Summary
Inborn errors of bile acid synthesis are rare genetic disorders that can present as neonatal cholestasis, neurologic disease or fat-soluble-vitamin deficiencies. There are nine known defects of bile acid synthesis, including oxysterol 7α-hydroxylase deficiency, Δ4-3-oxosteroid-5β-reductase deficiency, 3β-hydroxy-Δ5-C27-steroid dehydrogenase deficiency, cerebrotendinous xanthomatosis (also known as sterol 27-hydroxylase deficiency), α-methylacyl-CoA racemase deficiency, and Zellweger syndrome (also known as cerebrohepatorenal syndrome). These diseases are characterized by a failure to produce normal bile acids and an accumulation of unusual bile acids and bile acid intermediaries. Individuals with inborn errors of bile acid synthesis generally present with the hallmark features of normal or low serum bile acid concentrations, normal γ-glutamyl transpeptidase concentrations and the absence of pruritus. Failure to diagnose any of these conditions can result in liver failure or progressive chronic liver disease. If recognized early, many patients can have a remarkable clinical response to oral bile acid therapy.
Keywords: bile acid, cholestasis, fat soluble vitamins, liver failure, ursodeoxycholic acid
Introduction
Inborn errors of bile acid synthesis constitute an expanding category of rare genetic disorders that are responsible for up to 1–2% of cases of neonatal cholestasis,1 a condition defined by the development of conjugated or direct hyperbilirubinemia within the first few months of life. Most patients with inborn errors of bile acid synthesis have a remarkable clinical response to oral bile acid therapy.
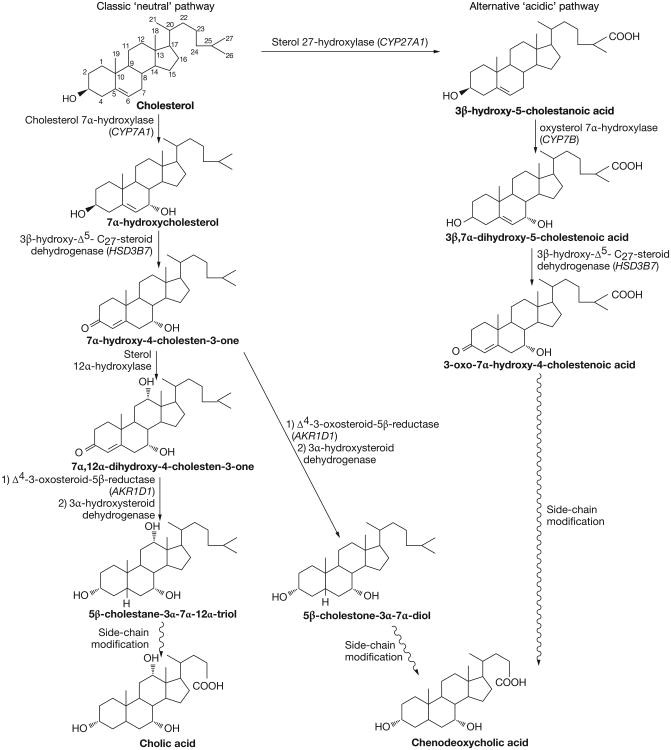
The primary bile acids cholic acid and chenodeoxycholic acid are synthesized by a sequence of enzymatic modifications to cholesterol that involves at least 14 enzymes, multiple subcellular compartments and two complementary chemical pathways (Figure 1). The classic ‘neutral’ pathway is considered the main pathway for bile acid synthesis and produces both cholic acid and chenodeoxycholic acid in approximately equal amounts. In this pathway, the rate-limiting enzymatic step is the steroid nucleus modification that takes place in hepatic microsomes and is catalyzed by cholesterol 7α-hydroxylase (the CYP7A1 gene product). The farnesoid X receptor (FXR) is vital for the regulation of CYP7A1 by bile acids. FXR is a member of the superfamily of ligand-activated transcription factors that acts on target genes as both a monomer and as a heterodimer with the retinoid X receptor (RXR).2–4 Bile acids are physiologic FXR ligands, with chenodeoxycholic acid the most potent activator of human FXR.5–7 FXR activation by primary bile acids leads to upregulation of the atypical nuclear receptor small heterodimer protein (SHP), which interacts with liver receptor homolog 1 (LRH-1) and inhibits the ability of LRH-1 to activate CYP7A1.8 LRH-1 is also important for SHP gene expression because formation of the SHP–LRH-1 complex reduces SHP expression, thereby decreasing the negative feedback signal.9
Figure 1.
Bile acid synthesis occurs by two pathways, the classic ‘neutral’ pathway and the alternative ‘acidic’ pathway. In the classic ‘neutral’ pathway, the rate-limiting step in bile acid formation is the conversion of cholesterol to 7α-hydroxycholesterol by cholesterol 7α-hydroxylase. Multiple sequential steps that modify both the steroid nucleus and the side chain produce cholic acid and chenodeoxycholic acid. The main enzymes in this process include cholesterol 7α-hydroxylase, 3β-hydroxy-Δ5-C27-steroid dehydrogenase (encoded by HSD3B7), sterol 12a-hydroxylase, Δ4-3-oxosteroid-5β-reductase (encoded by AKR1D1 [previously known as SRD5B1]) and 3α-hydroxysteroid dehydrogenase. A series of side-chain modifications then occurs to yield cholic and chenodeoxycholic acid. Alternatively, via the alternative ‘acidic’ pathway, cholesterol is converted to 3β-hydroxy-5-cholestanoic acid by sterol 27-hydroxylase (encoded by CYP27A1). This is followed by conversion to 3β, 7α-dihydroxy-5-cholestenoic acid by oxysterol 7α-hydroxylase (encoded by CYP7B).
An alternative ‘acidic’ pathway has also been described, in which C27-hydroxylation of cholesterol by sterol 27-hydroxylase is the initial step, followed by C7α-hydroxylation by oxysterol 7α-hydroxylase. This pathway leads to the production of primarily chenodeoxycholic acid. In both the classic and alternative pathways, side-chain modification takes place after steroid nucleus modification and is initiated in other organelles: C27-hydroxylation occurs in mitochondria, but further side-chain modification requires functioning peroxisomes. The final step in primary bile acid synthesis is the conjugation of cholic acid and chenodeoxycholic acid to taurine or glycine.10,11
In the past 20 years, deficiencies have been identified in the genes encoding the multiple enzymes involved in the bile acid synthesis pathways (Table 1). Such defects are proposed to cause liver disease in two ways. First, impaired hepatocyte production of primary bile acids reduces canalicular bile acid secretion, thereby hindering bile acid dependent bile flow. Second, potentially hepatotoxic atypical bile acid precursors accumulate in hepatocytes and cause cellular injury.12,13 These perturbations most commonly manifest in infants as cholestasis that can mimic other neonatal liver diseases, including biliary atresia.
Table 1.
Genetic, biochemical and clinical features of bile acid synthesis defects.
| Enzyme defect | Gene encoding the affected enzyme (reference) | Urine bile acid profile | serum bile acid profile | Clinical features |
|---|---|---|---|---|
| Oxysterol 7α-hydroxylase deficiency | CYP7B14 | ↑ Sulfate and glycosulfate conjugates of 3β-δ5-monohydroxy bile acids Absence of primary bile acids | Extremely high levels of bile acids, primarily 3β-δ5-monohydroxy bile acids | Neonatal hepatitis (single reported case; unrecognized cases could be due to prenatal or early-postnatal death) |
| Δ4-3-oxosteroid-5β-reductase deficiency | AKR1D1(SRD5B1)20 | ↑ 3-oxo-δ4 bile acids ↑ Allo bile acids ↓ Primary bile acids |
↑ 3-oxo-δ4 bile acids ↑ Allo bile acids ↓ Primary bile acids |
Neonatal hepatitis with rapid progression to liver failure Neonatal hemochromatosis |
| 3β-hydroxy-Δ5-C27-steroid dehydrogenase deficiency | HSD3B738,39 | ↑ Dihydroxy & trihydroxy cholenoic acids ↓ Primary bile acids |
↓ or absence of primary bile acids | Neonatal hepatitis Late-onset liver disease Malabsorption |
| Cerebrotendinous xanthomatosis (sterol 27-hydroxylase deficiency) | CYP27A148 | ↑ Plasma cholestanol: cholesterol ratio | ↑ Bile alcohol glucuronides | Progressive neurologic dysfunction in 2nd–3rd decade of life Chronic diarrhea Bilateral juvenile cataracts Neonatal cholestasis |
| Alpha methylacyl-CoA racemase deficiency | AMACR gene on chromosome 5p13.2-q11.171 | ↑ C27 trihydroxycholestanoic and pristanic acid ↓ Primary bile acids |
↑ C27 trihydroxycholestanoic and pristanic acid ↓ Primary bile acids Normal long-chain fatty acids and phytanic acid |
Adult onset peripheral neuropathy Neonatal cholestasis with considerable fat-soluble-vitamin deficiency |
| Zellweger syndrome (cerebrohepatorenal syndrome) | 12 PEX gene mutations; PEX1 mutations are the most common. |
Atypical monohydroxy, dihydroxy and trihydroxy C27 bile acids ↓ Primary bile acids |
↑ Long-chain fatty acids ↑ Cholestanoic and pipecolic acid ↑ C29 dicarboxylic acid ↓ Primary bile acids |
Craniofacial abnormalities Neuronal migration defects Polycystic kidneys Chronic liver disease Bony abnormalities |
| Bile acid conjugation defects | BAAT and BAL97,98 | Absence of glycine and/or taurine conjugates. Presence of cholic glucouronidates and/or sulfates and unconjugated cholic acid |
↑ Unconjugated cholic and deoxycholic acid Absence of chenodeoxycholic acid |
Transient neonatal cholestasis Fat-soluble-vitamin deficiencies |
Clinical Features
The clinical presentation of each of the nine known bile acid synthesis defects has been characterized, along with the liver histopathology, diagnostic procedures of choice and response to therapy (Table 1). Bile acid synthesis defects share three important clinical features that should raise clinical suspicion for these disorders. First, other cholestatic liver diseases are associated with elevated total serum bile acid concentrations, but levels are generally normal or low in infants with bile acid synthesis defects. Second, the serum level of γ-glutamyl transpeptidase (GGTP), which is often elevated in patients with other cholestatic liver diseases, is characteristically normal or minimally elevated in those with inborn errors of bile acid synthesis. Third, pruritus, which is a common and distressing feature of chronic cholestasis, is usually absent in infants with bile acid synthesis defects. A high index of suspicion is required to prevent the diagnosis of a bile acid synthesis defect being overlooked, as the clinical presentations of these rare disorders can be similar to those of other causes of neonatal liver failure, neonatal cholestasis and chronic liver disease. Importantly, many bile acid synthesis defects are readily treatable and therefore have an excellent prognosis if recognized and treated early in life. Moreover, these disorders represent ‘accidents of nature’ that have led to a more thorough understanding of basic biochemistry and biology, which can be applied to normal liver physiology and the pathophysiology of other diseases. In this Review, the current state of our understanding of bile acid synthesis defects is characterized, with emphasis on the translational and clinical investigations that have led to the emergence of this relatively new field within hepatology. It should be noted that because of the rarity of these diseases, many of the treatment recommendations mentioned are based on expert opinion rather than on firm data.
Oxysterol 7α-Hydroxylase Deficiency
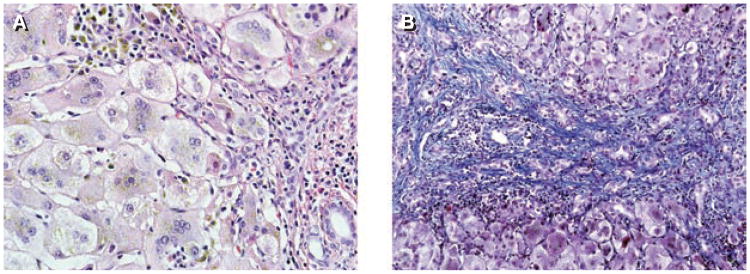
A deficiency in the enzyme oxysterol 7α-hydroxylase, which is encoded by the CYP7B gene, interrupts the alternative ‘acidic’ pathway for synthesis of the bile acid steroid nucleus. A single reported case of oxysterol 7α-hydroxylase deficiency has been described in the literature.14 The infant, the second child of a consanguineous Mexican couple, presented with cholestasis, acholic stools, liver synthetic failure, and hepatosplenomegaly. Liver biopsy showed lobular disarray with giant cell transformation, moderate portal inflammation and fibrosis, extramedullary hematopoiesis, and canalicular and bile duct plugging (Figure 2). Although the infant was initially believed to have biliary atresia, the findings of a normal serum GGTP level and low concentrations of total serum bile acids led to the diagnosis of a bile acid synthesis defect being considered.
Figure 2.
Liver histology in a patient aged 12 weeks with oxysterol 7α-hydroxylase deficiency. (A) Giant cell transformation with intrahepatocytic and focal canalicular cholestasis and bile-laden Kupffer cells are associated with moderate portal inflammation. Hematoxylin and eosin stain, magnification × 400. (B) Bile ductular proliferation associated with portal-to-portal fibrosis, highlighted in blue, is flanked by regenerative nodules. Masson trichrome stain, magnification × 200.
Fast-atom-bombardment mass spectrometry (FAB-MS) of the patient's urine revealed a lack of primary bile acids and high amounts of sulfate or glycosulfate conjugates of monohydroxycholenoic acids. Gas-chromatography mass spectrometry (GC-MS) analysis found that 96% of serum bile acids were 3-β-hydroxy-5-cholenoic acid and 3-β-hydroxy-5-cholestenoic acid. Serum analysis showed extremely high levels of 27-hydroxycholesterol and the absence of 7α-hydroxylated sterols. These features suggested a block at the level of oxysterol 7α-hydroxylase, which was found to be absent from the infant's liver tissue. Subsequent genetic evaluation of the patient revealed homozygosity for a cytosine to thymidine transition mutation at position 388 in exon 5 of CYP7B, and heterozygosity was demonstrated in both parents.14
Treatment with ursodeoxycholic acid worsened liver function test results, and oral cholic acid therapy was ineffective in reversing the liver failure. The baby underwent orthotopic liver transplantation, but subsequently died of a fulminant post-transplant lymphoproliferative disorder.14 It has been speculated that additional patients with oxysterol 7α-hydroxylase deficiency have not been identified because the severity of this disease potentially results in prenatal or early neonatal death. If additional patients are identified, early liver transplantation might be the only possible therapeutic option available.14
This case, combined with results from animal studies, has shed considerable light on the intricacies of the different pathways involved in the modification of cholesterol to bile acids. The expression of oxysterol 7α-hydroxylase, which is active in the alternative acidic pathway, is developmentally regulated in rodents.15–18 Mice in which the classic pathway is rendered nonfunctional by knockout of cholesterol 7α-hydroxylase (Cyp7a1−/−) are normal at birth but rapidly become ill; death occurs in the early postnatal period unless the mice are given supplemental vitamins and bile acids.19 Schwarz et al. found that adult Cyp7a1−/− mice can fully compensate for the lack of cholesterol 7α-hydroxylase by synthesizing 7α-hydroxylated bile acid products via the alternative acidic pathway.15 In rats in which cholesterol 7α-hydroxylase had been chemically ablated, 43% of expected bile acid synthesis still occurred, again supporting the importance of the alternative acidic pathway.17 Examination of the single reported case of human oxysterol 7α-hydroxylase deficiency similarly helped to establish the importance of the alternative acidic pathway for the synthesis of primary bile acids in humans, especially during infancy14
Δ4-3-Oxosteroid-5β-Reductase Deficiency
Deficiency of Δ4-3-oxosteroid-5β-reductase (also known as 5β-reductase deficiency) is an autosomal recessive condition that causes defective bile acid steroid nucleus synthesis.20,21 The enzyme Δ4-3-oxosteroid-5β-reductase, which is encoded by the gene AKR1D1 (previously known as SRD5B1), converts 7α-hydroxy-4-cholesten-3-one and 7α,12α-dihydroxy-4-cholesten-3-one into 3-oxo-5β analogs. Deficiency of this enzyme impairs the reduction of the double bond between C4 and C5 of the steroid nucleus. Consequently, low levels of normal primary bile acids are present in the urine and serum of affected patients, while intermediate products of bile acid synthesis accumulate and are readily detectable by FAB-MS.1
The 5β-reductase deficiency was first described by Setchell et al. in 1988 in a set of identical twins.22 The typical presentation is neonatal cholestasis and is characterized by increased concentrations of aminotransferases, a normal GGTP concentration, conjugated hyperbilirubinemia, and coagulopathy that worsens with disease progression.1,22,23 Without treatment, liver failure rapidly ensues; there is, therefore, a 50% mortality rate in infants for whom diagnosis is delayed.1 An alternative clinical presentation, neonatal liver failure resembling neonatal hemochromatosis, has also been described in patients with 5β-reductase deficiency, although none of these individuals were shown to have mutations in the AKR1D1 gene.24,25 The phenotypic overlap with neonatal hemochromatosis is postulated to be caused by an impairment in iron excretion, which is usually enhanced by bile acids.24,26,27
The enzyme Δ4-3-oxosteroid-5β-reductase is not expressed in fibroblasts or leukocytes, so cell-based enzyme studies are not useful for establishing the diagnosis of 5β-reductase deficiency Instead, spot urine samples are examined by FAB-MS and GC-MS for the presence of characteristic mass spectra of Δ4-3-oxo bile acids.1,23 High levels of Δ4-3-oxo bile acids are, however, present in various advanced liver diseases and might be nonspecific. Thorough evaluation of patients for advanced liver disease that can result in a secondary 5β-reductase deficiency is, therefore, important.28,29 In addition, molecular analysis of AKR1D1 to determine the presence of mutations is available in research laboratories and can be helpful in firmly establishing the diagnosis of primary 5β-reductase deficiency20,30
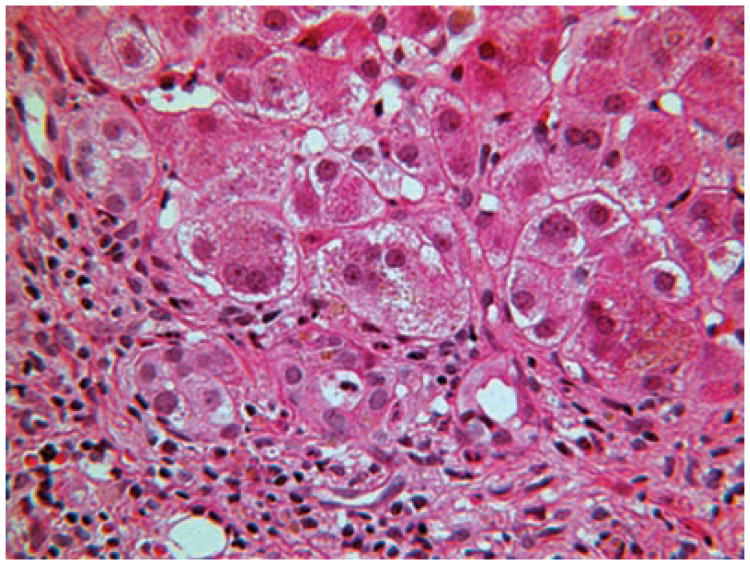
The histopathology of 5β-reductase deficiency is typical of that of neonatal hepatitis, with findings of giant cell hepatitis, pseudoacinar transformation, hepatocellular and canalicular cholestasis, and extramedullary hematopoesis (Figure 3). Electron microscopy shows a mix of normal and abnormal bile canaliculi, without dilation. The abnormal canaliculi have been described as having diverticuli, and many contain dense granular material.30,31
Figure 3.
Liver histology in a patient aged 6 weeks with Δ4-3-oxosteroid 5β-reductase deficiency. Multiple small ducts near the margin of the portal area are lined with degenerated epithelium and are accompanied by mild pericholangitis, a presumed toxic reaction to monohydroxy bile acids. The larger ducts are normal. The hepatocytes are irregularly ballooned. Hematoxylin and eosin stain, magnification × 250.
Therapy for 5β-reductase deficiency is directed at the replacement of primary bile acids to stimulate bile flow and limit the production of toxic bile acid precursors through feedback inhibition.13,26 Initially, both chenodeoxycholic acid and cholic acid were used as therapy26 Although treatment trials in humans are lacking, concerns over potential hepatotoxicity in animal models of the disease have curbed the use of chenodeoxycholic acid.1,32,33 Treatment with cholic acid (10–20 mg/kg daily) should be titrated to ensure that urinary excretion of Δ4-3-oxo bile acids ceases. Ursodeoxycholic acid has also been used as therapy in this setting because of its choleretic and hepatoprotective properties. Although ursodeoxycholic acid does help to stimulate bile flow, it does not, however, inhibit the first step in bile acid synthesis, which is mediated by cholesterol 7α-hydroxylase, and is, therefore, ineffective as sole therapy for this condition.1,26 Overall treatment response is good if the diagnosis of Δ4-3-oxosteroid-5β-reductase deficiency is made early in the course of the disease.31
3β-hydroxy-Δ5-C27-Steroid Dehydrogenase Deficiency
The most commonly reported defect of bile acid synthesis, 3β-hydroxy-Δ5-C27-steroid dehydrogenase (3βHSD) deficiency, is an autosomal recessive condition in which modification of the steroid nucleus is defective. The enzyme 3βHSD is necessary for the oxido-reduction of the 3-β-hydroxyl moiety of 7α-hydroxycholesterol, converting it to 7α-hydroxy-4-cholesten-3-one. A defect in this enzyme results in accumulation of both 7α-hydroxycholesterol and abnormal C24 bile acids that retain the 3-β-hydroxyl-Δ5 structure.34 Hepatic injury occurs as a consequence of the inadequate synthesis of the normal bile acids necessary to stimulate bile flow and the accumulation of abnormal toxic bile acids.
Most patients with 3βHSD deficiency present as neonates, although age at onset can be quite variable (3 months to 14 years), as can the clinical presentation.1 Typical features in the neonate include progressive jaundice, increased aminotransferase levels, a normal GGTP level, low or normal total serum bile acid concentrations, and conjugated hyperbilirubinemia.23,35,36 Hepatomegaly, with or without splenomegaly, is common, and pruritis can be present. In addition, malabsorption can occur, with resultant steatorrhea, fat-soluble-vitamin deficiency, poor growth, and rickets.23,36,37
Although total serum bile acid levels are quantitatively normal in this setting, they are qualitatively abnormal.1 The diagnosis of 3(3HSD deficiency can be made on the basis of FAB-MS analysis of a spot urine sample. Urine bile acids are abnormal, with a lack of the normal glycine and taurine conjugates of primary bile acids and the presence of sulfate and glycosulfate conjugates of dihydroxy and trihydroxy cholenoic acids, and levels are elevated.23,35 These abnormal bile acids are poorly transported across the canalicular membrane and interfere with ATP-dependent transport of conjugated bile acids.36 As 3βHSD is also expressed in fibroblasts, enzyme activity can be measured in cultured skin fibroblasts by using 7α-hydroxycholesterol as the substrate. No detectable enzyme activity is observed in affected patients, while heterozygotes have decreased but measurable enzyme activity37 Identification of mutations in the causative gene, HSD3B7, on chromosome 16p11.2–12 also allows for genetic diagnosis.38,39
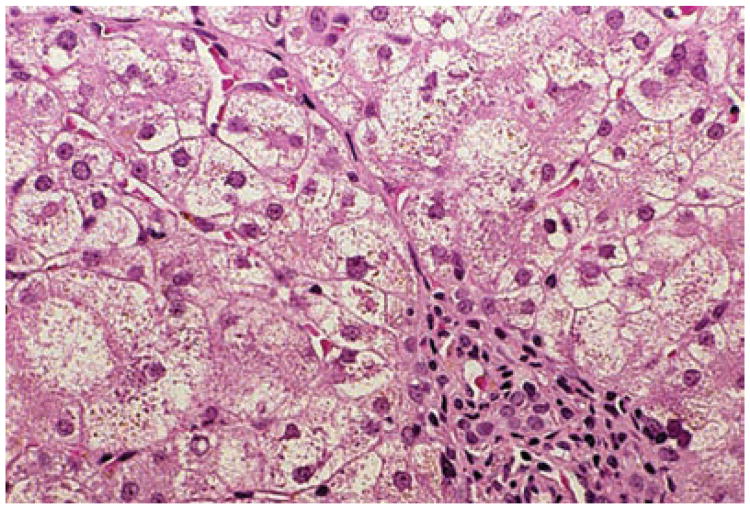
The histopathology of 3βHSD deficiency varies with patient age and correlates with the mode of presentation and rate of disease progression. Liver histology in the infant includes giant cell hepatitis, canalicular bile plugs, hepatocyte bile stasis, and portal tract inflammation with variable fibrosis (Figure 4).35,40–42 Liver biopsy samples from older infants and children can show less striking features of giant cell transformation and cholestasis; however, fibrosis becomes more prominent in the portal and periportal areas, and cirrhosis has been described.1,35,40,42 Variable levels of inflammation with lymphocytic predominance can be seen, mimicking chronic hepatitis. Although no specific ultrastructural changes have been described, findings include partial or complete loss of canalicular microvilli and granular or membranous material in canaliculi that is distinct from the granular material seen in progressive familial intrahepatic cholestasis type 1 (also known as FIC1 deficiency or Byler disease).1,42
Figure 4.
Liver histology in a patient aged 6 months with 3β-hydroxy-Δ5-C27 steroid dehydrogenase deficiency. Focal giant cell transformation of hepatocytes is seen in Zone 1 adjacent to the portal area. Hematoxylin and eosin stain, magnification × 250.
Treatment of 3βHSD deficiency is targeted at stimulating bile flow and downregulating 7α-hydroxylase activity to diminish or eliminate the production of hepatotoxic bile acids.12,13,41 We believe that this result is accomplished by administration of oral primary bile acid therapy combined with proper monitoring of the suppression of abnormal urine metabolites. In our opinion, oral bile acid therapy probably decreases the production of hepatotoxic bile acids by inducing negative feedback inhibition of 7α-hydroxylase transcription through FXR and SHP. Treatment with cholic acid (10–15 mg/kg daily) leads to improved liver function test results and resolution of jaundice.1 If advanced fibrosis is present, prognosis is not as good. Ursodeoxycholic acid has been used as a treatment for 3βHSD deficiency, but is ineffective without concomitant cholic acid therapy.43
Cerebrotendinous Xanthomatosis
Cerebrotendinous xanthomatosis (CTX) is a rare autosomal recessive lipid-storage disorder that results from abnormal side-chain modification of bile acids, which is caused by mitochondrial sterol 27-hydroxylase deficiency.44 Impaired oxidation of the cholesterol side chain causes accelerated cholesterol synthesis and metabolism, increased excretion of bile alcohol glucuronides, and abnormal cholestanol and cholesterol deposition in tissue, particularly in the nervous system and cardiovascular system.45–47 Various mutations in the sterol 27-hydroxylase gene CYP27A1, which is on the long arm of chromosome 2, cause CTX.48
CTX is typically detected in patients in the second or third decade of life as part of a neurologic evaluation, although young pediatric cases have been reported.49,50 The estimated prevalence of CTX is 1 in 70,000.51 Classic findings include progressive neurologic dysfunction, ataxia, dementia, cataracts, xanthomas of the brain, and atherosclerosis.44,45,52,53 Chronic diarrhea coupled with early bilateral juvenile cataracts can suggest the diagnosis of CTX before the onset of overt neurologic symptoms.49,50,54,55 Isolated neonatal cholestasis without neurologic symptoms, diarrhea or cataracts is a rare but reported presentation.23,56,57 Affected infants have a normal GGTP level and increased levels of aminotransferases and conjugated bilirubin, which gradually normalize by 6 months of age.23,57 One reported case of CTX was associated with fatal cholestasis in an infant.56 Early diagnosis is essential to limit or prevent the development of neurologic and cardiovascular complications.58
CTX can be differentiated from other conditions associated with xanthomata on the basis of serum cholesterol concentrations, which are generally elevated in other types of xanthomata-associated disorders.51 In patients with CTX, primary bile acid synthesis is reduced (chenodeoxycholic acid synthesis is affected more than cholic acid synthesis), bile alcohol glucuronide excretion in bile, urine and stools is increased, serum or plasma cholesterol levels are low or normal, plasma cholestanol levels are markedly elevated, and plasma cholestanol:cholesterol ratios are increased. The diagnosis of CTX is established by finding a greatly increased plasma cholestanol:cholesterol ratio or characteristic metabolites in urine (e.g. increased bile alcohol glucuronides detected by FAB-MS), followed by DNA sequencing of CYP27A1. Affected individuals have tissue (including central nervous system) deposition of cholesterol and cholestanol, which is responsible for neurologic injury and the associated symptomatology.23
The primary treatment of CTX is oral bile acid administration. Chenodeoxycholic acid (750 mg daily in adults) decreases plasma cholestanol levels and reduces urinary bile alcohol glucuronide excretion through feedback inhibition of cholesterol 7α-hydroxylase.59,60 Ursodeoxycholic acid, which does not inhibit cholesterol 7α-hydroxylase, is ineffective as a treatment.61 Cholic acid therapy, another treatment option, also decreases plasma cholestanol levels.62 Studies using 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitors, which lower cholesterol levels by inhibiting a rate-limiting step of endogenous cholesterol synthesis, have yielded mixed results for the treatment of CTX.61,63–65 A combination of oral bile acid therapy and an HMG-CoA reductase inhibitor, however, might be more effective at reducing plasma cholestanol concentrations than HMG-CoA reductase inhibitors alone.65,66 Concern has been raised that HMG-CoA reductase inhibitors could potentially worsen CTX by increasing LDL uptake by enhancing LDL receptor activity.44
Alpha Methylacyl-Coa Racemase Deficiency
Alpha methylacyl-CoA racemase (AMACR) deficiency is an autosomal recessive defect that inhibits cholesterol side-chain oxidation.1 AMACR is necessary for the racemization of trihydroxycholestanoic acid and pristanic acid into their stereoisomers.67 Conversion to these stereoisomers is necessary for the subsequent step of peroxisomal β-oxidation of the C27 bile acid side chain.1,68 Deficiency of AMACR leads to an accumulation of plasma pristanic acid and the bile acid intermediates dihydroxycholestanoic acid and trihydroxycholestanoic acid; therefore, AMACR deficiency affects both the bile acid and fatty acid synthesis pathways.69,70
Only five patients with this disorder have been described in the literature.71,72 Three of these patients were reported by Ferdinandusse et al. in 2000.71 Two patients were asymptomatic as children and presented with adult-onset peripheral neuropathy; one of these patients also had retinitis pigmentosa. A third patient had symptoms at 18 months of age that were consistent with Niemann-Pick disease type C. All three patients had a complete absence of AMACR enzyme activity in cultured fibroblasts. Subsequently, two mutations in the AMACR gene were identified that were present in all three patients.
Two additional patients with AMACR deficiency were later identified and described by Setchell and co-workers.72 The first was a 2-week-old infant with mild cholestasis, coagulopathy, and fat-soluble-vitamin deficiency. The infant's liver biopsy sample revealed the presence of giant cell transformation, moderate intralobular cholestasis, scattered necrotic hepatocytes, and areas with multinucleated hepatocytes. Electron microscopy showed a low number of peroxisomes, with those identified being described as abnormally small and mildly pleomorphic.1 A missense mutation in the AMACR gene was confirmed in this patient,72 and fibroblast studies also confirmed AMACR deficiency.73 Treatment with cholic acid therapy (15 mg/kg daily) and fat-soluble-vitamin supplementation normalized the infant's liver enzyme levels.72 Interestingly, the infant had a sibling who died at 5.5 months of age because of intracranial hemorrhage secondary to vitamin K deficiency, and who had become a liver transplant donor.72 AMACR deficiency was later confirmed in the recipient of the liver from this sibling, on the basis of an abnormal urine bile acid profile and an increased pristanic acid level.72 The liver transplant recipient, the second of the two additional patients, is receiving oral bile acid therapy.72
Zellweger Syndrome
Zellweger syndrome (also known as cerebrohepatorenal syndrome) is an autosomal recessive disorder that disrupts peroxisomal biogenesis, affecting bile acid synthesis pathways as well as other peroxisomal functions. The final steps of bile acid synthesis occur in peroxisomes, including beta-oxidation of the 2-methyl branched side chain of the C27 bile acids dihydroxycholestanoic acid and trihydroxycholestanoic acid, forming the C24 primary bile acids cholic acid and chenodeoxycholic acid.74,75 Mutations in 12 genes have been associated with Zellweger syndrome in humans: PEX1; PEX2; PEX3; PEX5; PEX6; PEX10; PEX12; PEX13; PEX14; PEX16; PEX19; and PEX26. PEX1 mutations are the most commonly identified mutations in individuals with Zellweger syndrome.76 The PEX family of genes encode proteins, called peroxins, that are essential for the proper assembly of functional peroxisomes.76
Zellweger syndrome is the most severe of the peroxisome biogenesis disorders; patients with neonatal adrenoleukodystrophy or infantile Refsum disease have less severe disease. Patients with neonatal adrenoleukodystrophy have deafness and progressive psychomotor delay, and commonly have seizures and hypotonia.76 Some patients have dysmorphic features. The liver disease associated with this disorder is very mild. Patients with Refsum disease are also universally deaf and have progressive psychomotor delay. Hypotonia and craniofacial abnormalities can occur. Hepatomegaly, neonatal cholestasis and abnormal liver tests are more common in patients with Refsum disease than in those with neonatal adrenoleukodystrophy.77
Zellweger syndrome manifests in multiple ways, reflecting the paramount role of peroxisomes throughout the body. Craniofacial abnormalities associated with the condition include a wide anterior fontanelle, a prominent forehead, epicanthal folds and midface hypoplasia.76 Patients have neuronal migration defects, with profound neurologic complications including hypotonia, areflexia, seizures, and little spontaneous movement. Affected individuals have a poor suck, with resultant difficulty feeding and failure to thrive. Polycystic kidneys are present and eye abnormalities (abnormal electroretinogram, cataracts, glaucoma, and optic atrophy) have been described. Bony abnormalities include characteristic stippling of the patella and other bones, and dislocated hips.76,78,79 Chronic liver disease is typical and manifests as hepatomegaly, conjugated hyperbilirubinemia, and abnormal liver function test results and, in some patients, as splenomegaly, cirrhosis, and portal hypertension.1,76,78 The progression of such liver disease is quite variable. Zellweger syndrome is usually fatal in the first 2 years of life; in most patients death occurs before the development of the serious complication of portal hypertension.1,80
The diagnosis of Zellweger syndrome is multitiered. Initial screening includes evaluating the patient for the presence of increased plasma concentrations of very long chain fatty acids and for low levels of erythrocyte plasmalogens.80,81 If the level of either of these is abnormal, further investigations can be made by checking the concentrations of plasma phytanic acid, pristanic acid, pipecolic acid and bile acids.80,81 Hyperpipecolic acidemia and increased levels of monohydroxy, dihydroxy and trihydroxy bile acids with elongated side chains (such as trihydroxycholestanoic acid) are characteristic of Zellweger syndrome.80,81 If the level of one of these types of bile acids is abnormal, confirmatory testing can include assessment of plasmalogen synthesis in cultured fibroblasts.80,81 Studies of peroxisome function in cultured fibroblasts can be helpful in determining whether genetic studies are needed. PEX gene mutation analysis is available for cases in which the biochemical or clinical profile is not classic for Zellweger syndrome.76 Prenatal diagnosis of Zellweger syndrome is also possible through either molecular or biochemical analysis.76
Histologic findings of Zellweger syndrome include an increase in the size of the hepatic stores of iron, mild cholestasis, and hepatocellular injury. Lesions of the cholangioles are also present and were originally termed biliary dysgenesis.1 Small plugs of inspissated bile can be seen in cholangioles, portal tracts, and small interlobular bile ducts.30 The biliary epithelium can be swollen, thin or absent in areas and bile duct proliferation can be present. Various nonspecific findings characteristic of metabolic liver diseases might be present, including canalicular and cytoplasmic cholestasis, sinusoidal fibrosis, and pseudoacinar transformation of hepatocytes. Intralobular and/or portal fibrosis can progress to cirrhosis in some patients.1 The hepatic ultrastructure in patients with this disorder has also been studied in great detail. Most striking is the absence of peroxisomes, which is virtually diagnostic of a peroxisomal biogenesis disorder and is one of the reasons to consider electron microscopy of liver biopsy samples when evaluating infants with cholestasis. Mitochondria can contain increased numbers of electrondense tubular cristae, enlarged matrix crystalloids and granules, or can be normal.1,82
Treatment of this fatal condition is largely supportive and palliative, and includes therapy for seizures and providing developmental support. Diets low in phytanic acid have been suggested, but have not proven helpful.76,78 Patients with Zellweger syndrome have been found to have particularly low concentrations of docosahexaenoic acid.83 Clinical trials of docosahexaenoic acid supplementation have yielded mixed results, although small series have shown improvements in eye abnormalities and liver function tests.83–85 Oral primary bile acid therapy has also been attempted with limited success and is not recommended for the treatment of Zellweger syndrome.86,87
D-Bifunctional Protein Deficiency
Deficiency of D-bifunctional protein causes abnormal peroxisomal fatty acid oxidation.88 The D-bifunctional protein is composed of two units, D-3-hydroxyacyl-CoA-dehydratase and D-3-hydroxyacyl-CoA-dehydrogenase. There are three variants of D-bifunctional protein deficiency, depending on which protein subunit is affected.89 Patients with the type I defect are deficient in both the dehydratase and the dehydrogenase subunits. Patients with the type II deficiency are deficient in the dehydratase subunit and those with the type III variant are deficient in the dehydrogenase subunit.89 The disease is caused by mutations in the HSD17B4 gene.90 Both peroxisomal beta-oxidation and oxidation of bile acid precursors are abnormal in patients with D-bifunctional protein deficiency.91 Most patients present with neonatal hypotonia, seizures and craniofacial abnormalities, and can have visual impairment and psychomotor retardation.91–93 Liver disease has also been reported in affected patients.92
Bile Acid Conjugation Defects
Conjugation of cholic acid and chenodeoxycholic acid to taurine or glycine is the final step in primary bile acid synthesis.10,11 The two enzymes that catalyze these final reactions are bile acid CoA ligase and bile acid CoA:amino acid N-acyltransferase,94–96 which are encoded by the genes BAL and BAAT.97,98 Patients affected by defects in bile acid conjugation present with marked malabsorption and deficiencies of fat-soluble vitamins. These symptoms occur as a consequence of decreased biliary secretion of conjugated bile acids, which leads to the inability to form mixed micelles because of rapid small intestinal absorption of the unconjugated bile acids.96 Conjugated hyperbilirubinemia, severe cholestasis, and liver failure have also been described in patients with bile acid conjugation defects.1,51 Similar to other bile acid synthesis defects, conjugation defects can be diagnosed using FAB-MS analysis of urine and serum.51 Administration of oral primary conjugated bile acids is a potential treatment for these disorders. There is not enough evidence available, however, to support a firm recommendation for the therapeutic use of these bile acids, and further data are needed.
Conclusions
Defects in bile acid synthesis comprise an expanding group of important hepatic disorders. Clinically, these conditions resemble many other causes of neonatal cholestasis and chronic liver disease, and a high index of clinical suspicion is required when making a diagnosis. Early diagnosis is important because most of these disorders can be treated effectively with bile acid replacement therapy. The current gold standard for definitive diagnosis, FAB-MS and GC-MS analyses of serum and urine, is technically demanding and only available in a few specialized referral laboratories. Our improved understanding of these disorders attests to the triumph of combining modern biochemical and molecular techniques for unraveling the etiology of rare liver diseases.
Note added in proof
A case report published in April 2008 has identified a second case of oxysterol 7α-hydroxylase deficiency, which presented as neonatal cholestatic cirrhosis in a Taiwanese infant who died of liver failure at 11 months of age.99
Review Criteria.
The literature cited in this article was located by searching PubMed using the terms “bile acid”, “bile acid synthesis”, “bile acid synthetic defect”, “ neonatal hepatitis”, “oxysterol 7 alpha hydroxylase deficiency”, “5 beta reductase deficiency”, “3 beta hydroxyl steroid dehydrogenase”, “alpha methyl coA racemase”, “Zellweger syndrome”, “cerebrotendinous xanthomatosis”, and “conjugation defect”, alone or in combination. Only full papers published in English language journals were considered. Reviews have been cited where appropriate to limit the number of references. The original search was performed through December 2007 and the reference list was updated in March 2008.
Key Points.
Defects in bile acid synthesis most commonly present as neonatal cholestasis or neonatal hepatitis, but can present as chronic liver disease in older children
Unlike most cholestatic diseases, patients with bile acid synthesis defects generally have the hallmark features of low or normal serum bile acid levels, normal or minimally increased γ-glutamyl transpeptidase levels and lack of pruritus
Deficiency of Δ4-3-oxosteroid-5β-reductase can present as either neonatal cholestasis or as liver failure that resembles neonatal hemochromatosis; it has a 50% mortality in infants when diagnosis is delayed
Deficiency of 3β-hydroxy-Δ5-C27-steroid dehydrogenase is the most common defect of bile acid synthesis, presenting both as neonatal cholestasis and as chronic liver disease in older patients
Cerebrotendinous xanthomatosis is a lipid storage disorder that presents with symptoms of progressive neurologic dysfunction in the second or third decade of life and occasionally as neonatal cholestasis; it can be treated with oral bile acids
Early recognition and diagnosis of bile acid synthesis defects is important as these disorders are often readily treatable with oral bile acid therapy
Acknowledgments
RJS and SSS are supported by NIH grants U54DK078377, UO1DK062453 and RR00069.
Footnotes
Competing interests: The authors declared no competing interests.
References
- 1.Bove KE, et al. Bile acid synthetic defects and liver disease: a comprehensive review. Pediatr Dev Pathol. 2004;7:315–334. doi: 10.1007/s10024-002-1201-8. [DOI] [PubMed] [Google Scholar]
- 2.Westin S, et al. FXR, a therapeutic target for bile acid and lipid disorders. Mini Rev Med Chem. 2005;5:719–727. doi: 10.2174/1389557054553802. [DOI] [PubMed] [Google Scholar]
- 3.Edwards PA, et al. BAREing it all: the adoption of LXR and FXR and their roles in lipid homeostasis. J Lipid Res. 2002;43:2–12. [PubMed] [Google Scholar]
- 4.Otte K, et al. Identification of farnesoid X receptor beta as a novel mammalian nuclear receptor sensing lanosterol. Mol Cell Biol. 2003;23:864–872. doi: 10.1128/MCB.23.3.864-872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makishima M, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 6.Parks DJ, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin B, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 9.Kuipers F, et al. The farnesoid X receptor (FXR) as modulator of bile acid metabolism. Rev Endocr Metab Disord. 2004;5:319–326. doi: 10.1023/B:REMD.0000045103.00467.9a. [DOI] [PubMed] [Google Scholar]
- 10.Vlahcevic ZR, et al. Regulation of bile acid biosynthesis. Gastroenterol Clin North Am. 1999;28:1–25. doi: 10.1016/s0889-8553(05)70041-8. [DOI] [PubMed] [Google Scholar]
- 11.Russell DW, et al. Bile acid biosynthesis. Biochemistry. 1992;31:4737–4749. doi: 10.1021/bi00135a001. [DOI] [PubMed] [Google Scholar]
- 12.Ichimiya H, et al. Bile acids and bile alcohols in a child with hepatic 3 beta-hydroxy-delta 5-C27-steroid dehydrogenase deficiency: effects of chenodeoxycholic acid treatment. J Lipid Res. 1991;32:829–841. [PubMed] [Google Scholar]
- 13.Ichimiya H, et al. Treatment of chronic liver disease caused by 3 beta-hydroxy-delta 5-C27-steroid dehydrogenase deficiency with chenodeoxycholic acid. Arch Dis Child. 1990;65:1121–1124. doi: 10.1136/adc.65.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Setchell KD, et al. Identification of a new inborn error in bile acid synthesis: mutation of the oxysterol 7α-hydroxylase gene causes severe neonatal liver disease. J Clin Invest. 1998;102:1690–1703. doi: 10.1172/JCI2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwarz M, et al. Disruption of cholesterol 7alpha-hydroxylase gene in mice: II. bile acid deficiency is overcome by induction of oxysterol 7alpha-hydroxylase. J Biol Chem. 1996;271:18024–18031. doi: 10.1074/jbc.271.30.18024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarz M, et al. Identification and characterization of a mouse oxysterol 7alpha-hydroxylase cDNA. J Biol Chem. 1997;272:23995–24001. doi: 10.1074/jbc.272.38.23995. [DOI] [PubMed] [Google Scholar]
- 17.Axelson M, Sjovall J. Potential bile acid precursors in plasma—possible indicators of biosynthetic pathways to cholic and chenodeoxycholic acids in man. J Steroid Biochem. 1990;36:631–640. doi: 10.1016/0022-4731(90)90182-r. [DOI] [PubMed] [Google Scholar]
- 18.Shoda J, et al. Formation of 7 alpha- and 7-beta-hydroxylated bile acid precursors from 27-hydroxycholesterol in human liver microsomes and mitochondria. Hepatology. 1993;17:395–403. [PubMed] [Google Scholar]
- 19.Ishibashi S, et al. Disruption of cholesterol 7alpha-hydroxylase gene in mice: I. postnatal lethality reversed by bile acid and vitamin supplementation. J Biol Chem. 1996;271:18017–18023. doi: 10.1074/jbc.271.30.18017. [DOI] [PubMed] [Google Scholar]
- 20.Gonzales E, et al. SRD5B1 (AKR1D1) gene analysis in delta(4)-3-oxosteroid 5beta-reductase deficiency: evidence for primary genetic defect. J Hepatol. 2004;40:716–718. doi: 10.1016/j.jhep.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 21.Lemonde HA, et al. Mutations in SRD5B1 (AKR1D1), the gene encoding delta(4)-3-oxosteroid 5beta-reductase, in hepatitis and liver failure in infancy. Gut. 2003;52:1494–1499. doi: 10.1136/gut.52.10.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Setchell KD, et al. Delta 4-3-oxosteroid 5 beta-reductase deficiency described in identical twins with neonatal hepatitis: a new inborn error in bile acid synthesis. J Clin Invest. 1988;82:2148–2157. doi: 10.1172/JCI113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heubi JE, et al. Inborn errors of bile acid metabolism. Semin Liver Dis. 2007;27:282–294. doi: 10.1055/s-2007-985073. [DOI] [PubMed] [Google Scholar]
- 24.Siafakas CG, et al. Abnormal bile acid metabolism and neonatal hemochromatosis: a subset with poor prognosis. J Pediatr Gastroenterol Nutr. 1997;25:321–326. doi: 10.1097/00005176-199709000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Shneider BL, et al. Delta 4-3-oxosteroid 5 beta-reductase deficiency causing neonatal liver failure and hemochromatosis. J Pediatr. 1994;124:234–238. doi: 10.1016/s0022-3476(94)70310-8. [DOI] [PubMed] [Google Scholar]
- 26.Clayton PT, et al. Delta 4-3-oxosteroid 5 beta-reductase deficiency: failure of ursodeoxycholic acid treatment and response to chenodeoxycholic acid plus cholic acid. Gut. 1996;38:623–628. doi: 10.1136/gut.38.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy P, et al. Acute infusions of bile salts increase biliary excretion of iron in iron-loaded rats. Gastroenterology. 1991;101:1673–1679. doi: 10.1016/0016-5085(91)90407-c. [DOI] [PubMed] [Google Scholar]
- 28.Clayton PT, et al. 3-Oxo-delta 4 bile acids in liver disease. Lancet. 1988;1:1283–1284. doi: 10.1016/s0140-6736(88)92104-6. [DOI] [PubMed] [Google Scholar]
- 29.Sumazaki R, et al. Gene analysis in delta 4-3-oxosteroid 5 beta-reductase deficiency. Lancet. 1997;349:329. doi: 10.1016/s0140-6736(05)62828-0. [DOI] [PubMed] [Google Scholar]
- 30.Bove KE, et al. Bile acid synthetic defects and liver disease. Pediatr Dev Pathol. 2000;3:1–16. doi: 10.1007/s100240050001. [DOI] [PubMed] [Google Scholar]
- 31.Daugherty CC, et al. Resolution of liver biopsy alterations in three siblings with bile acid treatment of an inborn error of bile acid metabolism (delta 4-3-oxosteroid 5 beta-reductase deficiency) Hepatology. 1993;18:1096–1101. [PubMed] [Google Scholar]
- 32.Bazzoli F, et al. Relationship between serum and biliary bile acids as an indicator of chenodeoxycholic and ursodeoxycholic acid-induced hepatotoxicity in the rhesus monkey. Dig Dis Sci. 1982;27:417–424. doi: 10.1007/BF01295650. [DOI] [PubMed] [Google Scholar]
- 33.Sarva RP, et al. Comparison of the effects between ursodeoxycholic and chenodeoxycholic acids on liver function and structure and bile acid composition in the Rhesus Monkey. Gastroenterology. 1980;79:629–636. [PubMed] [Google Scholar]
- 34.Wikvall K. Purification and properties of a 3 beta-hydroxy-delta 5-C27-steroid oxidoreductase from rabbit liver microsomes. J Biol Chem. 1981;256:3376–3380. [PubMed] [Google Scholar]
- 35.Clayton PT, et al. Familial giant cell hepatitis associated with synthesis of 3 beta, 7 alpha-dihydroxy-and 3 beta,7 alpha, 12 alpha-trihydroxy-5-cholenoic acids. J Clin Invest. 1987;79:1031–1038. doi: 10.1172/JCI112915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stieger B, et al. Differential interaction of bile acids from patients with inborn errors of bile acid synthesis with hepatocellular bile acid transporters. Eur J Biochem. 1997;244:39–44. doi: 10.1111/j.1432-1033.1997.00039.x. [DOI] [PubMed] [Google Scholar]
- 37.Buchmann MS, et al. Lack of 3 beta-hydroxy-delta 5-C27-steroid dehydrogenase/isomerase in fibroblasts from a child with urinary excretion of 3 beta-hydroxy-delta 5-bile acids: a new inborn error of metabolism. J Clin Invest. 1990;86:2034–2037. doi: 10.1172/JCI114939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarz M, et al. The bile acid synthetic gene 3beta-hydroxy-delta(5)-C(27)-steroid oxidoreductase is mutated in progressive intrahepatic cholestasis. J Clin Invest. 2000;106:1175–1184. doi: 10.1172/JCI10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng JB, et al. Molecular genetics of 3beta-hydroxy-delta5-C27-steroid oxidoreductase deficiency in 16 patients with loss of bile acid synthesis and liver disease. J Clin Endocrinol Metab. 2003;88:1833–1841. doi: 10.1210/jc.2002-021580. [DOI] [PubMed] [Google Scholar]
- 40.Jacquemin E, et al. A new cause of progressive intrahepatic cholestasis: 3 beta-hydroxy-C27-steroid dehydrogenase/isomerase deficiency. J Pediatr. 1994;125:379–384. doi: 10.1016/s0022-3476(05)83280-9. [DOI] [PubMed] [Google Scholar]
- 41.Horslen SP, et al. 3Beta-hydroxy-delta 5-C27-steroid dehydrogenase deficiency; effect of chenodeoxycholic acid therapy on liver histology. J Inherit Metab Dis. 1992;15:38–46. doi: 10.1007/BF01800342. [DOI] [PubMed] [Google Scholar]
- 42.Witzleben CL, et al. A new category of causes of intrahepatic cholestasis. Pediatr Pathol. 1992;12:269–274. doi: 10.3109/15513819209023305. [DOI] [PubMed] [Google Scholar]
- 43.Yamato Y, et al. 3Beta-hydroxy-delta5-C27-steroid dehydrogenase deficiency: diagnosis and treatment. J Paediatr Child Health. 2001;37:516519. doi: 10.1046/j.1440-1754.2001.00751.x. [DOI] [PubMed] [Google Scholar]
- 44.Moghadasian MH, et al. Cerebrotendinous xanthomatosis: a rare disease with diverse manifestations. Arch Neurol. 2002;59:527–529. doi: 10.1001/archneur.59.4.527. [DOI] [PubMed] [Google Scholar]
- 45.Gallus GN, et al. Clinical and molecular diagnosis of cerebrotendinous xanthomatosis with a review of the mutations in the CYP27A1 gene. Neurol Sci. 2006;27:143–149. doi: 10.1007/s10072-006-0618-7. [DOI] [PubMed] [Google Scholar]
- 46.Verrips A, et al. Clinical and molecular genetic characteristics of patients with cerebrotendinous xanthomatosis. Brain. 2000;123:908–919. doi: 10.1093/brain/123.5.908. [DOI] [PubMed] [Google Scholar]
- 47.Setchell KD, Street JM. Inborn errors of bile acid synthesis. Semin Liver Dis. 1987;7:85–99. doi: 10.1055/s-2008-1040568. [DOI] [PubMed] [Google Scholar]
- 48.Clayton PT, et al. Familial giant cell hepatitis with low bile acid concentrations and increased urinary excretion of specific bile alcohols: a new inborn error of bile acid synthesis? Pediatr Res. 1995;37:424–431. doi: 10.1203/00006450-199504000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Cruysberg JR, et al. Juvenile cataract associated with chronic diarrhea in pediatric cerebrotendinous xanthomatosis. Am J Ophthal. 1991;112:606–607. doi: 10.1016/s0002-9394(14)76874-6. [DOI] [PubMed] [Google Scholar]
- 50.Wevers RA, et al. Paediatric cerebrotendinous xanthomatosis. J Inherit Metab Dis. 1992;15:374–376. doi: 10.1007/BF02435980. [DOI] [PubMed] [Google Scholar]
- 51.Setchell KDR, et al. Disorders of bile acid synthesis and metabolism: a metabolic basis for liver disease. In: Suchy FJ, et al., editors. Liver Disease in Children. New York: Cambridge University Press; 2007. pp. 736–766. [Google Scholar]
- 52.Moghadasian MH. Cerebrotendinous xanthomatosis: clinical course, genotypes and metabolic backgrounds. Clin Invest Med. 2004;27:42–50. [PubMed] [Google Scholar]
- 53.Kuriyama M, et al. Cerebrotendinous xanthomatosis: clinical and biochemical evaluation of eight patients and review of the literature. J Neurol Sci. 1991;102:225–232. doi: 10.1016/0022-510x(91)90073-g. [DOI] [PubMed] [Google Scholar]
- 54.van Heijst AF, et al. Treatment and follow-up of children with cerebrotendinous xanthomatosis. Eur J Pediatr. 1998;157:313–316. doi: 10.1007/s004310050818. [DOI] [PubMed] [Google Scholar]
- 55.Bindl L, et al. Cerebrotendinous xanthomatosis presenting as “chologenic diarrhoea”. Acta Paediatr. 2001;90:828–829. [PubMed] [Google Scholar]
- 56.von Bahr S, et al. Mutation in the sterol 27-hydroxylase gene associated with fatal cholestasis in infancy. J Pediatr Gastroenterol Nutr. 2005;40:481–486. doi: 10.1097/01.mpg.0000150419.23031.2a. [DOI] [PubMed] [Google Scholar]
- 57.Clayton PT, et al. Mutations in the sterol 27-hydroxylase gene (CYP27A) cause hepatitis of infancy as well as cerebrotendinous xanthomatosis. J Inherit Metab Dis. 2002;25:501–513. doi: 10.1023/a:1021211520034. [DOI] [PubMed] [Google Scholar]
- 58.Egestad B, et al. Fast atom bombardment mass spectrometry in the diagnosis of cerebrotendinous xanthomatosis. Scand J Clin Lab Invest. 1985;45:443–446. doi: 10.3109/00365518509155241. [DOI] [PubMed] [Google Scholar]
- 59.Samenuk P, Koffman BM. Chenodeoxycholic treatment of cerebrotendinous xanthomatosis. Neurology. 2001;56:695–696. doi: 10.1212/wnl.56.5.695-a. [DOI] [PubMed] [Google Scholar]
- 60.Berginer VM, et al. Long-term treatment of cerebrotendinous xanthomatosis with chenodeoxycholic acid. N Engl J Med. 1984;311:1649–1652. doi: 10.1056/NEJM198412273112601. [DOI] [PubMed] [Google Scholar]
- 61.Batta AK, et al. Hydrophilic 7 beta-hydroxy bile acids, lovastatin, and cholestyramine are ineffective in the treatment of cerebrotendinous xanthomatosis. Metabolism. 2004;53:556–562. doi: 10.1016/j.metabol.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 62.Koopman BJ, et al. Bile acid therapies applied to patients suffering from cerebrotendinous xanthomatosis. Clin Chim Acta. 1985;152:115–122. doi: 10.1016/0009-8981(85)90182-2. [DOI] [PubMed] [Google Scholar]
- 63.Verrips A, et al. Effect of simvastatin in addition to chenodeoxycholic acid in patients with cerebrotendinous xanthomatosis. Metabolism. 1999;48:233–238. doi: 10.1016/s0026-0495(99)90040-9. [DOI] [PubMed] [Google Scholar]
- 64.Burnett JR, et al. Clinical and biochemical features, molecular diagnosis and long-term management of a case of cerebrotendinous xanthomatosis. Clin Chim Acta. 2001;306:63–69. doi: 10.1016/s0009-8981(01)00391-6. [DOI] [PubMed] [Google Scholar]
- 65.Kuriyama M, et al. Treatment of cerebrotendinous xanthomatosis: effects of chenodeoxycholic acid, pravastatin, and combined use. J Neurol Sci. 1994;125:22–28. doi: 10.1016/0022-510x(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura T, et al. Combined treatment with chenodeoxycholic acid and pravastatin improves plasma cholestanol levels associated with marked regression of tendon xanthomas in cerebrotendinous xanthomatosis. Metabolism. 1991;40:741–746. doi: 10.1016/0026-0495(91)90094-d. [DOI] [PubMed] [Google Scholar]
- 67.Ferdinandusse S, et al. Reinvestigation of peroxisomal 3-ketoacyl-CoA thiolase deficiency: identification of the true defect at the level of d-bifunctional protein. Am J Hum Genet. 2002;70:1589–1593. doi: 10.1086/340970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cuebas DA, et al. The role of alpha-methylacyl-CoA racemase in bile acid synthesis. Biochem J. 2002;363:801–807. doi: 10.1042/0264-6021:3630801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferdinandusse S, et al. Subcellular localization and physiological role of alpha-methylacyl-CoA racemase. J Lipid Res. 2000;41:1890–1896. [PubMed] [Google Scholar]
- 70.Ferdinandusse S, et al. Plasma analysis of di- and trihydroxycholestanoic acid diastereoisomers in peroxisomal alpha-methylacyl-CoA racemase deficiency. J Lipid Res. 2001;42:137–141. [PubMed] [Google Scholar]
- 71.Ferdinandusse S, et al. Mutations in the gene encoding peroxisomal alpha-methylacyl-CoA racemase cause adult-onset sensory motor neuropathy. Nat Gen. 2000;24:188–891. doi: 10.1038/72861. [DOI] [PubMed] [Google Scholar]
- 72.Setchell KD, et al. Liver disease caused by failure to racemize trihydroxycholestanoic acid: gene mutation and effect of bile acid therapy. Gastroenterology. 2003;124:217–232. doi: 10.1053/gast.2003.50017. [DOI] [PubMed] [Google Scholar]
- 73.Van Veldhoven PP, et al. Fibroblast studies documenting a case of peroxisomal 2-methylacyl-CoA racemase deficiency: possible link between racemase deficiency and malabsorption and vitamin K deficiency. Eur J Clin Invest. 2001;31:714–722. doi: 10.1046/j.1365-2362.2001.00877.x. [DOI] [PubMed] [Google Scholar]
- 74.Wanders RJ, et al. Measurement of peroxisomal fatty acid beta-oxidation in cultured human skin fibroblasts. J Inherit Metab Dis. 1995;18(suppl 1):S113–S124. doi: 10.1007/BF00711434. [DOI] [PubMed] [Google Scholar]
- 75.Keane MH, et al. Bile acid treatment alters hepatic disease and bile acid transport in peroxisome-deficient PEX2 Zellweger mice. Hepatology. 2007;45:982–997. doi: 10.1002/hep.21532. [DOI] [PubMed] [Google Scholar]
- 76.Steinberg SJ, et al. Peroxisome biogenesis disorders. Biochim Biophys Acta. 2006;1763:1733–1748. doi: 10.1016/j.bbamcr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 77.Watkins PA, et al. Peroxisomal Diseases. In: Suchy FJ, et al., editors. Liver Disease in Children. New York: Cambridge University Press; 2007. pp. 840–857. [Google Scholar]
- 78.Steinberg SJ, et al. Peroxisomal disorders: clinical and biochemical studies in 15 children and prenatal diagnosis in 7 families. Am J Med Genet. 1999;85:502–510. [PubMed] [Google Scholar]
- 79.Theil AC, et al. Clinical recognition of patients affected by a peroxisomal disorder: a retrospective study in 40 patients. Eur J Pediatr. 1992;151:117–120. doi: 10.1007/BF01958955. [DOI] [PubMed] [Google Scholar]
- 80.Wilson GN, et al. Zellweger syndrome: diagnostic assays, syndrome delineation, and potential therapy. Am J Med Genet. 1986;24:69–82. doi: 10.1002/ajmg.1320240109. [DOI] [PubMed] [Google Scholar]
- 81.Monnens L, et al. Disturbances in bile acid metabolism of infants with the Zellweger (cerebrohepato-renal) syndrome. Eur J Pediatr. 1980;133:31–35. doi: 10.1007/BF00444751. [DOI] [PubMed] [Google Scholar]
- 82.Mooi WJ, et al. Ultrastructure of the liver in the cerebrohepatorenal syndrome of Zellweger. Ultrastruct Pathol. 1983;5:135–144. doi: 10.3109/01913128309141833. [DOI] [PubMed] [Google Scholar]
- 83.Martinez M. Restoring the DHA levels in the brains of Zellweger patients. J Mol Neurosci. 2001;16:309–316. doi: 10.1385/JMN:16:2-3:309. [DOI] [PubMed] [Google Scholar]
- 84.Martinez M, et al. Therapeutic effects of docosahexaenoic acid ethyl ester in patients with generalized peroxisomal disorders. Am J Clin Nutr. 2000;71(suppl 1):S376–S385. doi: 10.1093/ajcn/71.1.376s. [DOI] [PubMed] [Google Scholar]
- 85.Martinez M, Vazquez E. MRI evidence that docosahexaenoic acid ethyl ester improves myelination in generalized peroxisomal disorders. Neurology. 1998;51:26–32. doi: 10.1212/wnl.51.1.26. [DOI] [PubMed] [Google Scholar]
- 86.Setchell KD, et al. Oral bile acid treatment and the patient with Zellweger syndrome. Hepatology. 1992;15:198–207. doi: 10.1002/hep.1840150206. [DOI] [PubMed] [Google Scholar]
- 87.Maeda K, et al. Oral bile acid treatment in two Japanese patients with Zellweger syndrome. J Pediatr Gastroenterol Nutr. 2002;35:227–230. doi: 10.1097/00005176-200208000-00025. [DOI] [PubMed] [Google Scholar]
- 88.van Grunsven EG, et al. Peroxisomal D-hydroxyacyl-CoA dehydrogenase deficiency: resolution of the enzyme defect and its molecular basis in bifunctional protein deficiency. Proc Natl Acad Sci USA. 1998;95:2128–2133. doi: 10.1073/pnas.95.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wanders RJA, et al. Single peroxisomal enzyme deficiencies. In: Scriver CR, et al., editors. The Molecular And Metabolic Bases Of Inherited Disease. New York: McGraw-Hill; 2001. pp. 3219–3256. [Google Scholar]
- 90.Suzuki Y, et al. D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxyacyl-CoA dehydrogenase bifunctional protein deficiency: a newly identified peroxisomal disorder. Am J Hum Genet. 1997;61:1153–1162. doi: 10.1086/301599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Watkins PA, et al. Peroxisomal bifunctional enzyme deficiency. J Clin Invest. 1989;83:771–777. doi: 10.1172/JCI113956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ferdinandusse S, et al. Clinical and biochemical spectrum of D-bifunctional protein deficiency. Ann Neurol. 2006;59:92–104. doi: 10.1002/ana.20702. [DOI] [PubMed] [Google Scholar]
- 93.Suzuki Y, et al. Novel subtype of peroxisomal acyl-CoA oxidase deficiency and bifunctional enzyme deficiency with detectable enzyme protein: identification by means of complementation analysis. Am J Hum Genet. 1994;54:36–43. [PMC free article] [PubMed] [Google Scholar]
- 94.Johnson MR, et al. Purification and characterization of bile acid-CoA: amino acid N-acyltransferase from human liver. J Biol Chem. 1991;266:10227–10233. [PubMed] [Google Scholar]
- 95.Wheeler JB, et al. Purification and characterization of a rat liver bile acid coenzyme A ligase from rat liver microsomes. Arch Biochem Biophys. 1997;348:15–24. doi: 10.1006/abbi.1997.0391. [DOI] [PubMed] [Google Scholar]
- 96.Carlton VE, et al. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Gen. 2003;34:91–96. doi: 10.1038/ng1147. [DOI] [PubMed] [Google Scholar]
- 97.Falany CN, et al. Glycine and taurine conjugation of bile acids by a single enzyme: molecular cloning and expression of human liver bile acid CoA:amino acid N-acyltransferase. J Biol Chem. 1994;269:19375–19379. [PubMed] [Google Scholar]
- 98.Falany CN, et al. Molecular cloning and expression of rat liver bile acid CoA ligase. J Lipid Res. 2002;43:2062–2071. doi: 10.1194/jlr.m200260-jlr200. [DOI] [PubMed] [Google Scholar]
- 99.Ueki I, et al. Neonatal cholestatic liver disease in an Asian patient with a homozygous mutation in the oxysterol 7α-hydroxylase gene. J Pediatr Gastroenterol Nutr. 2008;46:465–469. doi: 10.1097/MPG.0b013e31815a9911. [DOI] [PubMed] [Google Scholar]