Abstract
Here, we have translated from the rat to the non-human primate a unilateral lumbosacral injury as a model for cauda equina injury. In this morphological study, we have investigated retrograde effects of a unilateral L6-S2 ventral root avulsion (VRA) injury as well as the long-term effects of Wallerian degeneration on avulsed ventral roots at 6–10 months post-operatively in four adult male rhesus monkeys.
Immunohistochemistry for choline acetyl transferase and glial fibrillary acidic protein demonstrated a significant loss of the majority of the axotomized motoneurons in the affected L6-S2 segments and signs of an associated astrocytic glial response within the ventral horn of the L6 and S1 spinal cord segments. Quantitative analysis of the avulsed ventral roots showed that they exhibited normal size and were populated by a normal number of myelinated axons. However, the myelinated axons in the avulsed ventral roots were markedly smaller in caliber compared to the fibers of the intact contralateral ventral roots, which served as controls. Ultrastructural studies confirmed the presence of small myelinated axons and a population of unmyelinated axons within the avulsed roots. In addition, collagen fibers were readily identified within the endoneurium of the avulsed roots.
In summary, a lumbosacral VRA injury resulted in retrograde motoneuron loss and astrocytic glial activation in the ventral horn. Surprisingly, the Wallerian degeneration of motor axons in the avulsed ventral roots was followed by a repopulation of the avulsed roots by small myelinated and unmyelinated fibers. We speculate that the small axons may represent sprouting or axonal regeneration by primary afferents or autonomic fibers.
Keywords: Rhesus macaque, spinal cord, Wallerian degeneration, electron microscopy
INTRODUCTION
Conus medullaris and cauda equina injuries commonly result in a complex syndrome of motor, sensory, and autonomic impairments (Harrop et al., 2004; Havton and Carlstedt, 2009). Cauda equina injuries following trauma to the thoracolumbar spine may present with lesions also to the sacral spinal cord and result in a conus medullaris syndrome (Maynard et al., 1997). A cauda equina injury or an anatomically complete conus medullaris syndrome results in a lower motor neuron syndrome with denervation of peripheral targets, including skeletal muscle and autonomic ganglia.
Both the ventral and dorsal lumbosacral roots of the cauda equina may be affected by trauma to the lower spine. Transection, laceration, crush, or avulsion injuries to the ventral roots result in denervation of peripheral targets and lead to characteristic peripheral denervation and flaccid weakness of the affected end organ (Pavlakis et al., 1983; Hoang and Havton, 2006; Mauffrey et al., 2008). In addition, following a motor nerve or ventral root injury, motoneuron somata undergo a retrograde reaction and the distal segment of the motor axon undergoes Wallerian degeneration (Koliatsos and Price, 1996; Griffin, 2007).
Earlier studies in rats have demonstrated that a proximal ventral root avulsion (VRA) injury mimics many of the deficits encountered in a clinical conus medullaris syndrome and results in progressive and severe loss of motoneurons in the spinal cord (Koliatsos et al., 1994; Hoang et al., 2003, 2006). However, information on the response to a proximal ventral root injury in large mammalian models is sparse. Retrograde effects to injury may show differences between species, as e.g. immune and inflammatory reactions to nerve and spinal cord injury differ between different strains of rats and between species (Popovich et al., 1997; Sroga et al., 2003; Kigerl et al., 2006). Here, we investigated the long-term effects of a unilateral lumbosacral VRA injury in rhesus macaques. Our principal aim was to determine the response of axotomized motor neurons to a proximal ventral root lesion and late effects of Wallerian degeneration of avulsed ventral roots in a clinically relevant cauda equina injury model. An additional goal was to provide anatomical information about the potential suitability of avulsed ventral roots for delayed replantation procedures in the non-human primate.
We demonstrate that a lumbosacral VRA injury in the non-human primate resulted in a marked loss of axotomized motoneurons at 6 to 10 months post-operatively. An intramedullary astrocytic glial response was also detected in the ventral horn. The distal denervated segment of the ventral roots had undergone Wallerian degeneration. However, combined light and electron microscopic studies showed that the avulsed ventral root segments have been repopulated by numerous small myelinated and unmyelinated axons. It is possible that the small caliber fibers in the avulsed ventral roots may be of primary afferent or autonomic origin.
METHODS
All animal procedures were carried out at the California National Primate Research center at UC Davis. The facility is AAALAC-accredited, and all animals were maintained in accordance with provisions of the Animal Welfare Act (as Amended 2007.7 USC S̶2131- 2159) and the Guide for Care and Use of Laboratory Animals (National Institutes of Health Publications No. 86-23, revised 1985). All procedures were approved by the Institutional Animal Care and Use Committee at UC Davis. A total of four male rhesus monkeys (Macaca mulatta) were included in the study. The subjects were adults and weighed 8.8–11.7 kg at the time of surgery at the onset of the study.
Surgical procedures
All surgical procedures were performed under general anesthesia and sterile surgical conditions. All subjects were fasted overnight prior to surgery. Initial sedation and immobilization was obtained by administration of ketamine (10–15 mg/kg im) followed by 1–2% isoflurane (Abbott, North Illinois, IL) in 100% O2 via an inserted endotracheal tube for surgical anesthesia.
A longitudinal mid-line cutaneous incision was made over the L1–L5 spinous processes. The lumbar fascia was identified and cut slightly to the left of the midline. Using electrocautery as well as sharp and blunt subperiosteal dissection, the paraspinous muscles on the left side were gently separated to expose the dorsolateral surface of the laminae, facet joints, and pedicles of the spinal vertebrae. A left-sided hemi-laminectomy was performed using rongeurs and a high-speed diamond-bit drill under continuous saline irrigation. The hemi-laminectomy spared the spinous processes and extended from the caudal aspect of the L1 vertebra to the rostral aspect of the L3 vertebra.
A longitudinal incision was made to open the dura using a 15-blade dural scalpel and microscissors. Guided by vertebral landmarks and normal variations in ventral root caliber, the left L6-S2 ventral roots were identified by gentle micro-dissection using blunt instruments and a surgical microscope. The S2, S1, L7, and L6 ventral roots were individually avulsed from the surface of the spinal cord by applying traction along the normal course of the roots with a pair of fine forceps. The proximal stump of the avulsed roots were trimmed and left within the subdural space. The dura was closed using continuous Ethilon® 6-0 (Ethicon, New Brunswick, NJ) sutures. The muscle layer was closed over the laminectomy site. The skin was closed using intradermal resorbable Vicryl® 4-0 (Ethicon) sutures. All animals recovered well and received oxymorphone (0.15 mg/kg IM TID) for 3 days post-operatively.
At 6–10 months after the unilateral L6-S2 VRA injury, the subjects were placed under deep surgical anesthesia and transcardially perfused with saline followed by a 4% paraformaldehyde solution. After the perfusion procedure, the spinal cord and associated nerve roots were removed for morphological studies.
Immunohistochemical studies of spinal cord sections
The removed lumbosacral spinal cord segment was examined and the segmental levels for the L6-S2 VRA injuries were confirmed in all subjects. The L6, L7, S1, and S2 spinal cord segments were blocked and frozen individually. Serial 14 µm cryostat sections were cut and mounted on glass slides (Menzel-Gläzer, Braunschweig, Germany), air-dried and stored at −20 C°. For immunohistochemistry, commercially available primary antibodies were used for the detection of choline acetyltransferase (ChAT) and glial fibrillary acidic protein (GFAP) (Table 1).
Table 1.
Antibodies used in the present study
| Name | Immunogen | Manufacturer | Species and type | Dilution |
|---|---|---|---|---|
| ChAT | Choline Acetyltransferase | Millipore #AB144P | Goat polyclonal | 1:100 |
| GFAP | Glial Fibrillary Acidic Protein | Millipore #AB5804 | Rabbit polyclonal | 1:2000 |
References:
ChAT: JCN Antibody Database: Enjin et al, JCN 518: 2284, 2010; Puller et al JCN 519:759, 2011 http://antibodyregistry.org/AB_2079751
GFAP: JCN Antibody Database: Talos et al, JCN 497:42, 2006; Kim et al, JCN 511:581, 2008; Chung et al, JCN, 511:421, 2008. http://antibodyregistry.org/AB_92037
A goat polyclonal primary antibody against ChAT (AB144P; Millipore, Bedford, MA) was used to detect cholinergic neurons in the primate spinal cord. According to the manufacturer’s information, Western blot analysis using a 1:1000 dilution of this lot detected ChAT on 10 µg of mouse brain lysates. Immunohistochemical studies of the primate spinal cord tissues showed labeling of cholinergic neurons in a pattern similar to the established localization of various subsets of motor neurons in the murine spinal cord (Enjin et al., 2010).
A rabbit polyclonal antibody against GFAP (AB5804; Millipore) was used to detect glial cells in the primate spinal cord. According to the manufacturer’s information, purified bovine GFAP served as immunogen for this class-III intermediate filament, which is the main constituent of intermediate filaments in astrocytes. Immunohistochemical studies of the primate spinal cord shows labeling for astroglial cells and processes similar to astroglial labeling using this GFAP antibody in previous rodent studies on brain tissues (Talos et al., 2006; Kim et al., 2008).
For immunohistochemistry, the sections were briefly rinsed with PBS and incubated overnight with anti-ChAT (goat, 1:100 dilution, Millipore, Bedford, MA) or anti-GFAP (rabbit, 1:2,000 dilution, Millipore). Following a brief rinse in PBS, the sections were incubated at room temperature for 1 hour with biotinylated secondary antibodies of appropriate species. A light stable reaction product was obtained using an ABC detection kit (Vector Laboratories, Burlingame, CA). The slides were air dried and coverslipped with DPX Mountant (Sigma-Aldrich, St Louis, MO).
Established protocols were used for quantification of immunoreactivity in spinal cord sections (Bellander et al., 2001; Ohlsson and Havton, 2006; Ohlsson et al., 2006). Briefly, digital microphotographs were obtained of the bilateral ventral and dorsal horns of immuno-stained sections. Images of the contralateral side were used for control purposes. The photographs were analyzed using C-Imaging software (Compix, Cranberry Township, PA) by an observer blinded to section identity. The intensity of detectable staining was set at an equal level for both sides of a section and reflected the degree of immuno-reactivity. The measured area was calculated in pixels for both the ipsi- and contralateral sides of each section.
Plastic embedding and light microscopic analysis of ventral roots
Segments of the left-sided avulsed and right-sided intact L6-S2 ventral roots were collected for morphological studies. The root segments were immersed in 2% paraformaldehyde and 1% glutaraldehyde in PBS for 24–72 hours, rinsed in PBS, immersed in 1% osmium tetroxide in PBS (Ted Pella, Redding, CA) for 1.5 hours, dehydrated in a graded series of ethanol, placed in Propylene oxide (Ted Pella), and embedding in Epon® plastic resin (Ted Pella). Transverse semi-thin sectioning of the embedded ventral roots was performed using an RMC Products PowerTome ultramicrotome (Boeckeler Instruments, Tucson, AZ). The approximately 1 µm thick sections were stained with toluidine blue and cover-slipped on glass slides for light microscopy.
Transverse sections of the ventral roots were examined in a Nikon E600 light microscope at high magnification using a 100× lens. The microscope was also equipped with a motorized LEP 99S000 stage (Ludl Electronic Products, Hawthorne, NY) and a computerized stereological system (Stereo Investigator® 8, MBF Bioscience, Williston, VT). The Fractionator and Nucleator (4-ray) stereological probes were used to determine the 2D root surface area, fiber counts and cross-sectional areas of myelinated axons.
Electron microscopy of ventral roots
Ultrathin sections of 60–70 nm thickness were cut using an RMC Products PowerTome ultramicrotome (Boeckeler Instruments). The sections were collected on formvar-coated copper one-hole grids and counterstained with uranyl acetate and lead citrate. The ultrathin sections were examined using a JEOL 100 CX transmission electron microscope at 7,200 × primary magnification. Images were captured on photographic film and the negatives scanned into digital files using Adobe Photoshop® CS5 software (Adobe Systems, San Jose, CA).
Statistics
Quantitative data were expressed as the mean ± standard deviation (S.D.). Samples were compared using the Student’s t-test. A value of p<0.05 (two-tailed) was regarded as reflecting a statistically significant difference between groups.
RESULTS
We performed a quantitative examination of the long-term effects of a lumbosacral VRA injury on motoneuron counts, intramedullary glial response and Wallerian degeneration within the avulsed ventral roots in four rhesus monkeys at 6–10 months post-operatively. For this purpose, a unilateral L1–L2 laminectomy was initially performed to visualize the lumbosacral portion of the spinal cord and associated nerve roots. The ipsilateral L6, L7, S1, and S2 ventral roots were identified anatomically based on vertebral landmarks and normal differences in ventral root size between segments (Figure 1). At the termination of each experiment, the lumbosacral spinal cord as well as the ipsilateral and contralateral L6-S2 ventral roots were harvested and prepared for morphological studies.
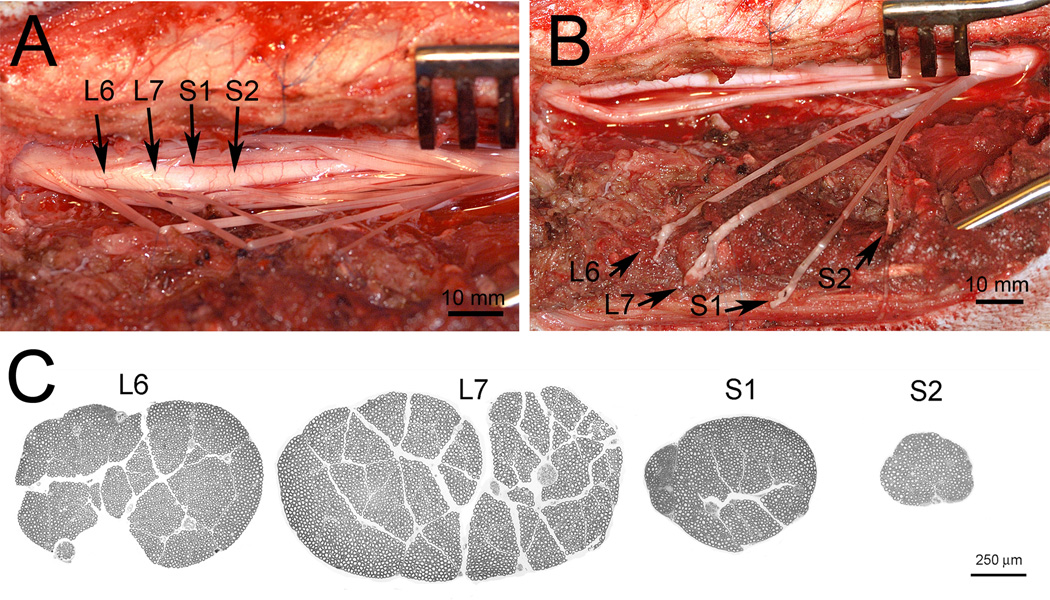
Figure 1.
Surgical display of left sided L6, L7, S1, and S2 ventral roots after a hemi-laminectomy and dural opening (A). The identification of the ventral roots were based on vertebral landmarks and characteristic differences in caliber for individual lumbosacral ventral roots. Sutures have been attached to each ventral root to assist with the identification and presentation. The L6-S2 ventral roots have been avulsed from the surface of the spinal cord (B). Note fine rootlets at the tip of the avulsed ventral root. Transverse sections of the L6, L7, S1, and S2 ventral roots harvested from the intact contralateral side at the end of experiment (C). Note the characteristic large size of the L6 and L7 ventral roots compared to the much smaller S1 and S2 ventral roots. Also note that ventral roots exhibit a very thin epineurium and that regions of densely packed myelinated fibers are separated by streaks of connective tissues.
Effects of VRA injury on motoneuron counts
Immunohistochemical studies of the L6-S2 spinal cord segments readily showed ChAT-IR expressed in medium and large sized neurons within the ventral motor nucleus bilaterally (Figure 2). Morphological studies of the L6, L7, S1, and S2 segments showed significant loss of ChAT-IR neurons in the ventral horn on the ipsilateral ventral horn for all affected segments after an L6-S2 VRA injury (n = 4 subjects).
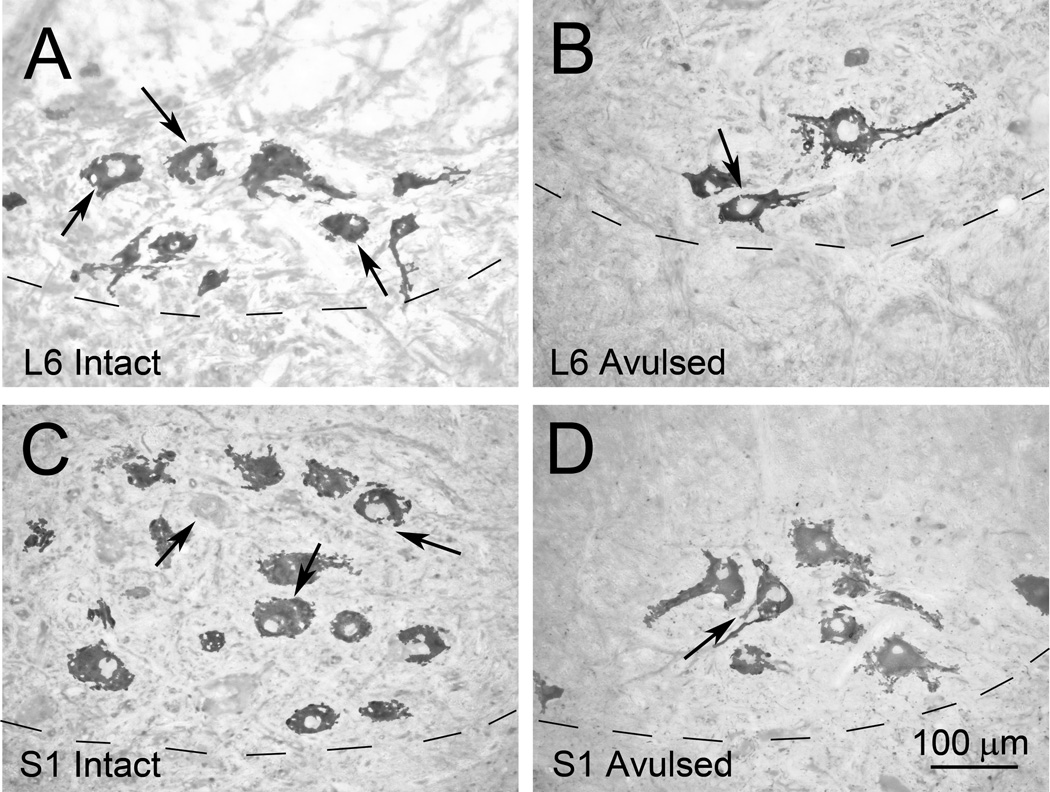
Figure 2.
Representative photomicrographs of the ventral horn of the L6 Intact (A) and L6 Avulsed (B) sides as well as the S1 Intact (C) and S1 Avulsed (D) sides in a Rhesus monkey at 6 months after a unilateral L6-S2 VRA injury. The sections have been processed for ChAT immunoreactivity. Note that the avulsed side appears more sparsely populated by cholinergic motor neurons.
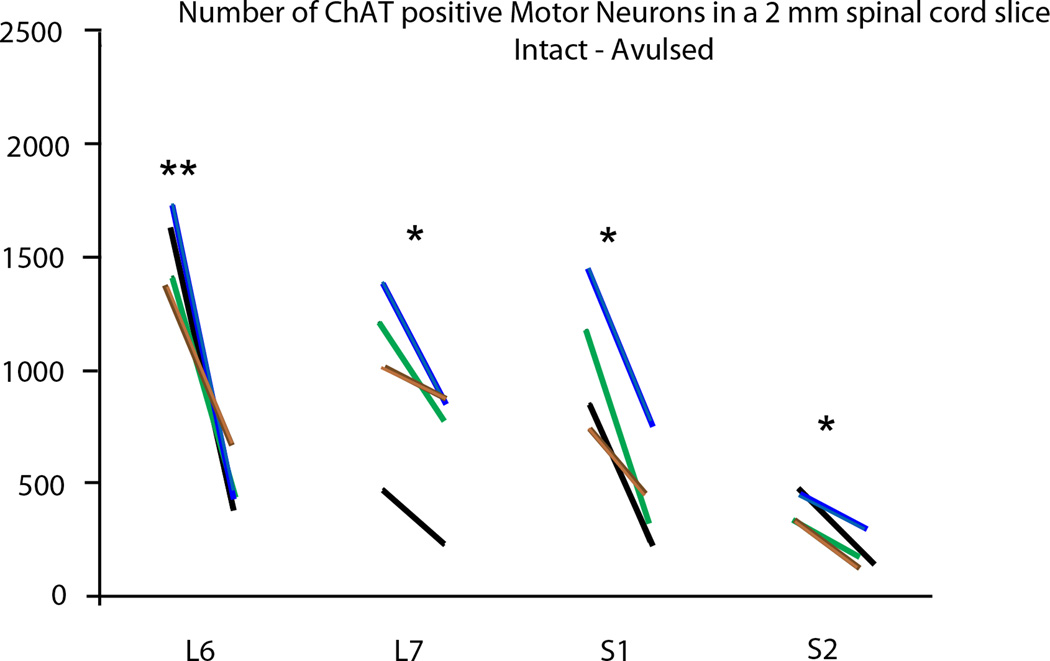
Counts of ChAT-IR motoneurons within a 2 mm longitudinal column within each segment demonstrated a significantly decreased number of labeled cells in the ipsilateral side of the L6 segment (526 ± 123; p<0.01), L7 segment (705 ± 313; p<0.05), S1 (464 ± 243; p<0.05), and the S2 segment (202 ± 80; p<0.05) compared to the contralateral side of the corresponding segments (1,542 ±181 for L6; 1031 ± 391 for L7, 1061 ± 326 for S1, and 400 ± 72 for S2) (Figure 3).
Figure 3.
Graph demonstrating the number of motor neurons in a 2 mm spinal cord slice for the L6-S2 segments. The left and right ends of each line represent the motor neuron count for the intact and avulsed sides, respectively. The black, blue, brown, and green color codes for the lines each represent a different subject. Note a significantly decreased number of motor neurons in all segments with * indicating p < 0.05 and ** indicating p < 0.01.
Astrocytic response to VRA injury
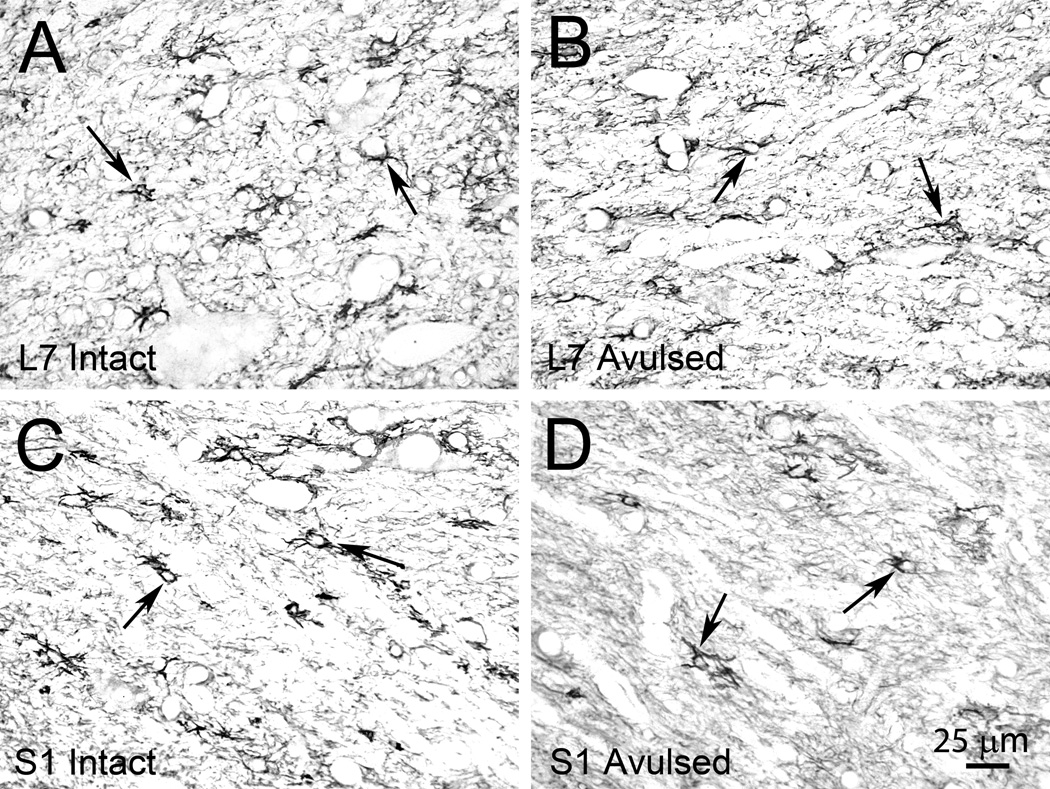
Immunohistochemical studies for GFAP readily detected GFAP-IR within the ventral horn of the L6-S2 segments bilaterally (n = 4 subjects) (Figure 4). Morphological studies demonstrated increased GFAP-IR within the ipsilateral L6 segment (98,976 ± 29,136 pixels; p<0.01) and S1 segment (83,078 ± 23,252 pixels; p<0.05) compared to the contralateral side of the corresponding segments (77,801 ± 25536 pixels for L6 and 68,805 ± 26,178 pixels for S1), whereas no side difference was detected for the L7 and S2 segments (Figure 5A). Additional analyses were performed to quantify the expression of GFAP in the dorsal horn, and no side difference was detected for the L6, L7, S1, and S2 segments (Figure 5B).
Figure 4.
Representative photomicrographs of the ventral horn of the L7 Intact (A) and L7 Avulsed (B) as well as the S1 Intact (C) and S1 Avulsed (D) sides in a Rhesus monkey at 6 months after a unilateral L6-S2 VRA injury. The sections have been processed for GFAP immunoreactivity, which is readily identified bilaterally.
Figure 5.
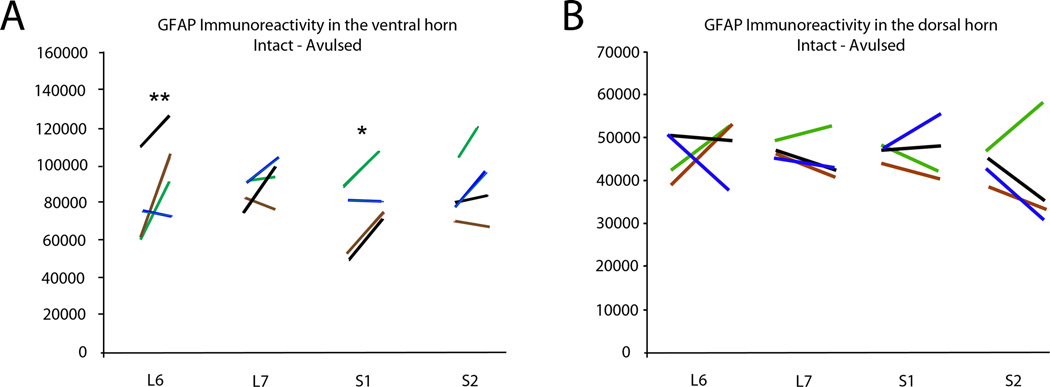
Graph demonstrating immunoreactivity for GFAP in the ventral horn (A) and dorsal horn (B) of the L6-S2 segments. The left and right ends of each line represent the number of pixels corresponding to GFAP labeling for the intact and avulsed sides, respectively. The black, blue, brown, and green color codes for the lines each represent a different subject. Note a significantly increased GFAP immunoreactivity in the ventral horn of the L6 and S1 segments with * indicating p < 0.05 and ** indicating p < 0.01.
Axonal innervation of avulsed ventral roots
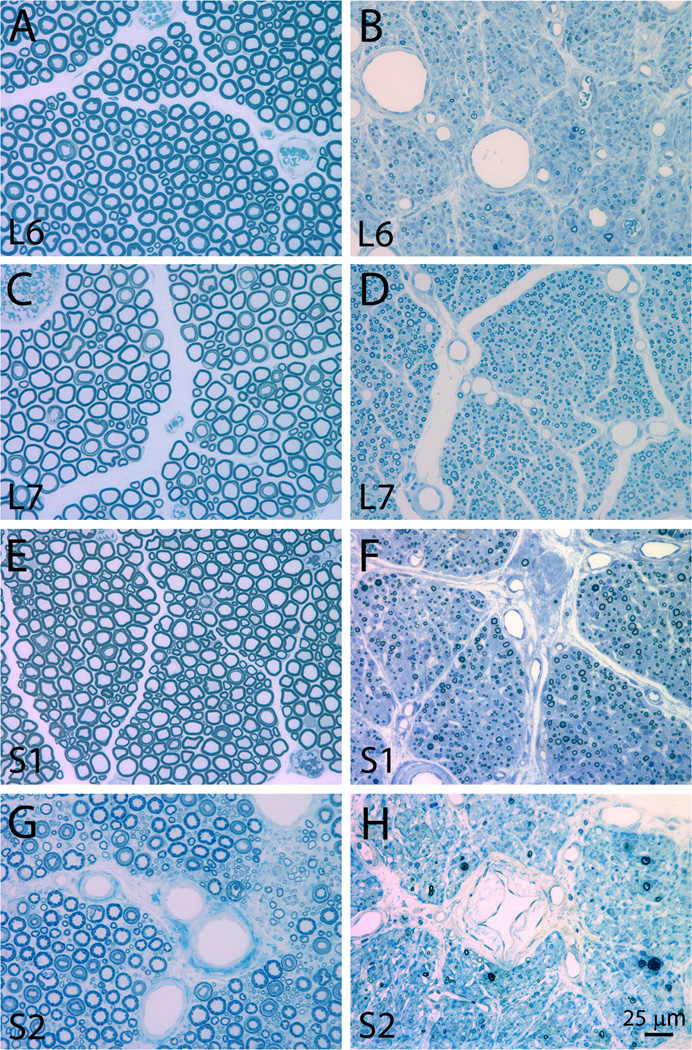
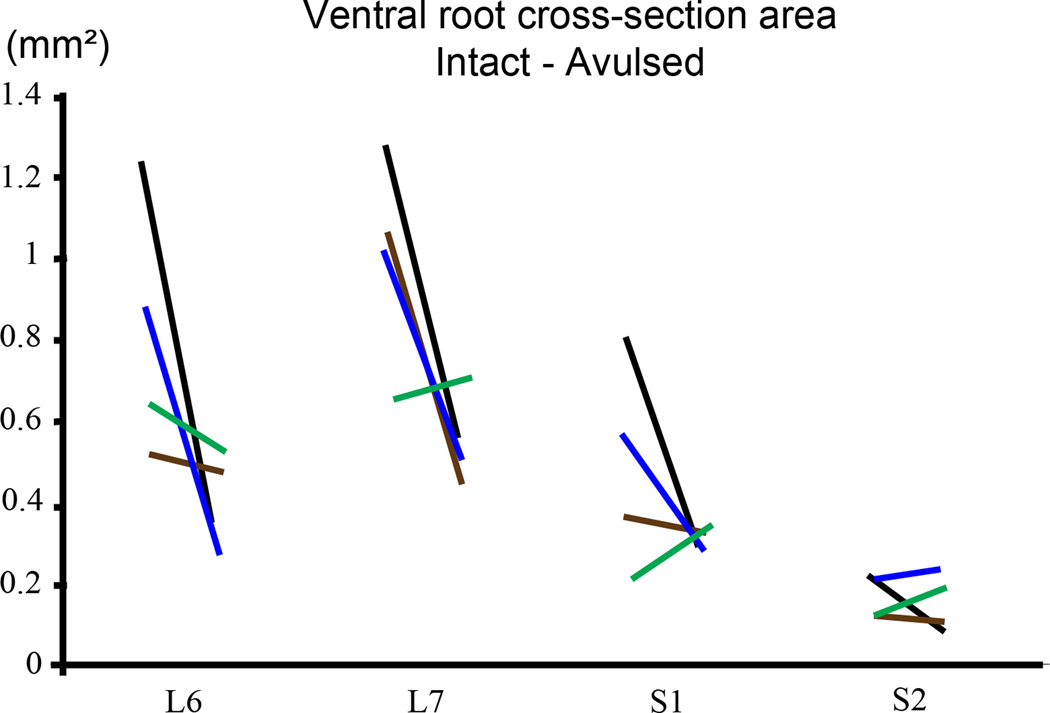
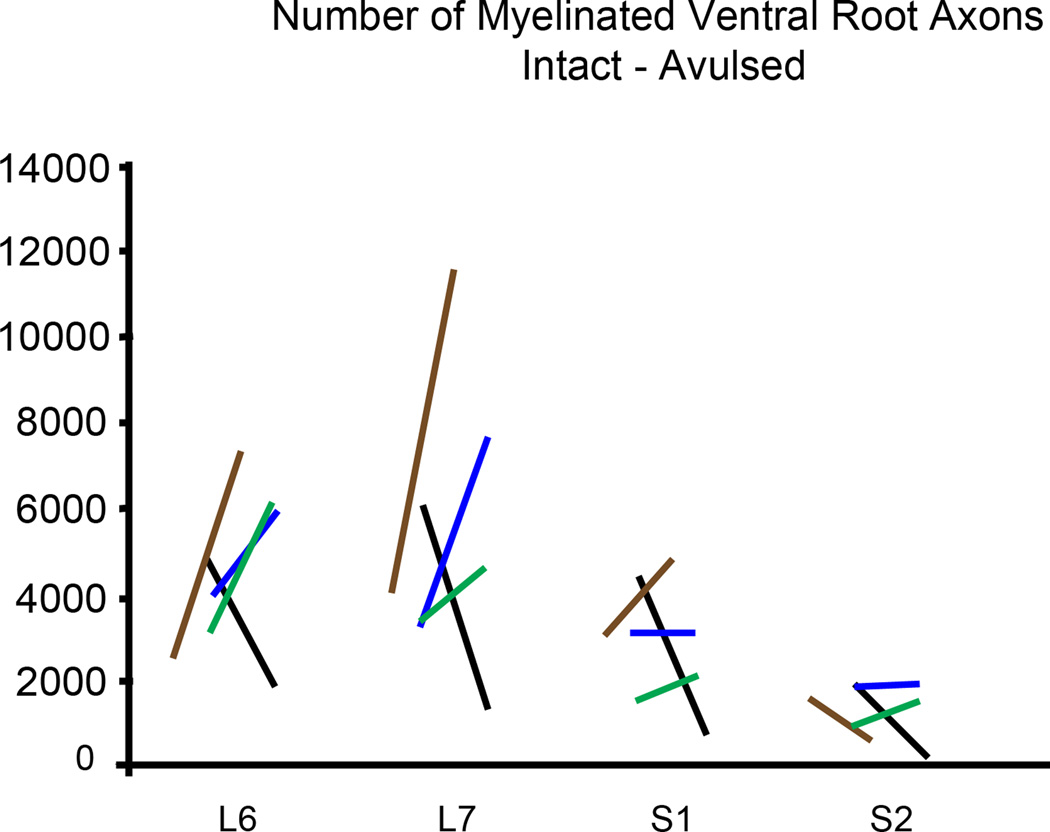
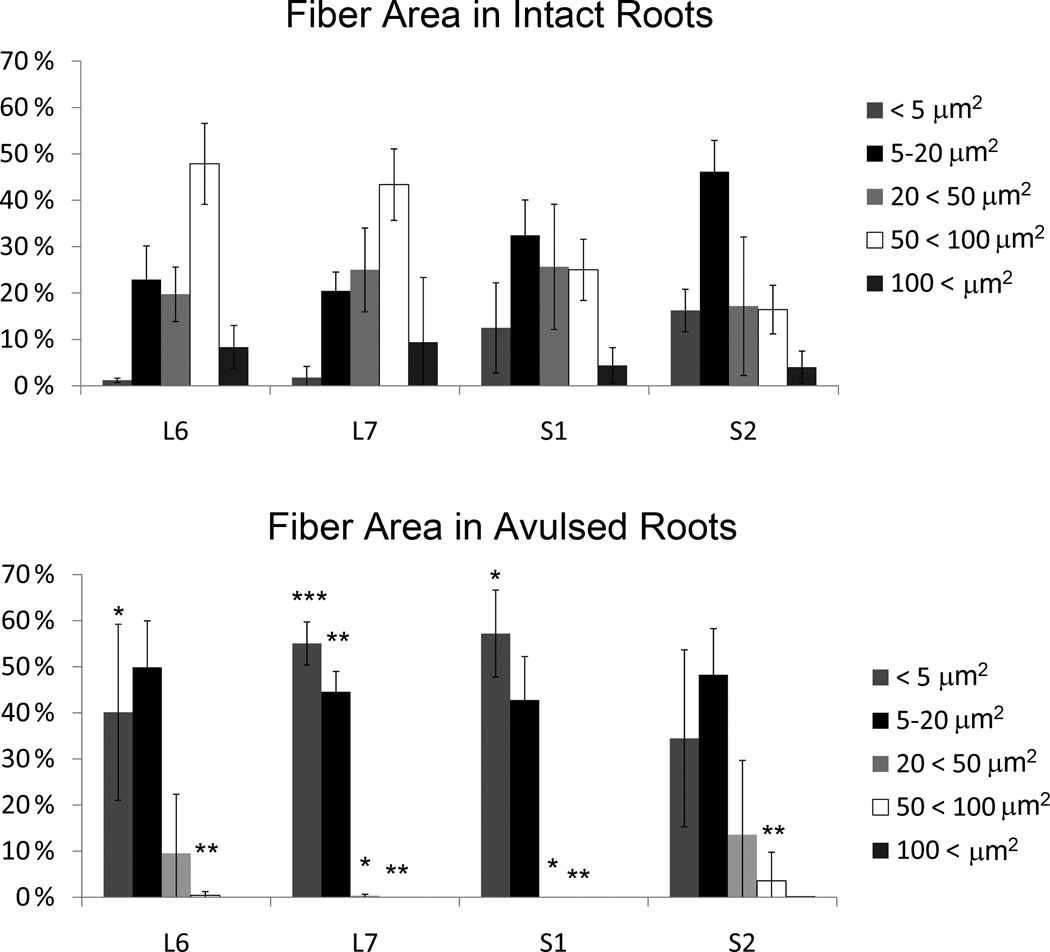
Plastic embedded ipsilateral avulsed and contralateral intact L6-S2 ventral roots were examined in the light microscope as toluidine blue stained semithin sections of about 1 µm thickness (n = 4 subjects) (Figure 6). Extensive variability existed for ventral root cross-section area between subjects, and quantitative analysis of the cross-sectional area of ventral roots showed absence of any demonstrable size difference between the two sides (Figure 7). Morphological studies on the number of myelinated axons in the L6-S2 ventral roots also showed an absence of any difference between the avulsed and intact ventral roots (Figure 7). However, there was a marked difference in the size of myelinated axons between the avulsed and intact L6-S2 ventral roots. Specifically, the fiber caliber was left-shifted for the avulsed roots with predominantly small myelinated axons occupying the avulsed ventral roots (Figure 8).
Figure 6.
Photomicrographs of representative plastic embedded and toluidine blue stained L6-S2 ventral roots at 6 months after a unilateral L6-S2 VRA injury. The intact L6, L7, S1, and S2 ventral roots are presented in A, C, E, and G, respectively. The avulsed L6, L7, S1, and S2 are presented in B, B, F, and H, respectively. Note the presence of a large number of small myelinated axons in the avulsed ventral roots.
Figure 7.
Graphs demonstrating ventral root cross-section area (upper graph) and number of ventral root axons (lower graph) in intact and avulsed L6-S2 ventral roots at 6–8.5 months after a unilateral L6-S2 VRA injury. The left and right ends of each line represent the values for the intact and avulsed roots, respectively. The black, blue, brown, and green color codes for the lines each represent a different subject.
Figure 8.
Graph demonstrating the size distribution for the cross sectional fiber area in intact (upper graph) and avulsed (lower graph) L6-S2 ventral roots at 6–8.5 months after a unilateral VRA injury. Note the predominance of small myelinated fibers in the avulsed ventral roots with * indicating p < 0.05, ** indicating p < 0.01, and *** indicating p < 0.001.
The avulsed L6 ventral roots showed a markedly increased proportion of small myelinated axons with a cross-sectional fiber area of < 5 µm2 (p < 0.05) and a decreased proportion of large myelinated axons with a fiber area of 50 < 100 µm2 (p < 0.01) compared to the corresponding contralateral intact L6 ventral roots (n=4 subjects).
The avulsed L7 ventral roots showed a significantly increased proportion of small myelinated axons with a cross-sectional fiber area of < 5 µm2 (p < 0.001) and 5–20 µm2 (p < 0.01) and a decreased proportion of large myelinated axons with a fiber area of 20 < 50 µm2 (p < 0.05), 50 < 100 µm2 (p < 0.01) compared to the corresponding contralateral intact L7 ventral roots (n=4 subjects).
The avulsed S1 ventral roots showed a significantly increased proportion of small myelinated axons with a fiber cross-sectional fiber area of < 5 um2 (p < 0.0.05) and a decreased proportion of large myelinated axons with a fiber area of 20 < 50 µm2 (p < 0.05), 50 < 100 µm2 (p < 0.01) compared to the corresponding contralateral intact S1 ventral roots (n=4 subjects).
The avulsed S2 ventral roots showed a significantly decreased proportion of large myelinated axons with a cross-sectional fiber area of 50 < 100 um2 (p < 0.01) compared to the corresponding contralateral intact S2 ventral roots (n=4 subjects).
Ultrastructural observations
Electron microscopic studies confirmed the presence of predominantly small and medium sized myelinated fibers as well as Schwann cell nuclei in the avulsed ventral roots (Figure 9A). In addition, small unmyelinated axons were encountered ultrastructurally and interspersed between the Schwann cells and myelinated axons. The distribution of unmyelinated fibers was patchy within the avulsed ventral roots and in the vicinity of small myelinated fibers. The avulsed roots also exhibited extensive areas of the endoneurium with deposits of collagen fibrils (Figure 9B).
Figure 9.
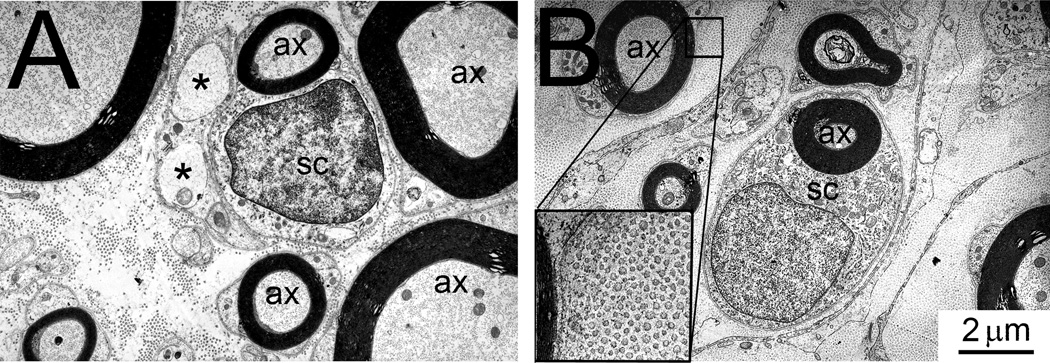
A: Electron micrograph of avulsed ventral root demonstrating several small and medium caliber myelinated axons (ax), a Schwann cell (SC), and unmyelinated axons, each indicated by an asterisk. B: Electron micrograph demonstrating a Schwann cell (SC) in close association with a small myelinated axon (ax). The endoneurium exhibits numerous collagen fibers (see insert).
DISCUSSION
The studies were performed to evaluate long-term effects of a unilateral VRA injury on the survival of motor neurons and morphology of ventral roots undergoing Wallerian degeneration in the non-human primate. The studies of the ventral roots were also completed to assess the potential suitability of avulsed ventral roots for delayed root replantation procedures. A lumbosacral VRA injury was performed to disconnect motor axons from their parent cell bodies in adult rhesus macaques. The motor axons were separated from the spinal cord surface, and the lesion took place at the interface between the central and peripheral nervous system. We demonstrated that axotomized motoneurons undergo retrograde degeneration with a significant long-term loss after an L6-S2 VRA injury in the non-human primate. The motoneuron loss was accompanied by glial activation in the ventral horn of the spinal cord. The severed ventral roots also demonstrated significant long-term post-injury changes. Surprisingly, the denervated ventral roots showed a large number of small myelinated and non-myelinated axons.
Loss of axotomized motoneurons
Injuries to motor axons from nerve transection or limb amputation in adult mammals have resulted in modest or no detectable signs of motoneuron degeneration and death (Carlson et al., 1979; Vanden Noven et al., 1993; Wu and Kaas, 2000; Ma et al., 2001). In contrast, a VRA injury with the separation of motor axons from the surface of the spinal cord is followed by a progressive and marked loss of the vast majority of axotomized motoneurons over several weeks after the lesion in rat models (Koliatsos et al., 1994; Novikov et al., 1995, 1997; Hoang et al., 2003, 2006). Specifically, a significant loss of axotomized motoneurons is detectable already within the first week after a VRA injury in rats, and the majority of loss takes place during the second and third week post-operatively (Koliatsos et al., 1994; Hoang et al., 2003). These prior studies suggest that a VRA-induced motoneuron loss is complete and reached a plateau by 4–6 weeks after the injury. In the present study, the four subjects were studied at 6–10 months after the VRA injury and represent animals with chronic intramedullary changes. The four animals were therefore pooled into one study group to study the long-term effects of VRA injury on motoneuron loss and glial activation.
A VRA injury may also induce retrograde loss of motoneurons in larger mammals. For instance, a marked loss of axotomized cervical motoneurons was seen after a C7 ventral root avulsion injury in rabbits (Lang et al., 2005). An intramedullary transection of motor axons within the ventral funiculus of the cat lumbar spinal cord also resulted in a significant and pronounced loss of motoneurons (Lindå et al., 2000). In the present study, the long-term loss of axotomized spinal motoneurons after a lumbosacral VRA injury in the non-human primate is therefore consistent with findings of previous studies on motoneuron responses after proximal motor axonal injuries in other mammalian species.
A potential technical limitation for the quantitative studies on cell survival is the use of ChAT-IR for motor neuron detection. An acute decrease in ChAT expression may take place in motor neurons after injury to their peripheral axons (Lams et al., 1988; Kou et al., 1995; Bussman and Sofroniew, 1999). However, this is a transient decrease in ChAT expression, and ChAT expression is again at detectable levels by 45–90 days after a peripheral nerve injury in rats (Armstrong et al., 1991; Rende et al., 1995). We have previously demonstrated in our rat model for VRA injury, using a combination of retrograde tracing and ChAT-IR, that axotomized motorneurons and preganglionic parasympathetic neurons show a significant decrease in ChAT expression at 1 week after a VRA injury, whereas the expression of ChAT is again detectable at control levels in the surviving motor neurons at 4 weeks postoperatively (Hoang et al., 2003). In the present study, quantitative studies on ChAT-expressing motor neurons were performed at 6–10 months after the VRA injury. Therefore, it seems reasonable that motor neurons would express a detectable cholinergic phenotype in this long-term injury model.
Glial responses
A glial scar formation commonly accompanies a traumatic injury to the central nervous system. Following a ventral root avulsion injury in rats, a retrograde loss of motoneurons is accompanied by a glial response within the spinal cord as demonstrated by microglial and macrophages (Koliatsos et al., 1994) and GFAP-immunoreactivity suggestive of astrocytic activation in the ventral horn (Ohlsson et al., 2006). The cellular inflammatory response patterns also differ at different time after injury. For instance, the microglial responses tend to peak within days whereas astrocytic reactions show the strongest reactivity at later stages after a traumatic spinal cord injury in rats (Popovich et al., 1997). Recent studies have demonstrated that the cellular immune response in the rat spinal cord after injury is characterized by a multiphasic patterns with both early and late peaks as well as persistence of increased microglia/macrophage, neutrophil, and T cell detection in the spinal cord up to at least 180 days after injury (Beck et al., 2010).
In the present study, we detected increased immunoreactivity for GFAP within the ventral horn of the lumbosacral spinal cord after a VRA injury. The detection of this glial response at the relatively late time point after the injury may reflect a direct response to motoneuron death. However, activated astrocytes have also been suggested to contribute to synapse displacement and collateral reinnervation after injury to the central nervous system (Aldskogius et al., 1999). Therefore, it remains uncertain to what degree the detected glial activation in the primate spinal cord after VRA injury may reflect part of a scar-forming lesion response or reflect a restorative process.
Wallerian degeneration and innervation of ventral roots
Our morphological studies included light and electron microscopic investigations of individual ventral roots of the L6-S2 segments after VRA injury with the contralateral intact ventral roots serving as an internal control. We noted a pronounced variability in the ventral root cross sectional area and number of myelinated fibers per ventral root between individual segments and subjects. Such variability in root size between lumbosacral segments is common across species and may result from a considerable variability in length of the spinal cord and rostro-caudal distribution of motor columns in control subjects (Sherrington, 1892; Romanes, 1951; VanderHorst and Holstege, 1997). The fiber size composition also varied between individual roots with the S2 ventral root exhibiting the largest proportion of small myelinated roots, likely a reflection of preganglionic parasympathetic neurons residing in the sacral spinal cord.
Ventral root avulsion injuries results in severed motor axons, which are separated from their somata in the spinal cord. The distal axonal segment undergoes Wallerian degeneration, which includes axonal self-destruction (Saxena and Caroni, 2007), fragmentation of myelin sheaths (Stoll et al., 1989), and recruitment of macrophages to assist Schwann cells with the removal of myelin and axonal debris (Brück et al., 1996; Stoll and Müller, 1999). The myelin debris is typically cleared by two weeks after the peripheral nerve injury (Stoll et al., 1989; Brück, 1997). Thus, the process of Wallerian degeneration is expected to markedly reduce or eliminate myelinated axons in avulsed ventral roots, which carry the distal axonal segment of axotomized motor neurons.
In the present study, we surprisingly encountered numerous small and myelinated axons within the avulsed ventral roots. The numbers of myelinated axons in the avulsed roots were similar to the corresponding numbers of myelinated axons extending into the intact ventral roots on the contralateral side of the spinal cord in the same subjects. However, the myelinated fibers in the avulsed ventral roots were much smaller than the corresponding fibers of the control roots. The avulsed roots remained separated from the spinal cord and there were no signs of any bridging between the spinal cord and the avulsed lumbosacral roots. Alternative explanations are therefore warranted.
Although there is a basic separation of efferent and afferent fibers within the ventral and dorsal roots, respectively, important exceptions exist. It is well established that small primary afferents, may extend into the ventral root from the dorsal root as U-turning fibers or to innervate the meninges (Coggeshall et al., 1973, 1980; Hildebrand et al., 1997). It is therefore possible that primary afferents, which were already present in the avulsed ventral roots, may have undergone sprouting and repopulated the denervated roots. Earlier studies have demonstrated that Wallerian degeneration in the peripheral nervous system is associated with the increased expression of growth-promoting neurotrophic factors and their receptors (Taniuchi et al., 1988; Funakoshi et al., 1993; Naveilhan et al., 1997; Höke et al., 2000, 2006). Based on our morphological data, we cannot exclude the possibility that also autonomic fibers, including postganglionic sympathetic fibers, may have contributed to the population of small myelinated fibers extending into the avulsed lumbosacral roots. However, no atypical clinical signs were encountered to suggest an altered autonomic function in these subjects.
Interestingly, a prior study has demonstrated that a neonatal, but not an adult, crush injury of the sciatic nerve results in the invasion of C-fibers into the L5 ventral root (Karlsson and Hildebrand, 1994). It is here of importance that a neonatal sciatic nerve injury is associated with a significant death of axotomized motoneurons (Schmalbruch, 1984; Kashihara et al., 1987; Kalmar et al., 2002), whereas a similar injury in adult animals results in no or modest loss of motoneurons (Carlson et al., 1979; Vanden Noven et al., 1993; Ma et al., 2001).
The functional significance of the extension of myelinated fibers into the denervated ventral root is not known. However, it may affect the utility of avulsed ventral roots for delayed replantation procedures after ventral root avulsion injuries. Interestingly, early replantation of avulsed ventral roots is more successful with regards to functional recovery than late replantation procedures in clinical cases of brachial plexus injury (Carlstedt et al., 2000).
Collagenosis of avulsed ventral roots
Wallerian degeneration of peripheral nerve segments distal to the site of a traumatic injury is associated with extensive endoneurial changes, including a marked increase in the collagen synthesis and deposits (Abercrombie and Johnson, 1946; Thomas, 1964; Eather et al., 1986; Eather and Pollock, 1987, 1988). The collagen deposits become part of the altered peripheral nerve architecture after chronic denervation. When reinnervation of the distal nerve segment is prevented after a transection injury, collagen fibrils are prominent in long-term studies of the denervated peripheral stump at 50 weeks after a sciatic nerve transection in rats (Röyttä and Salonen, 1988) and at 26 months after transection of the rabbit sciatic nerve (Bradley et al., 1998). The relative contribution of Schwann cells and fibroblasts to the production and accumulation of collagen fibrils in distal nerve segments undergoing Wallerian degeneration is not well understood. However, domains of dense collagen commonly exhibit a central Büngner band, which represent a continuous column of denervated Schwann cells and may accept regenerating axons (Bradley et al., 1998). In the present study, collagen fibrils were readily identified in avulsed ventral roots, which have been subject to Wallerian degeneration but also show signs of extensive reinnervation by myelinated axons.
Summary
Ventral root avulsion injury in the rhesus macaque resulted in a marked loss of axotomized motoneurons and an astroglial reaction in the ventral horn. The avulsed ventral roots undergoing initial Wallerian degeneration exhibited long-term changes, including the presence of a large number of small myelinated and unmyelinated axons, a finding suggestive of sprouting by primary sensory afferents or autonomic fibers within the denervated ventral root segment. The repopulation of the avulsed ventral roots by small myelinated fibers of presumed peripheral origin may affect the utility of the roots for late replantation procedures.
Highlights.
Late effects of a ventral root avulsion injury were studied in non-human primates.
There was a significant loss of axotomized lumbosacral motor neurons.
An astrocytic glial response was present in the ventral horn.
Many small myelinated axons were present in the avulsed ventral roots.
Ventral roots undergoing Wallerian degeneration showed repopulation by small fibers.
Acknowledgments
Grant support: The Dr. Miriam and Sheldon G. Adelson Medical Research Foundation; NIH/NCRR (RR000169)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Abercrombie M, Johnson ML. Collagen content of rabbit nerve during Wallerian degeneration. J. Neurol. Neurosurg. Psychiatry. 1946;9:113–118. doi: 10.1136/jnnp.9.4.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldskogius H, Liu L, Svensson M. Glial responses to synaptic damage and plasticity. J. Neurosci. Res. 1999;58:33–41. [PubMed] [Google Scholar]
- Armstrong DM, Brady R, Hersh LB, Hayes RC, Wiley RG. Expression of choline acetyltransferase and nerve growth factor receptor within hypoglossal motoneurons following nerve injury. J. Comp Neurol. 304:596–607. doi: 10.1002/cne.903040407. [DOI] [PubMed] [Google Scholar]
- Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellander BM, Singhrao SK, Ohlsson M, Mattsson P, Svensson M. Complement activation in the human brain after traumatic head injury. J. Neurotrauma. 2001;18:1295–1311. doi: 10.1089/08977150152725605. [DOI] [PubMed] [Google Scholar]
- Bradley JL, Abernethy DA, King RH, Muddle JR, Thomas PK. Neural architecture in transected rabbit sciatic nerve after prolonged nonreinnervation. J. Anat. 1998;192:529–538. doi: 10.1046/j.1469-7580.1998.19240529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück W. The role of macrophages in Wallerian degeneration. Brain Pathol. 1997;7:741–752. doi: 10.1111/j.1750-3639.1997.tb01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück W, Huitinga I, Dijkstra CD. Liposome-mediated monocyte depletion during Wallerian degeneration defines role of hematogenous phagocytes in myelin removal. J. Neurosci. Res. 1996;46:477–484. doi: 10.1002/(SICI)1097-4547(19961115)46:4<477::AID-JNR9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Bussmann KA, Sofroniew MV. Re-expression of P75NTR by adult motor neurons after axotomy is triggered by retrograde transport of a positive signal from axons regrowing through damaged or denervated peripheral nerve tissue. Neuroscience. 1999;91:273–281. doi: 10.1016/s0306-4522(98)00562-4. [DOI] [PubMed] [Google Scholar]
- Carlson J, Lais AC, Dyck PJ. Axonal atrophy from permanent peripheral axotomy in adult cat. J. Neuropathol. Exp. Neurol. 1979;38:579–585. doi: 10.1097/00005072-197911000-00002. [DOI] [PubMed] [Google Scholar]
- Carlstedt T, Anand P, Hallin R, Misra PV, Norén G, Seferlis T. Spinal nerve root repair and reimplantation of avulsed ventral roots into the spinal cord after brachial plexus injury. J. Neurosurg. 2000;93:237–247. doi: 10.3171/spi.2000.93.2.0237. [DOI] [PubMed] [Google Scholar]
- Chung EK, Chen LW, Chan YS, Yung KK. Downregulation of glial glutamate transporters after dopamine denervation in the striatum of 6-hydroxydopamine-lesioned rats. J. Comp. Neurol. 2008;511:421–437. doi: 10.1002/cne.21852. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Coulter JD, Willis WD., Jr Unmyelinated fibers in the ventral root. Brain Res. 1973;57:229–233. doi: 10.1016/0006-8993(73)90582-9. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Maynard CW, Langford LA. Unmyelinated sensory and preganglionic fibers in rat L6 and S1 ventral spinal roots. J. Comp. Neurol. 1980;193:41–47. doi: 10.1002/cne.901930103. [DOI] [PubMed] [Google Scholar]
- Eather TF, Pollock M. Collagen synthesis in axotomized peripheral nerve: evidence against Schwann cell involvement. Exp. Neurol. 1987;96:214–218. doi: 10.1016/0014-4886(87)90183-x. [DOI] [PubMed] [Google Scholar]
- Eather TF, Pollock M. Collagenosis in Wallerian degeneration depends on peripheral nerve injury type. Exp. Neurol. 1988;100:524–530. doi: 10.1016/0014-4886(88)90036-2. [DOI] [PubMed] [Google Scholar]
- Eather TF, Pollock M, Myers DB. Proximal and distal changes changes in collagen content of peripheral nerve that follows transection and crush lesion. Exp. Neurol. 1986;92:299–310. doi: 10.1016/0014-4886(86)90082-8. [DOI] [PubMed] [Google Scholar]
- Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Memic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, Eichele G, Whelan PJ, Kullander K. Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Ptx2 as markers for fast motor neurons and partition cells. J. Comp. Neurol. 518:2284–2304. doi: 10.1002/cne.22332. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Frisén J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J. Cell. Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JW. Augustus Waller and the case of the disappearing axon. Nat. Clin. Pract. Neurol. 2007;3:355. doi: 10.1038/ncpneuro0550. [DOI] [PubMed] [Google Scholar]
- Harrop JS, Hunt GE, Jr, Vaccaro AR. Conus medullaris and cauda equina syndrome as a result of traumatic injuries: management principles. Neurosurg. Focus. 2004;16(6):e4. doi: 10.3171/foc.2004.16.6.4. [DOI] [PubMed] [Google Scholar]
- Havton LA, Carlstedt T. Repair and rehabilitation of plexus and root avulsions in animal models and patients. Curr. Opin. Neurol. 2009;22:570–574. doi: 10.1097/WCO.0b013e328331b63f. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Karlsson M, Risling M. Ganglionic axons in motor roots and pia mater. Prog. Neurobiol. 1997;51:89–128. doi: 10.1016/s0301-0082(96)00052-4. [DOI] [PubMed] [Google Scholar]
- Hoang TX, Havton LA. Novel repair strategies to restore bladder function following cauda equina/conus medullaris injuries. Prog. Brain Res. 2006;152:195–204. doi: 10.1016/S0079-6123(05)52012-0. [DOI] [PubMed] [Google Scholar]
- Hoang TX, Nieto JH, Tillakaratne NJ, Havton LA. Autonomic and motor neuron death is progressive and parallel in a lumbosacral ventral root avulsion model. J. Comp. Neurol. 2003;467:477–486. doi: 10.1002/cne.10928. [DOI] [PubMed] [Google Scholar]
- Hoang TX, Pikov V, Havton LA. Functional reinnervation of the rat lower urinary tract after cauda equina injury and repair. J. Neurosci. 2006;26:8672–8679. doi: 10.1523/JNEUROSCI.1259-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höke A, Cheng C, Zochodne DW. Expression of glial cell line-derived neurotrophic factor family of growth factors in peripheral nerve injury in rats. NeuroReport. 2000;11:1651–1654. doi: 10.1097/00001756-200006050-00011. [DOI] [PubMed] [Google Scholar]
- Höke A, Redett R, Hameed H, Jari R, Zhou C, Li ZB, Griffin JW, Brushart TM. Schwann cells express motor and sensory phenotypes that regulate axon regeneration. J. Neurosci. 2006;26:9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar B, Burnstock G, Vrbová G, Urbanics R, Csermely P, Greensmith L. Upregulation of heat shock proteins rescues motoneurons from axotomy-induced cell death in neonatal rats. Exp. Neurol. 2002;176:87–97. doi: 10.1006/exnr.2002.7945. [DOI] [PubMed] [Google Scholar]
- Karlsson M, Hildebrand C. Invasion of the rat ventral root L5 by putative sympathetic C-fibers after neonatal sciatic nerve crush. Brain Res. 1994;667:39–46. doi: 10.1016/0006-8993(94)91711-6. [DOI] [PubMed] [Google Scholar]
- Kashihara Y, Kuno M, Miyata Y. Cell death of axotomized motoneurons in neonatal rats, and its prevention by peripheral reinnervation. J. Physiol. 1987;386:135–148. doi: 10.1113/jphysiol.1987.sp016526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Kim JE, Kwak SE, Choi KC, Kim DW, Kwon OS, Choi SY, Kang TC. Spatiotemporal characteristics of astroglial death in the rat hippocampo-entorhinal complex following pilocarpine-induced status epilepticus. J. Comp. Neurol. 2008;511:581–598. doi: 10.1002/cne.21851. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, McGaughy VM, Popovich PG. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J. Comp. Neurol. 2006;494:578–594. doi: 10.1002/cne.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos VE, Price DL. Axotomy as an experimental model of neuronal injury and cell death. Brain Pathol. 1996;6:447–465. doi: 10.1111/j.1750-3639.1996.tb00875.x. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Price WL, Pardo CA, Price DL. Ventral root avulsion: an experimental model of death of adult motor neurons. J. Comp. Neurol. 1994;342:35–44. doi: 10.1002/cne.903420105. [DOI] [PubMed] [Google Scholar]
- Kou SY, Chiu AY, Patterson PH. Differential regulation of motor neuron survival and cholinergic acetyltransferase expression following axotomy. J. Neurobiol. 27:561–572. doi: 10.1002/neu.480270410. [DOI] [PubMed] [Google Scholar]
- Lams BE, Isacson O, Sofroniew MV. Loss of transmitter-associated enzyme staining following axotomy does not indicate death of brainstem cholinergic neurons. Brain Res. 1988;475:401–406. doi: 10.1016/0006-8993(88)90635-x. [DOI] [PubMed] [Google Scholar]
- Lang EM, Schlegel N, Sendtner M, Asan E. Effects of root replantation and neurotrophic factor treatment on long-term motoneuron survival and axonal regeneration after C7 spinal root avulsion. Exp. Neurol. 2005;194:341–354. doi: 10.1016/j.expneurol.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Lindå H, Shupliakov O, Örnung G, Ottersen OP, Storm-Mathisen J, Risling M, Cullheim S. Ultrastructural evidence for a preferential elimination of glutamate-immunoreactive synaptic terminals from spinal motoneurons after intramedullary axotomy. J. Comp. Neurol. 2000;425:10–23. [PubMed] [Google Scholar]
- Ma J, Novikov LN, Wiberg M, Kellerth JO. Delayed loss of spinal motoneurons after peripheral nerve injury in adult rats: a quantitative morphological study. Exp. Brain Res. 2001;139:216–223. doi: 10.1007/s002210100769. [DOI] [PubMed] [Google Scholar]
- Mauffrey C, Randhawa K, Lewis C, Brewster M, Dabke H. Cauda equina syndrome: an anatomically driven review. Br. J. Hosp. Med. (Lond) 2008;69:344–347. doi: 10.12968/hmed.2008.69.6.29625. [DOI] [PubMed] [Google Scholar]
- Maynard FM, Jr, Bracken MB, Creasey G, Ditunno JF, Jr, Donovan WH, Ducker TB, Garber SL, Marino RJ, Stover SL, Tator CH, Waters RL, Wilberger JE, Young W. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord. 1997;35:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of m RNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur. J. Neurosci. 1997;9:1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Novikov L, Novikova L, Kellerth JO. Brain-derived neurotrophic factor promotes survival and blocks nitric oxide synthase expression in adult rat spinal motoneurons after ventral root avulsion. Neurosci. Lett. 1995;200:45–48. doi: 10.1016/0304-3940(95)12078-i. [DOI] [PubMed] [Google Scholar]
- Novikov L, Novikova L, Kellerth JO. Brain-derived neurotrophic factor promotes axonal regeneration and long-term survival of adult rat spinal motoneurons in vivo. Neuroscience. 1997;79:765–774. doi: 10.1016/s0306-4522(96)00665-3. [DOI] [PubMed] [Google Scholar]
- Ohlsson M, Havton LA. Complement activation after lumbosacral ventral root avulsion injury. Neurosci. Lett. 2006;394:179–183. doi: 10.1016/j.neulet.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Ohlsson M, Hoang TX, Wu J, Havton LA. Glial reactions in a rodent cauda equina injury and repair model. Exp. Brain Res. 2006;170:52–60. doi: 10.1007/s00221-005-0188-6. [DOI] [PubMed] [Google Scholar]
- Pavlakis AJ, Siroky MB, Goldstein I, Krane RJ. Neurourologic findings in conus medullaris and cauda equina injury. Arch. Neurol. 1983;40:570–573. doi: 10.1001/archneur.1983.04050080070014. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Puller C, Ondreka K, Haverkamp S. Bipolar cells of the ground squirrel retina. J. Comp. Neurol. 2011;519:759–774. doi: 10.1002/cne.22546. [DOI] [PubMed] [Google Scholar]
- Rende M, Giambanco I, Buratta M, Tonali P. Axotomy induces a different modulation of both low-affinity nerve growth factor receptor and choline acetyltransferase between adult rat spinal and brainstem motoneurons. J. Comp. Neurol. 1995;363:249–263. doi: 10.1002/cne.903630207. [DOI] [PubMed] [Google Scholar]
- Romanes GJ. The motor cell columns of the lumbo-sacral spinal cord of the cat. J. Comp. Neurol. 1951;94:313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- Röyttä M, Salonen V. Long-term endoneurial changes after nerve transection. Acta Neuropathol. 1988;76:35–45. doi: 10.1007/BF00687678. [DOI] [PubMed] [Google Scholar]
- Saxena S, Caroni P. Mechanisms of axonal degeneration: from development to disease. Prog. Neurobiol. 2007;83:174–191. doi: 10.1016/j.pneurobio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Notes on the arrangement of some motor fibers in the lumbo-sacral plexus. J. Physiol. (London) 1892;13:621–772. doi: 10.1113/jphysiol.1892.sp000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch H. Motoneuron death after sciatic nerve section in newborn rats. J. Comp. Neurol. 1984;224:252–258. doi: 10.1002/cne.902240206. [DOI] [PubMed] [Google Scholar]
- Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J. Comp. Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- Stoll G, Griffin JW, Li CY, Trapp BD. Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J. Neurocytol. 1989;18:671–683. doi: 10.1007/BF01187086. [DOI] [PubMed] [Google Scholar]
- Stoll G, Müller HW. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol. 1999;9:313–325. doi: 10.1111/j.1750-3639.1999.tb00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos DM, Fishman RE, Park H, Folkerth RD, Follett PL, Volpe JJ, Jensen FE. Developmental regulation of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. I. Rodent cerebral white matter and cortex. J. Comp. Neurol. 2006;497:42–60. doi: 10.1002/cne.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniuchi M, Clark HB, Schweitzer JB, Johnson EM., Jr Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves: ultrastructural location, suppression of axonal contact, and binding properties. J. Neurosci. 1988;8:664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PK. Changes in the endoneurial sheaths of peripheral myelinated nerve fibers during Wallerian degeneration. J. Anat. 1964;98:175–182. [PMC free article] [PubMed] [Google Scholar]
- Vanden Noven S, Wallace N, Muccio D, Turtz A, Pinter MJ. Adult spinal motoneurons remain viable despite prolonged absence of functional synaptic contact with muscle. Exp. Neurol. 1993;123:147–156. doi: 10.1006/exnr.1993.1147. [DOI] [PubMed] [Google Scholar]
- VanderHorst VG, Holstege G. Organization of lumbosacral motneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J. Comp. Neurol. 1997;382:46–76. [PubMed] [Google Scholar]
- Wu CW, Kaas JH. Spinal cord atrophy and reorganization of motoneuron connections following long-standing limb loss in primates. Neuron. 2000;28:967–978. doi: 10.1016/s0896-6273(00)00167-7. [DOI] [PubMed] [Google Scholar]