Abstract
The innate immune system is a prewired set of cellular and humoral components that has developed to sense perturbations in normal physiology and trigger responses to restore the system back to baseline. It is now understood that many of these components can also sense the physiologic changes that occur with obesity and be activated. While the exact reasons for this chronic immune response to obesity are unclear, there is strong evidence to suggest that innate inflammatory systems link obesity and disease. Based on this, anti-inflammatory therapies for diseases like type 2 diabetes and metabolic syndrome may form the core of future treatment plans. This review will highlight the components involved in the innate immune response and discuss the evidence that they contribute to the pathogenesis of obesity-associated diseases.
Keywords: Obesity, inflammation, macrophages, type 2 diabetes, insulin resistance, metabolic syndrome
1. Introduction
The ability of organisms to mount a response to infectious challenge without prior exposure is regulated the coordinated interaction of components of the innate immune system. This preformed system is important to respond to exogenous stimuli such as bacterial, viral, and fungal infections. Beyond the initial response to a stressor, the innate immune system coordinates the resolution of inflammation, tissue repair, and the activation of the adaptive immune system to provide memory for future challenges.
While much of our understanding of innate immunity comes from models of infection, it is also clear that immune responses can be triggered by endogenous stimuli. Such mechanisms play a wide role in health and disease from the response to tissue injury, the direction of tissue remodeling, and the response to tumors. Resident tissue leukocytes such as macrophages and dendritic cells found in almost every tissue are key sensors of the microenvironment (Pollard, 2009). In addition, rapid responses can be mounted from circulating cells such as monocytes that patrol tissues and amplify the inflammatory response (Auffray et al., 2007). In many settings, these responses may be a part of normal physiology as opposed to pathophysiology. For example, the repair of damaged skeletal muscle is intricately linked to monocytes that trigger myogenesis (Arnold et al., 2007). With a short term fast, macrophages are rapidly recruited to adipose tissue and provide signals that suppress lipolysis and improve nutrient storage (Kosteli et al., 2010).
This broadened view of innate immunity presents a challenge to traditional nomenclature and concepts of the nature of inflammation. With this in mind, I will present a overview of the evidence that obesity is associated with the activation of the innate immune system. The exciting developments in this field have come out of the need to respond to the epidemic of obesity-associated diseases that threatens to overwhelm health care systems across the world (Ogden et al., 2006; Wang and Dietz, 2002). The complexity of the components necessitates a measured approach to the problem, so this review will present a reductionist view of how obesity alters the components innate immunity. In many ways, we are still in a descriptive phase of trying to understand obesity-induced inflammation. However, the future challenge in the field will be to construct integrated models of how these components communicate and interact in different settings. With this background, it is my hope that researchers and clinicians with an interest in the health effects of obesity can better understand the nature of the links between obesity and inflammation relevant to disease.
2. The Innate Immune Response to Obesity
The increased prevalence of obesity and overweight in adults continues to rise and contributes to morbidity and mortality that is estimated to cost $147 billion dollars a year in the U.S. (Finkelstein et al., 2009) and up to 0.6% of the gross domestic product of European countries (Muller-Riemenschneider et al., 2008). More ominous is the high rates of childhood obesity which is a strong predictor of adult obesity (Lee et al., 2009). This has also shifted the prevalence of adult diseases such as type 2 diabetes and pre-diabetes into childhood and has generated new treatment and prevention challenges (Lee, 2006; Lee et al., 2006). Relevant to this review, increases in inflammatory bio-markers such as C-reactive Protein (CRP) and neutrophilia are seen in obese children as young as 3 years of age (Skinner et al., 2010). This indicates that many of the origins of obesity-induced inflammation may actually be initiated during childhood. Therefore, many people will face a lifetime threat to health from obesity.
The long term duration of obesity-induced inflammation makes it challenging to describe this unique type of inflammatory activation based on classical models of innate immunity. Applying such models may be inaccurate and insufficient to encompass the events that are triggered with obesity in metabolic tissues such as fat. Furthermore, it is clear that the inflammation generated by obesity is not as high in amplitude as those seen in acute infectious settings (Hotamisligil, 2006). These unique challenges have led to the coining of the term “metainflammation” to describe the chronic low grade inflammatory events that occur in obesity and its associated diseases.
A frequently asked question is why would obesity trigger an immune response? For the most part, this question remains unanswered, but one answer to this may lie in the fact that many of the key regulators of metabolism also play critical roles in regulating inflammatory responses. The venn diagram between nutrient control and inflammatory responses overlaps much more extensively than previously appreciated. For example, the paradigm of anti-inflammatory alternative macrophage activation (M2) appears to be intrinsically linked to the activity of peroxisomal proliferator activator receptors (PPARs) and which are also key regulators of mitochondrial function and lipid metabolism (Kang et al., 2008; Odegaard et al., 2007; Odegaard et al., 2008). Liver X receptors (LXRs) also play dual roles in the control of lipid metabolism and inflammation (Hong et al., 2011). Such overlap may be related to the high energy requirements needed by immune cells to generate reactive oxygen species, mobilize lipid membranes for phagocytosis, produce inflammatory cytokines, and activate cell migration. Furthermore, hormones that are dysregulated in obesity can have direct effects upon leukocyte function (e.g. Insulin receptor (Baumgartl et al., 2006) and leptin receptor (Mancuso et al., 2004)).
3. Inflammation as a link between obesity and disease
The interest in obesity-induced inflammation relates to the understanding that inflammatory mechanisms are central to the pathogenesis of diseases such as heart disease that are modified by obesity. The scope of all of these diseases is too broad to adequately cover so I will focus attention on the inflammatory mechanisms of fatty liver disease and type 2 diabetes - related diseases with fundamental alterations in nutrient control derived from pro-inflammatory inputs. This will set the stage for future discussion of the innate immune components activated in obesity. I will highlight both clinical and pre-clinical studies in animal models of obesity that have built our understanding of the mechanisms that drive obesity-associated diseases.
3.1 Non-alcoholic Fatty Liver Disease (NAFLD)
The liver plays a critical role in the regulation of glucose and lipids levels in the blood. Obesity generate a number of physiologic changes in hepatocyte glucose production as well as lipid oxidation and storage. Aberrant hepatic lipid accumulation is closely linked to many obesity-associated morbidities that include non-alcoholic fatty liver disease (NAFLD) and metabolic syndrome (Cohen et al., 2011; Fabbrini et al., 2010). The metabolic changes that occur with hepatic lipid accumulation include hepatic insulin resistance which is related to inflammatory cytokine signals. TNF and IL-6 generated by resident Kupffer cells (KC) and recruited inflammatory macrophages contribute to hepatocyte dysfunction (Baffy, 2009). Depletion of KC in the context of high fat diet (HFD) feeding can prevent steatosis and hepatic insulin resistance by attenuating TNF production (Huang et al., 2010). While this suggests that KC play a negative role in liver function, there is also evidence that KC may mediate beneficial effects on hepatic metabolism by secretion of anti-inflammatory cytokines such as IL-10 (Clementi et al., 2009). In addition, new evidence suggests that adiponectin can have beneficial effects on hepatic insulin signaling by the acute stimulation of IL-6 production from macrophages (Awazawa et al., 2011).
Besides the resident KC, new macrophages are recruited to the liver with obesity and contribute to steatosis (Obstfeld et al., 2010). Such recruitment is dependent upon the chemokine CCL2 (C-C chemokine ligand 2; monocyte chemoattractant protein 1 (MCP-1)) which is secreted by the liver to recruit CCR2 (C-C chemokine receptor 2) expressing monocytes. The progression from steatosis to non-alcoholic steatohepatitis (NASH) involves inflammatory responses to hepatocyte damage and fibrosis mediated by cytokines which are induced with obesity such as TNF and IL-6(Feldstein, 2010; Tilg, 2010). Besides KC, other leukocytes are found in the steatotic liver including NK cells (discussed below) that likely cooperate with KC to regulate liver inflammation.
3.2 Type 2 Diabetes (T2D)
The regulation of glucose metabolism is tightly coordinated between nutrient inputs regulated by the liver and gut, nutrient utilization and storage in muscle and fat, insulin secretion by the pancreas, and central signals from the hypothalamus that coordinate these responses (Saltiel and Kahn, 2001). The dysregulation of almost all of these processes with obesity is now known to be associated with the activation of innate pro-inflammatory pathways (Gregor and Hotamisligil, 2011; Hotamisligil, 2006). The net result of this is the generation of systemic insulin resistance and hyperglycemia. While the autoimmune targeting of pancreatic β cells underlies the pathogenesis of type 1 diabetes, there is growing evidence that islet inflammation also occurs with obesity to limit insulin secretion and promote β cell failure - a key event in the progression from pre-diabetes to diabetes (Dobrian et al., 2011; Ehses et al., 2007). The activation of pro-inflammatory signaling pathways in the hypothalamus such as NF B can also have significant effects upon glucose metabolism and weight gain (Ozcan et al., 2009; Zhang et al., 2008).
When energy consumption outpaces energy expenditure, the only tissue capable of expanding to store large amounts excess nutrients is adipose tissue. This adipocentric view of obesity derives from the hypothesis that many of the chronic health effects of obesity are secondary to the breakdown of the ability of adipose tissue to properly store lipids. This places subsequent pressure on non-adipose organs to deal with the excess lipid load (Kim et al., 2007b; Unger and Scherer, 2010). It is now clear that adipose tissue dysfunction is closely linked to the innate inflammatory responses generated by inflammatory leukocytes that make up a large proportion of the non-adipocyte cells in fat (e.g. Macrophages, T cells, B cells). There are now many examples in the literature that show that attenuation of pro-inflammatory pathways decreases adipose tissue inflammation and subsequently improve global insulin sensitivity by producing healthy fat. This perspective may explain much of the variation in obesity phenotypes and the risk of developing diabetes and its complications (Albu et al., 2010; Sluik et al., 2011).
4. Cellular Effectors of Innate Immunity in Obesity
The inflammatory effects of obesity are seen in many tissues and are generated by a combination of systemic inflammatory signals, dynamic cellular events, and tissue specific responses to nutrient excess. To cope with these multiple challenges to homeostasis, it appears that the innate immune response is equally diverse and dynamic.
These factors make it difficult to generalize on how obesity alters the function of different components of the innate immune response. In the next section, we will try to summarize what is known about how obesity effects the cellular mediators of innate immunity. We will highlight examples of how these innate immune cells contribute to NAFLD, atherosclerosis, and T2D in obesity with a focus on adipose tissue inflammation (Figure 1). While obesity contributes to the progression of all these disease processes through inflammatory inputs, the nature of the inflammation is unique for each of these cases. For example, while CCL2/MCP-1 mediates the recruitment of monocytes to atherosclerotic plaques and the liver with diet induced obesity (DIO), there is mixed data regarding if it is required for the accumulation of adipose tissue macrophages (ATMs) in fat (Kanda et al., 2006; Kirk et al., 2008; Obstfeld et al., 2010).
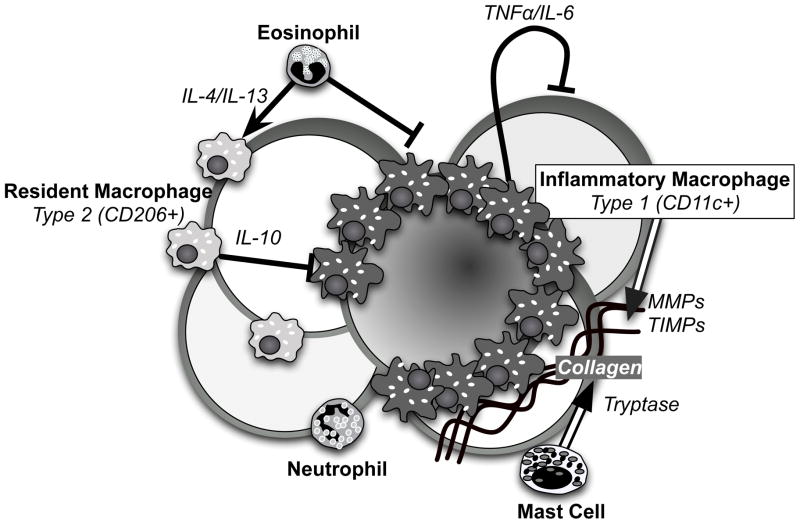
Figure 1. Innate immune responders in adipose tissue.
The cartoon depicts the leukocytes involved in adipose tissue inflammation in obesity. Resident macrophages (Type 2) present in lean states are retained and manifest an alternative activation state under the influence of eosinophils. Recruited inflammatory macrophages (Type 1) form crown-like structures (CLS) around dead and dying adipocytes and secrete inflammatory cytokines which can induced insulin resistance on neighboring adipocytes. Mast cells are found in association with the CLS in regions with high collagen deposition. Neutrophils contribute to the early immune response to obesity. Amplifying the function of Type 2 ATMs and eosinophils can attenuate the pro-inflammatory signals.
4.1 Macrophages
The macrophage sits at the junction of many innate immune responses to initiate inflammation and to lead to its resolution. An extensive population of resident macrophages exist in nearly all tissues with unique properties depending on the context (e.g. KCs vs alveolar macrophages vs ATMs) (Pollard, 2009). Adding to this complexity is the newly appreciated ability of macrophages to proliferate upon activation within tissues (Jenkins et al., 2011).
On top of the resident macrophages, new macrophages can be recruited from circulating monocytes and traffic to sites of injury. Regulation of inflammation is partially driven by distinct subtypes of monocytes in the circulation: inflammatory monocytes (Ly-6chi in mice; CD16+ in humans) which generate pro-inflammatory macrophages and patrolling monocytes (Ly-6clo in mice) which play a role in immune surveillance as opposed to responses (Robbins and Swirski, 2010). Obesity leads to an increase in inflammatory monocytes in the circulation in mice (Tsou et al., 2007). In humans, CD16+ monocytes are elevated in obese diabetic patients and their numbers precipitously decline with surgical weight loss in concert with improvements in vascular inflammation (Poitou et al., 2011). Therefore, many of the inflammatory origins of obesity may derive from bone marrow and splenic monocyte pools that give rise to circulating monocytes. Experimental evidence for this possibility comes from studies of mice deficient in C-C chemokine receptor 2 (CCR2), the receptor for the chemokine CCL2/MCP1. Ccr2 knockout mice have low levels of inflammatory monocytes in the circulation have are protected from many of pathologic features associated with obesity including steatosis, atherosclerosis, and adipose tissue inflammation (Boring et al., 1998; Obstfeld et al., 2010; Weisberg et al., 2005). Pre-clinical studies show promise that CCR2 antagonists to block monocyte trafficking may be useful to treat obesity-associates diseases (Kang et al., 2011; Tamura et al., 2010).
The importance of macrophage activation in obesity and metabolic disease is supported by genome wide association studies. Analysis of gene expression variation in fat and metabolic measures identified a signature gene expression pattern enriched for macrophage activation genes that predicted risk for the features of metabolic syndrome (Chen et al., 2008; Emilsson et al., 2008). While this suggests that macrophages are deleterious to adipose tissue function, this does not fully explain why an extensive network of resident macrophages exist in fat and liver in lean insulin sensitive states. In addition, macrophage deficient mice show multiple abnormalities in metabolism and bone development (Banaei-Bouchareb et al., 2004; Bergmann et al., 2006).
These observations argue that macrophages may have both protective and deleterious functions towards metabolic regulation. A paradigm used to explain such diversity in macrophages is based on the differential responses of macrophages to Th1 (M1) or Th2 (M2) polarizing signals (Mantovani et al., 2004). The M1/M2 paradigm is not a simple binary system as many in vitro subsets have been defined (e.g. M2a, M2b) (Martinez et al., 2008). In addition, in vivo, macrophages exist along the M1/M2 spectrum and can have mixed M1 and M2 properties. However, this paradigm has been a useful starting point to understand macrophage diversity in obesity especially for adipose tissue macrophages (Dalmas et al., 2011; Morris et al., 2011). Due to the limitation of the M1/M2 paradigm, we have proposed a new nomenclature for ATMs based on surface marker expression, localization in fat, and kinetics of accumulation with obesity (Morris et al., 2011).
Multiple studies have demonstrated that obesity-induced inflammation correlates with an increase in markers of M1 genes and a decrease in M2 genes in adipose tissue (Fujisaka et al., 2009; Shaul et al., 2010a; Zeyda et al., 2007; Lumeng et al., 2007; Wentworth et al., 2010). This phenotypic shift in the inflammatory environment in fat is linked to an increase in the ratio of inflammatory (M1-like) to non-inflammatory (M2-like) ATMs. Type 2 resident ATMs express surface markers characteristic of alternative M2 activation in mice and humans such as the mannose receptor (CD206) and macrophage galactose-type C-type lectin 1 (MGL1/CD301) (Lumeng et al., 2008; Spencer et al., 2010; Wentworth et al., 2010). Obesity induces the recruitment and appearance of pro-inflammatory Type 1 ATMs that express the dendritic cell marker CD11c and sub-populations with different inflammatory gene expression exist within this population (Fujisaka et al., 2009; Shaul et al., 2010a; Zeyda et al., 2007). In addition, F4/80+ macrophages which lack significant CD206 or CD11c expression have been identified in obesity with anti-inflammatory gene expression profiles (Zeyda et al., 2010).
Studies extending from the M1/M2 paradigm have revealed key connections between genes that regulate macrophage activation state and metabolism. Disruption of M2 activation by knockout of PPAR or PPAR in macrophages aggravates obesity-induced inflammation and worsens insulin resistance (Kang et al., 2008; Odegaard et al., 2007; Odegaard et al., 2008; Ricardo-Gonzalez et al., 2010) The maintenance of an M2-like polarization state in resident Type 2 ATMs appears to be governed by local production of IL-4 in adipose tissue and possibly derived from eosinophils (Wu et al., 2011). In addition, ablation of inflammatory CD11c+ ATMs leads to a rapid decrease in adipose tissue inflammation and an improvement in insulin sensitivity (Patsouris et al., 2008).
How the balance of ATMs gets shifted is unclear although monocyte recruitment is clearly important (Weisberg et al. 2007; Westcott et al. 2009). Adipocyte death is associated with the formation of crown-like structures made up of Type 1 ATMs suggesting that this is a primary trigger (Cinti et al., 2005). However, a recent study demonstrated that it is possible to uncouple adipocyte death from ATM accumulation as well as local tissue inflammation (Feng et al., 2011). This suggests that the coupling of ATMs to adipocyte dysfunction may involve two-way communication or involve other inflammatory partners such as B and T cells which are activated in adipose tissue in concert with ATMs (Nishimura et al., 2009; Winer et al., 2011; Winer et al., 2009). It is also clear that ATMs can respond to the microenvironment independent of new macrophage recruitment. For example, acute lipid infusion induces the expression of plasminogen activator inhibitor 1 (PAI-1) in ATMs without altering their number (Kishore et al., 2010). Weight loss is able to deactivate inflammatory gene expression in ATMs without significantly changing the number of CD11c+ Type 1 ATMs (Li et al., 2010). New studies have also highlighted the link between physiologic adipose tissue adaptation and ATM responses. With short term fasting, ATMs accumulate in fat and may help dampen lipolytic signals and restore the system back to a normal baseline (Kosteli et al., 2010; Mottillo et al., 2007).
Overall, we are in a phase where we are still cataloging the diversity of ATM and their function. Ultimately, we may revise our models to classify ATMs by function (e.g. Classically activated, wound healing, regulatory (Mosser and Edwards, 2008)) but we currently lack all of the experimental tools to phenotype ATM functional properties well. There remain many questions as to the range of function of ATMs in obesity as both initiators and effectors of the innate immune response to obesity. Outstanding issues in the field are the precise functions for ATMs in different fat depots and in different physiologic and pathologic contexts. As the predominant leukocyte in adipose tissue, future studies on how ATMs communicate with the other innate immune cellular and molecular components remain a challenge for the future.
4.2 Natural killer (NK) Cells
NK cells are large granular lymphocytes that derive from the same common lymphoid progenitor as B and T cells. However, NK cells lack T cell receptors and CD3 and derive their name from their potent ability to activate cytolytic activity in response to chemokines generated in settings such as viral infections (Paust and von Andrian, 2011).
NK cells are found in many metabolic tissues including the liver and adipose tissue. Evidence for quantitative changes in NK cells with obesity have been mixed and tissue dependent. In mouse models of obesity, NK cells are found more prominently in visceral fat compared to subcutaneous fat depots. With obesity, they appear to decrease or be unchanged in number (Caspar-Bauguil et al., 2005; Duffaut et al., 2009). Other leukocytes in fat may regulate their number, as B and T cell deficient mice have a coordinated increase of NK cells as well as ATMs in fat (Duffaut et al., 2009). In human obesity, there is evidence that adipose tissue NK cells are a potent constitutive source of inflammatory cytokines such as IFN and may be increased in omental fat with obesity (O’Rourke et al., 2009; O’Rourke et al., 2011).
It should be noted that NK cells represent a single branch of a family of innate lymphoid cells that include lymphoid tissue inducer (LTi) cells and cells with a spectrum of inflammatory phenotypes (Spits and Di Santo, 2011). This large family of lymphoid cells have protective roles and maintain tissue homeostasis by enhancing tissue remodeling via cytokine production. The possibility that LTis are present in fat or liver and regulate metabolism has yet to be examined comprehensively.
4.3 Natural Killer T (NKT) Cells
Natural killer T (NKT) cells are unique in that they express invariant T cell receptors as well as markers of NK cells (NK1.1) (Godfrey et al., 2010). The interest in the intersection between NKT cells and nutrient metabolism extend from the unique ability of NKT cells to be recognize lipid species presented on antigen presenting cells in the context of the CD1d receptor. This coordinated regulation is seen in omental fat in humans which contain large numbers of NKT cells along with CD1d+ cells (Lynch et al., 2009). Omental NKT cells produce both Th1 (IFN) and Th2 (IL-4) chemokines and decrease in quantity with obesity in humans. This observation conflicts with mouse models of obesity which show that NKT cells increase in fat with obesity (Mantell et al., 2011; Ohmura et al., 2010). In contrast, NKT cells are suppressed in the liver with DIO and genetic obesity models (Li et al., 2002; Li et al., 2005; Mantell et al., 2011; Syn et al., 2010). The mechanism for this suppression is unclear but may involve cytokine signals between tissue macrophages (e.g. KCs) and NKT cells (Li et al., 2002).
Several studies have modified NKTs in mice to examine the effect of NKT cell deficiency on metabolism and obesity. Beta2-microglobulin deficient mice which lack NKT cells as well as CD8+ T cells are protected from insulin resistance with DIO despite similar weight gain as controls (Ohmura et al., 2010). This protection correlated with a significant reduction in ATM infiltration. Similarly, treatment of obese mice with -galactosylceramide led to an expansion of NKT cells in fat, increased inflammation, and led to a small, but significant, worsening of glucose tolerance. A complementary approach examined CD1d-deficient mice which block the generation of NKT cells but retain normal CD8+ cell numbers. These mice had no significant alterations in glucose tolerance, adipose tissue inflammation, or hepatic inflammation (Mantell et al., 2011). This argues that NKT-CD1d signals do not contribute significantly to obesity-induced inflammation in mice.
Further research will be required to resolve these disparate results especially given the differences in NKT and CD1 regulation in mice and humans (Godfrey et al., 2010). However, an open mind will be required as we consider how metabolism intersects with NKT cel inflammation. For example, a recent case report observed a rapid improvement in psoriasis in a patient with type 2 diabetes shortly after starting therapy with a glucagon-like peptide (GLP-1) agonist used to improve glycemic control (Hogan et al., 2011). Subsequent studies demonstrated that the improvement in psoriatic inflammation was related to the suppression of inflammatory cytokine release from NKT cells via the GLP-1 receptor. This is one of many examples of how existing anti-diabetes drugs may have anti-inflammatory mechanism at the heart of their beneficial effects on glucose homeotasis. Such findings have led to the reevaluation of the mechanisms of action of drugs such as PPAR agonists (Hevener et al., 2007) and inhibitors of dipeptidyl peptidase 4 (DPP4) (Dobrian et al., 2011; Shirakawa et al., 2011).
4.4 Mast Cells
Mast cells are critical effectors of the anaphylactic response to allergens found in all tissues (Rao and Brown, 2008). They also play a central role in wound healing at all stages of inflammation and secrete growth factors that regulate collagen deposition and breakdown. Mast cell granules contain preformed inflammatory lipids and cytokines as well as neutral proteases such as tryptase that can rapidly respond to activation. In chronic diseases such as arthritis, renal injury, and cardiovascular disease, mast cells accumulate and contribute to disease progression and tissue remodeling. For example in vascular remodeling and atherosclerosis, mast cell derived cytokines and proteases regulate the activity of matrix metalloproteinases (MMPs) remodel the extracellular matrix (ECM) (Levick et al., 2011; Sun et al., 2007).
Adipose tissue mast cells are found between adipocytes and co-localize with ATMs in crown like structures in mouse models of obesity (Altintas et al., 2011; Liu et al., 2009). In human obesity, mast cells are distributed along micro vessels as well as fibrotic regions in fat. Mast cell trypase levels are elevated with obesity suggesting that an increase in mast cell activity is relevant in obese states. As with other leukocytes, the distribution of mass cells differ between fat depot with more found in visceral fat compared to subcutaneous fat (Altintas et al., 2011). The origins of mast cells in adipose tissue are unclear. While mast cells are bone marrow derived, there is evidence that adipose tissue contains a population of mast cell progenitor cells (Poglio et al., 2010).
The importance of mast cells in the regulation of obesity was demonstrated by studies of mast cell deficient mice (Liu et al., 2009). These mice were protected from HFD induced obesity related to defects in adipose tissue angiogenesis. Mast cell derived expression of IL-6 and IFN contributed this weight loss phenotype. Mast cell stabilizing drugs (disodium cromoglycate (DSCG)) also led to weight loss with subsequent improvement in glucose metabolism.
The weight loss phenotype observed in these studies suggest that mast cells may be important in allowing adipose tissue to dynamically expand in response to excess nutrients. This may be linked to the remodeling function of mast cells. The regulation of collagen deposition and breakdown in adipose tissue is critical for normal fat development and the physiological response of fat expansion (Chun et al., 2006; Khan et al., 2009). This has led to a growing interest in how the extracellular compartments of fat are regulated and can influence metabolism. The cellular effectors that regulate the ECM in fat are unclear, but may involve a balance between macrophage derived MMPs and mast cells proteases as seen in other settings.
4.5 Eosinophils
Eosinophils are effectors of allergic inflammation and partner with mast cells to respond to elevations in IgE. Surprisingly there is evidence that these components are also present in adipose tissue and may regulate metabolism. Eotaxin, a chemokine for eosinophils, is elevated in adipose tissue and serum in obese humans and animal models (Vasudevan et al., 2006). This suggested an inflammatory activation of allergic innate immune responses with obesity. Such a process may relate to the associations seen between obesity and asthma, although clinical studies supporting an inflammatory link between asthma and obesity have been mixed (Farah et al., 2011; Lang et al., 2011).
A recent study carefully examined the role that eosinophils play in adipose tissue inflammation with obesity (Wu et al., 2011). Eosinophils are present in adipose tissue in very low numbers that decline with DIO. Eosinophil deficient mice have increased body weight and worse insulin resistance suggesting that eosinophils help preserve normal metabolic regulation. The mechanism of this finding was related to eosinophil derived Th2 cytokines (IL-4 and IL-13) that maintain resident ATMs in an alternatively activated M2 state. Supporting the principle that Th2 signals can counteract many of the harmful effects of obesity, helaminth infection was shown to increase adipose tissue eosinophils and lead to a sustained improvement in glucose tolerance and decrease fat mass. This finding matches the observations that Th2 biased mouse strains such as BALB/c mice are relatively protected from the effects of DIO (Fearnside et al., 2008).
4.6 Neutrophils
Classic innate immune responses are characterized by an early transient phase of neutrophil infiltration into inflamed tissues (Mantovani et al., 2011). Neutrophils are capable of communication with multiple components of both innate and adaptive immunity and are closely linked to macrophage immune function in pathologic setting and at steady state (Gordy et al., 2011). The role of neutrophils in obesity-induced inflammation is incompletely understood, but there are clear associations between neutrophils and obesity. Mild elevations in circulating neutrophils are observed in obese adults and children and correlate with inflammatory cytokine levels (Herishanu et al., 2006; Zaldivar et al., 2006). Neutrophil counts are also associated with BMI, waist circumference and total adipose tissue in obese female adolescents (Kim and Park, 2008). Neutrophils are found in adipose tissue in small numbers, but migrate to fat even with short term HFD exposure (Elgazar-Carmon et al., 2008). Adhesion between adipocytes and neutrophils is mediated by interactions between CD11b on neutrophils and ICAM-1 on adipocytes. Byproducts of neutrophil activation are elevated in obesity and some markers (e.g. Calprotectin) are decreased with bariatric surgery suggesting that neutrophils are chronically activated in obesity. The location of neutrophil activation remains unclear as one study did not find evidence of myeloperoxidase induction in fat or muscle with morbid obesity (Elgazar-Carmon et al., 2008).
Neutrophils contribute to inflammatory infiltrates in alcoholic fatty liver disease but are less prominent a feature of inflammation in NAFLD (Tiniakos, 2009). Another setting where the innate immune response is important is in wound healing, a process which is abnormal in type 2 diabetics and is the cause of significant morbidity and mortality. Obese mouse models demonstrate abnormal kinetics of early and late infiltration of wounds with macrophages and neutrophils which may contribute to the microvascular disease complications seen in diabetes (Seitz et al., 2010).
In addition, there is new interest in neutrophils as mediators of atherosclerosis progression (Noels and Weber, 2011). Neutrophil egress from the bone marrow is induced with hypercholesterolemia. In addition, neutropenia significantly limits the appearance of macrophages in atherosclerotic plaques (Drechsler et al., 2010). Evidence for the importance of neutrophils in obesity and metabolism may already exist in the literature. Many studies that modulate macrophage gene expression utilize the Cre-recombinase under the expression of the lysozyme M (Lyzs) gene (Hevener et al., 2007; Odegaard et al., 2007). While LysM-Cre is expressed in macrophages, it is also potently expressed in neutrophils (Clausen et al., 1999), therefore, we have to assume that many of these models have inactivated genes in neutrophils as well.
5. Humoral Effectors of Innate Immunity
Partnering with the cellular components of the innate immune system are humoral factors in serum and tissue fluids. These include inflammatory cytokines and chemokines that participate in the initiation, amplification, and resolution of immune responses. It also includes innate inflammatory molecules such as C-reactive protein, defensins, and the complement system which promote phagocytosis, inflammation, and cell lysis when activated. Much of our knowledge of the interaction between obesity and inflammation derives from the study of these humoral factors in clinical populations and in experimental contexts.
5.1 Inflammatory Cytokines
Inflammatory cytokines are the prominent amplifiers of the immune response that trigger leukocyte activation. The studies that demonstrate associations between inflammatory cytokines and obesity are vast and cannot be fully captured in this review. I would point readers to one of the many excellent reviews on this topic in the context of metabolic syndrome, diabetes, and NAFLD (Donath and Shoelson, 2011; Espinola-Klein et al., 2011; Tilg, 2010). In obesity, the production of many of these cytokines may originate from visceral adipose tissue and are coupled with adipose tissue expansion. While adipocytes were once thought to be the primary engine for inflammatory cytokine production in fat, it is now appreciated that the stromal cells in fat are the primary source of most inflammatory cytokines in fat (Bastelica et al., 2002; Bruun et al., 2005; Weisberg et al., 2003; Xu et al., 2003). Mechanistically, pro-inflammatory cytokines such as TNF, IL-1, and IFN appear to contribute to the link between inflammation and insulin resistance as disruption of these signals improves glucose homeostasis in mouse models (Hotamisligil et al., 1993; McGillicuddy et al., 2011; Rocha et al., 2008)
5.2 Complement
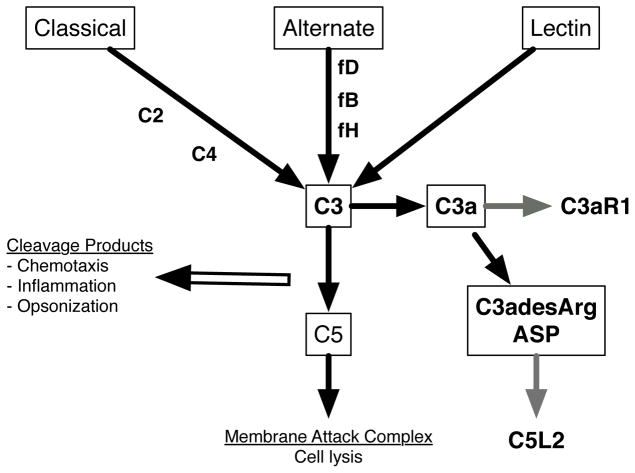
The complement system is an evolutionarily conserved system designed to defend against infection and tissue damage. The complement system can be triggered by multiple pathways (Figure 2): classical pathways involve antibody dependent identification of foreign agents, alternate pathways involve antibody-independent sensing pathogens, and the lectin binding pathway is dependent upon mannose binding lectin (MBL) as a sensor (Ehrnthaller et al., 2011). These pathways ultimately trigger to the formation of the membrane attack complex (MAC) which forms pores in the membrane of the target cells. Along the way, pro-inflamamtory cleavage products are generated that promote the opsonization of foreign particles for clearance and partner with macrophages and neutrophils to regulate the resolution of inflammation.
Figure 2. Complement contribution to inflammation and obesity.
Overview of the complement inputs that activate immunity. Components elevated in obesity, contributing to inflammation, or secreted from obese adipose tissue are noted in Bold type.
The levels of core complement components C2, C3, and C4 have been shown to be elevated in obese subjects and in adipose tissue specifically (Gabrielsson et al., 2003; Muscari et al., 2007; Pomeroy et al., 1997; Yang et al., 2006). The association is particularly robust for serum C3 levels which is strongly associated with measures of insulin resistance (van Greevenbroek et al., 2011). This suggests that complement activation may be yet another link between obesity and disease. Complement is also associated with cardiovascular disease risk (Muscari et al., 1995). Animal models suggest that the classical complement pathway that is important in early atherogenesis as C1q deficient mice demonstrated protection from atherosclerotic plaque formation (Bhatia et al., 2007).
Supporting a role of complement in obesity are results of a non-biased genome-wide association studies. In a screen for genes that regulate omental fat pad weight in mice, the C3a receptor (C3ar1) was identified as a regulator of visceral fat mass (Schadt et al., 2005). C3ar1 knockout mice are protected from insulin resistance and obesity- induced inflammation with DIO (Mamane et al., 2009). This phenotype was linked to the suppression of ATM infiltration in fat and a link between complement and Type 1 ATMs. The targets of the complement response in obesity are unclear. Perivascular adipose tissue generates large amounts of C3 which may regulate the remodeling events in blood vessels in hypertension and other vascular diseases (Miao and Li, 2011; Ruan et al., 2010).
Besides classical complement components, expression of alternate pathway proteins are elevated in obesity and diabetes. The source of this appears to be adipose tissue which secretes complement factors B, D (adipsin), and H (fB and fH) into the circulation that are associated with insulin resistance (Moreno-Navarrete et al., 2010; Choy and Spiegelman, 1996; Fain et al., 2007). The function of these components may be tied into the regulation of acylation-stimulation protein (ASP/C3adesArg) which was identified as an adipokine that induces triglyceride synthesis via the C5L2 receptor. ASP is generated from the alternative complement pathway after the cleavage of C3 to C3a and its further modification by carboxypeptidase B (Cianflone et al., 2003). C3 knockout mice lack ASP and have a lean phenotype despite hyperphagia due to increased energy expenditure (Paglialunga et al., 2008; Xia et al., 2004). How ASP expression in adipose tissue ties into the adipose tissue inflammation and leukocytes is unclear at this time, but microarray experiments have identified associations between ASP expression and genes important in adipose tissue remodeling in obese subjects (MacLaren et al., 2010).
5. Molecular Sensors of Inflammation in Obesity
A possible answer to why inflammation is triggered with obesity is that inflamamtion is a normal response to tissue expansion (e.g. Adipose tissue expansion and ATMs (Kosteli et al., 2010)). However, it is hard to understand how this might have been evolutionarily advantageous. In times of low nutrient supply, it would likely be a disadvantage to coordinately upregulate energy consuming inflammatory responses which increase energy utilization if the goal is to build up nutrient stores.
A second possibility is that the obese state is triggering inflammatory pathways “accidentally” by co-opting signaling pathways already firmly established to guard against infection. A core component of innate immunity is the ability to sense the danger signals that indicate the presence of microbes. The innate discrimination between self and foreign microbes has evolved to rely on unique chemical structures present in only in bacterial structures (e.g. Peptidoglycan and lipolysaccharide (LPS; endotoxin). Collectively these compounds are referred to as pathogen-associated molecular patterns (PAMPs) and include unique peptides and proteins (e.g. Muramyl dipeptides, flaggelin), carbohydrates, lipoproteins, and nucleic acid species (e.g. CpG-DNA, dsRNA) (Kumar et al., 2011; Zhang and Mosser, 2008).
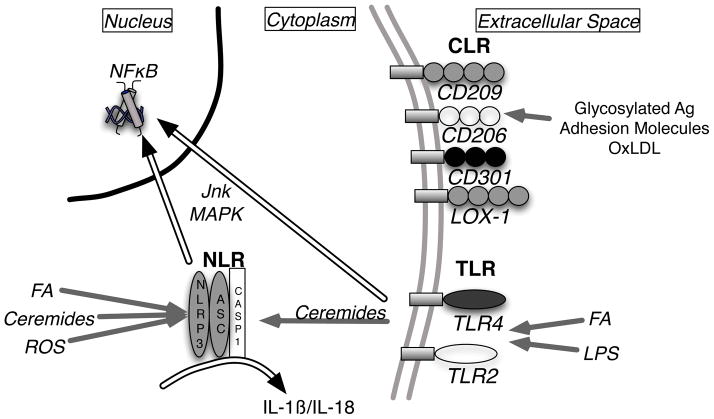
Mammals have co-evolved a set of pattern recognition receptors (PRRs) designed to sense and trigger a response to PAMPs they encounter in extracellular and intracellular compartments (Figure 3). What has become clear is that many of these PRRs are involved in the inflammatory response to obesity. While the range of PRRs continues to expand, they can be broadly grouped into several evolutionarily conserved receptor classes: Toll-like receptors (TLRs), Nod-like receptors (NLRs), C-type lectin receptors (CLRs), and Rig-1-like receptors (RLRs).
Figure 3. Metabolic sensors of the innate immune system.
The major classes of pattern recognition receptors (PRRs) are shown highlighting those implicated in obesity-associated inflammation. Proposed endogenous ligands for the Nod-like receptors (NLR), Toll-like receptors (TLR), and C-type lectin receptors (CLR) are shown. Downstream transduction pathways activated upon receptor ligation are shown as double arrows.
There is evidence that TLRs, NLRs, and CLRs participate in obesity-induced inflammation. In most cases, the endogenous ligands that trigger these pathways are incompletely understood, but it is apparent that obesity may trigger the production of metabolites capable of being sensed by these receptors. In some ways, it could be said that the inflammatory response to obesity is a reaction to “non-self” cues generated with nutrient excess and metabolic dysregulation. Clearly, this is an extreme view of the situation, however it provides a starting point from which we can explore the intersection between metabolism and PRR’s.
5.1 Toll-like Receptors (TLRs)
A critical set of membrane-bound PRRs are those of the Toll-like receptor (TLR) family which consists of 10 receptors in humans. Named due to their homology to the Toll gene in drosophila, TLRs have conserved intracellular signaling domains with homology to the IL-1 receptor (TLR/IL-1 Domain; TIR) that activate nuclear factor kappa b (NF- B), mitogen-activated protein (MAP) kinases, and interferon response factor (IRF) signaling pathways (Iwasaki and Medzhitov, 2004; Tsan and Gao, 2004). The PAMPs that maximally activate each TLRs are distinct, but many TLRs can be activated by free fatty acids especially saturated fatty acids (Fessler et al., 2009; Shi et al., 2006). In addition, TLRs can trigger ceramide production that is linked to NF B activation (Holland et al., 2011). These mechanisms may form critical links between metabolism and inflammation.
5.1.1 TLR4
As a sensor of LPS produced by gram negative bacteria, TLR4 is one of the most studied TLRs due to its role in sepsis and its ability to activate nearly every leukocyte type. The link between obesity and TLR4 may be as simple as elevations in circulating LPS that has been seen in experimental and clinical obesity (Basu et al., 2011; Cani et al., 2007; Sun et al., 2010). Even shortly after a high fat meal, LPS levels in the blood increase to levels that are sufficient to activate monocytes and endothelial cells (Erridge et al., 2007). The source of this LPS is likely from gut microorganisms which dynamically respond to diet and are known to contribute to weight and metabolic regulation (Manco et al., 2010; Rabot et al., 2010; Tilg and Kaser, 2011). The close relationship between the gut microbiota, TLRs, and obesity was demonstrated in TLR5 deficient mice which demonstrate hyperphagia, obesity, and insulin resistance mediated the gut microbiome (Vijay-Kumar et al., 2010)
Several studies have demonstrated that TLR4 knockout mice are protected from insulin resistance with HFD feeding (Davis et al., 2008; Kim et al., 2007a; Shi et al., 2006). Unlike TLR5, many of these studies found the significant effects of TLR4 deficiency on prevention of obesity with HFD which complicates interpretations of glucose metabolism. TLR4 deficiency specifically in hematopoietic cells was shown to be sufficient to protect mice from insulin resistance without weight loss (Saberi et al., 2009). In addition, the deletion of CD14, a TLR2/TLR4 co-receptor for LPS, can confer protection from insulin resistance with HFD feeding (Cani et al., 2007; Roncon-Albuquerque et al., 2008).
While the TLR4 expression in hematopoietic cells appears to mediate most of the effects of inflammation on glucose metabolism (Saberi et al., 2009), an expanded role for TLR4 in metabolic tissues is apparent. TLR4 is expressed in skeletal muscle fibers and can regulate substrate utilization (Frisard et al., 2010). It is also expressed on hepatocytes and adipocytes and upon activation can modify insulin sensitivity via MyD88 dependent pathways (Davis et al., 2009; Raetzsch et al., 2009). Such pathways may be important in lipid-infusion induced insulin resistance which is dependent on TLR4 and relates to intracellular ceramide production (Holland et al., 2011).
It should be noted that some have observed minimal effects of TLR4 deficiency on glucose metabolism in obese rodent models (Coenen et al., 2009). In addition, disrupting a major downstream signaling pathway from TLR by deletion of MyD88 resulted in mice with worse insulin resistance with DIO (Hosoi et al., 2010). While there are MyD88-independent pathways from TLR4, these results suggest that in some cases TLR4 may not contribute to obesity-induced chronic inflammation. This is supported by observations that the inflammatory effects of saturated fatty acids may be independent of TLR4 in certain cells (Erridge and Samani, 2009). This study urged caution in in vitro experiments as LPS contamination in fatty acid preparations can confound results.
5.1.2 TLR2
Other TLRs besides TLR4 can sense dietary fatty acids. Like TLR4, TLR2 is sensitive to activation by saturated, but not polyunsaturated fatty acids (Lee et al., 2004). Evaluation of TLR2 knockout mice support this link as several studies have shown that loss of TLR2 provides protection from DIO-induced insulin resistance (Davis et al., 2011b; Ehses et al., 2010; Himes and Smith, 2010). This protection is coupled to the decrease in ATM accumulation in fat, as well as a decrease in pro-inflammatory cytokine production from adipose tissue. Some studies have observed a protection from weight gain with DIO as contributing to the protected phenotype. Variation in the gutimmune interface and husbandry conditions may possibly explain much of the variation in DIO studies involving TLR modified mice.
5.2 Nod-like Receptors (NLRs)
Besides sensing extracellular threats, the innate immune system has mechanisms to survey the cytoplasmic compartment for danger. This is a primary function of the Nod-like receptor (NLR) family of PRRs. While they have not been shown to bind to any cognate microbial products, NLRs partner with other PRRs to integrate signals from pathogenic activators (e.g. Bacterial toxins, viral RNA, fungal glycoproteins) as well as “sterile” activators of immunity (e.g. Asbestos, amyloid) (Chen et al., 2009; Davis et al., 2011a). This latter function may overlap with obesity as many studies have shown that the NLR system is activated in obesity and contributes to insulin resistance.
Much of the recent attention has been put on NLRP3 (cryopyrin/NALP3) which forms a complex known as the inflammasome. The inflammasome can be activated by a number of endogenous and exogenous stimuli to translate danger signals into the production of proinflammatory cytokines IL-1 and IL-18 by Caspase-1. This process is highly active in macrophages and plays a critical role in the intestinal inflammation, infectious responses, and tumorigenesis (Davis et al., 2011a). Relevant to obesity is the ability of the inflammasome to be activated by ceramides, saturated fatty acids, and reactive oxygen species and negatively regulate insulin receptor signaling (Lukens et al., 2011; Wen et al., 2011)
Interest in the intersection of the NLRP3 inflammasome and obesity derives from correlations between IL-1 and diabetes measures, IL-1 gene polymorphisms that are associated with weight variation, and the ability of IL-1 to induce insulin resistance in adipocytes (Boni-Schnetzler et al., 2008; Carter et al., 2008; Masters et al., 2011; Misaki et al., 2010) From a treatment perspective, IL-1 receptor blockade can improve glycemic control in T2D patients (Larsen et al., 2007). In addition, the anti-diabetic drug glyburide can inhibits NLRP3 and therefore may function partially via ani-inflammatory mechanisms (Lamkanfi et al., 2009).
Mouse models demonstrated that Nlrp3 and Casp1 knockout mice were resistant to insulin resistance with DIO and had reduced inflammation in adipose tissue (Stienstra et al., 2010; Vandanmagsar et al., 2011). Nlrp3 knockout mice had the reduced expression in visceral fat of markers of M1 activation and an increase in alternative activation (M2) gene expression. This occurred without a significant quantitative changes in ATMs, suggesting that the inflammasome may regulate ATM activation state. While the inflammasome is primarily understood in leukocyte inflammation, there is also the possibility that it has important function in metabolic cells such as adipocytes. Caspase-1 deficient mice demonstrate defects in adipogenesis and insulin sensitivity of adipocytes (Stienstra et al., 2010). Future work into how markers of intracellular lipotoxicity such as ceramides interact with the inflammasome in metabolic tissues will likely reveal further connections between metabolism and inflammation.
5.3 C-type Lectin Receptors (CLRs)
C-type lectin receptors (CLRs) represent a class of receptors prominently expressed on antigen presenting cells such as dendritic cells and macrophages (Osorio and Reis e Sousa, 2011; van Vliet et al., 2008). These receptors bind to and mediate the internalization of glycosylated antigens from self and non-self sources. Their function in antigen uptake is tightly coupled to their ability to transfer the antigen to endosomal compartments and enable their presentation by major histocompatibility complex (MHC) I and II. In combination with TLR activation signals, CLRs-mediated antigen internalization and processing to promote T cell activation. The CLRs are diverse with close to 1000 members that share a C-type lectin-like domain (CTLD) that it is found in one or more copies in the extracellular domain (Osorio and Reis e Sousa, 2011; van Kooyk, 2008). The intracellular signaling domains vary widely between the family members. Receptor binding can drive responses towards the initiation of immunity or the dampening of inflammatory responses.
Many CLRs have been studied in the context of obesity and metabolic disease. The oxidized low-density lipoprotein receptor 1 (OLR1/LOX-1/CLEC8A) is a CLR found on endothelial cells, macrophages, platelets, and smooth muscle cells (Ogura et al., 2009). Expression of LOX-1 is strongly induced in atherosclerotic lesions as well as in obese adipose tissue (Takanabe-Mori et al., 2010). While it plays an important role in the endocytosis of oxidized LDL, it can also induce macrophage and endothelial cell activation via NF B and MAP kinase pathways (Cominacini et al., 2000; Li and Mehta, 2000). The overexpression of LOX-1 is able to induce widespread vascular inflammation (Inoue et al., 2005).
Elevations in circulating LOX-1 have been identified in obese rodent models as well as in obese humans. Adipose tissue LOX-1 correlates with BMI and measures of insulin resistance (Brinkley et al., 2008; Kelly et al., 2008; Rasouli et al., 2009). LOX-1 knockout mice gain weight on a HFD, but have a blunted induction of inflammatory cytokine genes (Ccl2, Mip1a, and Il6) in visceral fat compared to controls (Takanabe-Mori et al., 2010). This suggests that LOX-1 may serve as a metabolic sensor that mediates the inflammatory activation in obesity. Further work is required to discern which cells in fat express LOX-1 and how uptake of oxidized LDL relates to ATM activation state.
Several prominent CLRs are expressed in ATMS and have been studied in the context of adipose tissue inflammation. These include the mannose receptor (CD206/MRC1/CLEC13D), dendritic dell-specific intercellular adhesion molecule-3-grabbing non- integrin (CD209/DC-SIGN/CLEC4L), macrophage galactose-type C-type lectin 1 (CD301/MGL1/CLEC10A), and Mincle (CLEC4E). CD206, CD209, and CD301 are markers of alternatively activated macrophage in mice and have been used to delineate distinct populations of ATMs as described above. CD206 and CD301 are strongly expressed on resident Type 2 ATMs found in all adipose tissue depots in lean mice (Lumeng et al., 2008; Wentworth et al., 2010; Zeyda et al., 2010). The Type 1 CD11c+ ATM population contains subtypes that express little or moderate levels of CD301 that mark a unique population of ATMs with remodeling properties (Shaul et al., 2010b). The function of these receptors in ATMs not fully understood. CD301 knockout mice demonstrated protection from adipose tissue inflammation as they had blunted recruitment of inflammatory ATMs to fat and improved insulin sensitivity and glucose tolerance (Westcott et al., 2009). The mechanism of this was related to the lack of induction of inflammatory monocytes in the circulation with DIO. CD301 is expressed on inflammatory Ly-6chi monocytes. Loss of CD301 decreases monocyte adhesion to adipocytes, suggesting that CD301 bind targets on adipocytes. These receptors may also function in the clearance of dead adipocytes as induction of adipocyte apoptosis triggers the recruitment of CD206+ ATMs with M2 features (Fischer-Posovszky et al., 2011).
Overall, expression many of these CLRs in fat negatively correlates with inflammation and correlates with anti-inflammatory therapies. The angiotensin II receptor blocker telmisartan as well as the PPAR agonist pioglitizone decrease inflammatory profiles of adipose tissue and concurrently induce the expression of CD209/DC-SIGN (Fujisaka et al., 2011; Hirata et al., 2011). CD206 expression in epicardial adipose tissue was shown to negatively correlate with measures of coronary artery disease suggesting regional connections between adipose tissue inflammation and atherosclerosis (Hirata et al., 2011).
A possible exception to this trend is Mincle/CLEC4E. Mincle was identified as a CLR induced in obese adipose tissue in mice and humans. In macrophages, Mincle is induced upon co-cultured with adipocytes and exposure to saturated fatty acids (Ichioka et al., 2011). This latter effect is TLR4 dependent and linked to M1 macrophage activation, which contrasts with the other CLRs mentioned above.
Future studies will likely expand our understanding of how CLRs interact with obesity-induced inflammatory pathways. To date, little is known about CLRs in hepatic inflammation in NAFLD or in atherosclerosis. Given their role in linking innate to adaptive immunity and the growing interest in adaptive immune responses in obesity, there is likely much more to learn.
6. Anti-inflammatory Treatments for Obesity Associated Diseases
Opportunities for drug development for T2D and metabolic syndrome may lie in the design of anti-inflammatory strategies to break the link between obesity and disease. Fortunately, the potential targets for intervention are growing. Salsalate is a prodrug of salicylate that blocks activation of the IKKβ/NF B pathway (Hundal et al., 2002). Pre-clinical studies demonstrate that it can attenuate obesity induced inflammation (Yuan et al., 2001). In clinical studies, it has shown efficacy in improving glycemic control in patients with type 2 diabetes over 14 weeks without significant adverse effects (Goldfine et al., 2010). Long-term studies are underway, as are studies evaluating the efficacy of salicylates in cardiovascular disease (Goldfine et al., 2011).
The strong links between inflammatory cytokines and obesity suggest that long-term blockade of these chemokines may treat or prevent obesity-induced inflammation. Overall, pre-clinical studies in rodent models of obesity have show promise in using such strategies, while clinical trails in humans have had mixed success. TNF was one of the first cytokines identified as being increased in association with BMI and insulin resistance (Hotamisligil and Spiegelman, 1994). The use of TNF blocking antibodies (etanercept) in diabetic patients has not shown significant benefits in improving glycemic control or insulin sensitivity (Bernstein et al., 2006; Dominguez et al., 2005). However, there is evidence that TNF blockade may have potential benefits to other morbidities such as cardiovascular disease by decreasing inflammatory markers, increasing adiponectin levels, and down-regulating endothelial adhesion molecules (Stanley et al., 2011; Zanni et al., 2010).
Work continues to evolve on the potential use of agents that antagonize the action of IL-1 in type 2 diabetes. Preclinical studies show that IL-1 is induced islets and contributes to their dysfunction in type 2 diabetes (Boni-Schnetzler et al., 2008; Ehses et al., 2009). Anakinra, an IL-1 receptor antagonist, improves glycemic control, β cell function, and decreases systemic inflammatory markers in diabetic subjects (Larsen et al., 2007).
While looking for the future for clues on how to treat obesity-induced inflammation, we have also learned something about the past. Modulation of PPAR activity with agonists such as thiazolidinediones (TZDs) has been a longstanding component of diabetes treatment and can have anti-inflammatory effects by antagonizing NF B (Ohga et al., 2007; Welch et al., 2003). In insulin resistance, it has been shown that much of the anti-diabetic effect of TZDs are mediated by modification of PPAR activity in macrophages (Hevener et al., 2007). Part of this effect may involve the role that PPAR (as well as PPAR) play in M2 alternative activati vation on. The continued on how PPAR specifically modulates genes related to lipid metabolism and inflammation may open further doors for taking advantage of the beneficial effects of PPAR (Choi et al., 2010).
Finally, future anti-inflammatory approaches to obesity-associated disease may take advantage of the advances in the understanding of beneficial nutrients. 3 fatty acids have profound anti-inflammatory and lipid lowering effects. Many of the molecular mechanisms behind this were unclear until the recent identification of a macrophage/dendritic cell receptor for 3 fatty acids, GPR120 (Oh et al., 2010). Activation of GPR120 led to deactivation of inflammatory macrophages in adipose tissue and an associated improvement in insulin sensitivity. GPR120 knockout mice were unable to respond to the beneficial effects of 3 fatty acids demonstrating that this is an attractive future target for drug discovery.
7. Summary
Research into obesity and its associated diseases has increased the overlap between the mechanisms that control metabolism and inflammation. Hopefully, this review has provided an overview of obesity and inflammation that can wrap and reframe the problem around the core aspects of innate immunity. As may be apparent, the shoe fits well in many instances, but may not be the right size in others. The future challenge will be to retool many of these core concepts to make them understandable in the context of the chronic metainflammation.
Beyond this temporal challenge, the complexity of metabolic regulation makes it difficult to examine inflammation in every tissue specific context necessary to comprehensively understand the system and models used. For example, while studies of adipose tissue inflammatory mechanisms have advanced the field, they have largely ignored the possibility that the knockout and pharmacological interventions used may be actually influencing hypothalamic or β cell inflammation to provide beneficial effects. Few individual labs have the expertise to turn over every stone and in the future combined efforts may be required to truly advance the field.
This field provides unique opportunities for those interested in metabolism to partner with experts in inflammation and immunity and to advance the field. The future challenges are many and there remain more questions than answers. However, from this challenge lies many opportunities to identify ways to prevent and treat obesity-associated morbidities that will likely consume enormous resources over the next generation of physicians and scientists.
Acknowledgments
Support for this work came from grants from the National Institutes of Health (NIH) DK078851 and DK090262.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albu JB, Lu J, Mooradian AD, Krone RJ, Nesto RW, Porter MH, Rana JS, Rogers WJ, Sobel BE, Gottlieb SH. Relationships of obesity and fat distribution with atherothrombotic risk factors: baseline results from the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial. Obesity (Silver Spring) 2010;18 (5):1046–1054. doi: 10.1038/oby.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altintas MM, Azad A, Nayer B, Contreras G, Zaias J, Faul C, Reiser J, Nayer A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J Lipid Res. 2011;52 (3):480–488. doi: 10.1194/jlr.M011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204 (5):1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317 (5838):666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Awazawa M, Ueki K, Inabe K, Yamauchi T, Kubota N, Kaneko K, Kobayashi M, Iwane A, Sasako T, Okazaki Y, Ohsugi M, Takamoto I, Yamashita S, Asahara H, Akira S, Kasuga M, Kadowaki T. Adiponectin Enhances Insulin Sensitivity by Increasing Hepatic IRS-2 Expression via a Macrophage-Derived IL-6-Dependent Pathway. Cell Metab. 2011;13 (4):401–412. doi: 10.1016/j.cmet.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51 (1):212–223. doi: 10.1016/j.jhep.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaei-Bouchareb L, Gouon-Evans V, Samara-Boustani D, Castellotti MC, Czernichow P, Pollard JW, Polak M. Insulin cell mass is altered in Csf1op/Csf1op macrophage-deficient mice. J Leukocyte Biol. 2004;76 (2):359–367. doi: 10.1189/jlb.1103591. [DOI] [PubMed] [Google Scholar]
- Bastelica D, Morange P, Berthet B, Borghi H, Lacroix O, Grino M, Juhan-Vague I, Alessi MC. Stromal cells are the main plasminogen activator inhibitor-1-producing cells in human fat: evidence of differences between visceral and subcutaneous deposits. Arterioscler Thromb Va c Biol. 2002;22 (1):173–178. doi: 10.1161/hq0102.101552. [DOI] [PubMed] [Google Scholar]
- Basu S, Haghiac M, Surace P, Challier JC, Guerre-Millo M, Singh K, Waters T, Minium J, Presley L, Catalano PM, Hauguelde Mouzon S. Pregravid obesity associates with increased maternal endotoxemia and metabolic inflammation. Obesity (Silver Spring) 2011;19 (3):476–482. doi: 10.1038/oby.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartl J, Baudler S, Scherner M, Babaev V, Makowski L, Suttles J, McDuffie M, Fazio S, Kahn CR, Hotamisligil GS, Krone W, Linton M, Bruning JC. Myeloid lineage cell-restricted insulin resistance protects apolipoprotein E-deficient mice against atherosclerosis. Cell Metab. 2006;3 (4):247–256. doi: 10.1016/j.cmet.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann CE, Hoefer IE, Meder B, Roth H, van Royen N, Breit SM, Jost MM, Aharinejad S, Hartmann S, Buschmann IR. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukocyte Biol. 2006;80 (1):59–65. doi: 10.1189/jlb.0206087. [DOI] [PubMed] [Google Scholar]
- Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med. 2006;166 (8):902–908. doi: 10.1001/archinte.166.8.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia VK, Yun S, Leung V, Grimsditch DC, Benson GM, Botto MB, Boyle JJ, Haskard DO. Complement C1q reduces early atherosclerosis in low-density lipoprotein receptor-deficient mice. The American journal of pathology. 2007;170 (1):416–426. doi: 10.2353/ajpath.2007.060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, Pattou F, Halban PA, Weir GC, Donath MY. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93 (10):4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394 (6696):894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- Brinkley TE, Kume N, Mitsuoka H, Phares DA, Hagberg JM. Elevated soluble lectin-like oxidized LDL receptor-1 (sLOX-1) levels in obese postmenopausal women. Obesity (Silver Spring) 2008;16 (6):1454–1456. doi: 10.1038/oby.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun JM, Lihn AS, Pedersen SB, Richelsen B. MCP-1 Release is Higher in Visceral than Subcutaneous Human Adipose Tissue. Implication of Macrophages resident in the Adipose Tissue. J Clin Endocrinol Metab. 2005;90 (4):2282–2289. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56 (7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Carter KW, Hung J, Powell BL, Wiltshire S, Foo BT, Leow YC, McQuillan BM, Jennens M, McCaskie PA, Thompson PL, Beilby JP, Palmer LJ. Association of Interleukin-1 gene polymorphisms with central obesity and metabolic syndrome in a coronary heart disease population. Hum Genet. 2008;124 (3):199–206. doi: 10.1007/s00439-008-0540-6. [DOI] [PubMed] [Google Scholar]
- Caspar-Bauguil S, Cousin B, Galinier A, Segafredo C, Nibbelink M, Andre M, Casteilla L, Penicaud L. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 2005;579 (17):3487–3492. doi: 10.1016/j.febslet.2005.05.031. [DOI] [PubMed] [Google Scholar]
- Chen G, Shaw MH, Kim YG, Nunez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009;4:365–398. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhu J, Lum PY, Yang X, Pinto S, MacNeil DJ, Zhang C, Lamb J, Edwards S, Sieberts SK, Leonardson A, Castellini LW, Wang S, Champy MF, Zhang B, Emilsson V, Doss S, Ghazalpour A, Horvath S, Drake TA, Lusis AJ, Schadt EE. Variations in DNA elucidate molecular networks that cause disease. Nature. 2008;452 (7186):429–435. doi: 10.1038/nature06757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, Griffin PR, Spiegelman BM. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARgamma by Cdk5. Nature. 2010;466 (7305):451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy LN, Spiegelman BM. Regulation of alternative pathway activation and C3a production by adipose cells. Obesity Res. 1996;4 (6):521–532. doi: 10.1002/j.1550-8528.1996.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125 (3):577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Cianflone K, Xia Z, Chen LY. Critical review of acylation-stimulating protein physiology in humans and rodents. Biochim Biophys Acta. 2003;1609 (2):127–143. doi: 10.1016/s0005-2736(02)00686-7. [DOI] [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46 (11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8 (4):265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- Clementi AH, Gaudy AM, van Rooijen N, Pierce RH, Mooney RA. Loss of Kupffer cells in diet-induced obesity is associated with increased hepatic steatosis, STAT3 signaling, and further decreases in insulin signaling. Biochim Biophys Acta. 2009;1792 (11):1062–1072. doi: 10.1016/j.bbadis.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen KR, Gruen ML, Lee-Young RS, Puglisi MJ, Wasserman DH, Hasty AH. Impact of macrophage toll-like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia. 2009;52 (2):318–328. doi: 10.1007/s00125-008-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332 (6037):1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cominacini L, Pasini AF, Garbin U, Davoli A, Tosetti ML, Campagnola M, Rigoni A, Pastorino AM, Lo Cascio V, Sawamura T. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000;275 (17):12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- Dalmas E, Clement K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32 (7):307–314. doi: 10.1016/j.it.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Davis BK, Wen H, Ting JP. The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu Rev Immunol. 2011a;29 doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JE, Braucher DR, Walker-Daniels J, Spurlock ME. Absence of Tlr2 protects against high-fat diet-induced inflammation and results in greater insulin-stimulated glucose transport in cultured adipocytes. J Nutr Biochem. 2011b;22 (2):136–141. doi: 10.1016/j.jnutbio.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity. 2008;16 (6):1248–1255. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. The c-Jun N-terminal kinase mediates the induction of oxidative stress and insulin resistance by palmitate and toll-like receptor 2 and 4 ligands in 3T3-L1 adipocytes. Hormone and metabolic research = Hormonund Stoffwechselforschung = Hormones et metabolisme. 2009;41 (7):523–530. doi: 10.1055/s-0029-1202852. [DOI] [PubMed] [Google Scholar]
- Dobrian AD, Ma Q, Lindsay JW, Leone KA, Ma K, Coben J, Galkina EV, Nadler JL. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab. 2011;300 (2):E410–421. doi: 10.1152/ajpendo.00463.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez H, Storgaard H, Rask-Madsen C, Steffen Hermann T, Ihlemann N, Baunbjerg Nielsen D, Spohr C, Kober L, Vaag A, Torp-Pedersen C. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42 (6):517–525. doi: 10.1159/000088261. [DOI] [PubMed] [Google Scholar]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11 (2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122 (18):1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun. 2009;384 (4):482–485. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Ehrnthaller C, Ignatius A, Gebhard F, Huber-Lang M. New insights of an old defense system: structure, function, and clinical relevance of the complement system. Mol Med. 2011;17 (3–4):317–329. doi: 10.2119/molmed.2010.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N, Irminger JC, Kergoat M, Portha B, Homo-Delarche F, Donath MY. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci U S A. 2009;106 (33):13998–14003. doi: 10.1073/pnas.0810087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, Wielinga PY, Schraenen A, Lemaire K, Debray S, Van Lommel L, Pospisilik JA, Tschopp O, Schultze SM, Malipiero U, Esterbauer H, Ellingsgaard H, Rutti S, Schuit FC, Lutz TA, Boni-Schnetzler M, Konrad D, Donath MY. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diab tologia. 2010;53 (8):1795–1806. doi: 10.1007/s00125-010-1747-3. [DOI] [PubMed] [Google Scholar]
- Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56 (9):2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49 (9):1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- Emilsson V, Thorleifsson G, Zhang B, Leonardson AS, Zink F, Zhu J, Carlson S, Helgason A, Walters GB, Gunnarsdottir S, Mouy M, Steinthorsdottir V, Eiriksdottir GH, Bjornsdottir G, Reynisdottir I, Gudbjartsson D, Helgadottir A, Jonasdottir A, Styrkarsdottir U, Gretarsdottir S, Magnusson KP, Stefansson H, Fossdal R, Kristjansson K, Gislason HG, Stefansson T, Leifsson BG, Thorsteinsdottir U, Lamb JR, Gulcher JR, Reitman ML, Kong A, Schadt EE, Stefansson K. Genetics of gene expression and its effect on disease. Nature. 2008;452 (7186):423–428. doi: 10.1038/nature06758. [DOI] [PubMed] [Google Scholar]
- Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. The American journal of clinical nutrition. 2007;86 (5):1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Atertio Thromb Vasc Biol. 2009;29 (11):1944–1949. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- Espinola-Klein C, Gori T, Blankenberg S, Munzel T. Inflammatory markers and cardiovascular risk in the metabolic syndrome. Front Biosci. 2011;16:1663–1674. doi: 10.2741/3812. [DOI] [PubMed] [Google Scholar]
- Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51 (2):679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain JN, Nesbit AS, Sudlow FF, Cheema P, Peeples JM, Madan AK, Tichansky DS. Release in vitro of adipsin, vascular cell adhesion molecule 1, angiotensin 1-converting enzyme, and soluble tumor necrosis factor receptor 2 by human omental adipose tissue as well as by the nonfat cells and adipocytes. Metabolism. 2007;56 (11):1583–1590. doi: 10.1016/j.metabol.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Farah CS, Kermode JA, Downie SR, Brown NJ, Hardaker KM, Berend N, King GG, Salome CM. Obesity Is a Determinant of Asthma Control, Independent of Inflammation and Lung Mechanics. Chest. 2011 doi: 10.1378/chest.11-0027. [DOI] [PubMed] [Google Scholar]
- Fearnside JF, Dumas ME, Rothwell AR, Wilder SP, Cloarec O, Toye A, Blancher C, Holmes E, Tatoud R, Barton RH, Scott J, Nicholson JK, Gauguier D. Phylometabonomic patterns of adaptation to high fat diet feeding in inbred mice. PLoS One. 2008;3 (2):e1668. doi: 10.1371/journal.pone.0001668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein AE. Novel insights into the pathophysiology of nonalcoholic fatty liver disease. Semin Liver Dis. 2010;30 (4):391–401. doi: 10.1055/s-0030-1267539. [DOI] [PubMed] [Google Scholar]
- Feng D, Tang Y, Kwon H, Zong H, Hawkins M, Kitsis RN, Pessin JE. High-fat diet-induced adipocyte cell death occurs through a cyclophilin d intrinsic signaling pathway independent of adipose tissue inflammation. Diabetes. 2011;60 (8):2134–2143. doi: 10.2337/db10-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler MB, Rudel LL, Brown JM. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr Opin Lipidol. 2009;20 (5):379–385. doi: 10.1097/MOL.0b013e32832fa5c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff. 2009;28 (5):w822–831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- Fischer-Posovszky P, Wang QA, Asterholm IW, Rutkowski JM, Scherer PE. Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated m2 macrophages. Endocrinology. 2011;152 (8):3074–3081. doi: 10.1210/en.2011-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisard MI, McMillan RP, Marchand J, Wahlberg KA, Wu Y, Voelker KA, Heil-bronn L, Haynie K, Muoio B, Li L, Hulver MW. Toll-like receptor 4 modulates skeletal muscle substrate metabolism. Am J Physiol Endocrinol Metab. 2010;298 (5):E988–998. doi: 10.1152/ajpendo.00307.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58 (11):2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisaka S, Usui I, Kanatani Y, Ikutani M, Takasaki I, Tsuneyama K, Tabuchi Y, Bukhari A, Yamazaki Y, Suzuki H, Senda S, Aminuddin A, Nagai Y, Takatsu K, Kobayashi M, Tobe K. Telmisartan improves insulin resistance and modulates adipose tissue macrophage polarization in high-fat-fed mice. Endocrinology. 2011;152 (5):1789–1799. doi: 10.1210/en.2010-1312. [DOI] [PubMed] [Google Scholar]
- Gabrielsson BG, Johansson JM, Lonn M, Jernas M, Olbers T, Peltonen M, Larsson I, Lonn L, Sjostrom L, Carlsson B, Carlsson LM. High expression of complement components in omental adipose tissue in obese men. Obesity Res. 2003;11 (6):699–708. doi: 10.1038/oby.2003.100. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11 (3):197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152 (6):346–357. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfine AB, Fonseca V, Shoelson SE. Therapeutic approaches to target inflammation in type 2 diabetes. Clin Chem. 2011;57 (2):162–167. doi: 10.1373/clinchem.2010.148833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordy C, Pua H, Sempowski GD, He YW. Regulation of steady-state neutrophil homeostasis by macrophages. Blood. 2011;117 (2):618–629. doi: 10.1182/blood-2010-01-265959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Herishanu Y, Rogowski O, Polliack A, Marilus R. Leukocytosis in obese individuals: possible link in patients with unexplained persistent neutrophilia. Eur J Haematol. 2006;76 (6):516–520. doi: 10.1111/j.1600-0609.2006.00658.x. [DOI] [PubMed] [Google Scholar]
- Hevener AL, Olefsky JM, Reichart D, Nguyen MT, Bandyopadyhay G, Leung HY, Watt MJ, Benner C, Febbraio MA, Nguyen AK, Folian B, Subramaniam S, Gonzalez FJ, Glass CK, Ricote M. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117 (6):1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes RW, Smith CW. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. FASEB J. 2010;24 (3):731–739. doi: 10.1096/fj.09-141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Tabata M, Kurobe H, Motoki T, Akaike M, Nishio C, Higashida M, Mikasa H, Nakaya Y, Takanashi S, Igarashi T, Kitagawa T, Sata M. Coronary atherosclerosis is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol. 2011;58 (3):248–255. doi: 10.1016/j.jacc.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Hogan AE, Tobin AM, Ahern T, Corrigan MA, Gaoatswe G, Jackson R, O’Reilly V, Lynch L, Doherty DG, Moynagh PN, Kirby B, O’Connell J, O’Shea D. Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diab tologia. 2011 doi: 10.1007/s00125-011-2232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid–induced ceramide biosynthesis in mice. J Clin Invest. 2011 doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Walczak R, Dhamko H, Bradley MN, Marathe C, Boyadjian R, Salazar JV, Tontonoz P. Constitutive activation of LXR in macrophages regulates metabolic and inflammatory gene expression: identification of ARL7 as a direct target. J Lipid Res. 2011;52 (3):531–539. doi: 10.1194/jlr.M010686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi T, Yokoyama S, Matsuo S, Akira S, Ozawa K. Myeloid differentiation factor 88 (MyD88)-deficiency increases risk of diabetes in mice. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444 (7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259 (5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43 (11):1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O’Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59 (2):347–357. doi: 10.2337/db09-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109 (10):1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichioka M, Suganami T, Tsuda N, Shirakawa I, Hirata Y, Satoh-Asahara N, Shimoda Y, Tanaka M, Kim-Saijo M, Miyamoto Y, Kamei Y, Sata M, Ogawa Y. Increased expression of macrophage-inducible C-type lectin in adipose tissue of obese mice and humans. Diabetes. 2011;60 (3):819–826. doi: 10.2337/db10-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Arai Y, Kurihara H, Kita T, Sawamura T. Overexpression of lectin-like oxidized low-density lipoprotein receptor-1 induces intramyocardial vasculopathy in apolipoprotein E-null mice. Circul Res. 2005;97 (2):176–184. doi: 10.1161/01.RES.0000174286.73200.d4. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5 (10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332 (6035):1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116 (6):1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 2008;7 (6):485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YS, Cha JJ, Hyun YY, Cha DR. Novel C-C chemokine receptor 2 antagonists in metabolic disease: a review of recent developments. Expert Opin Investig Drugs. 2011;20 (6):745–756. doi: 10.1517/13543784.2011.575359. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Wu P, Patterson CE, Temm C, Dominguez JH. LOX-1 and inflammation: a new mechanism for renal injury in obesity and diabetes. Am J Physiol Renal Physiol. 2008;294 (5):F1136–1145. doi: 10.1152/ajprenal.00396.2007. [DOI] [PubMed] [Google Scholar]
- Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29 (6):1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circul Res. 2007a;100 (11):1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- Kim JA, Park HS. White blood cell count and abdominal fat distribution in female obese adolescents. Metabolism. 2008;57 (10):1375–1379. doi: 10.1016/j.metabol.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007b;117 (9):2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk EA, Sagawa ZK, McDonald TO, O’Brien KD, Heinecke JW. Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue [corrected] Diabetes. 2008;57 (5):1254–1261. doi: 10.2337/db07-1061. [DOI] [PubMed] [Google Scholar]
- Kishore P, Li W, Tonelli J, Lee DE, Koppaka S, Zhang K, Lin Y, Kehlenbrink S, Scherer PE, Hawkins M. Adipocyte-derived factors potentiate nutrient-induced production of plasminogen activator inhibitor-1 by macrophages. Sci Transl Med. 2010;2 (20):20ra15. doi: 10.1126/scitranslmed.3000292. [DOI] [PubMed] [Google Scholar]
- Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120 (10):3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30 (1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187 (1):61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang JE, Hossain J, Dixon AE, Shade D, Wise RA, Peters SP, Lima JJ. Does Age Impact the Obese Asthma Phenotype?: Longitudinal Asthma Control, Airway Function and Airflow Perception among Mild Persistent Asthmatics. Chest. 2011 doi: 10.1378/chest.11-0675. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. The New England journal of medicine. 2007;356 (15):1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- Lee JM. Insulin resistance in children and adolescents. Rev Endocr Metab Disord. 2006;7 (3):141–147. doi: 10.1007/s11154-006-9019-8. [DOI] [PubMed] [Google Scholar]
- Lee JM, Herman WH, McPheeters ML, Gurney JG. An epidemiologic profile of children with diabetes in the U.S. Diabetes Care. 2006;29 (2):420–421. doi: 10.2337/diacare.29.02.06.dc05-2182. [DOI] [PubMed] [Google Scholar]
- Lee JM, Pilli S, Gebremariam A, Keirns CC, Davis MM, Vijan S, Freed GL, Herman WH, Gurney JG. Getting heavier, younger: trajectories of obesity over the life course. Int J Obes (Lond) 2009 doi: 10.1038/ijo.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279 (17):16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- Levick SP, Melendez GC, Plante E, McLarty JL, Brower GL, Janicki JS. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc Res. 2011;89 (1):12–19. doi: 10.1093/cvr/cvq272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Mehta JL. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation. 2000;101 (25):2889–2895. doi: 10.1161/01.cir.101.25.2889. [DOI] [PubMed] [Google Scholar]
- Li P, Lu M, Nguyen MTA, Bae E, Chapman J, Feng D, Hawkins M, Pessin JE, Sears DD, Nguyen AK, Amidi A, Watkins SM, Nguyen U, Olefsky JM. Functional heterogeneity of CD11C positive adipose tissue macrophages in diet-induced obese mice. J Biol Chem. 2010;285 (20):15333–15345. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Lin H, Yang S, Diehl AM. Murine leptin deficiency alters Kupffer cell production of cytokines that regulate the innate immune system. Gastroenterology. 2002;123 (4):1304–1310. doi: 10.1053/gast.2002.35997. [DOI] [PubMed] [Google Scholar]
- Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42 (4):880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, Doria A, Libby P, Blumberg RS, Kahn BB, Hotamisligil GS, Shi GP. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15 (8):940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukens JR, Dixit VD, Kanneganti TD. Inflammasome activation in obesity-related inflammatory diseases and autoimmunity. Discov Med. 2011;12 (62):65–74. [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117 (1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57 (12):3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG, O’Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol. 2009;39 (7):1893–1901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- MacLaren RE, Cui W, Lu H, Simard S, Cianflone K. Association of adipocyte genes with ASP expression: a microarray analysis of subcutaneous and omental adipose tissue in morbidly obese subjects. BMC Med Genomics. 2010;3:3. doi: 10.1186/1755-8794-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamane Y, Chung Chan C, Lavallee G, Morin N, Xu LJ, Huang J, Gordon R, Thomas W, Lamb J, Schadt EE, Kennedy BP, Mancini JA. The C3a anaphylatoxin receptor is a key mediator of insulin resistance and functions by modulating adipose tissue macrophage infiltration and activation. Diabetes. 2009;58 (9):2006–2017. doi: 10.2337/db09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31 (6):817–844. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- Mancuso P, Canetti C, Gottschalk A, Tithof PK, Peters-Golden M. Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2gamma) protein expression. Am J Physiol Lung Cell Mol Physiol. 2004;287 (3):L497–502. doi: 10.1152/ajplung.00010.2004. [DOI] [PubMed] [Google Scholar]
- Mantell BS, Stefanovic-Racic M, Yang X, Dedousis N, Sipula IJ, O’Doherty RM. Mice Lacking NKT Cells but with a Complete Complement of CD8 T-Cells Are Not Protected against the Metabolic Abnormalities of Diet-Induced Obesity. PLoS One. 2011;6 (6):e19831. doi: 10.1371/journal.pone.0019831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nature reviews Immunology. 2011;11 (8):519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25 (12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Masters SL, Latz E, O’Neill LA. The inflammasome in atherosclerosis and type 2 diabetes. Science translational medicine. 2011;3 (81):81ps17. doi: 10.1126/scitranslmed.3001902. [DOI] [PubMed] [Google Scholar]
- McGillicuddy FC, Harford KA, Reynolds CM, Oliver E, Claessens M, Mills KH, Roche HM. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes. 2011;60 (6):1688–1698. doi: 10.2337/db10-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao CY, Li ZY. The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki Y, Miyauchi R, Mochizuki K, Takabe S, Shimada M, Ichikawa Y, Goda T. Plasma interleukin-1beta concentrations are closely associated with fasting blood glucose levels in healthy and preclinical middle-aged nonoverweight and overweight Japanese men. Metabolism. 2010;59 (10):1465–1471. doi: 10.1016/j.metabol.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Martinez-Barricarte R, Catalan V, Sabater M, Gomez-Ambrosi J, Ortega FJ, Ricart W, Bluher M, Fruhbeck G, Rodriguez de Cordoba S, Fernandez-Real JM. Complement factor H is expressed in adipose tissue in association with insulin resistance. Diabetes. 2010;59 (1):200–209. doi: 10.2337/db09-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DL, Singer K, Lumeng CN. Adipose tissue macrophages: phenotypic plasticity and diversity in lean and obese states. Current Opinion in Clinical Nutrition and Metabolic Care. 2011;14 (4):341–346. doi: 10.1097/MCO.0b013e328347970b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8 (12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottillo EP, Shen XJ, Granneman JG. Role of hormone-sensitive lipase in beta-adrenergic remodeling of white adipose tissue. Am J Physiol Endocrinol Metab. 2007;293 (5):E1188–1197. doi: 10.1152/ajpendo.00051.2007. [DOI] [PubMed] [Google Scholar]
- Muller-Riemenschneider F, Reinhold T, Berghofer A, Willich SN. Health-economic burden of obesity in Europe. Eur J Epidemiol. 2008;23 (8):499–509. doi: 10.1007/s10654-008-9239-1. [DOI] [PubMed] [Google Scholar]
- Muscari A, Antonelli S, Bianchi G, Cavrini G, Dapporto S, Ligabue A, Ludovico C, Magalotti D, Poggiopollini G, Zoli M. Serum C3 is a stronger inflammatory marker of insulin resistance than C-reactive protein, leukocyte count, and erythrocyte sedimentation rate: comparison study in an elderly population. Diabetes Care. 2007;30 (9):2362–2368. doi: 10.2337/dc07-0637. [DOI] [PubMed] [Google Scholar]
- Muscari A, Bozzoli C, Puddu GM, Sangiorgi Z, Dormi A, Rovinetti C, Descovich GC, Puddu P. Association of serum C3 levels with the risk of myocardial infarction. The American journal of medicine. 1995;98 (4):357–364. doi: 10.1016/S0002-9343(99)80314-3. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15 (8):914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Noels H, Weber C. Catching up with important players in atherosclerosis: type I interferons and neutrophils. Curr Opin Lipidol. 2011;22 (2):144–145. doi: 10.1097/MOL.0b013e328344780b. [DOI] [PubMed] [Google Scholar]
- O’Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin II, Jobe BA, Roberts CT, Jr, Slifka MK, Marks DL. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 2009;33 (9):978–990. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke RW, White AE, Metcalf MD, Olivas AS, Mitra P, Larison WG, Cheang EC, Varlamov O, Corless CL, Roberts CT, Jr, Marks DL. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diab tologia. 2011;54 (6):1480–1490. doi: 10.1007/s00125-011-2103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Ables EV, Ferrante AW., Jr C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes. 2010;59 (4):916–925. doi: 10.2337/db09-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447 (7148):1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7 (6):496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295 (13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Ogura S, Kakino A, Sato Y, Fujita Y, Iwamoto S, Otsui K, Yoshimoto R, Sawamura T. Lox-1: the multifunctional receptor underlying cardiovascular dysfunction. Circ J. 2009;73 (11):1993–1999. doi: 10.1253/circj.cj-09-0587. [DOI] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142 (5):687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohga S, Shikata K, Yozai K, Okada S, Ogawa D, Usui H, Wada J, Shikata Y, Makino H. Thiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-kappaB activation. Am J Physiol Renal Physiol. 2007;292 (4):F1141–1150. doi: 10.1152/ajprenal.00288.2005. [DOI] [PubMed] [Google Scholar]
- Ohmura K, Ishimori N, Ohmura Y, Tokuhara S, Nozawa A, Horii S, Andoh Y, Fujii S, Iwabuchi K, Onoe K, Tsutsui H. Natural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese mice. Atertio Thromb Vasc Biol. 2010;30 (2):193–199. doi: 10.1161/ATVBAHA.109.198614. [DOI] [PubMed] [Google Scholar]
- Osorio F, Reis e Sousa C. Myeloid C-type lectin receptors in pathogen recognition and host defense. Immunity. 2011;34 (5):651–664. doi: 10.1016/j.immuni.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9 (1):35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Paglialunga S, Fisette A, Yan Y, Deshaies Y, Brouillette JF, Pekna M, Cianflone K. Acylation-stimulating protein deficiency and altered adipose tissue in alternative complement pathway knockout mice. Am J Physiol Endocrinol Metab. 2008;294 (3):E521–529. doi: 10.1152/ajpendo.00590.2007. [DOI] [PubMed] [Google Scholar]
- Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8 (4):301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;12 (6):500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- Poglio S, De Toni-Costes F, Arnaud E, Laharrague P, Espinosa E, Casteilla L, Cousin B. Adipose tissue as a dedicated reservoir of functional mast cell progenitors. Stem Cells. 2010;28 (11):2065–2072. doi: 10.1002/stem.523. [DOI] [PubMed] [Google Scholar]
- Poitou C, Dalmas E, Renovato M, Benhamo V, Hajduch F, Abdennour M, Kahn JF, Veyrie N, Rizkalla S, Fridman H, Sautes-Fridman C, Clement K, Cremer I. CD14dimCD16+ and CD14+CD16+ Monocytes in Obesity and During Weight Loss: Relationships With Fat Mass and Subclinical Atherosclerosis. Atertio Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9 (4):259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy C, Mitchell J, Eckert E, Raymond N, Crosby R, Dalmasso AP. Effect of body weight and caloric restriction on serum complement proteins, including Factor D/adipsin: studies in anorexia nervosa and obesity. Clin Exp Immunol. 1997;108 (3):507–515. doi: 10.1046/j.1365-2249.1997.3921287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabot S, Membrez M, Bruneau A, Gerard P, Harach T, Moser M, Raymond F, Mansourian R, Chou CJ. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24 (12):4948–4959. doi: 10.1096/fj.10-164921. [DOI] [PubMed] [Google Scholar]
- Raetzsch CF, Brooks NL, Alderman JM, Moore KS, Hosick PA, Klebanov S, Akira S, Bear JE, Baldwin AS, Mackman N, Combs TP. Lipopolysaccharide inhibition of glucose production through the Toll-like receptor-4, myeloid differentiation factor 88, and nuclear factor kappa b pathway. Hepatology. 2009;50 (2):592–600. doi: 10.1002/hep.22999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KN, Brown MA. Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann N Y Acad Sci. 2008;1143:83–104. doi: 10.1196/annals.1443.023. [DOI] [PubMed] [Google Scholar]
- Rasouli N, Yao-Borengasser A, Varma V, Spencer HJ, McGehee RE, Jr, Peter-son CA, Mehta JL, Kern PA. Association of scavenger receptors in adipose tissue with insulin resistance in nondiabetic humans. Atertio Thromb Vasc Biol. 2009;29 (9):1328–1335. doi: 10.1161/ATVBAHA.109.186957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, Mukundan L, Wu D, Locksley RM, Chawla A. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107 (52):22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins CS, Swirski FK. The multiple roles of monocyte subsets in steady state and inflammation. Cell Mol Life Sci. 2010;67 (16):2685–2693. doi: 10.1007/s00018-010-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, Libby P. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103 (5):467–476. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncon-Albuquerque R, Jr, Moreira-Rodrigues M, Faria B, Ferreira AP, Cerqueira C, Lourenco AP, Pestana M, von Hafe P, Leite-Moreira AF. Attenuation of the cardiovascular and metabolic complications of obesity in CD14 knockout mice. Life Sci. 2008;83 (13–14):502–510. doi: 10.1016/j.lfs.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Ruan CC, Zhu DL, Chen QZ, Chen J, Guo SJ, Li XD, Gao PJ. Perivascular adipose tissue-derived complement 3 is required for adventitial fibroblast functions and adventitial remodeling in deoxycorticosterone acetate-salt hypertensive rats. Atertio Thromb Vasc Biol. 2010;30 (12):2568–2574. doi: 10.1161/ATVBAHA.110.215525. [DOI] [PubMed] [Google Scholar]
- Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G. Verma, liorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 10(5):419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414 (6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Schadt EE, Lamb J, Yang X, Zhu J, Edwards S, Guhathakurta D, Sieberts SK, Monks S, Reitman M, Zhang C, Lum PY, Leonardson A, Thieringer R, Metzger JM, Yang L, Castle J, Zhu H, Kash SF, Drake TA, Sachs A, Lusis AJ. An integrative genomics approach to infer causal associations between gene expression and disease. Nat Genet. 2005;37 (7):710–717. doi: 10.1038/ng1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz O, Schurmann C, Hermes N, Muller E, Pfeilschifter J, Frank S, Goren I. Wound healing in mice with high-fat diet- or ob gene-induced diabetes-obesity syndromes: a comparative study. Exp Diabetes Res. 2010;2010:476969. doi: 10.1155/2010/476969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010a;59 (5):1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-Like Remodeling Phenotypes of CD11c+ Adipose Tissue Macrophages During High-Fat Diet-Induced Obesity in Mice. Diabetes. 2010b;59 (5):1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116 (11):3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa J, Fujii H, Ohnuma K, Sato K, Ito Y, Kaji M, Sakamoto E, Koganei M, Sasaki H, Nagashima Y, Amo K, Aoki K, Morimoto C, Takeda E, Terauchi Y. Diet-induced adipose tissue inflammation and liver steatosis are prevented by DPP-4 inhibition in diabetic mice. Diabetes. 2011;60 (4):1246–1257. doi: 10.2337/db10-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner AC, Steiner MJ, Henderson FW, Perrin EM. Multiple markers of inflammation and weight status: cross-sectional analyses throughout childhood. Pediatrics. 2010;125 (4):e801–809. doi: 10.1542/peds.2009-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluik D, Boeing H, Montonen J, Pischon T, Kaaks R, Teucher B, Tjonneland A, Halkjaer J, Berentzen TL, Overvad K, Arriola L, Ardanaz E, Bendinelli B, Grioni S, Tumino R, Sacerdote C, Mattiello A, Spijkerman AM, van der AD, Beulens JW, van der Schouw YT, Nilsson PM, Hedblad B, Rolandsson O, Franks PW, Nothlings U. Associations between general and abdominal adiposity and mortality in individuals with diabetes mellitus. Am J Epidemiol. 2011;174 (1):22–34. doi: 10.1093/aje/kwr048. [DOI] [PubMed] [Google Scholar]
- Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peter-son CA, Kern PA. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299 (6):E1016–1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12 (1):21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H, Khor VK, Ahima RS, Grinspoon SK. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. The Journal of clinical endocrinology and metabolism. 2011;96 (1):E146–150. doi: 10.1210/jc.2010-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, Kersten S, Muller M, van den Berg WB, van Rooijen N, Wabitsch M, Kullberg BJ, van der Meer JW, Kanneganti T, Tack CJ, Netea MG. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12 (6):593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J, Shi GP. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13 (6):719–724. doi: 10.1038/nm1601. [DOI] [PubMed] [Google Scholar]
- Sun L, Yu Z, Ye X, Zou S, Li H, Yu D, Wu H, Chen Y, Dore J, Clement K, Hu FB, Lin X. A marker of endotoxemia is associated with obesity and related metabolic disorders in apparently healthy Chinese. Diabetes Care. 2010;33 (9):1925–1932. doi: 10.2337/dc10-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syn WK, Oo YH, Pereira TA, Karaca GF, Jung Y, Omenetti A, Witek RP, Choi SS, Guy CD, Fearing CM, Teaberry V, Pereira FE, Adams DH, Diehl AM. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51 (6):1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanabe-Mori R, Ono K, Sowa N, Wada H, Takaya T, Horie T, Satoh-Asahara N, Shimatsu A, Fujita M, Sawamura T, Hasegawa K. Lectin-like oxidized low-density lipoprotein receptor-1 is required for the adipose tissue expression of proin-flammatory cytokines in high-fat diet-induced obese mice. Biochem Biophys Res Commun. 2010;398 (3):576–580. doi: 10.1016/j.bbrc.2010.06.123. [DOI] [PubMed] [Google Scholar]
- Tamura Y, Sugimoto M, Murayama T, Minami M, Nishikaze Y, Ariyasu H, Akamizu T, Kita T, Yokode M, Arai H. C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. J Atheroscler Thromb. 2010;17 (3):219–228. doi: 10.5551/jat.3368. [DOI] [PubMed] [Google Scholar]
- Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Dig Dis. 2010;28 (1):179–185. doi: 10.1159/000282083. [DOI] [PubMed] [Google Scholar]
- Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. The Journal of clinical investigation. 2011;121 (6):2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiniakos DG. Liver biopsy in alcoholic and non-alcoholic steatohepatitis patients. Gastroenterol Clin Biol. 2009;33 (10–11):930–939. doi: 10.1016/j.gcb.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76 (3):514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117 (4):902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome: a road-map to lipotoxicity. Trends Endocrinol Metab. 2010;21 (6):345–352. doi: 10.1016/j.tem.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Greevenbroek MM, Jacobs M, van der Kallen CJ, Vermeulen VM, Jansen EH, Schalkwijk CG, Ferreira I, Feskens EJ, Stehouwer CD. The cross-sectional association between insulin resistance and circulating complement C3 is partly explained by plasma alanine aminotransferase, independent of central obesity and general inflammation (the CODAM study) Eur J Clin Invest. 2011;41 (4):372–379. doi: 10.1111/j.1365-2362.2010.02418.x. [DOI] [PubMed] [Google Scholar]
- van Kooyk Y. C-type lectins on dendritic cells: key modulators for the induction of immune responses. Biochem Soc Trans. 2008;36 (Pt 6):1478–1481. doi: 10.1042/BST0361478. [DOI] [PubMed] [Google Scholar]
- van Vliet SJ, Garcia-Vallejo JJ, van Kooyk Y. Dendritic cells and C-type lectin receptors: coupling innate to adaptive immune responses. Immunol Cell Biol. 2008;86 (7):580–587. doi: 10.1038/icb.2008.55. [DOI] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan AR, Wu H, Xydakis AM, Jones PH, Smith EO, Sweeney JF, Corry DB, Ballantyne CM. Eotaxin and obesity. J Clin Endocrinol Metab. 2006;91 (1):256–261. doi: 10.1210/jc.2005-1280. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328 (5975):228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Dietz WH. Economic burden of obesity in youths aged 6 to 17 years: 1979–1999. Pediatrics. 2002;109 (5):E81–81. doi: 10.1542/peds.109.5.e81. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2005;116 (1):115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112 (12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc Natl Acad Sci U S A. 2003;100 (11):6712–6717. doi: 10.1073/pnas.1031789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12 (5):408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O’Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59 (7):1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott DJ, Delproposto JB, Geletka LM, Wang T, Singer K, Saltiel AR, Lumeng CN. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. J Exp Med. 2009;206 (13):3143–3156. doi: 10.1084/jem.20091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17 (5):610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, Maezawa Y, Drucker D, Endleman E, Winer D, Dosch HM. Normalization of Obesity-Associated Insulin Resistance through Immunotherapy: CD4+ T Cells Control Glucose Homeostasis. Nat Med. 2009;15 (8):921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan H, Bando JK, Chawla A, Locksley RM. Eosinophils Sustain Adipose Alternatively Activated Macrophages Associated with Glucose Homeostasis. Science. 2011 doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Stanhope KL, Digitale E, Simion OM, Chen L, Havel P, Cianflone K. Acylation-stimulating protein (ASP)/complement C3adesArg deficiency results in increased energy expenditure in mice. J Biol Chem. 2004;279 (6):4051–4057. doi: 10.1074/jbc.M311319200. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112 (12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lu HL, Zhang J, Yu HY, Wang HW, Zhang MX, Cianflone K. Relationships among acylation stimulating protein, adiponectin and complement C3 in lean vs obese type 2 diabetes. Int J Obesity. 2006;30 (3):439–446. doi: 10.1038/sj.ijo.0803173. [DOI] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293 (5535):1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- Zaldivar F, McMurray RG, Nemet D, Galassetti P, Mills PJ, Cooper DM. Body fat and circulating leukocytes in children. Int J Obes (Lond) 2006;30 (6):906–911. doi: 10.1038/sj.ijo.0803227. [DOI] [PubMed] [Google Scholar]
- Zanni MV, Stanley TL, Makimura H, Chen CY, Grinspoon SK. Effects of TNF-alpha antagonism on E-selectin in obese subjects with metabolic dysregulation. Clin Endocrinol (Oxf) 2010;73 (1):48–54. doi: 10.1111/j.1365-2265.2009.03741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyda M, Farmer D, Todoric J, Aszmann O, Speiser M, Gyori G, Zlabinger GJ, Stulnig TM. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31 (9):1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Gollinger K, Kriehuber E, Kiefer FW, Neuhofer A, Stulnig TM. Newly identified adipose tissue macrophage populations in obesity with distinct chemokine and chemokine receptor expression. Int J Obes (Lond) 2010;34 (12):1684–1694. doi: 10.1038/ijo.2010.103. [DOI] [PubMed] [Google Scholar]
- Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214 (2):161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135 (1):61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]