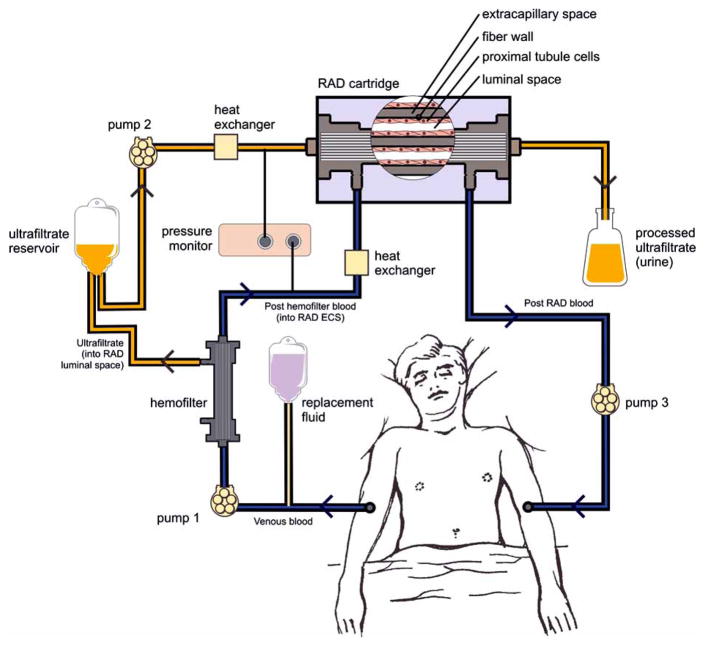
The bioartificial kidney consists of a filtration device (a conventional high-flux hemofilter) followed in series by the RAD [26–28]. Specifically, blood is pumped out of the patient and enters the fibers of the hemofilter, where UF is formed and delivered to the fibers of the tubule lumens within the RAD downstream to the hemofilter. Processed UF exiting the RAD is collected and discarded as “urine.” The filtered blood exiting the hemofilter enters the RAD through the extracapillary space port and disperses among the fibers of the device. On exiting the RAD, the processed blood is returned to the patient’s body by a third pump (Fig. 1).
Fig. 1.
Schematic of the extracorporeal bioartificial kidney circuit consisting of a synthetic hemofilter and the RAD cartridge.
(Previously published in: Tiranathanagul K, Eiam-Ong S, Humes HD. The future of renal support: High flux dialysis to bioartificial kidneys. In: Kellum J, ed. Critical Care Nephrology, Critical Care Clinics. Vol. 21, no. 2. Philadelphia: W.B. Saunders, 2005).
The RAD maintains viability because metabolic substrates and low-molecular weight growth factors are delivered to the tubule cells from the ultrafiltration unit and the blood in the extracapillary space. At the same time, immunoprotection of the cells grown within the hollow fibers is achieved because of the impenetrability of these fibers to immunoglobulins and immunologically competent cells. Besides the immunoisolating effect, this approach allows recovery of the implant, a key fail-safe condition if unwanted events arise. This strategy is more controllable than indiscriminate direct injections of genes or stem cells, or direct implantation of heterologous, transformed cells into the patient with the inability to control the final destination of the cells or to retrieve the genetically modified cells from the body should their removal become medically desirable [29, 30].
The arrangement of the RAD and hemofilter within the extracorporeal circuit allows the filtrate to enter the internal compartments of the hollow fiber network, lined with confluent monolayers of renal tubule cells for regulated transport and metabolic function. This circuit design reconstructs the functional structure of the nephron, comprising both glomerulus and tubule. Replacement of the functions of the tubule cells during the episode of ATN will provide almost full renal replacement therapy in conjunction with hemofiltration. This extracorporeal circuit containing the RAD was initially tested on uremic dogs with bilateral nephrectomies [26]. The animals were treated with either a RAD or a sham control cartridge daily for either 7 or 9 h for 3 successive days or for 24 h continuously. The RADs maintained viability and functionality throughout the study period. Fluid and small solutes, including blood urea nitrogen (BUN), creatinine (Cr), and electrolytes, were adequately controlled in both groups, but potassium and BUN levels were more easily controlled by RAD treatment. Furthermore, active reabsorption of K+, HCO3−, and glucose and excretion of ammonia were accomplished only in RAD treatments. Glutathione reclamation from UF exceeded 50% in the RAD. Finally, uremic animals receiving cell therapy attained normal 1,25-(OH)2-vitD3 levels, whereas sham treatment resulted in a further decline from the already low plasma levels.
EFFECT OF BIOARTIFICIAL KIDNEY IN ANIMAL MODEL OF AKI AND SEPSIS
A series of additional animal experiments investigated the impact of RAD treatment on the mortality of sepsis complicated by AKI. Studies in both dogs and pigs have demonstrated that RAD treatment in a bioartificial kidney circuit improves cardiovascular performance associated with changes in cytokine profiles and confers a significant survival advantage in endotoxin or bacterial septic shock in acutely uremic animals. In endotoxin (LPS)-challenged nephrectomized Mongrel dogs, simulating gram-negative septic shock and AKI, the RAD in conjunction with continuous venovenous hemofiltration (CVVH) improved hemodynamics and altered cytokine response compared to a sham cartridge with CVVH [28]. Mean peak levels of an antiinflammatory cytokine, IL-10, and mean arterial pressures (MAPs) were significantly higher in RAD-treated animals. This result was confirmed in a subsequent experiment in ARF with gram-negative bacterial peritonitis-induced septic shock [31]. In E. coli-challenged nephrec-tomized dogs, RAD treatment maintained better cardiovascular performance, as determined by MAP and cardiac output, for longer periods than sham-RAD therapy. All sham-treated animals expired 2 to 10 hours after bacteria administration, whereas all cell-RAD-treated animals survived longer than 10 hours. Plasma levels of IL-10 were significantly elevated in the RAD group. The RAD maintained renal metabolic activity, as determined by 1,25-(OH)2-vitD3 level, throughout the septic period.
In another study, pigs with normal kidney function were administered E. coli intraperitoneally [32]. All animals developed ARF with anuria 2 to 4 hours after bacteria administration. RAD treatment maintained better cardiovascular performance for longer periods than sham therapy. Consistently, the RAD group survived longer than the controls (10 ± 2 hours versus 5 ± 1 hour, respectively). RAD treatment was associated with significantly lower plasma circulating levels of IL-6 and interferon (INF)-γ. These data demonstrate that treatment with the bioartificial kidney improves cardiovascular performance associated with changes in cytokine profiles and confers a significant survival advantage.
FABRICATION OF HUMAN-CELL BIOARTIFICIAL RAD
Early RAD studies utilized porcine cells. However, recent reports of the ability of porcine endogenous retroviruses (PERVs) to infect human cells in co-culture in vitro raised concerns of the potential, but currently unquantifiable, risk of transmission of viral elements between species with the use of porcine tissue in xenotransplantation or cell therapy devices [33, 34]. Accordingly, human renal tubule cells were isolated from kidneys donated for cadaveric transplantation but found unsuitable for such purpose because of anatomic or fibrotic defects. The experiments demonstrated that renal tubule cells can be isolated from human kidneys, expanded and seeded into a hemofiltration cartridge to produce a device containing over 109 cells suitable for clinical study. The human-cell RAD maintained excellent viability in an extracorporeal hemoperfusion circuit in series with a synthetic hemofilter during CVVH [27]. With these results, the FDA approved the first investigator-initiated Phase I/II clinical trial of the RAD and bioartificial kidney in patients with ARF.
EFFICACY AND SAFETY OF THE BIOARTIFICIAL KIDNEY IN HUMAN CLINICAL STUDIES
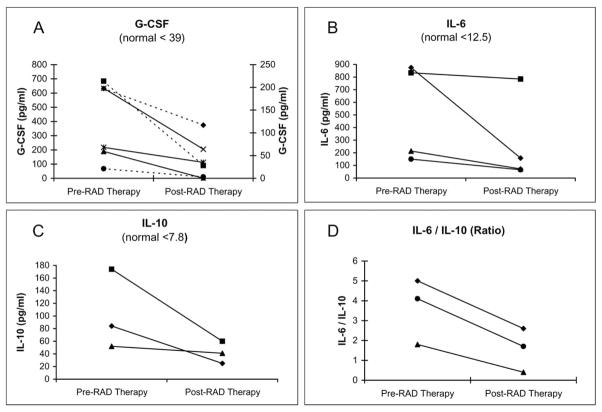
The first human clinical study of the bioartificial kidney containing human cells was carried out in 10 ICU patients with AKI receiving CVVH [35]. This study demonstrated that the RAD can be used safely for up to 24 hours. Cardiovascular stability was maintained, and increased native renal function, as determined by elevated urine outputs, temporally correlated with RAD treatment. All patients were critically ill with AKI and MOF, with predicted hospital mortality rates between 80% and 95%, and 6 of the 10 treated patients survived past 30 days, with mortality reduced to 40%. The human renal tubule cells contained in the RAD demonstrated differentiated metabolic and endocrinologic activity in this ex vivo condition, including glutathione degradation and endocrinologic conversion of 25-OH-vitD3 to 1,25-(OH)2-vitD3 (Fig. 2). Plasma cytokine levels suggest that RAD therapy produces dynamic and individualized responses in patients depending on their unique pathophysiologic conditions. For the subset of patients who had excessive proinflammatory levels, RAD treatment resulted in significant declines in granulocyte-colony stimulating factor (G-CSF), IL-6, IL-10, and especially IL-6/IL-10 ratio, suggesting a greater decline in IL-6 relative to IL-10 levels and a less proinflammatory state (Fig. 3).
Fig. 2.
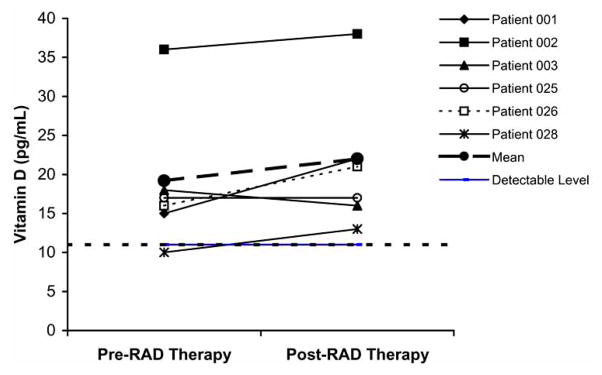
Plasma 1,25-(OH)2-vitD3 levels in patients with detectable levels and treated with the bioartificial kidney and RAD for >20 hours [35].
Fig. 3.
Plasma cytokine levels and ratios for excessively proinflamed patients before and after treatment with the bioartificial kidney and RAD [35]. In (A), dashed lines refer to the left axis, and solid lines refer to the right axis.
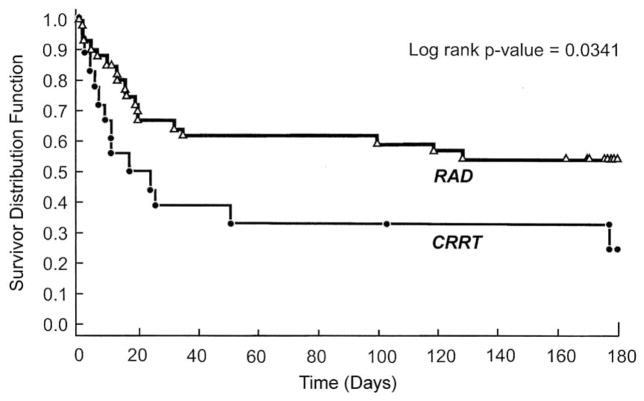
These favorable Phase I/II trial results led to a randomized, controlled, open-label Phase II trial conducted at 12 clinical sites in the U.S. [36]. Fifty-eight patients with ARF requiring CVVH in the ICU were randomized (2:1) to receive CVVH + RAD (n = 40) or CVVH alone (n = 18). Despite the critical nature and life-threatening illnesses of the patients enrolled in this study, the addition of the RAD to CVVH resulted in a substantial clinical impact on survival compared with the conventional CVVH-treatment group. RAD treatment for up to 72 h promoted a statistically significant survival advantage over 180 d of follow-up in ICU patients with AKI and demonstrated an acceptable safety profile. Cox proportional hazards models suggested that the risk of death was approximately 50% of that observed in the CRRT-alone group (Fig. 4). A follow-up Phase IIb study to evaluate a commercial manufacturing process was not completed due to difficulties with the manufacturing process and clinical study design. This approach will be further evaluated when an improved scale-up manufacturing process is established.
Fig. 4.
Kaplan-Meier estimates of survival among patients in the RAD and conventional CRRT groups [36].
IMMUNOMODULATORY EFFECT OF THE RAD
As described previously, RAD treatment altered systemic circulating cytokine levels in animal and human experiments. In endotoxin-challenged and gram-negative peritonitis uremic dog models [28, 31], plasma levels of IL-10 were significantly higher in RAD-treated animals. The role of IL-10 in regulating immune response continues to be elucidated, but data suggest that IL-10 levels influence outcome in endotoxin shock and gram-negative sepsis. Several reports have demonstrated that administration of recombinant IL-10 is protective against gram-negative septic shock in murine sepsis models [37–39]. Another study in a similar model demonstrated that administration of antibodies to IL-10 was associated with higher mortality [40]. The mechanism underlying the link between proximal tubule function and IL-10 levels remains to be detailed, but preliminary data suggest that renal production of IL-6 induces liver production of IL-10 [41].
In gram-negative septic pigs without nephrectomy [32], RAD treatment significantly reduced plasma circulating levels of IL-6 and INF-γ. The difference in IL-6 concentrations is especially noteworthy, since the plasma elevations of this proinflammatory cytokine have been directly correlated to clinical outcome in patients with SIRS [42]. The lower concentration of plasma INF-γ may be important due to its central role in the inflammatory response. INF-γ stimulates B-cell antibody production, enhances polymorpholeukocyte phagocytosis, and activates monocytes and macrophages to release proinflammatory cytokines [43–45]. Excessive rates of INF-γ production by NK cells have correlated with progression to lethal endotoxin shock in mice [46].
Further support for an immunomodulatory role of renal tubule cells has been suggested in the Phase I/II clinical trial of the RAD containing human renal tubule cells [35]. The patients treated in this study had a wide spectrum of plasma cytokine levels. The subset of patients who presented with very high plasma cytokine levels and who were treated for an adequate period showed that RAD treatment resulted in significant reductions in G-CSF, IL-6, and IL-10 levels. The greater relative reduction in IL-6/IL-10 ratio suggests renal tubule cell therapy may rebalance the excessive proinflammatory response with the concurrent anti-inflammatory response. These results are consistent with an immunomo-dulatory role for the RAD in patients with ATN and multiorgan failure.
To further evaluate the RAD’s influence on local inflammation in tissue and distant organ dysfunction, especially in the lungs, a recent study compared bronchoalveolar lavage (BAL) fluid from cell-RAD-treated and non-cell, sham-treated groups in a pig model with septic shock with AKI. The levels of total protein in BAL were significantly higher in sham control animals than in the RAD group (143 ± 11 compared to 78 ± 10 μg/mL, respectively; p > 0.05). Pro-inflammatory cytokines, including IL-6 and IL-8, were markedly elevated in the non-cell group. These results demonstrate an important role for renal epithelial cells in ameliorating multiorgan injury in sepsis by influencing microvascular injury and the local proinflammatory response [47].
FURTHER STRATEGIES DIRECTED TO DISRUPTING SIRS AND MULTIORGAN DYSFUNCTION
A more promising direction to improve outcome of AKI is to better understand and interrupt the pathophysiologic processes that are activated in AKI, resulting in distant multi-organ dysfunction and eventually death. AKI results in a profound inflammatory response state resulting in micro-vascular dysfunction in distant organs [15, 48]. Leukocyte activation plays a central role in these acute inflammatory states. Disruption of the activation process of circulating leukocytes may limit microvascular damage and multi-organ dysfunction [49]. The RAD appears to influence systemic leukocyte activation and the balance of inflammatory cyto-kines and may alter the proinflammatory state of AKI and, ultimately, improve morbidity and mortality. Our group has recently developed a novel synthetic membrane embedded in an extracorporeal device to bind and inhibit circulating leukocytes. This “selective cytopheretic inhibitory device” (SCD) mimics immunomodulation and duplicates RAD efficacy. The SCD improved septic shock survival times in pre-clinical animal models and improved the survival outcome of ICU patients with multiorgan failure in a small exploratory, randomized, double-blinded, multicenter trial [50, 51].
CONCLUSION
Despite refinements in dialysis therapy, AKI remains an unmet medical need largely because dialysis substitutes for only the filtration function of the kidney. A bioartificial kidney combining a conventional hemodialysis filter and living renal proximal tubule cells may advance AKI therapies by providing more complete renal replacement than the renal clearance achieved by dialysis. Differentiated activities of the RAD have been demonstrated experimentally both in vitro and ex vivo. Data from Phase I/II and Phase II clinical studies have shown that treatment with the bioartificial kidney resulted in a significant clinical impact on survival with an acceptable safety profile. AKI may lead to a profound systemic inflammatory response, distant multi-organ dysfunction, and eventually death, and methods to disrupt this process have been lacking. The bioartificial kidney and SCD may improve morbidity and mortality by altering the proinflammatory state in patients with renal failure. Technologies directed toward preventing multiorgan dysfunction may well represent the next major improvement in the clinical outcome of these critically ill patients.
Acknowledgments
This work was supported by the U.S. Army Medical Research and Materiel Command, Contract W81XWH-05-2-0010, and the Small Business Innovation Research program of the National Institutes of Health, Grant NIDDK R43 DK074289.
Footnotes
DISCLOSURES
HDH is a shareholder of Innovative BioTherapies, Inc., and Nephrion, Inc., biotechnology spin-out companies of the University of Michigan.
References
- 1.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–8. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 2.Bates DW, Su L, Yu DT, Chertow GM, Seger DL, Gomes DRJ, et al. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis. 2001;32:686–93. doi: 10.1086/319211. [DOI] [PubMed] [Google Scholar]
- 3.Humes HD, MacKay SM, Funke AJ, Buffington DA. Tissue engineering of a bioartificial renal tubule assist device: In vitro transport and metabolic characteristics. Kidney Int. 1999;55:2502–14. doi: 10.1046/j.1523-1755.1999.00486.x. [DOI] [PubMed] [Google Scholar]
- 4.Stadnyk AW. Cytokine production in epithelial cells. FASEB J. 1994;8:1041–7. doi: 10.1096/fasebj.8.13.7926369. [DOI] [PubMed] [Google Scholar]
- 5.Deneke SM, Fanburg BL. Regulation of cellular glutathione. Am J Physiol. 1989;257:L163–73. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- 6.Tannen RL, Sastrasinh S. Response of ammonia metabolism to acute acidosis. Kidney Int. 1984;25:1–10. doi: 10.1038/ki.1984.1. [DOI] [PubMed] [Google Scholar]
- 7.Maack T. Renal handling of proteins and polypeptides. In: Windhager EE, editor. Handbook of Physiology. New York: Oxford University Press; 1992. pp. 2039–118. [Google Scholar]
- 8.Bishop GA, Waugh JA, Hall BM. Expression of HLA antigens on renal tubular cell stimulation of lymphocyte activation and on their vulnerability to cell-mediated lysis. Transplantation. 1988;46:303–10. doi: 10.1097/00007890-198808000-00022. [DOI] [PubMed] [Google Scholar]
- 9.Wahl P, Schoop R, Bilic G, Neuweiler J, Le Hir M, Yoshinaga SK, et al. Renal tubular epithelial expression of the costimulatory molecule B7RP-1 (Inducible Costimulator Ligand) J Am Soc Nephrol. 2002;13:1517–26. doi: 10.1097/01.asn.0000017901.77985f. [DOI] [PubMed] [Google Scholar]
- 10.Prodjosudjadi W, Gerritsma JS, Klar-Mohamed N, Gerritsen AF, Bruijn JA, Daha MR, et al. Production and cytokine-mediated regulation of monocyte chemoattractant protein-1 by human proximal tubular epithelial cells. Kidney Int. 1995;48:1477–86. doi: 10.1038/ki.1995.437. [DOI] [PubMed] [Google Scholar]
- 11.Humes HD. Bioartificial kidney for full renal replacement therapy. Semin Nephol. 2000;20:71–82. [PubMed] [Google Scholar]
- 12.Kelly KJ. Acute renal failure: Much more than a kidney disease. Semin Nephrol. 2006;26:105–13. doi: 10.1016/j.semnephrol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Breen D, Bihari D. Acute renal failure as a part of multiple organ failure: The slippery slope of critical illness. Kidney Int. 1998;53(Suppl 66):S25–33. [PubMed] [Google Scholar]
- 14.Humes HD, MacKay SM, Funke AJ, Buffington DA. The bioartificial renal tubule assist device to enhance CRRT in acute renal failure. Am J Kidney Dis. 1997;30(Suppl):S28–31. doi: 10.1016/s0272-6386(97)90539-4. [DOI] [PubMed] [Google Scholar]
- 15.Okusa MD. The inflammatory cascade in acute ischemic renal failure. Nephron. 2002;90:133–8. doi: 10.1159/000049032. [DOI] [PubMed] [Google Scholar]
- 16.Humes HD, MacKay SM, Funke AJ, Buffington DA. Acute renal failure: Growth factors, cell therapy, gene therapy. Proc Assoc Am Phys. 1997;109:547–57. [PubMed] [Google Scholar]
- 17.Bellomo R. Do we know the optimal dose for renal replacement therapy in the intensive care unit? Kidney Int. 2005;70:1202–120. doi: 10.1038/sj.ki.5001827. [DOI] [PubMed] [Google Scholar]
- 18.Pannu N, Klarenbach S, Wiebe N. Renal replacement therapy in patients with acute renal failure: A systematic review. JAMA. 2008;299:793–805. doi: 10.1001/jama.299.7.793. [DOI] [PubMed] [Google Scholar]
- 19.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, et al. VA/NIH Acute Renal Failure Trial Network: Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bone RC, Grodzin CJ, Balk RA. Sepsis. A new hypothesis for pathogenesis of the disease process. Chest. 1997;112:235–43. doi: 10.1378/chest.112.1.235. [DOI] [PubMed] [Google Scholar]
- 21.Gage FH. Cell therapy. Nature. 1998;392:518–24. [PubMed] [Google Scholar]
- 22.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–26. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe FD, Mullon CJ, Hewitt WR, Arkadopoulos N, Kahaku E, Eguchi S, et al. Clinical experience with a bioartificial liver in the treatment of severe liver failure: A phase I clinical trial. Ann Surg. 1997;225:484–94. doi: 10.1097/00000658-199705000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolovski J, Gulari E, Humes HD. Design engineering of a bioartificial renal tubule cell therapy device. Cell Transplant. 1999;8:351–64. doi: 10.1177/096368979900800403. [DOI] [PubMed] [Google Scholar]
- 25.MacKay SM, Funke AJ, Buffington DA, Humes HD. Tissue engineering of a bioartificial renal tubule. ASAIO J. 1998;44:179–83. doi: 10.1097/00002480-199805000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Humes HD, Buffington DA, MacKay SM, Funke AJ, Weitzel W. Replacement of renal function in uremic animals with a tissue-engineered kidney. Nat Biotechnol. 1999;17:451–5. doi: 10.1038/8626. [DOI] [PubMed] [Google Scholar]
- 27.Humes HD, Fissell WH, Weitzel WF, Buffington DA, Westover AJ, MacKay SM, et al. Metabolic replacement of kidney function in uremic animals with a bioartificial kidney containing human cells. Am J Kidney Dis. 2002;39:1078–87. doi: 10.1053/ajkd.2002.32792. [DOI] [PubMed] [Google Scholar]
- 28.Fissell WH, Dyke DB, Weitzel WF, Buffington DA, Westover AJ, MacKay SM, et al. Bioartificial kidney alters cytokine response and hemodynamics in endotoxin-challenged uremic animals. Blood Purif. 2002;20:55–60. doi: 10.1159/000046986. [DOI] [PubMed] [Google Scholar]
- 29.Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999;401:517–8. doi: 10.1038/43977. [DOI] [PubMed] [Google Scholar]
- 30.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. New Engl J Med. 2003;348:255–6. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 31.Fissell WH, Lou L, Abrishami S, Buffington DA, Humes HD. Bioartificial kidney ameliorates gram negative bacteria-induced septic shock in uremic animals. J Am Soc Nephrol. 2003;14:454–61. doi: 10.1097/01.asn.0000045046.94575.96. [DOI] [PubMed] [Google Scholar]
- 32.Humes HD, Buffington DA, Lou L, Abrishami S, Wang M, Xia J, et al. Cell therapy with a tissue-engineered kidney reduces the multiple-organ consequences of septic shock. Crit Care Med. 2003;31:2421–8. doi: 10.1097/01.CCM.0000089644.70597.C1. [DOI] [PubMed] [Google Scholar]
- 33.Le Tissier P, Stoye JP, Takeuchi Y, Patience C, Weiss RA. Two sets of human-tropic pig retrovirus. Nature. 1997;389:681–2. doi: 10.1038/39489. [DOI] [PubMed] [Google Scholar]
- 34.Paradis K, Langford G, Long Z, Heneine W, Sandstrom P, Switzer WM, et al. Search for cross-species transmission of porcine endogenous retrovirus in patients treated with living pig tissue. Science. 1999;285:1236–41. doi: 10.1126/science.285.5431.1236. [DOI] [PubMed] [Google Scholar]
- 35.Humes HD, Weitzel WF, Bartlett RH, Swaniker FC, Paganini EP, Luderer JR, et al. Initial clinical results of the bioartificial kidney containing human cells in ICU patients with acute renal failure. Kidney Int. 2004;66:1578–88. doi: 10.1111/j.1523-1755.2004.00923.x. [DOI] [PubMed] [Google Scholar]
- 36.Tumlin J, Wali R, Williams W, Murray P, Tolwani AJ, Vinnikova AK, et al. Efficacy and safety of renal tubule cell therapy for acute renal failure. J Am Soc Nephrol. 2008;19:1034–40. doi: 10.1681/ASN.2007080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lally K, Cruz E, Xue H. The role of anti-tumor necrosis factor-α and interleukin-10 in protecting murine neonates from Escherichia coli sepsis. J Pediatr Surg. 2000;35:852–4. doi: 10.1053/jpsu.2000.6862. [DOI] [PubMed] [Google Scholar]
- 38.Walley K, Lukacs N, Standiford T, Streiter R, Kunkel S. Balance of inflammatory cytokines related to severity and mortality of murine sepsis. Infect Immun. 1996;64:4733–8. doi: 10.1128/iai.64.11.4733-4738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto T, Tateda K, Miyazaki S, Furuya N, Ohno A, Ishii Y, et al. Effect of interleukin-10 on gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob Agents Chemother. 1998;42:2853–7. doi: 10.1128/aac.42.11.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marchant A, Bruyns C, Vandenabeele P, Ducarne M, Gerard C, Delvaux A, et al. Interleukin-10 controls interferon-γ and tumor necrosis factor production during experimental endotoxemia. Eur J Immunol. 1994;24:1167–71. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- 41.Kielar M, Jeyarajah DR, Lu CY. The regulation of ischemic acute renal failure by extrarenal organs. Curr Opin Nephrol Hypertens. 2002;11:451–7. doi: 10.1097/00041552-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Pinsky MR, Vincent JL, Deviere J, Alegre M, Kahn RJ, Dupont E. Serum cytokine levels in human septic shock. Chest. 1993;103:565–76. doi: 10.1378/chest.103.2.565. [DOI] [PubMed] [Google Scholar]
- 43.Bone RC. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–69. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 44.Joyce DA, Gibbons DP, Green P, Steer JH, Feldmann M, Brennan FM. Two inhibitors of pro-inflammatory cytokine release, interleukin-10 and interleukin-4, have contrasting effects on release of soluble p75 tumor necrosis factor receptor by cultured monocytes. Eur J Immunol. 1994;24:2699–705. doi: 10.1002/eji.1830241119. [DOI] [PubMed] [Google Scholar]
- 45.Redmond HP, Chavin KD, Bromberg JS, Daly JM. Inhibition of macrophase-activating cytokines is beneficial in the acute septic response. Ann Surg. 1991;214:502–8. doi: 10.1097/00000658-199110000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emoto M, Miyamoto M, Yoshizawa I, Emoto Y, Schaible UE, Kita E, et al. Critical role of NK cells rather than V-α14+NKT cells in lipopolysaccharide-induced lethal shock in mice. J Immunol. 2002;169:1426–32. doi: 10.4049/jimmunol.169.3.1426. [DOI] [PubMed] [Google Scholar]
- 47.Humes HD, Buffington DA, Lou L, Wang M, Abrishami S. Renal cell therapy ameliorates pulmonary abnormalities in a large animal model of septic shock and acute renal injury. J Am Soc Nephrol. 2007;18:A382. [Google Scholar]
- 48.Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, et al. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004;65:1357–65. doi: 10.1111/j.1523-1755.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 49.Maroszynska I, Fiedor P. Leukocytes and endothelium interaction as rate limiting step in the inflammatory response and a key factor in the ischemia-reperfusion injury. Ann Transplant. 2000;5:5–11. [PubMed] [Google Scholar]
- 50.Ding F, Song JH, Lou L, Rojas A, Reoma JL, Cook KE, et al. A novel selective cytopheretic inhibitory device (SCD) inhibits circulating leukocyte activation and ameliorates multiorgan dysfunction in a porcine model of septic shock. (Abstract.) J Am Soc Nephrol. 2008;19:458A. [Google Scholar]
- 51.Humes HD, Dillon J, Tolwani A, Cremisi H, Wali R, Murray P, et al. A novel selective cytopheretic inhibitory device (SCD) improves mortality in ICU patients with acute kidney injury (AKI) and multiorgan failure (MOF) in a phase II clinical study. (Abstract.) J Am Soc Nephrol. 2008;19:458A. [Google Scholar]