Abstract
Intelligence quotient (IQ) scores tend to remain stable across the lifespan. Nevertheless, in some healthy individuals, significant decreases or increases in IQ have been observed over time. It is unclear whether such changes reflect true functional change or merely measurement error. Here, we applied surface-based corticometry to investigate vertex-wise cortical surface area and thickness correlates of changes in Full Scale IQ (FSIQ), Peformance IQ (PIQ) and Verbal IQ (VIQ) in a representative sample of children and adolescents (n = 188, mean age = 11.59 years) assessed two years apart as part of the NIH Study of Normal Brain Development. No significant associations between changes in IQ measures and changes in cortical surface area were observed, whereas changes in FSIQ, PIQ, and VIQ were related to rates of cortical thinning, mainly in left frontal areas. Participants who showed reliable gains in FSIQ showed no significant changes in cortical thickness on average, whereas those who exhibited no significant FSIQ change showed moderate declines in cortical thickness. Importantly, individuals who showed large decreases in FSIQ displayed the steepest and most significant reductions in cortical thickness. Results support the view that there can be meaningful cognitive ability changes that impact IQ within relatively short developmental periods and show that such changes are associated with the dynamics of cortical thickness development.
Keywords: Intelligence, IQ, Brain development, Brain plasticity, Cortical thickness, Verbal IQ, Performance IQ
Introduction
Intelligence quotient (IQ) is one of the most relevant human psychological characteristics, as highlighted by epidemiologic studies that document long-term predictive relationships between early cognitive ability and adult physical and mental health outcomes, including longevity (Whalley and Deary, 2001; Jokela et al., 2009). Brain imaging research has shown that general cognitive ability is also associated with several features of the human brain, such as grey matter morphology (Colom et al., 2009; Gläscher et al., 2010; Haier et al., 2009; Karama et al., 2009, 2011; Burgaleta et al., 2013), trajectories of cortical development (Shaw et al., 2006), functional efficiency (Haier et al., 1988; Neubauer & Fink, 2009; van den Heuvel, Stam, Kahn, & Pol, 2009), and integrity of white matter connections (Chiang et al., 2009; Tamnes et al., 2010; Yu et al., 2008).
Contrary to other cognitive measures, IQ is an index of relative general performance, as it summarizes how well an individual performs in a cognitive battery with respect to a reference group of same-age peers. Therefore, although an individual’s absolute level of performance will strongly vary across development (McArdle et al. 2002), IQ scores, which are age-adjusted, will tend to remain relatively stable (Deary et al. 2000). In keeping with this, in the current manuscript, the terms ‘cognitive ability’ and ‘general cognitive ability’ should be understood as referring to age-standardized rather than absolute measures of cognitive performance. Thus change scores, even absolute difference scores, indicate relative rather than absolute change.
Empirical data confirms that IQ is highly stable developmentally; for instance, Deary et al. (2000) reported a mean test-retest correlation between ages 11 and 77 of r = .73. However, although high, such a correlation nonetheless allows for occurrence of increases and decreases, sometimes significant in magnitude. Along these lines, a recent report from the NIH Study of Normal Brain Development (Waber et al., 2012) revealed that although the test-retest correlation for Full Scale IQ (FSIQ) was high across a 2-year interval (r = 0.81), 25% of these healthy children and adolescents showed changes of 9 points or more (nearly 2/3 standard deviation) across this interval.
Such fluctuations, which are not well understood, have often been ascribed to measurement error (Flynn, 2007), which some most certainly are. However, in keeping with recent evidence about brain plasticity and higher-order cognition (Draganski et al., 2004; Haier et al., 2009; LöLövdén et al., 2010; Takeuchi et al., 2010; Mackey et al., 2012), some of these fluctuations could also represent true changes in cognitive abilities. Documentation of association between IQ changes and morphometric variations in neural structure would strongly support such a view. In this vein, Ramsden et al. (2011) studied 33 adolescents using voxel-based morphometry and found changes in two aspects of FSIQ, Verbal and Performance IQ (VIQ; PIQ), to be associated with changes in regional grey matter in sensorimotor areas. While these findings strongly suggest that IQ changes can indeed be genuine, generalization is somewhat limited by small sample size, use of different IQ tests at different ages, and sample peculiarities (e.g., about half appeared to meet criteria for dyslexia).
Here, we investigated the structural correlates of longitudinal changes in IQ in participants of the NIH MRI Study of Normal Brain Development. Individuals included in the present study (N=188) contributed structural MRIs and concurrent IQ testing with the Wechsler Abbreviated Scale of Intelligence (WASI) at a 2-year interval. To better characterize structural changes in grey matter, corticometric methods were applied to generate two independent indices of cortical morphology: cortical thickness (CTh) and cortical surface area (CSA). Each of these metrics is known to reflect different components of cortical structure. Cortical surface area is related to the number and spacing of minicolumnar units of cells whereas cortical thickness is thought to index the number of neurons per column as well as glial support and dendritic arborization (Rakic, 1988; Chklovskii et al., 2004; Thompson et al., 2007; la Fougere et al., 2011). Changes in IQ were regressed against maps of changes in cortical thickness and surface area.
Given previous findings (Ramsden et al., 2011), we hypothesized that changes in VIQ would be positively related to grey matter structural changes in sensorimotor areas. Furthermore, because of our larger sample and hence greater statistical power, we hypothesized that we would observe additional associations with VIQ and PIQ, as well as with FSIQ. We anticipated such associations in parieto-frontal areas previously shown to be involved with individual differences in cognitive ability (Jung and Haier, 2007).
Materials and Methods
Sample
Data were obtained from the Pediatric MRI Data Repository (Objective 1) created for the National Institute of Mental Health MRI Study of Normal Brain Development (Evans and Brain Development Cooperative Group, 2006), a multi-site longitudinal project aimed at providing a normative database to characterize healthy brain maturation in relation to behavior; 431 subjects underwent cognitive evaluation and MRI acquisition, distributed in six different sites (a listing of the participating sites and of the study investigators can be found at: http://www.bic.mni.mcgill.ca/nihpd/info/participating_centers.html). The sample was demographically representative of the normative US population based on age, gender, ethnicity, and socioeconomic status (Waber et al., 2007). Participants with prior history of psychiatric disorders, neurological, or other medical illnesses with central nervous system implications were excluded. Some of the participants (24%) underwent one single MRI session; 39% were scanned twice; and 37% were scanned three times. Data for visit 3 were excluded for those participants with more than two time points in order to build a simple pre-post design. Participants with only one MRI scan, missing IQ scores or failing processed MRI quality control at one or more time points were excluded. Further visual quality control (blinded to cognitive ability scores) of the native cortical surfaces detected obvious problems in a total of 36 scans (e.g., frontal lobe truncation due to failed automatic brain masking, fused gyri or clearly aberrant cortical thickness values due to ringing artifacts), that were also excluded of the analyses. The final sample analyzed retained a total of 188 subjects (mean age ± SD at the time 1: 11.59 years ± 3.46; range 6.01 to 20.01 years; 59% of participants were females; and the mean inter-scan lapse was 1.96 years. See Table 1 for further characteristics of the sample used).
Table 1.
Descriptive statistics.
| Age | FSIQ | VIQ | PIQ | Mean CTh | Mean CSA | ||
|---|---|---|---|---|---|---|---|
| Time 1 | Mean (SD) Min, Max |
11.59 (3.46) 6.09, 20.05 |
112.04 (12.94) 78, 160 |
111.12 (13.82) 74, 156 |
110.29 (13.04) 72, 157 |
3.59 (0.17) 3.18, 3.99 |
2.29 (0.18) 1.82, 2.74 |
| Time 2 | Mean (SD) Min, Max |
13.55 (3.49) 7.74, 21.87 |
113.12 (12.62) 75, 150 |
111.27 (13.09) 80, 147 |
112.12 (12.94) 74, 147 |
3.55 (0.17) 3.06, 3.97 |
2.29 (0.19) 1.78, 2.73 |
| Longitudinal correlation | – | 0.83 | 0.74 | 0.80 | 0.86 | 0.98 | |
| Change | Mean (SD) Min, Max |
1.96 (0.42) 0.85, 3.81 |
1.07 (7.51) −18, 24 |
0.15 (9.62) −29, 30 |
1.82 (8.46) −25, 24 |
−0.04 (0.09) −0.28, 0.25 |
0 (0.04) −0.09, 0.13 |
FSIQ = Full scale IQ. VIQ = Verbal IQ. PIQ = Performance IQ. CTh = cortical thickness. CSA = cortical surface area. Change = Time 2 – Time 1. All correlations are significant at p < 0.01.
Cognitive measures
The Wechsler Abbreviated Scale of Intelligence (WASI) was used for all subjects (Wechsler, 1999). The WASI includes the Vocabulary, Similarities, Matrix Reasoning, and Block Design subtests. FSIQ as well as VIQ and PIQ scores were obtained for each participant at each visit. See Table 1 for a summary of these data.
MRI acquisition protocol
A 3D T1-weighted Spoiled Gradient Recalled (SPGR) echo sequence from 1.5 Tesla scanners was acquired for each participant at each visit, with 1mm isotropic data acquired sagittally (whole head); TR = 22–25 ms, TE = 10–11 ms. Excitation pulse = 30°, refocusing pulse = 180°. FOV = AP 256 mm, LR 160–180 mm. Matrix size = AP 256 mm, LR for 1 mm isotropic. Slice thickness of ~1.5 mm for GE scanners (with a limit of 124 slices) was allowed to guarantee whole head coverage.
MRI processing
MRI images were processed by applying a fully automated in-house pipeline, CIVET 1.1.9 (MacDonald et al., 2000; Kim et al., 2005; Ad-Dab'bagh et al., 2006) for the measurement of regional cortical thickness. CIVET was developed at the Montreal Neurological Institute and comprises several steps, extensively detailed elsewhere (Karama et al., 2009): linear registration of native T1 images to the ICBM152 template (Mazziotta et al., 1995); non-uniformity correction; tissue classification into grey matter, white matter cerebrospinal fluid and background; pial and white matter surface fitting (40962 vertices per hemisphere); non-linear surface registration to a high-resolution surface template in ICBM152 space; inverse registration of the surfaces into native space; cortical thickness calculation at each vertex with the t-link metric (Lerch and Evans, 2005); cortical thickness smoothing applying a 20 mm FWHM surface-based smoothing kernel; surface area calculation at each vertex as one third of the total area of all triangular facets adjoining it; and surface area smoothing using a 40 mm FWHM surface-based smoothing kernel (Lyttelton et al., 2009).
Longitudinal design and statistical analyses
We computed IQ difference scores (time 2 minus time 1; ΔIQ), as well as individual maps of cortical changes (time 2 minus time 1; ΔCTh or ΔCSA depending on the variable under study). Vertex-wise analyses were carried out via permutation-based inference (Nichols and Holmes, 2002), implemented using the randomise tool of the FSL package (FMRIB, Oxford, UK; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise). For this purpose, the surface data were projected onto an ICBM152 2mm template by means of the appropriate function of the SurfStat Toolbox (http://www.math.mcgill.ca/keith/surfstat/), so that each vertex value was assigned to its nearest voxel while respecting the standard space of reference. Five thousand permutations were performed for each analysis. The association between ΔIQ and ΔCTh or each analysis. The association CSA was analyzed at each vertex, controlling for the effects of age at time 1, gender, scanner, IQ at time 1, and inter-scan lapse and mean CTh (when looking at CTh changes) or total CSA (when looking at CSA changes) at time 1. Controlling for mean CTh or total CSA effects allowed improved sensitivity to detect local areas of significance unrelated to potential overall cortical effects. More specifically, the fitted regression equation at each vertex was:
ΔCTh ~ b0 + b1Age_T1 + b2Gender + b3Scanner + b4MeanCTh_T1 + b5IQ_T1 + b6TimeLapse + b7ΔIQ + ε,
where Age_T1 is age in years at time 1, MeanCTh_T1 is mean cortical thickness at time 1, IQ_T1 is IQ at time 1, and TimeLapse is the exact interval between time 1 and time 2 in years. An equivalent regression equation was fitted for ΔCSA.
Regression coefficients for changes in CTh or CSA were estimated for changes in FSIQ, PIQ, or VIQ, depending on the focus of analysis. Corollary t-statistic maps were produced at each vertex of the cortical surface. Threshold-Free Cluster Enhancement (Smith and Nichols, 2009) was applied to detect cluster-wise statistical signal while avoiding the setting of arbitrary cluster-forming thresholds. Finally, statistical maps were thresholded at P < 0.05 corrected for multiple comparisons (family-wise error rate below 5%).
Results
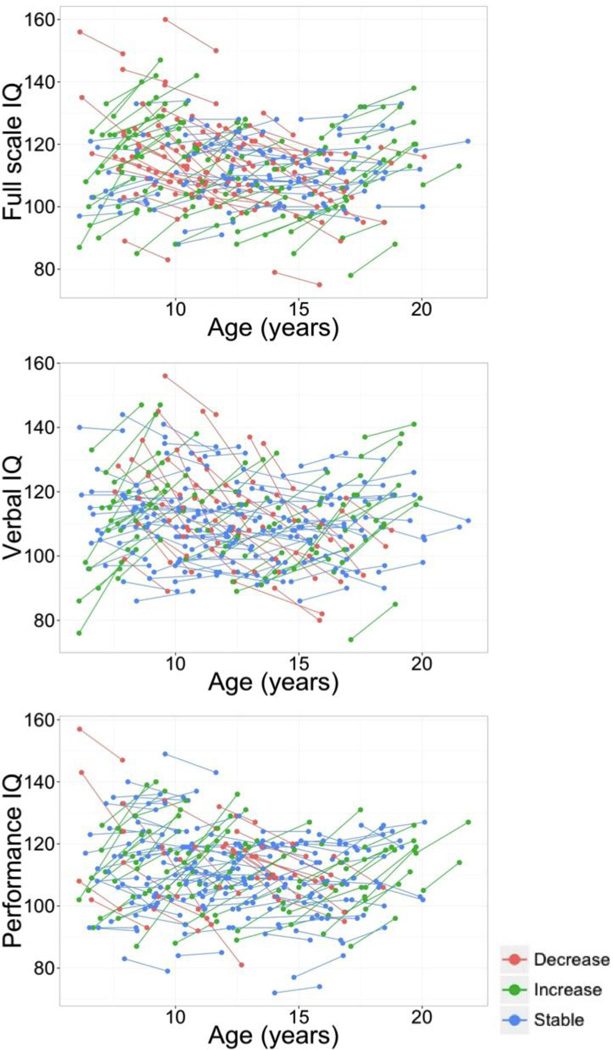
Descriptive statistics for the main variables of interest are shown in Table 1. Mean IQs for the sample were substantially above the population norm of 100 at both time points, and standard deviations were slighty smaller than the norm of 15. Figure 1 displays the individual trajectories of change for FSIQ, VIQ and PIQ. According to the published 90% confidence intervals (Wechsler, 1999), 51.6% of the subjects showed clear changes in FSIQ, 46.3% in VIQ, and 44.2% in PIQ. There were no significant correlations between change scores in FSIQ/VIQ/PIQ and age, mean CTh or mean CSA at either time point. There were also no significant gender differences. However, change in FSIQ correlated with initial FSIQ (r = −.33, p < .01) as well as with subsequent FSIQ (r = .25, p < .01). Similar patterns were found for VIQ (initial: r = −.43, p < .01; subsequent: r = .29, p < .01) and PIQ (initial: r = −.33, p < .01; subsequent: r = .31, p < .01). These patterns show the expected regressions to the mean.
Figure 1.
Longitudinal plots for the intra-individual changes in IQ (full-scale, verbal and performance) and cortical thickness between time 1 and time 2. Participants are color-coded based on the magnitude and direction of their change in FSIQ scores (90% confidence interval).
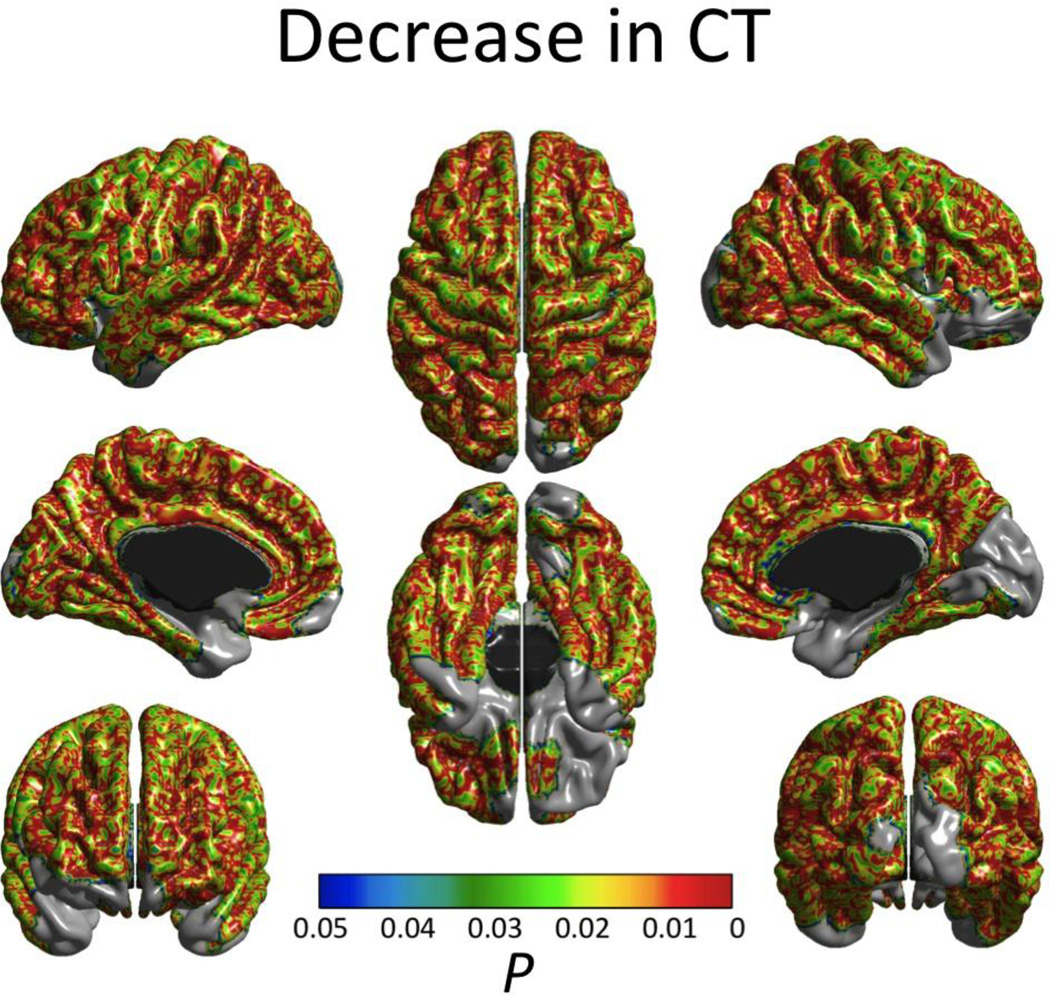
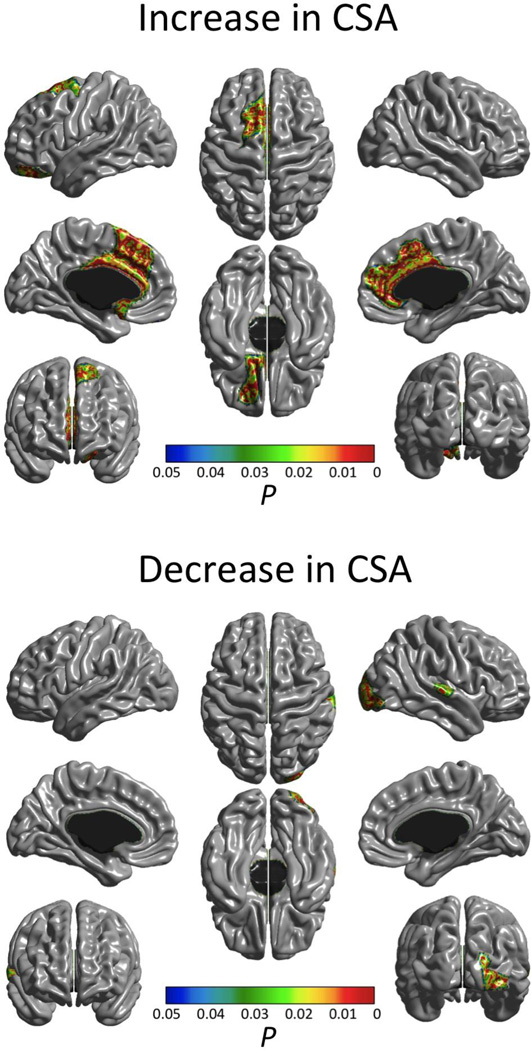
In keeping with previously reported findings on this dataset (Brain Development Cooperative Group, 2012; Nguyen et al., in press), only first-order linear (i.e. no quadratic or cubic effects) cortical thinning effects of age were found across most of the cortex (Fig. 2) and no significant increases in CTh were detected. On the other hand, decreases in CSA (also exclusively of a first-order linear type) were found only in the right occipital pole and right superior temporal gyrus, whereas increases were detected bilaterally in the anterior cingulate cortex (Fig. 3).
Figure 2.
Significant (FWE-corrected P < 0.05) age effect on cortical thickness. Only first order linear cortical thinning age effects were observed. Effects of age at time 1, sex, scanner and inter-scan lapse were controlled.
Figure 3.
Significant (FWE-corrected P < 0.05) age effect on cortical surface area. Upper image shows regions of cortical surface area decreases while bottom image shows regions of increases. Effects of age at time 1, sex, scanner and inter-scan lapse were controlled.
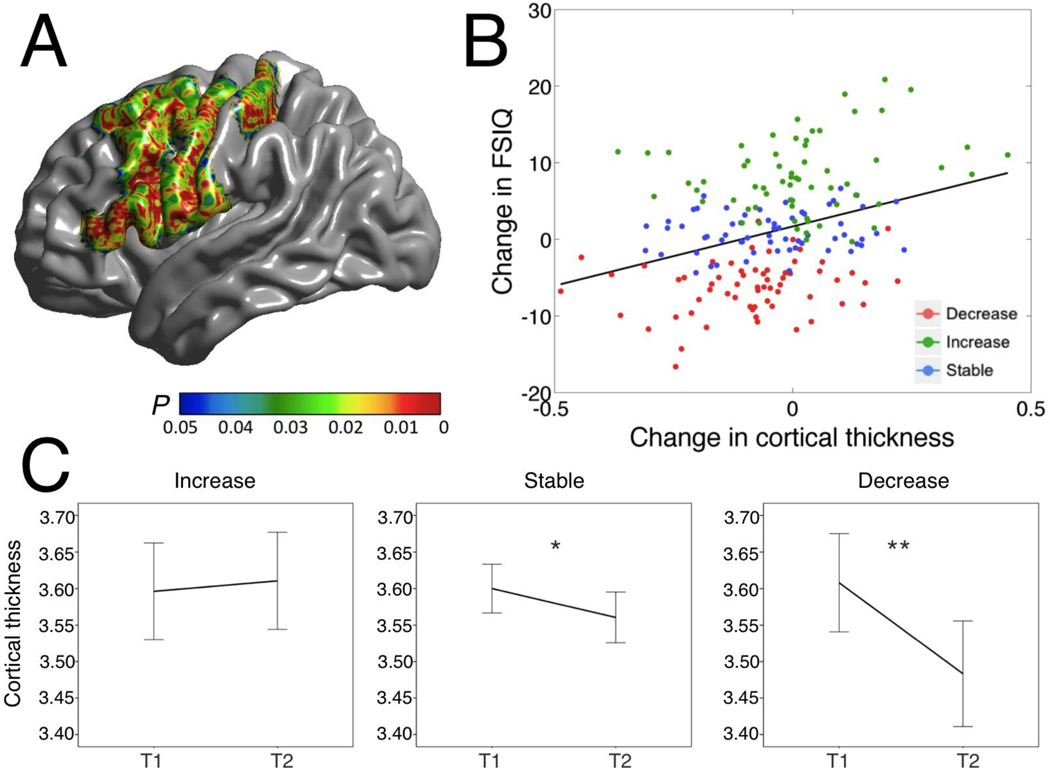
Importantly, changes in FSIQ scores were significantly related (FWE < 0.05) to changes in CTh (ΔCTh) in regions of the left hemisphere that included the sensorimotor cortex (left pre- and post-central gyri) as well as the superior, middle, and inferior frontal gyri, and pars opercularis and triangularis regions (Figure 4A). Correlations ranged from 0.11 to 0.33 within the significant region detected by the TFCE algorithm, with the local maximum of significance found in the left inferior pre-central gyrus (changes for CTh at the peak vertex are also shown in Figure 5). No significant associations between changes in CSA and changes in FSIQ were found. When inspecting the maximum and minimum values for FSIQ, PIQ and VIQ at both time points, we identified two subjects with particularly high scores at time 1 (z score > 3; see longitudinal plots in Figure 1). We repeated our analyses after excluding those subjects and obtained essentially identical results to those shown in Figure 4. Finally, we also repeated our analyses for CTh after applying a FWHM smoothing kernel of 40 mm, equaling the one applied to CSA. Again, this modification had very little impact on our results and had no repercussions on our conclusions.
Figure 4.
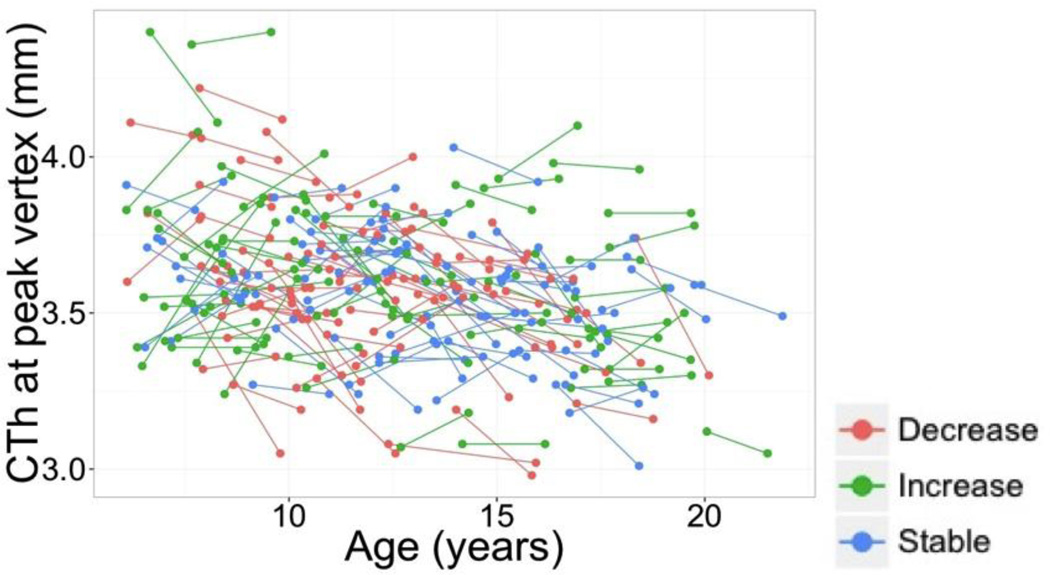
A. Cortical regions where change in cortical thickness was associated with change in FSIQ (FWE < 0.05). B. Scatter plot for the relation between IQ changes and CTh changes at the peak vertex (inferior pre-central gyrus; r = 0.33, p < 0.001). Participants are color-coded based on the magnitudes and directions of their changes in FSIQ scores in relation to the 90% confidence interval around mean change in FSIQ). C. Changes in cortical thickness at the same peak vertex represented in panel B, separately for each group of change in IQ: ‘increase’ (N=58), ‘no change’ (N=92), and ‘decrease’ (N=38). T1 = time 1. T2 = time 2. * p < 0.005. ** p < 0.001.
Figure 5.
Longitudinal plots for the intra-individual changes in CTh at the peak vertex for the association between FSIQ changes and CTh changes (see Figure 4). Participants are color-coded based on the magnitude and direction of their change in FSIQ scores (90% confidence interval).
To illustrate the main IQ results, participants were partitioned into three groups according to their ΔFSIQ scores. As did Ramsden et al. (2011), we used the 90% CI to ensure that FSIQ changes were not due to measurement error. Further, because difference scores can be to some extent unreliable, using the confidence intervals allowed us to study brain changes for those participants showing reliable IQ increases, reliable IQ decreases, and no reliable IQ changes. Thus, groups were defined as follows: ‘increase’ (FSIQ at time 2 ≥ upper endpoint of the FSIQ 90% confidence interval; N = 58), ‘stable’ (FSIQ at time 2 within the FSIQ 90% CI; N = 92), and ‘decrease’ (FSIQ at time 2 ≤ 90% CI lower endpoint; N = 38). At the peak vertex for the association between FSIQ change and CTh change, the ‘increase’ group showed non-significant cortical thickening (t = 0.658, p = 0.513), whereas the ‘decrease’ group displayed the steepest rates of reduction in CTh (t = − 4.827, p = 0.00001; Figure 4C). Subjects in the ‘stable’ group showed milder decreases in cortical thickness (t = −2.578, p = 0.012) (Figure 4C).
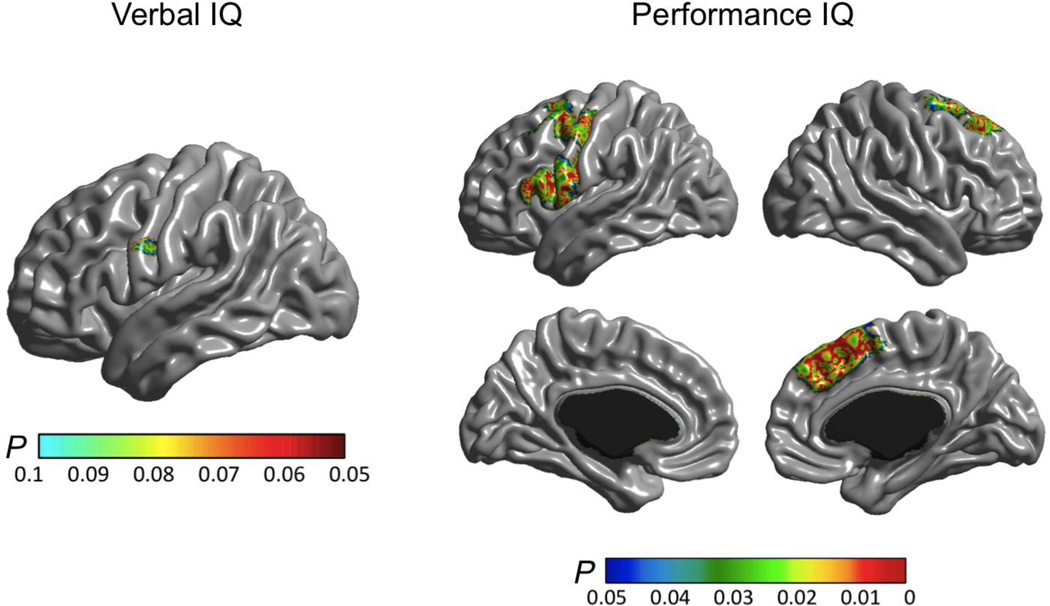
Finally, changes in PIQ were associated (FWE < 0.05) with changes in CTh in the left pre-central and inferior frontal gyri (as found for FSIQ), in regions of the right posterior superior frontal cortex including the superior precentral gyrus as well as in the right paracingulate / medial superior frontal gyrus including the supplementary motor area. For VIQ, only a trend association was observed (FWE < 0.1) in a small region located in the inferior precentral gyrus (Figure 5). This was in very close vicinity to the peak region where associations with FSIQ and PIQ changes were observed.
Discussion
The primary finding of this study was that individual differences in the rate of change in cortical thickness were related to changes in three aspects of IQ, indices of relative general cognitive ability that summarize how well an individual performs in a cognitive battery with respect to a population-based reference group. More specifically, we showed that i) changes in CTh, and not CSA, were associated with FSIQ, VIQ, and PIQ changes; ii) relevant CTh changes took place in regions that are known to be associated with general cognitive ability; and iii) children and adolescents displaying FSIQ losses displayed the steepest cortical thinning rates, and FSIQ gains were not accompanied, on average, by significant cortical thickening. Taken together, these findings, which were based on a sizeable sample of children and adolescents, underscore the dynamic nature of intelligence-brain relations and, more importantly, support the idea that changes in IQ across development can reflect meaningful general cognitive ability changes and have a neuroanatomical substrate.
Consistency with previous literature on brain-cognition associations
Changes in cognitive ability were significantly associated with changes in CTh in the frontoparietal regions of the left hemisphere, mainly comprising posterior areas of the frontal cortex. Crucially, these cortical regions have previously been related to individual differences in general cognitive ability in cross-sectional studies (Jung and Haier, 2007; Karama et al., 2009; Barbey et al., 2012). Changes in PIQ were associated with changes in the left pre-central gyrus and left posterior superior and inferior frontal cortices, areas that roughly overlapped with regions that correlated with changes in FSIQ. Change in PIQ was additionally associated with changes in CTh in the caudal and rostral supplementary motor areas of the right hemisphere. In contrast, changes in VIQ yielded only trend-level correlations with CTh changes (FWE < 0.1) in the left pre-central gyrus. Post-hoc analyses helped illustrate the nature of the observed associations. These analyses revealed that, on average, participants with substantial gains in FSIQ scores exhibited no significant changes in cortical thickness, although those with the largest FSIQ gains tended to show increases in cortical thickness, as shown in Figure 4B. Participants who exhibited no change in FSIQ tended to show modest decreases in cortical thickness, of a magnitude consistent with previous observations of normative developmental change in cortical thickness for the age group under study (Shaw et al., 2008; Panizzon et al., 2009; Raznahan et al., 2011). Finally, those individuals who experienced declines in FSIQ displayed the steepest reductions in cortical thickness. Contrasted with our systematic findings for cortical thickness, we did not find evidence of associations between changes in measures of cognitive ability and changes in cortical surface area. These findings are consistent with reports that developmental changes in surface area are less significant (Østby et al., 2009) and less steep (Raznahan et al., 2011) than those for cortical thickness.
Our results are somewhat consistent with, and also extend, those of Ramsden et al. (2011). Indeed, our strongest region of association with changes in FSIQ, PIQ, and VIQ, was in the left pre-central gyrus; the same region where Ramsden et al. (2011) noted an association with VIQ change. They did not report associations for FSIQ, however, whereas we found a clear pattern of association. Moreover, they observed an association between grey matter density and PIQ in the cerebellum – a structure that is not yet amenable to automated cortical thickness quantification due to its high degree of gyrification. Differences between voxel and surface-based morphometry techniques, as well as other methodological differences (e.g., sample size, cognitive tests used, sample representativeness) are likely partly to account for the observed discrepancies. Importantly, and contrary to Ramsden et al.’s (2011) conclusions, the association between cortical and cognitive changes in our data was found in brain areas that are relevant for individual differences in general intelligence.
The associations we observed in sensorimotor areas deserve further study, although they are consistent with extensive evidence showing that the sensorimotor cortex is involved in abstract reasoning, a main subcomponent of general cognitive ability. Indeed, a recent meta-analysis of 28 fMRI studies on deductive reasoning (Prado et al., 2011) described reliable association with a left-lateralized cortical network which includes, among other regions, the inferior frontal gyrus, middle frontal gyrus, pre-central gyrus, and medial frontal cortex. Activity of the left pre-central gyrus during reasoning tasks has been interpreted as indicative of the relevance of motor and attentional processes to cognitive processing (Acuna et al., 2002). Furthermore, the sensorimotor cortex is part of a broad working memory system that includes the prefrontal cortex, anterior cingulate cortex and hippocampus (Cohen and Servan-Schreiber, 1992; O'Reilly et al., 1999; Braver and Cohen, 2000; Miller and Cohen, 2001). Along these lines, recent research on the impact of brain lesions on cognition has suggested a left-lateralized frontoparietal network for working memory, with the peak for the lesion-deficit relationship located in the vicinity of the left paracentral gyri (Gläscher et al., 2010). Importantly, working memory capacity has been proposed as germane to individual differences in reasoning and general cognitive ability (Stauffer et al., 1996; Engle et al., 1999; Colom et al., 2003, 2004). Therefore, one could speculate about developmental changes in working memory capacity, with corollary impacts on IQ. However, this hypothesis remains to be tested.
Finally, the benefits of a reduced rate of cortical thinning across childhood and adolescence have been shown for another cognitive domain by Shaw et al. (2013), who recently reported that children with ADHD whose symptoms persisted into adulthood displayed higher rates of cortical thinning across development when compared to normal controls (bilateral cingulate gyrus, medial prefrontal cortex, and precuneus, as well as right dorsolateral prefrontal cortex), while those that remitted exhibited a slower rate of cortical thinning – and in some cases, even cortical thickening – than normal controls.
Cellular underpinnings of cognitive ability-related changes in cortical thickness
The fact that IQ changes were significantly associated with CTh changes, but not CSA changes, may have interesting implications regarding the microstructural underpinnings of the reported longitudinal fluctuations in IQ. At the cellular level, the number of neurons, the amount of glial and capillary support, and the level of dendritic arborization can account for most of the variability in CTh, whereas CSA is presumably related to the number and spacing of mini-columnar units of cells (Rakic, 1988; Chklovskii et al., 2004; Thompson et al., 2007; la Fougere et al., 2011). This fits well with the fact that IQ changes were related to CTh changes – likely indexing cellular events that are sensitive to postnatal development and experience-, but not to CSA changes – which are likely dependent on neurogenesis and neuronal migration, two processes that are almost complete by term gestation (Hill et al., 2010).
Nevertheless, CSA may also index indirect processes by which the distance between cortical mini-columns is increased or decreased, such as the mechanistic pressures exerted by the size and complexity of the dendritic arbors (Hill et al., 2010; Mountcastle, 1997; Meyer, 1987). CSA might also be sensitive to the size of intracortical elements or to the volume of white matter adjacent to a given gyrus or sulcus (Feczko et al., 2009). This would be consistent with the fact that we observed significant longitudinal changes in CSA during childhood and adolescence. With regard to the associations found for CTh changes, there is no evidence of neuronal proliferation in the human cortex during most of post-natal development, which makes it unlikely that the observed CTh changes are due to changes in the number of cortical neurons (Zatorre, Fields, & Johansen-Berg, 2012). A more plausible alternative is that CTh changes stem from modifications in amount of glial and capillary support, as well as in dendritic arborization (Chklovskii et al., 2004; Sur and Rubenstein, 2005; Thompson et al., 2007). Indeed, there is evidence showing that gliogenesis occurs as a consequence of learning and experience (Dong and Greenough, 2004) and is considered an important candidate mechanism for experience-related changes in grey matter morphology (Zatorre, Fields, & Johansen-Berg, 2012). In addition, animal studies show learning-related increases in number of synapses, glial cells, as well as in cortical capillary density (Black et al., 1990; Isaacs et al., 1992; Anderson et al., 1994; Kleim et al., 1996; Anderson et al., 2002). However, it is not clear whether decrements in these microstructural parameters would also account for cortical thinning processes, or whether they may instead underpin cortical thickening only.
On the other hand, cortical thinning in the age range studied here can be partly explained by synaptic pruning (Huttenlocher and Dabholkar, 1997; Paus, 2005; Petanjek et al., 2011). Based on this evidence, we speculate that an excessive loss of neuronal connections (perhaps with associated glia and capillary modifications) might be behind the steeper cortical thinning found in those participants in whom cognitive ability decreased, thus preventing or delaying adequate circuitry specializations relevant for cognition (Hensch, 2004; Knudsen, 2004). This is consistent with cross-sectional studies showing that higher IQ levels are associated with greater numbers of dendrites (Jacobs, Schall & Scheibel, 1993), and that individuals with very low IQ display reduced dendritic branching compared to the normal population (Huttenlocher, 1991).
Putative underpinnings of the association between changes in cognitive ability and rate of cortical thinning
The reported changes in cognitive ability may reflect individual differences in relative rates of cognitive (and brain) development. That is, some participants may develop earlier or later compared to their peers, causing their age-related rank orders in cognitive ability to shift upward or downward. Such shifts could be transitory or, instead, have long-lasting effects and persist in adulthood. A second possibility, consistent with the hypothesized excess of dendritic and spine elimination suggested above, is that a non-pathological relative decrease in cognitive ability may be partly caused by insufficient educational and social stimulation during a sensitive period (Hensch, 2004; Knudsen, 2004), among other factors known to influence cognitive ability, such as lifestyle, diet and nutrition (Deary et al., 2009). Indeed, previous reports have suggested that dendritic and spine rearrangement and elimination occurring during childhood and adolescence are likely dependent on social and educational interactions (Petanjek et al., 2008). Moreover, it has been shown that schooling has an effect on IQ (Ceci, 1991; Ceci and Williams, 1997).
Nevertheless, the processes that underlie cognitive development are known to involve complex genetic and experiential interactions (Lenroot & Giedd, 2008). A fundamental question that arises, therefore, is how these complex interactions between genetic and environmental influences contribute to the observed developmental trajectories. Along these lines, van Soelen et al. (2012) recently found that cortical thinning rates in children were under moderate to high genetic influence, and independent genetic factors influenced different cortical areas. This does not necessarily mean, however, that cognitive ability levels and developmental trajectories in general are largely genetically determined via some genetic influence on cortical structure. For example, experimental evidence in both animals and humans suggests that cortical morphology is malleable to experience (Draganski et al., 2004; Haier et al., 2009; Lerch et al., 2011). If the outcome of practice with intellectual skills is genetically influenced and in turn influences CTh development, the genetic influences on such practice will appear over time as genetic influences on CTh even if access to practice with intellectual skills were completely social dictated. Moreover, the genetic contribution to variance in cognitive ability can vary considerably depending upon the environmental context; for instance, in less advantaged circumstances, environmental factors can play more potent roles, apparently obscuring genetic influences (Turkheimer et al., 2003). Furthermore, shared environmental influences account for substantial variance in cognitive ability during childhood (Haworth et al., 2010), but they decrease with age and are offset by increasing genetic influences – a pattern similar to that observed for cortical thickness development (Lenroot & Giedd, 2008) that could also be explained by gene-environment correlation that passes from passive to active (Johnson, 2010). We thus speculate that environmental (e.g., social and educational) factors may be of relevance, although their interactions with the genetic endowment would obviously be critical for phenotypic alterations in relative cognitive ability.
Limitations and future research
It has long been proposed that MRI methods could yield apparent cortical thinning during adolescence that is partly explained by developmental myelination at the grey-white matter boundary (white matter encroachment; Gogtay and Thompson, 2010; Panizzon et al., 2009; Sowell et al., 2001, 2004; Thompson et al., 2007). Such a process is thought to increase the T1-weighted MRI signal of lower cortical layers, making it more likely for those boundary voxels to be classified as white matter. While such a process likely occurs, it would be unlikely to account for the tendency observed here for cortical thickening in individuals whose FSIQ scores increased. Further, Tamnes et al. (2010) showed, by means of concurrent estimation of developmental trajectories for cortical thickness and white matter volume and integrity, that longitudinal increases in white matter structure did not account for the observed cortical thinning. Therefore, although indirect effects of wiring and myelin proliferation at the lower cortical layers might affect MRI signal and tissue segmentation, in the present case it is more likely that the cognitive changes were related to true change in the microstructure and thickness of the cortical mantle, and not only in surrounding white matter.
A further methodological consideration is that difference scores such as we used can be to some extent unreliable, due to the compounding of error variance in both time 1 and time 2 measures as well as the tendency for extreme scorers to regress towards the mean. Moreover, our sample was rather restricted in range of cognitive ability, with average FSIQ scores around .8 standard deviations above the population mean at both time points, and standard deviations about 20% smaller than in the population at large. In general, these problems should act to attenuate the associations in which we were interested. Albeit we addressed this by considering separate groups of participants who likely showed reliable increases in FSIQ, no reliable change, and reliable decreases, it remains to be answered whether the associations observed here, as well as their magnitude, would be amplified in more heterogeneous samples. We showed, on the other hand, that our results were not sensitive to the presence of outliers and the use of different smoothing kernels, which supports the robustness of the findings.
Despite these limitations, this research supports the view that developmental fluctuations in IQ scores are reflected in changes in brain structure. Specifically, here we show, for the first time, that longitudinal general cognitive ability changes were associated with cortical thickness changes in brain structures that are known to be related to individual differences in intelligence in cross-sectional correlational studies. Future research should investigate observed changes in adulthood and include other MRI-based indices that provide information about potential changes in subcortical morphology, white matter integrity and functional connectivity, given that these have been shown to correlate with general cognitive ability in cross-sectional studies (Burgaleta et al., 2013; Karama et al., 2009, 2011; Chiang et al., 2009; Tamnes et al., 2010; Yu et al., 2008). Furthermore, this research could be complemented by studying longitudinal changes in specific cognitive skills, independent of general intelligence (e.g., by studying changes in residual scores obtained after regressing out FSIQ upon VIQ and PIQ). Although we reported results for VIQ and PIQ in an effort to increase comparability with the study of Ramsden et al. (2011), these measures are highly correlated and load strongly on a general factor of intelligence, and thus should not be regarded as proper indices of specific skills. Finally, in order to better understand the underpinnings of general cognitive ability changes, future research on how social, educational, and genetic factors influence changes in intelligence via their impact on brain development is necessary.
Figure 6.
Correlations between change in CTh and change in verbal IQ (left), and performance IQ (right).
Highlights.
Intelligence quotient (IQ) scores tend to remain stable across the lifespan
-
-
Despite high stability, some individuals display strong longitudinal changes in IQ
-
-
These have been frequently interpreted as measurement error
-
-
Here, changes in IQ were associated with local rates of cortical thinning
-
-
Some IQ changes are therefore genuine and have a neuroanatomical substrate
Acknowledgments
This project was conducted by the Brain Development Cooperative Group and supported by the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract #s N01-HD02-3343, N01-MH9-0002, and N01-NS-9-2314, -2315, -2316, -2317, -2319 and -2320). Special thanks to the NIH contracting officers for their support. S.K. was supported by a Fellowship from the Fonds de Recherche en Santé du Québec. M.B. was supported by the Spanish Ministry of Education and Science (grant FPU-AP2006). We also acknowledge the important contribution and remarkable spirit of John Haselgrove, Ph.D. (deceased).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For the Brain Development Cooperative Group (http://www.brain-child.org/brain_group.html)
Conflicts of interest: Authors declare that they have no conflicting financial interests.
References
- Acuna BD, Eliassen JC, Donoghue JP, Sanes JN. Frontal and parietal lobe activation during transitive inference in humans. Cerebral Cortex. 2002;12:1312–1321. doi: 10.1093/cercor/12.12.1312. [DOI] [PubMed] [Google Scholar]
- Ad-Dab'bagh Y, Lyttelton O, Muehlboeck J-S, Lepage C, Einarson D, Mok K, Ivanov O, Vincent RD, Lerch J, Fombonne E, Evans AC. The CIVET Image-Processing Environment: A Fully Automated Comprehensive Pipeline for Anatomical Neuroimaging Research. In: Corbetta M, editor. Proceedings of the 12th Annual Meeting of the Organization for Human Brain Mapping. Italy: Florence; 2006. p. S45. [Google Scholar]
- Anderson BJ, Eckburg PB, Relucio KI. Learning & memory. Vol. 9. Cold Spring Harbor, NY: 2002. Alterations in the thickness of motor cortical subregions after motor-skill learning and exercise; pp. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BJ, Li X, Alcantara AA, Isaacs KR, Black JE, Greenough WT. Glial hypertrophy is associated with synaptogenesis following motor-skill learning, but not with angiogenesis following exercise. Glia. 1994;11:73–80. doi: 10.1002/glia.440110110. [DOI] [PubMed] [Google Scholar]
- Barbey AK, Colom R, Solomon J, Krueger F, Forbes C, Grafman J. An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain. 2012;135:1154–1164. doi: 10.1093/brain/aws021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain Development Cooperative Group. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cereb Cortex. 2012;22:1–12. doi: 10.1093/cercor/bhr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen J. On the control of control: The role of dopamine in regulating prefrontal function and working memory. In: Monsell S, Driver J, editors. Attention and performance XVIII: Control of cognitive processing. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- Burgaleta M, MacDonald PA, Martínez K, Román FJ, Álvarez-Linera J, Ramos González A, Karama S, Colom R. Subcortical regional morphology correlates with fluid and spatial intelligence. Human Brain Mapping. 2013 doi: 10.1002/hbm.22305. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceci S. Schooling and intelligence. Psychological Science Agenda. 1992;5:7–9. [Google Scholar]
- Ceci S, Williams W. Schooling, intelligence, and income. American Psychologist. 1997;52(10):1051–1058. [Google Scholar]
- Chiang M, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, Wright MJ, Srivastava A, Balov N, Thompson PM. Genetics of brain fiber architecture and intellectual performance. Journal of Neuroscience. 2009;29:2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chklovskii DB, Mel BW, Svoboda K. Cortical rewiring and information storage. Nature. 2004;431:782–788. doi: 10.1038/nature03012. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychological review. 1992;99:45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- Colom R, Flores-Mendoza C, Rebollo I. WM and Intelligence. Personality and Individual Differences. 2003;34 [Google Scholar]
- Colom R, Rebollo I, Palacios A, Juan-Espinosa M, Kyllonen P. WM is (almost) perfectly predicted by g. Intelligence. 2004;32:277–296. [Google Scholar]
- Colom R, Haier RJ, Head K, Álvarez-Linera J, Quiroga MA, Shih PC, Jung RE. Gray matter correlates of fluid, crystallized, and spatial intelligence: Testing the P-FIT model. Intelligence. 2009;37:124–135. [Google Scholar]
- Deary IJ, Whalley LJ, Lemmon H, Crawford JR, Starr JM. The stability of individual differences in mental ability from childhood to old age: follow-up of the 1932 Scottish Mental Survey. Intelligence. 2000;28:49–55. [Google Scholar]
- Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, Penke L, Rafnsson SB, Starr JM. Age-associated cognitive decline. Br. Med. Bull. 2009;92(1):135–152. doi: 10.1093/bmb/ldp033. [DOI] [PubMed] [Google Scholar]
- Dong WK, Greenough WT. Plasticity of nonneuronal brain tissue: roles in developmental disorders. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10:85–90. doi: 10.1002/mrdd.20016. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway AR. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. Journal of experimental psychology General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Evans AC Brain Development Cooperative Group. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Feczko E, Augustinack JC, Fischl B, Dickerson BC. An MRI-based method for measuring volume, thickness and surface area of entorhinal, perirhinal, and posterior parahippocampal cortex. Neurobiology of Aging. 2009;30:420–431. doi: 10.1016/j.neurobiolaging.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JR. What is intelligence? Cambridge: Cambridge University Press; 2007. [Google Scholar]
- Gläscher J, Rudrauf D, Colom R, Paul LK, Tranel D, Damasio H, Adolphs R. Distributed neural system for general intelligence revealed by lesion mapping. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4705–4709. doi: 10.1073/pnas.0910397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Thompson PM. Mapping gray matter development: Implications for typical development and vulnerability to psychopathology. Brain and Cognition. 2010;72:6–15. doi: 10.1016/j.bandc.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Karama S, Leyba L, Jung RE. MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Res Notes. 2009;2:174. doi: 10.1186/1756-0500-2-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier RJ, Siegel B, Jr, Nuechterlein KH, Hazlett E, Wu JC, Paek J, Browning HL, Buchsbaum MS. Cortical glucose metabolic rate correlates of abstract reasoning and attention studied with positron emission tomography. Intelligence. 1988;12:199–210. [Google Scholar]
- Haworth CM, Wright MJ, Luciano M, Martin NG, de Geus EJ, van Beijsterveldt CE, Bartels M, Posthuma D, Boomsma DI, Davis OS, Kovas Y, Corley RP, Defries JC, Hewitt JK, Olson RK, Rhea SA, Wadsworth SJ, Iacono WG, McGue M, Thompson LA, Hart SA, Petrill SA, Lubinski D, Plomin R. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular Psychiatry. 2010;15(11):1112–1120. doi: 10.1038/mp.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Ann Rev of Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hill J, Inder T, Neil J, Dierker D, Harwell J, Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1001229107. published ahead of print July 12, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of comparative neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Dendritic and synaptic pathology in mental retardation. Pediatric Neurol. 1991;7:79–85. doi: 10.1016/0887-8994(91)90001-2. [DOI] [PubMed] [Google Scholar]
- Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1992;12:110–119. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- Jacobs B, Schall M, Scheibel AB. A quantitative dendritic analysis of Wernicke’s area in humans. II. Gender, hemispheric, and environmental factors. J Comparative Neurol. 1993;327:97–111. doi: 10.1002/cne.903270108. [DOI] [PubMed] [Google Scholar]
- Johnson W. Undertstanding the genetics of intelligence: Can height help? Can corn oil? Current Directions in Psychological Science. 2010;19:177–182. [Google Scholar]
- Jokela M, Batty GD, Deary IJ, Gale CR, Kivimaki M. Low childhood IQ and early adult mortality: the role of explanatory factors in the 1958 British Birth Cohort. Pediatrics. 2009;124:e380–e388. doi: 10.1542/peds.2009-0334. [DOI] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: Converging neuroimaging evidence. The Behavioral and brain sciences. 2007;30:135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Karama S, Ad-Dab'bagh Y, Haier RJ, Deary IJ, Lyttelton OC, Lepage C, Evans AC. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 2009;37:145–155. doi: 10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Colom R, Johnson W, Deary IJ, Haier RJ, Waber DP, Lepage C, Ganjavi H, Jung R, Evans AC The Brain Development Cooperative Group. Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in health children aged 6 to 18. NeuroImage. 2011;55(4):1–11. doi: 10.1016/j.neuroimage.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab'bagh Y, MacDonald D, Lee JM, Kim SI, Evans AC. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cognitive Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- la Fougere C, Grant S, Kostikov A, Schirrmacher R, Gravel P, Schipper HM, Reader A, Evans A, Thiel A. Where in-vivo imaging meets cytoarchitectonics: the relationship between cortical thickness and neuronal density measured with high-resolution [18F]flumazenil-PET. Neuroimage. 2011;56:951–960. doi: 10.1016/j.neuroimage.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. The changing impact of genes and environment on brain development during childhood and adolescence: Initial findings from a neuroimaging study of pediatric twins. Developmental Psychopathology. 2008;20(4):1161–1175. doi: 10.1017/S0954579408000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Yiu AP, Martinez-Canabal A, Pekar T, Bohbot VD, Frankland PW, Henkelman RM, Josselyn SA, Sled JG. Maze training in mice induces MRI-detectable brain shape changes specific to the type of learning. Neuroimage. 2011;54:2086–2095. doi: 10.1016/j.neuroimage.2010.09.086. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Bodammer NC, Kühne S, Kaufmann J, Schütze H, Tempelmann C, Heinze HJ, Düzel E, Schmiedek F, Lindenberger U. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010;48:3878–3883. doi: 10.1016/j.neuropsychologia.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Lyttelton OC, Karama S, Ad-Dab'bagh Y, Zatorre RJ, Carbonell F, Worsley K, Evans AC. Positional and surface area asymmetry of the human cerebral cortex. Neuroimage. 2009;46:895–903. doi: 10.1016/j.neuroimage.2009.03.063. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Whitaker KJ, Bunge SA. Experience-dependent plasticity in white matter microstructure: reasoning training alters structural connectivity. Frontiers in Neuroanatomy. 2012;6:32. doi: 10.3389/fnana.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- Meyer G. Forms and spatial arrangement of neurons in the primary motor cortex of man. J Comp Neurol. 1987;262:402–428. doi: 10.1002/cne.902620306. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Developmental Psychology. 2002;38(1):115–142. [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual review of neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Neubauer AC, Fink A. Intelligence and neural efficiency: Measures of brain activation versus measures of functional connectivity in the brain. Intelligence. 2009;37:223–229. [Google Scholar]
- Nguyen TV, McCracken J, Ducharme S, Botteron KN, Mahabir M, Johnson W, Israel M, Evans AC, Karama S. Testosterone-Related Cortical Maturation Across Childhood and Adolescence. Cereb Cortex. In Press doi: 10.1093/cercor/bhs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly RC, Braver TS, Cohen JD. A biologically-based computational model of working memory. In: Miyake A, Shah P, editors. Models of working memory: Mechanisms of active maintenance and executive control. New York, NY: Cambridge University Press; 1999. [Google Scholar]
- Østby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB. Heterogeneity in subcortical brain development: A structural magnetic resonance imaging study of brain maturation from 8 to 30 years. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HBM, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. PNAS. PNAS. 2011;108(32):13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Kostovic I, Uylings HBM. Lifespan Alterations of Basal Dendritic Trees of Pyramidal Neurons in the Human Prefrontal Cortex: A Layer-Specific Pattern. Cerebral Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- Prado J, Chadha A, Booth JR. The brain network for deductive reasoning: a quantitative meta-analysis of 28 neuroimaging studies. Journal of cognitive neuroscience. 2011;23:3483–3497. doi: 10.1162/jocn_a_00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Ramsden S, Richardson FM, Josse G, Thomas MS, Ellis C, Shakeshaft C, Seghier ML, Price CJ. Verbal and non-verbal intelligence changes in the teenage brain. Nature. 2011;479:113–116. doi: 10.1038/nature10514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN. How does your cortex grow? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental Trajectories of the Human Cerebral Cortex. The Journal of Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Trajectories of Cerebral Cortical Development in Childhood and Adolescence and Adult Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.04.007. Epub ahead of print. http://dx.doi.org/10.1016/j.biopsych.2013.04.007. [DOI] [PMC free article] [PubMed]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localization in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer JM, Ree MJ, Caretta TR. Cognitive components tests are not much more than g: An extension of Kyllonen's analysis. The Journal of general psychology. 1996;123:193–205. [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R. Training of working memory impacts structural connectivity. Journal of Neuroscience. 2010;30:3297–3303. doi: 10.1523/JNEUROSCI.4611-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Walhovd KB, Westlye LT, Due-Tønnessen P, Fjell AM. Intellectual abilities and white matter microstructure in development: A diffusion tensor imaging study. Human Brain Mapping. 2010;31:1609–1625. doi: 10.1002/hbm.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Dutton RA, Chiang MC, Leow AD, Sowell ER, De Zubicaray G, Becker JT, Lopez OL, Aizenstein HJ, Toga AW. Tracking Alzheimer's disease. Annals of the New York Academy of Sciences. 2007;1097:183–214. doi: 10.1196/annals.1379.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D'Onofrio B, Gottesman II. Socioeconomic status modifies heritability of IQ in young children. Psychol Sci. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Kahn RS, Pol H. Efficiency of functional brain networks and intellectual performance. Journal of Neuroscience. 2009;29:7619–7624. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soelen IL, Brouwer RM, van Baal GC, Schnack HG, Peper JS, Collins DL, Evans AC, Kahn RS, Boomsma DI, Hulshoff Pol HE. Genetic influences on thinning of the cerebral cortex during development. Neuroimage. 2012;59:3871–3880. doi: 10.1016/j.neuroimage.2011.11.044. [DOI] [PubMed] [Google Scholar]
- Waber D, De Moor C, Forbes P, Almli C, Botteron K, Leonard G, Milovan D, Paus T, Rumsey J, Group BDC. The NIH MRI study of normal brain development: Performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. Journal of the International Neuropsychological Society. 2007;13:729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- Waber DP, Forbes PW, Almli CR, Blood EA. Four-year longitudinal performance of a population-based sample of healthy children on a neuropsychological battery: the NIH MRI study of normal brain development. J Int Neuropsychol Soc. 2012;18:179–190. doi: 10.1017/S1355617711001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio: Harcourt Brace and Company; 1999. [Google Scholar]
- Whalley LJ, Deary IJ. Longitudinal cohort study of childhood IQ and survival up to age 76. Bmj. 2001;322:819. doi: 10.1136/bmj.322.7290.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Li J, Liu Y, Qin W, Li Y, Shu N, Jiang T, Li K. White matter tract integrity and intelligence in patients with mental retardation and healthy adults. NeuroImage. 2008;40:1533–1541. doi: 10.1016/j.neuroimage.2008.01.063. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen-Berg H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nature Neuroscience. 2012;15(4):528–536. doi: 10.1038/nn.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]