Abstract
Stromal keratitis (SK) is a chronic immunopathological lesion of the eye caused by herpes simplex virus-1 (HSV-1) infection and a common cause of blindness in humans. The inflammatory lesions are primarily perpetuated by neutrophils with the active participation of CD4+ T cells. Therefore, targeting these immune cell types represents a potentially valuable form of therapy to reduce the severity of disease. Resolvin E1 (RvE1), an endogenous lipid mediator, was shown to promote resolution in several inflammatory disease models. In the present report, we determined if RvE1 administration begun at different times after ocular infection of mice with HSV could influence the severity of SK lesions. Treatment with RvE1 significantly reduced the extent of angiogenesis and SK lesions that occurred. RvE1 treated mice had fewer numbers of inflammatory cells that included Th1 and Th17 cells as well as neutrophils in the cornea. The mechanisms by which RvE1 acts appear to be multiple. These included reducing the influx of neutrophils and pathogenic CD4+ T cells, increasing production of the anti-inflammatory cytokine IL-10, and inhibitory effects on the production of pro-inflammatory mediators and molecules such as IL-6, IFN-γ, IL-17, KC, VEGF-A, MMP-2 and MMP-9, that are involved in corneal neovascularization and SK pathogenesis. These findings are the first to show that RvE1 treatment could represent a novel approach to control lesion severity in a virally induced immunopathological disease.
Keywords: Resolvins, Inflammation, Herpes stromal Keratitis
Introduction
Ocular herpes simplex virus (HSV) infection of the eye is the most frequent infectious cause of vision impairment in the industrialized world (1). Recurrent episodes may lead to a chronic inflammatory reaction in the corneal stroma that is characterized by vascularization of the normally avascular cornea, which together with stromal opacification impedes vision and can result in blindness (2). Management of stromal keratitis (SK) usually relies on a combination of anti-viral and anti-inflammatory drugs, with the latter often needed for lengthy periods to prevent a perpetuating inflammatory reactivation of the lesions (3, 4). The current main anti-inflammatory adjunct therapy to existing antiviral drugs are glucocorticoids that are usually topically administered. However, glucocorticoid treatment of chronic eye inflammation is limited by the high risk of cataract formation and elevated intra-ocular pressure, in themselves potential threats to vision (5). Accordingly there is a need for alternative therapies to control SK. One approach would be to exploit the host’s own counter-regulatory functions to balance an ongoing inflammatory response and accelerate its resolution towards tissue homeostasis. The resolvins represent one such group of molecules (6), but no information is currently available about their potential to control the immune response during a viral infection and prevent long term sequelae such as SK following HSV infection of the eye.
Resolvins are endogenous lipid mediators that are derived from the omega-3 poly unsaturated fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (6). The currently investigated resolvin E1 (RvE1; 5S,12R,18R-trihydroxyeicosapentaenoic acid) was the first resolvin to be identified in exudates of resolving local inflammations, and following its full structural elucidation and organic synthesis was confirmed to actively dampen acute inflammation (7, 8, 9). Events that are associated with inflammation and controlled by resolvins include prevention of diapedesis, notably neutrophil migration (6), regulation of dendritic cell co-stimulatory factors leading to reduced T-cell activation (10), increased macrophage phagocytosis of apoptotic neutrophils (11), and inhibition of host tissue inflammatory responses, with the release of chemokines and cytokines (11). In addition to regulating inflammation RvE1 was recently shown to actively promote tissue repair and prevent host tissue cell death during stress (12, 13). Consequently, various resolvins have proved to be efficacious in a range of experimental models of inflammatory diseases including, inflammation of the lung (14), colitis (15), periodontitis (16), retinal angiogenesis (17), and very recently dry eye (18). More recently resolvins have also been demonstrated to dampen the immune response during acute bacterial infections without being immunosuppressive, but led to improved bacterial control and enhanced survival (19). Current observations with resolvins suggest that they would be efficacious in controlling SK, since the inflammatory events known to be counter-regulated by resolvins are necessary steps in the initial pathogenic development of SK, in particular, the rapid neovascularization which permits neutrophils to enter the normally avascular cornea (20). This possibility was investigated in the presently reported experiments using a well established mouse model of SK in which primary ocular infection with HSV regularly results in chronic, unresolving inflammatory SK lesions that is orchestrated by T-cells (21, 22).
In this report we demonstrate the efficacy of topical therapy with resolvin E1 (RvE1) in controlling ocular disease caused by HSV. The results show that RvE1 administration markedly diminished SK lesion severity and corneal neovascularization. We show that RvE1 therapy decreased the influx of effector CD4+ T cells, neutrophils and the production of proinflammatory cytokines and molecules involved in ocular neovascularization, an essential component of SK pathogenesis. Our results indicate that RvE1 therapy may represent a novel approach to control HSV-induced ocular immunopathological lesions, the most common infectious cause of blindness in humans.
Materials and Methods
Mice and Virus
Female BALB/c mice were purchased from Harlan Sprague-Dawley (Indianapolis, IN). The animals were housed in American Association of Laboratory Animal Care approved facilities at the University of Tennessee, Knoxville. All investigations followed guidelines of the Institutional Animal Care and Use Committee. HSV-l RE strain (obtained from the laboratory of Robert Hendricks, University of Pittsburgh, Pittsburgh, PA, USA) was used in all procedures. Virus was grown in Vero cell monolayers (American Type Culture Collection, Manassas, VA), titrated, and stored in aliquots at −80°C until used.
Corneal HSV infection
Corneal infections of all mouse groups (6–10 week old) were conducted under a deep anesthesia (avertin). The mice were scarified lightly on their corneas with a 30 gauge needle, and a 3-µl drop containing 2 × 105 plaque-forming units (PFU) of HSV-1 RE was applied to the eye.
RvE1 adminstration
The methyl ester prodrug of RvE1 (RX-10005, Resolvyx, Bedford, MA) was topically applied to the cornea (300 ng per eye; 3µl drop) twice daily from day 1 until day 12 post infection (dpi) for treatments starting early or was applied twice daily from day 6 to day13 for treatment starting late unless otherwise specified. The vehicle control group received an equal volume of phosphate buffered saline (PBS). For Systemic administration mice were given RvE1 intra peritonealy at a dose of 1.0µg/mice (0.05mg/Kg body weight) daily once from day 6 to day 13. This particular dosage was selected based on previous studies by other groups (9, 23, 24).
Clinical observations
The eyes were examined on different days after infection for the development of clinical lesions by slit-lamp biomicroscopy (Kawa Co., Nagoya, Japan), and the clinical severity of keratitis of individually scored mice was recorded by a blinded observer. The scoring system was as follows: 0, normal cornea; +1, mild corneal haze; +2, moderate corneal opacity or scarring; +3, severe corneal opacity but iris visible; +4, opaque cornea and corneal ulcer; +5, corneal rupture and necrotizing stromal keratitis. The severity of angiogenesis was recorded as described previously (25). In reference to the angiogenic scoring system, the method relied on quantifying the degree of neovessel formation based on three primary parameters: 1) the circumferential extent of neovessels (as the angiogenic response is not uniformly circumferential in all cases); 2) the centripetal growth of the longest vessels in each quadrant of the circle; and 3) the longest neovessel in each quadrant was identified and graded between 0 (no neovessel) and 4 (neovessel in the corneal center) in increments of 0.4 mm (radius of the cornea is 1.5 mm). According to this system, a grade of 4 for a given quadrant of the circle represents a centripetal growth of 1.5 mm toward the corneal center. The score of the four quadrants of the eye were then summed to derive the neovessel index (range, 0 to 16) for each eye at a given time point.
Immunohistochemical Staining
At the termination of the experiment (14dpi), eyes were removed from control and RvE1 treated mice and snap-frozen in OCT compound (Miles, Elkhart, IN). Six-micron thick sections were cut and stained with hematoxylin and eosin. Sections were observed for thickness of cornea, presence of inflammatory infiltrates, ulceration and epithelial erosions as described previously (26).
Flow cytometric analysis
At day15 corneas were excised, pooled group wise, and digested with 60 U/ml Liberase (Roche Diagnostics) for 45 min at 37°C in a humidified atmosphere of 5% CO2. After incubation, the corneas were disrupted by grinding with a syringe plunger on a cell strainer and a single-cell suspension was made in complete RPMI 1640 medium. The single-cell suspensions obtained from corneal samples were stained for different cell surface molecules for FACS analyses. All steps were performed at 4°C. Briefly, a total of 1 × 106 cells were first blocked with an unconjugated anti-CD32/CD16 mAb for 30 min in FACS buffer. After washing with FACS buffer, the cells were stained with respective fluorochrome-labeled Abs for 30 min. Finally, the cells were washed three times and resuspended in 1% paraformaldehyde. To enumerate the number of IFN-γ, IL-2 and IL-17-producing T cells, intracellular cytokine staining was performed. In brief, corneal cells were stimulated with anti-CD3 (3µg/ml) and anti-CD28 (1µg/ml) for 5 hrs in the presence of brefelidin A (5µg/ml) in U-bottom 96-well plates. After this period, cell surface staining was performed, followed by intracellular cytokine staining using a Cytofix/Cytoperm kit (BD Pharmingen) in accordance with the manufacturer’s recommendations. The Abs used were anti-CD4 APC, anti-IFN-γ-PE, IL-17 Percp cy5.5 and anti-IL-2-FITC. The fixed cells were resuspended in 1% paraformaldehyde. For stimulation with virus, cells were left untreated or stimulated with 1 MOI of UV-inactivated HSV-1 and incubated overnight at 37°C in 5% CO2. Brefeldin A (5 µg/ml) was added for the last 5 h of the culture period. After this period, cell surface staining was performed, followed by intracellular cytokine staining using a Cytofix/Cytoperm kit (BD Pharmingen) in accordance with the manufacturer’s recommendations as mentioned above. The stained samples were acquired with a FACS Calibur (BD Biosciences) and the data were analyzed using the FlowJo software.
Protein quantification of corneal lysates by ELISA
The corneal samples were pooled groupwise (6 corneas/group) and homogenized using a tissue homogenizer (Pellet Pestle mortar; Kontes). The concentrations of various cytokines and VEGF was measured by sandwich ELISA kits from eBioscience (IL-6, IL-10, IFN-γ, IL-17) and R&D (VEGF-A, KC) as per the manufacturer’s instructions.
MK/T-1 cell assay
MK/T-1 cells, immortalized keratocytes from the corneal stroma of C57BL/6 mouse (kindly provided by Dr. Dana Reza, Harvard medical School, Boston, MA) were stimulated in vitro with 10ng/ml recombinant murine IL-6 in the presence of various concentrations of RvE1 (25nM, 75nM, 250nM and 750 nM) in DMEM supplemented with 10% fetal bovine serum for 24 hr at 37°C in 5% CO2. Following 24 hr of stimulation, the supernatants were collected and stored at −80°C until further use. Supernatants were analyzed for VEGF-A production using VEGF-A quantikine sandwich ELISA kit (R&D) as per manufacturer’s instructions.
Quantitative PCR (QPCR)
At 15 days after ocular infection, the corneas were isolated, and 4 corneas per group were pooled, corneal cells were lysed and total mRNA was extracted using TRIzol LS reagent (Invitrogen). Total cDNA was made with 1 µg of RNA using oligo(dT) primer. Quantitative PCR was performed using SYBR Green PCR Master Mix (Applied Biosystem, Foster City, CA) with iQ5 real-time PCR detection system (Bio Rad, Hercules, CA). The expression levels of different molecules were normalized to β-actin using ΔCt calculation. Relative expression between control and experimental groups were calculated using the 2-ΔΔCt formula. The PCR primers used were as follows VEGF-A, forward 5'-GTTCATGGATGTCTACCAGCGAAG-3' and reverse 5'-GAAGATGTACTCTATCTCGTCGGG-3'; MMP2 forward 5'-CCGATCTACACCTACACCAAGAAC-3' and reverse 5'-CCAGTACCAGTGTCAGTATCAG-3'; MMP-9, forward 5'-CTCTACAGAGTCTTTGAGTCCG-3' and reverse 5'-CCTGTAATGGGCTTCCTCTATG-3'; Beta actin forward 5'-CCTTCTTGGGTATGGAATCCTG-3' and Beta actin reverse 5'-GGCATAGAGGTCTTTACGGATG-3'.
Western blot analysis
At 15 days after ocular infection, the corneas were isolated, and 4 corneas per group were pooled The supernatants from lysed corneal cells were quantified using BCA protein Assay kit (Thermo scientific, Waltham, MA) using BSA as a standard and samples with equal protein concentrations were denatured by boiling in Laemmli buffer. Polypeptides were resolved by SDS-PAGE and transferred onto a PVDF membrane. The membrane was blocked with 5% BSA in Tris-buffered saline with Tween20 (20 mM Tris [pH 7.4], 137 mM NaCl, and 0.1% Tween20) overnight at 40°C and probed with specific primary and secondary antibodies. Proteins were detected using chemiluminiscent HRP substrate (Millipore, Billerica, MA). The membrane was kept in stripping buffer for 10 min and re-probed using anti-β-actin antibody. The antibodies used were mouse anti-VEGF-A (EE02; Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti MMP-2 (8B4; Santa Cruz Biotechnology, Santa Cruz, CA), goat anti mouse MMP-9 (C-20; Santa Cruz Biotechnology, Santa Cruz, CA), and mouse anti-β-actin (AC74; Sigma-Aldrich, St Louis MO), goat anti rat IgG-HRP (R & D Systems), goat anti-mouse IgG-HRP (Santa Cruz Biotechnology) and donkey anti goat IgG-HRP (Santa Cruz Biotechnology).
Statistical analysis
Statistical significance was determined by Student’s t test unless otherwise specified. A p value of <0.05 was regarded as a significant difference between groups *, p≤ 0.05; **, p ≤ 0.001; ***, p ≤0.0001. For some experiments, as mentioned in the figure legends, one-way analysis of variance (ANOVA) with Dunnett’s post test was used. GraphPad Prism software was used for statistical analysis.
Results
RvE1 reduces the initial Leukocyte and PMN response to HSV infection
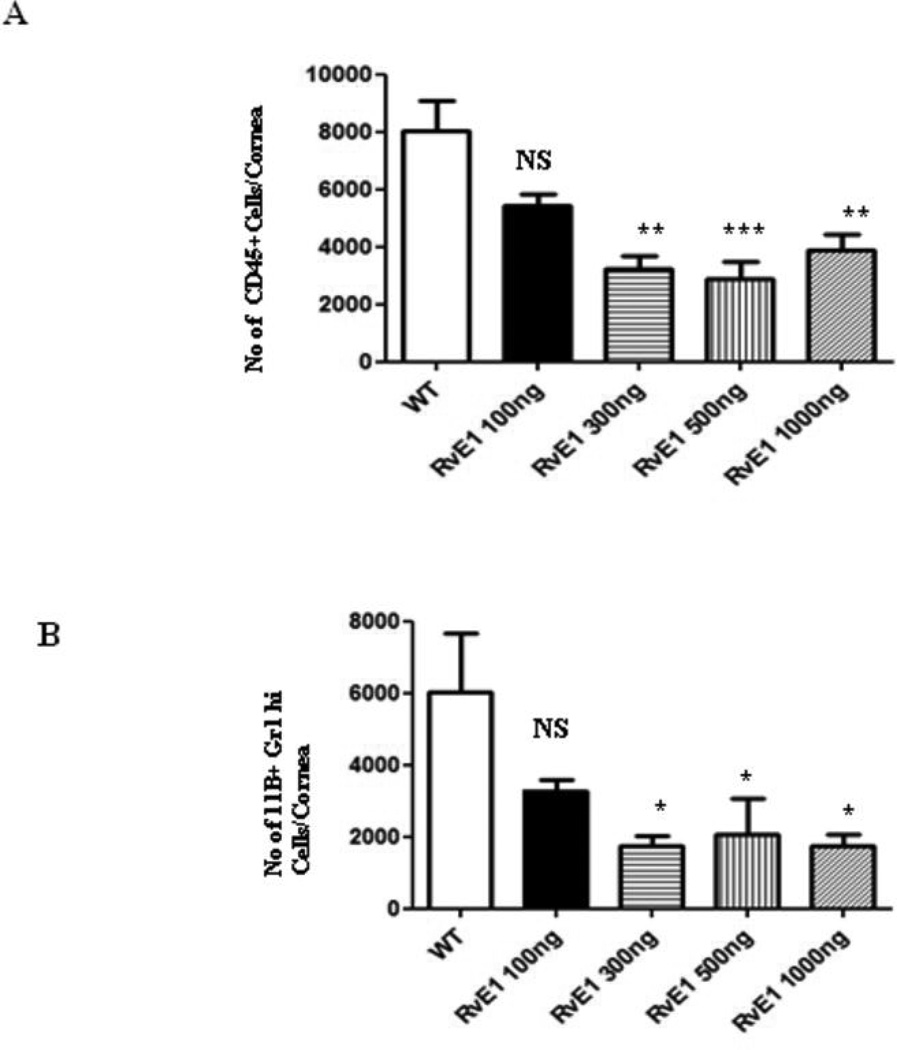
Following infection of the corneal surface with HSV, virus replicates in epithelial cells and a prompt, mainly neutrophil influx occurs in the underlying stroma that peaks around 48hrs pi (26). Analysis of single cell suspensions from digested corneas obtained from mice sacrificed at 48hrs pi (after 3 dosing) confirmed that RvE1 dose-dependently reduced corneal infiltration measured either as total leukocytes (CD45) or neutrophils (11b+Gr1hi) (Figure 1). In the dose range investigated, 100–1000ng, a maximal response of 60% reduction in either cell population was evident at the 300ng dose, with no further reduction at higher doses. Figure 1 also showed, as expected at this early stage of infection, that the leukocyte population mainly consisted of cells expressing phenotypic markers characteristic of neutrophils.
Figure 1. RvE1 reduces cellular infiltration into the cornea of ocularly infected mice in a dose dependent manner.
Balb/c mice were ocularly infected with 2×105 of HSV-1 RE. Different doses (100ng, 300ng, 500ng, 1000ng) of RvE1 were applied topically at 18hrs, 24hrs and 36hrs post infection. The animals were sacrificed at 48hrs post infection and pooled corneal samples were analyzed for surface expression of CD45, 11b and Gr1 by flow cytometry. A. Number of leukocytes (CD45+) per cornea and B, Number of neutrophils (11b+ Gr1hi) per cornea at indicated time point is shown. Data shown is from one representative experiment. Experiments were repeated two times. One-way ANOVA was used to calculate the level of significance
Topical RvE1 treatment inhibits the severity of SK lesions and extent of neoangiogenesis
Typically SK becomes evident clinically around 1 week after HSV infection, at a time when the replicating virus has disappeared, but lesions continue to progress in severity for 7–10 days. To investigate the effects of RvE1 treatment on the clinical consequences, groups of animals were treated twice daily with RvE1 or vehicle starting at day 1 pi or day 6 pi and continued until 2 days before experiments were terminated on day 15pi. Animals were examined at 2 day intervals to record severity of SK lesions as well as the extent of angiogenesis.
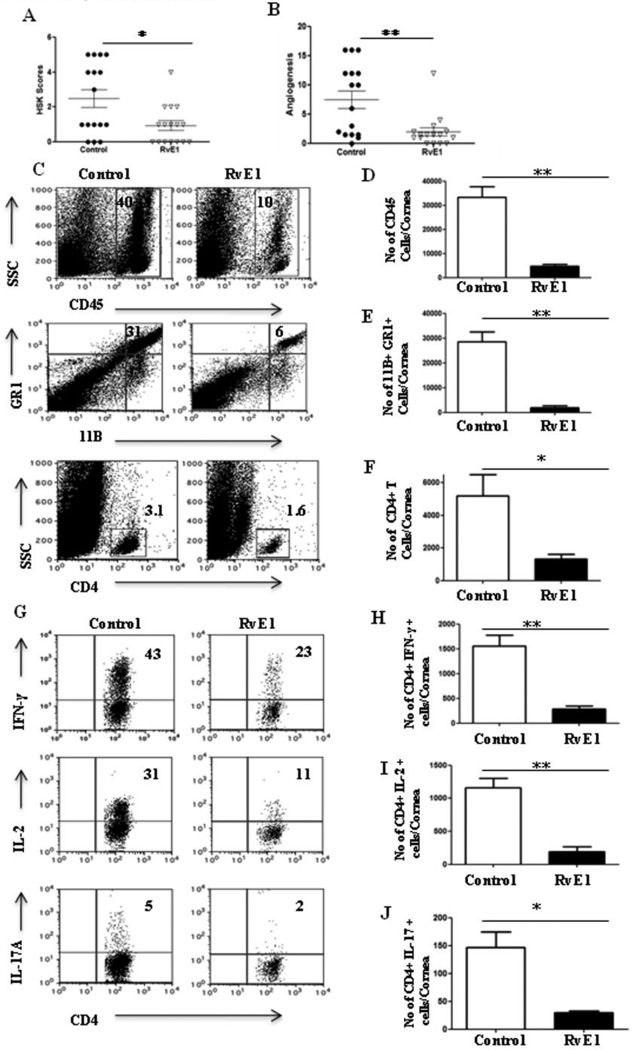
In animals begun treatment starting at day 1, only 55% of corneas of those receiving RvE1 showed positive lesions compared to 80% of controls (p≤ 0.009) with the extent of neovascularization being similarly reduced (p≤ 0.004) (Figure 2A and B). Not only the frequency of lesions but also their severity was reduced with 6% of RvE1 treated animals showing a lesion score of ≥3 compared to 50 % in control animals. Angiogenesis was also significantly reduced in the treated animals compared to controls (Figure 2B). At termination on day15pi, corneas were pooled and isolated cells were identified and enumerated by flow cytometry. The numbers of total leukocytes, neutrophils and CD4+ T cells were reduced by 86%, 93% and 74% respectively in the RvE1 treated group compared to the control group, which was highly significant for all three cell populations (Figure 2C–F). Importantly, when the frequency of subsets of CD4+ T cells producing IFN-γ, IL-2 or IL-17 was investigated after stimulating with CD3/CD28, RvE1 was shown to markedly reduce both Th1 and Th17 populations by 82% and 79% respectively (Figure 2H–J).
Figure 2. Effect of RvE1 started during the pre-clinical phase (day 1 pi) on SK severity and cellular infiltration.
Balb/c mice infected with 2×105 PFU of HSV-1 RE were given RvE1 topically twice daily starting from day 1 until day 12. The disease severity and immune parameters were analyzed at day 15. A–B. SK lesion severity and angiogenesis at day 14 are shown. C. Representative histograms show percentage of leukocytes (CD45+), neutrophils (11b+Gr1hi) and CD4+ T cells in the inflamed cornea of control and RvE1 treated animals at day 15. D–F. Average no. of (D) CD45+ cells, (E) 11b+ Gr1hi and (F) CD4+ T cells per cornea at indicated time point are shown. G. Representative plots show percentage of CD4 cells producing IFN-γ, IL-2 or IL-17 following stimulation with CD3/CD28 in the corneas of infected animals. Plots shown were gated on CD4+ T cells. H–J. Average no. of CD4 cells producing (H) IFN-γ, (I) IL-2 and (J) IL-17 in the cornea are shown. Experiments were repeated three times and the level of significance was determined by Student’s t test (unpaired).
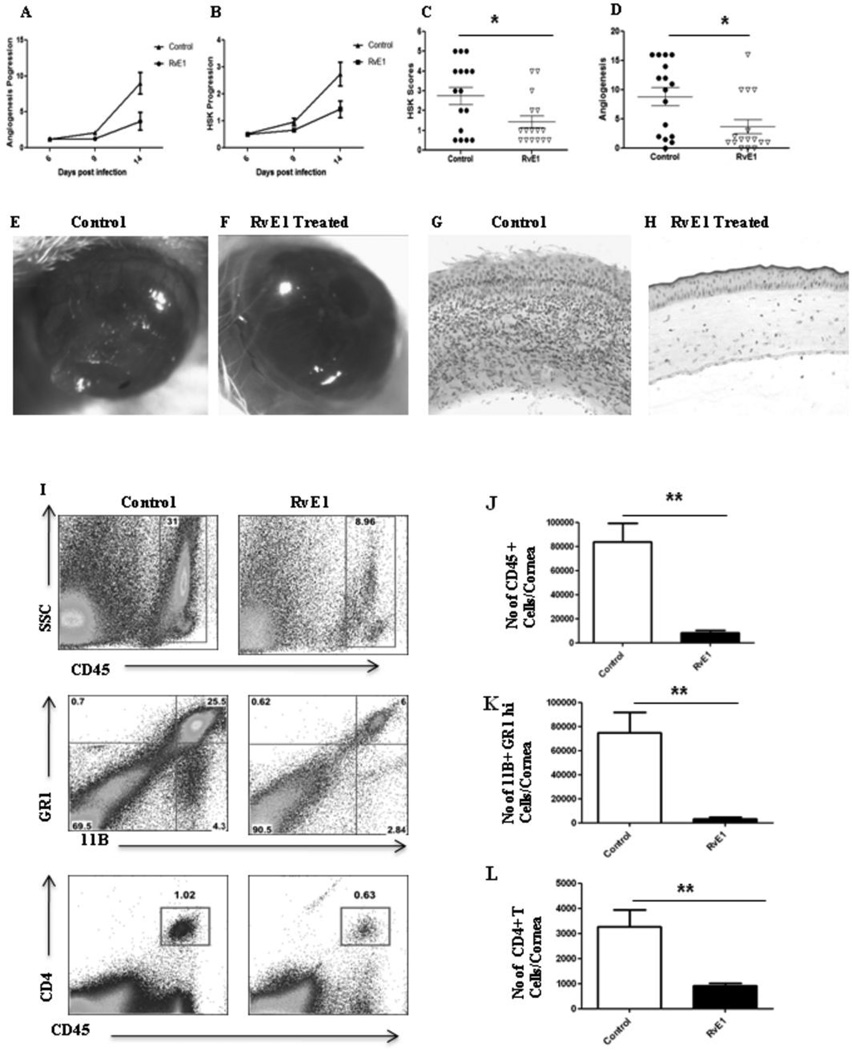
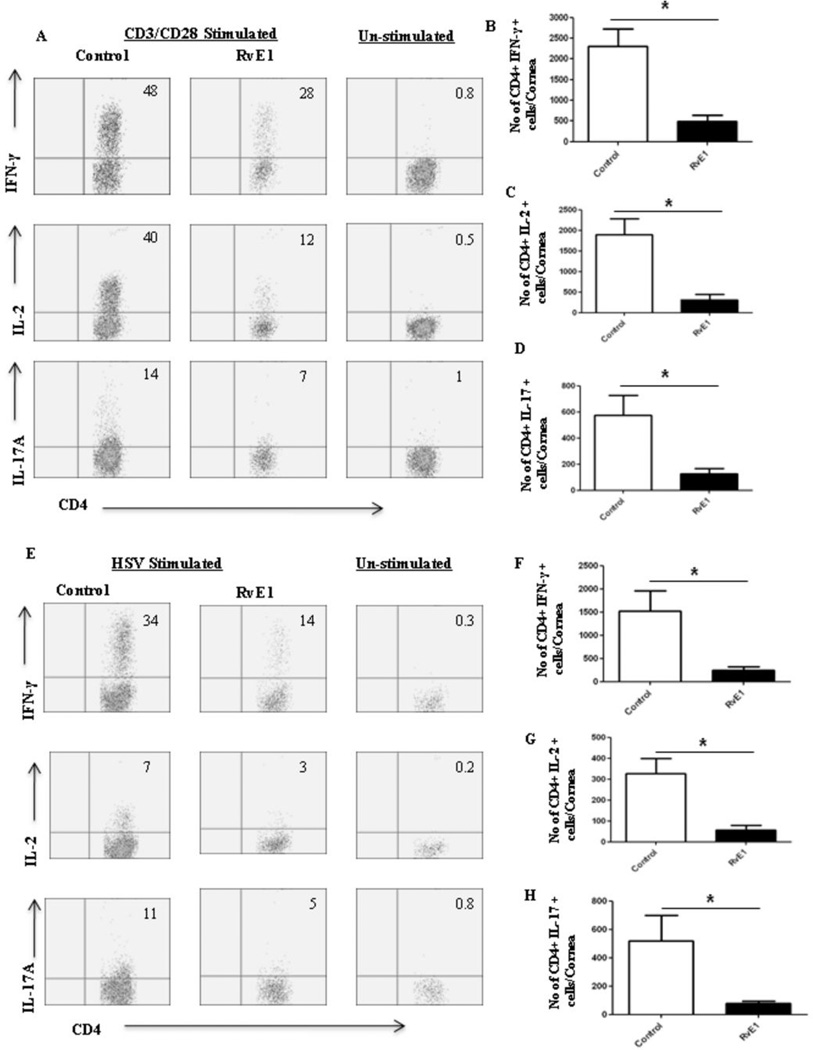
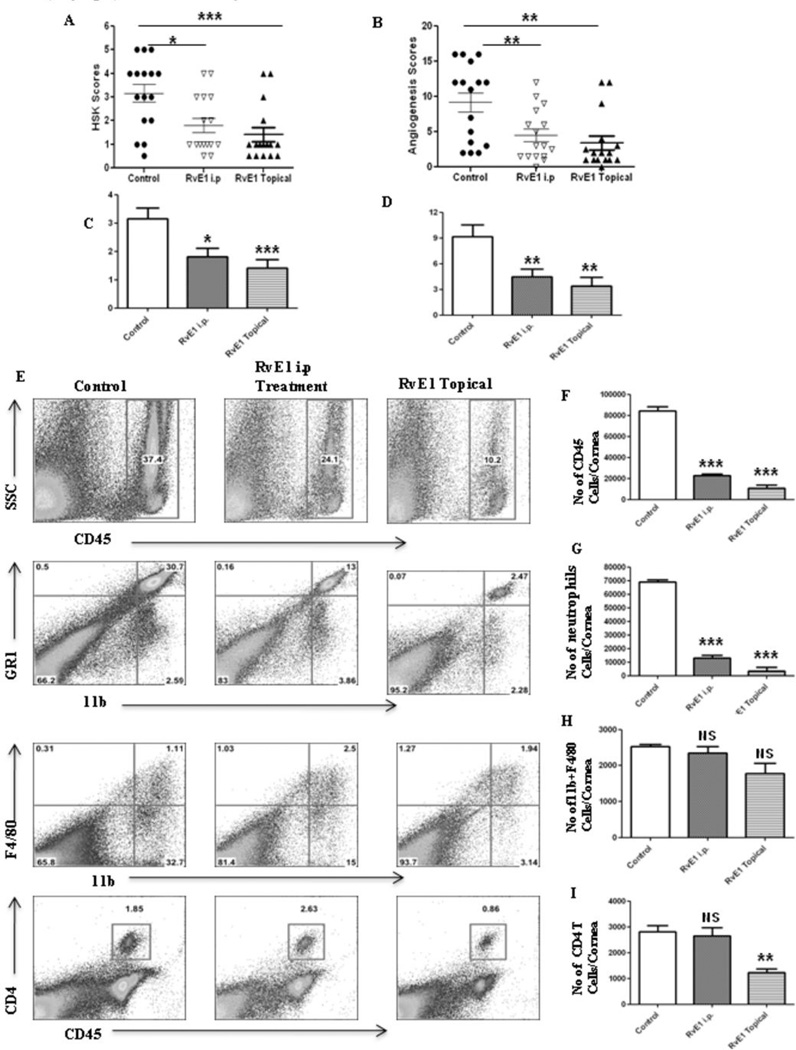
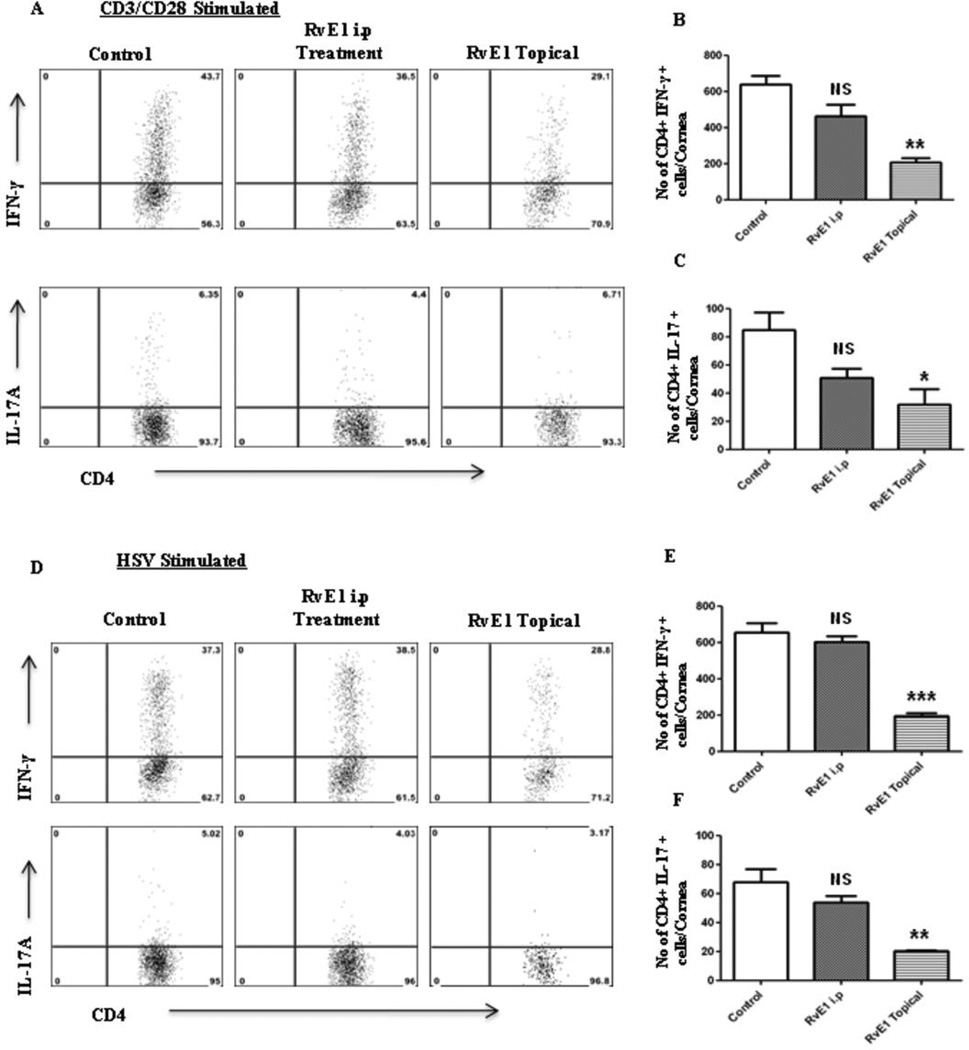
In the second series of experiments, RvE1 treatment was begun at day 6pi, a time point when replicating virus was no longer detected on the HSV infected corneas (data not shown). This time point also corresponds to the beginning of the chronic stage of SK with infiltration of pathogenic CD4+ T cells that orchestrate SK lesion development. Monitoring the progression of disease over the subsequent 10 day, a delayed onset of neovascularization and SK was seen in the RvE1 treated group, which then progressed at a slower rate (Figure 3A–B). At the termination of the experiment on day 15pi, there was a significant reduction in both the extent of neovascularization and the severity of SK in RvE1 treated animals (Figure 3C–F). Nearly 60% of eyes from untreated animals exhibited severe lesions (score≥3) compared to 20% in the RvE1 treated group (p≤0.02). Immunohistochemical staining showed that cornea from untreated mice were swollen and contained a massive infiltrate of inflammatory cells when compared with RvE1 treated mice (Figure 3G–H). The numbers of infiltrating leukocytes and neutrophils in infected corneas at the day of termination were reduced by almost 90% in mice treated with RvE1 (Figure 3I–K). Additionally, the numbers of CD4+ T cells were reduced by almost 70% compared to control animals. Also with a delayed onset of treatment, the numbers of CD4+ T cells of both Th1 and Th17 subtypes were found to be reduced in the group treated with RvE1 when the corneal cell suspensions from different treatment groups were stimulated with either CD3/CD28 or HSV-1 antigens (Figure 4A–H).
Figure 3. Effect of RvE1 started during the clinical phase (day 6 pi) on corneal inflammation and cellular infiltration.
Balb/c mice infected with 2×105 PFU of HSV-1 RE were given RvE1 topically twice daily starting from day 6 until day 13. The disease progression and immune parameters were analyzed. A–B. Disease progression of lesions and angiogenesis are shown. C–D. SK lesion severity and angiogenesis at day 14 are shown. E–F. Eye pictures of control and RvE1 treated animals showing lesion severity at 14 days post infection from a representative experiment are shown. Images were captured by stereomicroscopy and an imaging system. Magnification, 20×. E–F. Mice were terminated at day 14 p.i. and eyes were processed for cryosection. H&E staining was performed on 6-µm sections. The figure shows the pictures of the sections taken at 20×, magnification. I. Representative histograms show percentage of leukocytes (CD45+), neutrophils (11b+Gr1hi) and CD4+ T cells in the inflamed cornea of control and RvE1 treated animals at day 15. J–L. Average no. of (J) CD45+ cells, (K) 11b+ Gr1hi and (L) CD4+ T cells per cornea at indicated time point are shown. Experiments were repeated three times and the level of significance was determined by Student’s t test (unpaired).
Figure 4. Effect of RvE1 started during the clinical phase (day 6 pi) on Th1 and Th17 cell infiltration into the inflamed cornea at day 15.
Balb/c mice infected with 2×105 PFU of HSV-1 RE were given RvE1 topically twice daily starting from day 6 until day 13.A. Representative plots show percentage of CD4 cells producing IFN-γ, IL-2 or IL-17 following stimulation with CD3/CD28 in the corneas of infected animals. Plots shown were gated on CD4+ T cells. B–D. Average no. of CD4 cells producing (B) IFN-γ, (C) IL-2 and (D) IL-17 in the cornea at day15 are shown. E. Representative plots show percentage of CD4 cells producing IFN-γ, IL-2 or IL-17 following stimulation with HSV-1 in the corneas of infected animals. Plots shown were gated on CD4+ T cells. F–H. Average no. of CD4 cells producing (F) IFN-γ, (G) IL-2 and (H) IL-17 in the cornea at day15 are shown. Experiments were repeated three times and the level of significance was determined by Student’s t test (unpaired).
Effect of RvE1 treatment on mediator levels involved in SK pathogenesis
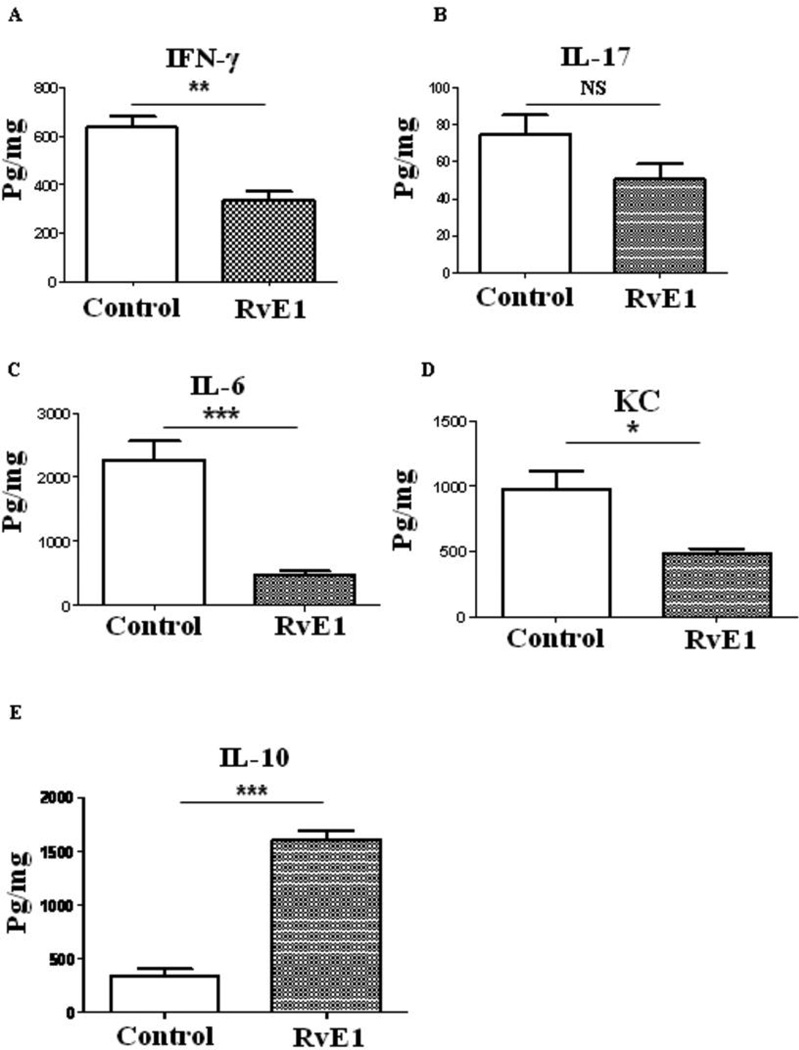
Pooled corneal extracts were collected on day 15pi from control and from RvE1 treated (started 6 day pi) animals and sample pools were analyzed for IFN-γ, IL-17, IL-6, CXCL1, IL-10 and VEGF-A protein levels using ELISA. The data show that RvE1 treatment reduced the production of IFN-γ by 47% in the RvE1 treated mice (p≤0.002) (Figure 5A). Although the levels of IL-17 were lower in the RvE1 treated group, the differences were not significant (Figure 5B). Importantly the levels of IL-6, a key pro-inflammatory cytokine involved in SK pathology (27) were substantially reduced by 80% in RvE1 treated mice compared to control the control group (Figure 5C). The levels of KC, a chemokine which has a major role in neutrophil early migration, were reduced by 50% in the RvE1 treated group compared to untreated mice (Figure 5D). A striking finding was that RvE1 treatment induced production of IL-10 with a 7 fold higher level observed over that in corneal tissue from control animals (Figure 5E).
Figure 5. Effect of RvE1 treatment on cytokine and chemokine production in the corneas of HSV-1 infected animals.
Balb/c mice infected with 2×105 PFU of HSV-1 RE were given RvE1 topically twice daily starting from day 6 until day 13. Mice were sacrificed at day 15 and corneal extracts were measured by sandwich ELISA. (A) IFN-γ (B) IL-17 (C) IL-6 (D) KC and (E) IL-10) protein levels in four different pooled corneal samples each consisting of four cornea/group in control and RvE1 treated animals are shown. The level of significance was determined by Student’s t test (unpaired).
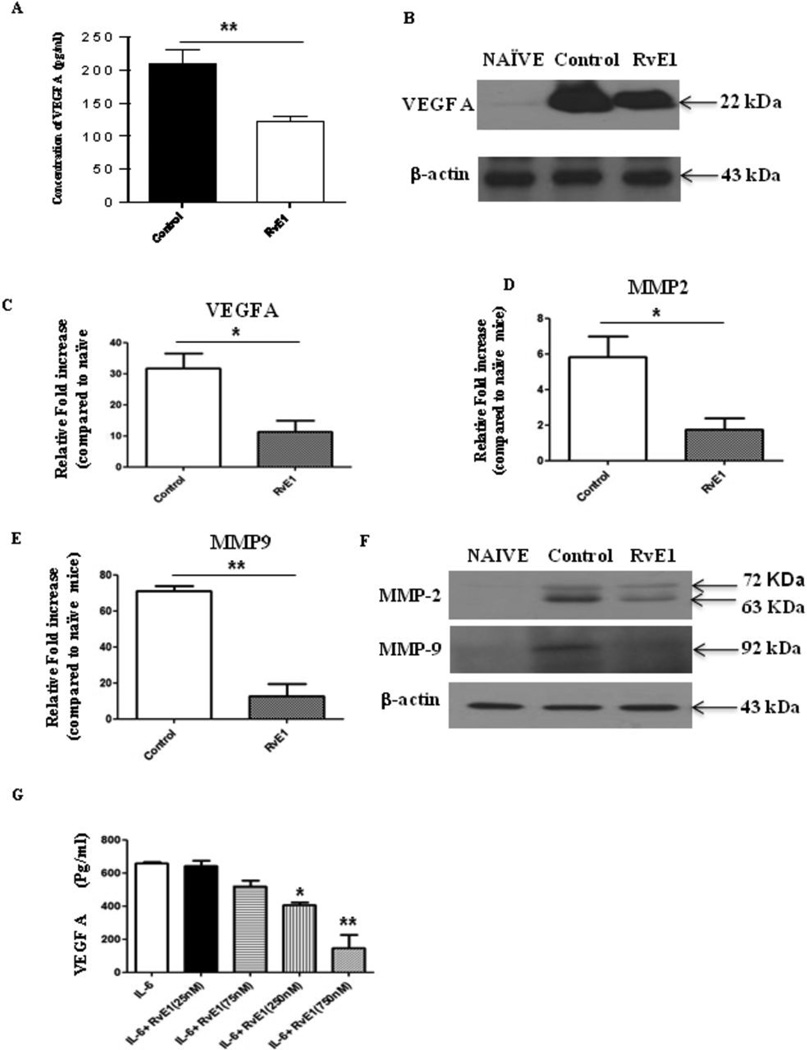
Consistent with a reduction in new vessel formation in corneas from RvE1 treated animals, the VEGF-A levels were reduced by 41% (p≤0.006) (Figure 6A–C). There was also a change in the levels of MMP-2 and MMP-9, molecules also associated with the neovascularization process in the eye (28). RvE1 effectively blunted the increased levels of MMP-2 and MMP-9 in corneal tissues from control mice (Figure 6D–F). The levels of MMP-2 were reduced by 3 fold and MMP-9 by 7 fold in the corneas tissue from RvE1 treated group when compared with the control group. Additionally, we also measured the in vitro production of VEGF-A in MKT-1, a murine stromal fibroblast cell line. For this purpose MKT-1 cells were stimulated for 24hrs in the presence of IL-6 alone or IL-6 in combination with different doses of RvE1 and the supernatant analyzed for VEGF-A production using ELISA. As is evident in Figure 6G, VEGF-A production was suppressed by RvE1 in a dose dependent manner. The effect of RvE1 on VEGF-A production was significantly reduced at both 250 nM and 750 nM doses of RvE1 when compared with cells stimulated with IL-6 alone. Taken together our results indicate that RvE1 treatment served to diminish pathological lesions by a number of mechanisms.
Figure 6. Effect of RvE1 treatment on pro-angiogenic factors.
Balb/c mice infected with 2×105 PFU of HSV-1 RE were given RvE1 topically twice daily starting from day 6 until day 13. Mice were sacrificed at day 15 and (A) VEGF-A protein levels in four different pooled corneal samples each consisting of four cornea/group in control and RvE1 treated animals were quantified using quantikine sandwich ELISA kit (R&D). B. Immunoblot showing VEGF-A expression in the pooled corneal homogenates from cornea obtained from naïve and HSV-1 infected control and RvE1 treated animals. C–E. Q PCR was used measure the expression of (C) VEGF-A (D) MMP-2 and (E) MMP-9 in pooled corneal samples four cornea/group in control and RvE1 treated animals. The experiments were repeated 3 times. (F) Immunoblot showing MMP-2 and MMP-9 expression in the pooled corneal homogenates from cornea obtained from naive and HSV-1 infected control and RvE1 treated animals. The experiments were repeated two times and the level of significance was determined by Student’s t test (unpaired). G. Effects of RvE1 on VEGF-A production. MK/T-1 cells were stimulated with recombinant murine IL-6 in the presence of various doses of RvE1 for 24 hr period. VEGF-A protein levels in the supernatants were measured using sandwich ELISA. One-way ANOVA was used to calculate the level of significance.
Systemic administration of RvE1 reduced neutrophil infiltration and SK pathology
Normally the cornea is an avascular tissue which contributes to its immune privilege, but this breaks down when angiogenesis occurs following HSV-1 infection (28). It was therefore of interest to investigate if systemic administration of RvE1 would be efficacious in established disease. For this purpose the effect of RvE1 on HSV induced lesions was investigated measured following intra-peritoneal (ip) administration starting at day 6 pi. In this experiment, the efficacy of systemic administration was compared to a parallel group receiving topical RvE1 treatment (300ng), as well as an untreated control group. The dose of ip administered RvE1 was chosen at 1µg per mouse (0.05mg/kg body wt) based on past experience in other models of inflammation (9, 23, 24). As with the previous topical experiments, the mice were scored for extent of disease on day 14pi and sacrificed on day 15 pi for analysis of cell and mediator content. Administration of RvE1 systemically reduced SK immunopathology compared to untreated control mice almost as efficaciously as topical administration, with control mice having a frequency of severe lesions of 69% (Score>3) compared to 31% for systemic and 19% for topical administration (Figure 7A–D). While there was a 70% (p≤0.002) reduction in neutrophil content in corneas from mice administered RvE1 ip and to a same magnitude as seen with topical administration, a notable finding was the less marked attenuation of corneal CD4+ cells with ip compared to topical treatment (Figure 7F–I). This was further confirmed when isolated cell suspensions from different treatment groups were stimulated with either CD3/CD28 or HSV, and consistently a lesser effect was seen with systemic administration over topical on cells stained for CD4 and IFN-γ or IL-17(Figure 8A–F). There was only a marginal difference in the influx of macrophages (11b+ F4/80+) between the RvE1 treated and control groups (Figure 7H). These results show that although both systemic and topical treatments were effective, topical treatment proved to be the better approach.
Figure 7. Effect of systemic administration of RvE1 started during the clinical phase (day 6 pi) on corneal inflammation and cellular infiltration.
Balb/c mice infected with 2×105 PFU of HSV-1 RE were given RvE1 either intra peritonealy or topically twice daily starting from day 6 until day 13. A–B. SK lesion severity and angiogenesis at day 14 are shown. C–D. Cumulative lesion and angiogenesis scores are shown. E. Representative histograms show percentage of leukocytes (CD45+), neutrophils (11b+Gr1hi) and CD4+ T cells in the inflamed cornea of control and RvE1 treated animals at day 15. F–H. Average no. of (F) CD45+ cells, (G) 11b+ Gr1hi and (H) CD4+ T cells per cornea at indicated time point are shown. Experiment was repeated two times. One-way ANOVA was used to calculate the level of significance.
Figure 8. Effect of systemic administration of RvE1 started during the clinical phase (day 6 pi) on Th1 and Th17 cell infiltration into the inflamed cornea at day 15.
Balb/c mice infected with 2×105 PFU of HSV-1 RE were given RvE1 either intra peritonealy or topically twice daily starting from day 6 until day 13.A. Representative plots show percentage of CD4 cells producing IFN-γ or IL-17 following stimulation with CD3/CD28 in the corneas of infected animals. Plots shown were gated on CD4+ T cells. B–D. Average no. of CD4 cells producing (B) IFN-γ (C) IL-17 in the cornea at day15 are shown. D. Representative plots show percentage of CD4 cells producing IFN-γ or IL-17 following stimulation with HSV-1 in the corneas of infected animals. Plots shown were gated on CD4+ T cells. E–F. Average no. of CD4 cells producing (E) IFN-γ (F) IL-17 in the cornea at day15 are shown. Experiments were repeated two times. One-way ANOVA was used to calculate the level of significance.
Discussion
A troublesome consequence of HSV infection of the eye is a chronic inflammatory reaction in the corneal stroma that may persist after clearance of virus and can lead to impaired vision and eventually blindness (3). Management of stromal keratitis lesions usually relies on a combination of antiviral and anti-inflammatory drugs with the later commonly needed for lengthy periods, which, with corticosteroids as a preferred choice, can have unwanted side effects, notably elevated intraocular pressure and cataract. In the present report, we have used a novel approach to control SK lesions in a mouse model of SK using RvE1, which is endogenously biosynthesized through oxidation of the omega-3 PUFA EPA and shown to regulate resolution of inflammation (6). Administration of RvE1 was efficacious whether begun soon after or at a later stage after infection. Administered topically as its methyl ester prodrug (prepared by full organic synthesis), RvE1 inhibited several key events in SK pathogenesis. These included inhibitory effects on the infiltration of inflammatory cells, particularly neutrophils and CD4+ T cells into the inflamed cornea. There was also a reduction in the levels of inflammatory cytokines, and an increase in IL-10, an anti-inflammatory cytokine associated with the resolution of SK lesions (29, 30). The now demonstrated in vivo response to RvE1 with an elevation in tissue IL-10 levels supports earlier in vitro findings using isolated cell systems, suggesting that this may be a feature shared by proresolving lipid derived mediators, including lipoxins and protectins (31, 32). RvE1 treatment also resulted in reduced ocular neovascularization, likely because of inhibitory effects on the production of the angiogenic factor, VEGF-A, and metalloproteinases, MMP-2 and 9 that are involved in angiogenesis (28, 33, 34). RvE1 therapy could represent a useful approach for the management of HSV-induced inflammatory lesions.
Stromal keratitis is an example of a chronic virally induced immunopathological lesion (20). Studies in mice have demonstrated that SK is orchestrated principally by CD4+ T cells that are mainly of the Th1 subset with an ancillary role for Th17 cells later in the disease process (21). The actual damage to the eye and the resultant vision impairment is caused mainly by inflammatory cells, particularly neutrophils and their products that are recruited to the eye by mediators released by CD4+ T cells (26). Without treatment, the mouse eye lesion usually does not regress even though replicating virus is cleared from the cornea within a week after infection (34). RvE1 treatment represents a logical therapeutic approach for SK since several studies have now established that resolvins play a natural role in resolving inflammatory lesions and that a principal target for RvE1 activity are neutrophils, the cell type that dominates inflammatory reactions in SK (6, 26). One prominent effect in targeting neutrophils is the stimulation of macrophage-mediated phagocytosis to enhance the removal of apoptotic neutrophils (11). Additionally, resolvins may exert inhibitory effects on inflammatory cell recruitment to damaged tissue sites as well as to angiogenesis (11, 35), although the actual mechanisms by which these effects are mediated are currently not fully understood. In own studies, we could show that the influx of neutrophils was markedly attenuated with RvE1 treatment. Several present findings may explain the regulation of neutrophils in the HSV-infected cornea. Firstly, RvE1 attenuated both, Th1 and Th17 responses, with the former well documented to orchestrate the perpetuating immune response in stromal keratitis (21, 26). However, regulation of Th17 activity measured both ex vivo and as tissue levels of IL-17 were reduced to the degree seen for Th1 activation. Th17/IL-17 has increasingly been suggested as a regulatory hub between the innate and the adaptive immune response arms with IL-17 regulating both CXCL1 (KC) and CXCL8 responses, which are prominent regulators of neutrophil migration (36). In our model we could show that RvE1 treatment attenuated levels of KC. Furthermore, human data suggest that IL-17 is expressed in corneas from patients with SK, and that IL-17 is constitutively expressed in corneal fibroblasts, supporting IL-17 as an important therapeutic target (37). The current in vivo experiments cannot separate between a direct or indirect effect in dampening the T-cell responses. However, previous observations suggest that RvE1 can prevent upregulation of co-stimulatory molecules on antigen presenting cells, and also that mixed cell populations and not purified T-cells are required for the dampening of both Th1 and Th17 responses (10). In this model antigen presentation and T-cell activation is early suggesting that other mechanisms may account for the dampened T-cell responses seen with treatment initiation at day 6 pi. A novel finding in our infectious model investigating actions of resolvins was the profound upregulation of IL-10, which is also considered a master switch in the control of keratitis (29), and a major regulator of T-helper cell activation (38). The production of IL-10 could explain the efficacy of late RvE1 treatment (start day 6 pi) in the SK model.
Another, a key event in regulating the severity of keratitis is control of IL-6 (27). In agreement with earlier observations with RvE1 the IL-6 response was blunted (14). IL-6 not only contributes to the regulation of neutrophil diapedesis into inflamed tissues but is also required for expansion of Th17 cells (36). The source of IL-6 may be either leukocytic or could derive from the virus infected corneal epithelium (27). Previous data on human corneal epithelial cells (HCEC) indicate that RvE1 prevents the release of hyperosmotic stress induced IL-6 and IL-8 secretion indicating that actions of RvE1 in dampening a pathological immune response is not limited to actions on bone marrow derived cells, as was initially described.
An important aspect of switching an active inflammatory event towards resolution to reestablish tissue homeostasis is clearance of apoptotic neutrophils through the activation of resolution-phase macrophages (39). Several resolvins, including RvE1, were recently documented to stimulate macrophage phagocytosis of apoptotic neutrophils (31), and the time course of their activation following RvE1 exposure described (32). We did not specifically attempt to quantify subsets of macrophages as either of predominantly pro-inflammatory or pro-resolution phenotype, but it was interesting to note that in animals treated with RvE1 the levels of macrophages at day 15 pi were proportionately less reduced than either neutrophils or T-helper cells. It should also be noted that resolution type macrophages are reported to secrete IL-10 in response to RvE1 exposure (32).
Ocular neovascularization is a key event in the pathogenesis of SK with the lesion being limited in severity by procedures that inhibit the production of, or response to, molecules involved in new blood vessel development (40). Angiogenesis is increasingly seen as an inflammation-triggered VEGF mediated response, whether in the cornea or retina (41) and not unexpectedly corneal neovascularization following HSV-inoculation was inhibited in RvE1 treated mice. Several factors in addition to inhibition of VEGF-A release likely contributed to the marked inhibition, including control of MMP-2, MMP-9 and angiopoietin (data not shown for angiopoietin) and may be attributed to the reduced levels of IL-6 measured in corneal tissue of RvE1 treated mice. IL-6 is considered a major stimulus of VEGF-A formation in HSV-induced neovascularization through activation of stromal resident cells as an additional source to migrating leukocytes for VEGF-A (27). Thus RvE1 regulation of both epithelial release of IL-6 and leukocyte infiltration, could together explain the reduced levels of VEGF-A. From our in vitro studies, we showed that RvE1 reduced VEGF-A protein levels in a producing cells line. Conceivably RvE1 acts similarly in vivo to inhibit angiogenesis factor production in one or more producing cell types, but this needs to be formally demonstrated. In addition, control of corneal neovascularization by resolvins has previously been demonstrated using the suture model to induce an inflammatory response (35), or in the retina, using the oxygen induced retinopathy model (17). Further studies are underway to establish how RVE1 acts mechanistically to limit the extent of angiogenesis in the SK model.
Systemic administration of RvE1 seemed somewhat less efficacious than topical administration. It may be argued that we did not explore a dose-response relationship for systemic administration, as was done for topical delivery, but a dose level selected for analysis is known from other systems of inflammation to give maximal RvE1 inhibition of an inflammatory response (9, 23, 24). However, it is noteworthy that there was no effect on CD4+ cells following systemic administration of RvE1. This may indicate that in spite of neovascularization, parts of the cornea may remain inaccessible to systemic drug delivery and/or that newly formed pathological vessels do not function optimally for drug delivery.
In humans, SK represents an important cause of visual problems and current treatment modalities are not considered ideal. Corticosteroids, for example, which are used for prolonged periods, have several side-effects that include additional effects on vision such as cataract formation and increased susceptibility to glaucoma (5). Since resolvins, as well as some other groups of molecules derived from omega 3 fatty acids, such as the protectins (6), are endogenous molecules responsible for resolving inflammatory reactions under normal circumstances, they are expected to have limited side-effects. In our reported studies, we only administered resolvins in the early phases of SK. However the effects of treatment at later stages, which is technically difficult as later stage lesions become severe in mouse models, is warranted, since this may better reflect the situation presented by human SK. Resolvins show initial high promise for the use to control ocular inflammatory disease and is further supported by very recent reports demonstrating that resolvin treatment may have value in the dry eye syndrome (18) as well as in inflammatory lesions that affect the retina (17). In conclusion, the use of resolvins that were without evident toxicity could represent a useful addition to the therapeutic management of virus induced ocular inflammatory lesions, and could conceivably, due to their origin as pro-resolving mediators that are enzymatically produced from omega-3 PUFAs, represent a safe treatment alternative.
Acknowlegements
We thank Shravan Sehrawat, Tamara Veiga Parga, Shalini Sharma, Greg Spencer and Eric Schwartz for their assistance during research and manuscript preparation.
This work was supported by National Institute of Allergy and Infectious Diseases Grant AI 063365 and National Institutes of Health Grant EY 005093.
References
- 1.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Colin J. Ganciclovir ophthalmic gel, 0.15%: a valuable tool for treating ocular herpes. Clin. Ophthalmol. 2007;1:441–453. [PMC free article] [PubMed] [Google Scholar]
- 3.Deshpande S, Banerjee K, Biswas PS, Rouse BT. Herpetic eye disease: immunopathogenesis and therapeutic measures. Expert Rev. Mol. Med. 2004;6:1–14. doi: 10.1017/S1462399404007604. [DOI] [PubMed] [Google Scholar]
- 4.Knickelbein JE, Hendricks RL, Charukamnoetkanok P. Management of herpes simplex virus stromal keratitis: An evidence-based review. Surv. Ophthalmol. 2009;54:226–234. doi: 10.1016/j.survophthal.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 5.McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25:33–55. doi: 10.2165/00002018-200225010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp. Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, Blumberg RS, Serhan CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vassiliou EK, Kesler OM, Tadros JH, Ganea D. Bone marrow-derived dendritic cells generated in the presence of resolvin E1 induce apoptosis of activated CD4+ T cells. J. Immunol. 2008;181:4534–4544. doi: 10.4049/jimmunol.181.7.4534. [DOI] [PubMed] [Google Scholar]
- 11.Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim. Biophys. Acta. 2010;1801:1260–1273. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F, Yang H, Pan Z, Wang Z, Wolosin JM, Gjorstrup P, Reinach PS. Dependence of resolvin-induced increases in corneal epithelial cell migration on EGF receptor transactivation. Invest. Ophthalmol. Vis. Sci. 2010;51:5601–5609. doi: 10.1167/iovs.09-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keyes KT, Ye Y, Lin Y, Zhang C, Perez-Polo JR, Gjorstrup P, Birnbaum Y. Resolvin E1 protects the rat heart against reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H153–H164. doi: 10.1152/ajpheart.01057.2009. [DOI] [PubMed] [Google Scholar]
- 14.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat. Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishida T, Yoshida M, Arita M, Nishitani Y, Nishiumi S, Masuda A, Mizuno S, Takagawa T, Morita Y, Kutsumi H, Inokuchi H, Serhan CN, Blumberg RS, Azuma T. Resolvin E1, an endogenous lipid mediator derived from eicosapentaenoic acid, prevents dextran sulfate sodium-induced colitis. Inflamm. Bowel Dis. 2010;16:87–95. doi: 10.1002/ibd.21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 17.Connor KM, Paul J, Giovanni S, Lofqvist C, Aderman CM, Chen J, Higuchi A, Hong S, Pravda EA, Majchrzak S, Carper D, Hellstrom A, Kang JX, Chew EY, Salem N, Serhan CN, Smith LEH. Increased dietary intake of ω3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li N, He J, Schwartz CE, Gjorstrup P, Bazan HE. Resolvin E1 Improves Tear Production and Decreases Inflammation in a Dry Eye Mouse Model. J. Ocul. Pharmacol. Ther. 2010;26:431–439. doi: 10.1089/jop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seki H, Fukunaga K, Arita M, Arai H, Nakanishi H, Taguchi R, Miyasho T, Takamiya R, Asano K, Ishizaka A, Takeda J, Levy BD. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J. Immunol. 2010;184:836–843. doi: 10.4049/jimmunol.0901809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deshpande SP, Zheng M, Lee S, Rouse BT. Mechanisms of pathogenesis in herpetic immunoinflammatory ocular lesions. Vet. Microbiol. 2002;86:17–26. doi: 10.1016/s0378-1135(01)00487-4. [DOI] [PubMed] [Google Scholar]
- 21.Niemialtowski MG, Rouse BT. Predominance of Th1 cells in ocular tissues during herpetic stromal keratitis. J. Immunol. 1992;149:3035–3039. [PubMed] [Google Scholar]
- 22.Hendricks RL, Tumpey TM. Contribution of virus and immune factors to herpes simplex virus type I-induced corneal pathology. Invest. Ophthalmol. Vis. Sci. 1990;31:1929–1939. [PubMed] [Google Scholar]
- 23.Aoki H, Hisada T, Ishizuka T, Utsugi M, Ono A, Koga Y, Sunaga N, Nakakura T, Okajima F, Dobashi K, Mori M. Protective effect of resolvin E1 on the development of asthmatic airway inflammation. Biochem. Biophys. Res. Commun. 2010;10:128–133. doi: 10.1016/j.bbrc.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Campbell EL, MacManus CF, Kominsky DJ, Keely S, Glover LE, Bowers BE, Scully M, Bruyninckx WJ, Colgan SP. Resolvin E1-induced intestinal alkaline phosphatase promotes resolution of inflammation through LPS detoxification. Proc. Natl. Acad. Sci. U S A. 2010;107:14298–14303. doi: 10.1073/pnas.0914730107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim B, Sarangi PP, Lee Y, Deshpande S, Lee S, Rouse BT. Depletion of MCP-1 increases development of herpetic stromal keratitis by innate immune modulation. J. Leukocyte Biol. 2006;80:1405–1415. doi: 10.1189/jlb.0406295. [DOI] [PubMed] [Google Scholar]
- 26.Thomas J, Gangappa S, Kanangat S, Rouse BT. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J. Immunol. 1997;158:1383–1139. [PubMed] [Google Scholar]
- 27.Biswas PS, Banerjee K, Kinchington PR, Rouse BT. Involvement of IL-6 in the paracrine production of VEGF in ocular HSV-1 infection. Exp. Eye Res. 2006;82:46–54. doi: 10.1016/j.exer.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J. Clin. Invest. 2002;110:1105–1111. doi: 10.1172/JCI15755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarangi PP, Sehrawat S, Suvas S, Rouse BT. IL-10 and natural regulatory T cells: two independent anti-inflammatory mechanisms in herpes simplex virus-induced ocular immunopathology. J. Immunol. 2008;180:6297–6306. doi: 10.4049/jimmunol.180.9.6297. [DOI] [PubMed] [Google Scholar]
- 30.Tumpey TM, Elner VM, Chen SH, Oakes JE, Lausch RN. Interleukin-10 treatment can suppress stromal keratitis induced by herpes simplex virus type 1. J. Immunol. 1994;153:2258–2265. [PubMed] [Google Scholar]
- 31.Schwab, Chiang JMN, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution pathways. Nature. 2007;447:869–875. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarro-Xavier RA, Newson J, Silveira VLF, Farrow SN, Gilroy DW, Bystrom J. A new strategy for the identification of novel molecules with targeted proresolution of inflammation properties. J. Immunol. 2010;184:1516–1525. doi: 10.4049/jimmunol.0902866. [DOI] [PubMed] [Google Scholar]
- 33.Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J. Virol. 2001;75:9828–9835. doi: 10.1128/JVI.75.20.9828-9835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biswas PS, Rouse BT. Early events in HSV keratitis--setting the stage for a blinding disease. Microbes Infect. 2005;7:799–810. doi: 10.1016/j.micinf.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Jin Y, Arita M, Zhang Q, Saban DR, Chauhan SK, Chiang N, Serhan CN, Reza D. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest. Ophthalmol. Vis. Sci. 2009;50:4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinman L. A brief history of Th17, the first major revision in the Th1/Th2 hypothesis of T-cell mediated tissue damage. Nat. Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 37.Maetzdorf J, Albert DME, Osterhaus G, Verjans MGM. IL-17 expression in human herpes stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J. Immunol. 2002;169:5897–5903. doi: 10.4049/jimmunol.169.10.5897. [DOI] [PubMed] [Google Scholar]
- 38.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J. Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 39.Bystrom J, Evans I, Newson J, Stables M, Toor I, van Rooijen N, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. Resolution-phase macrophages possess a unigue inflammatory phenotype that is controlled by cAMP. Blood. 2008;112:4117–4127. doi: 10.1182/blood-2007-12-129767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng M, Schwarz MA, Lee S, Kumaraguru U, Rouse BT. Control of stromal keratitis by inhibition of neovascularization. Am. J. Pathol. 2001;159:1021–1029. doi: 10.1016/S0002-9440(10)61777-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penn JS, Madhan A, Caldwell RB, Bartoli M, Caldwell RW, Harnett ME. Vascular endothelial growth factor in eye disease. Progr Retinal Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]