Abstract
T cells can be redirected to overcome tolerance to cancer by engineering with integrating vectors to express a chimeric antigen receptor (CAR). In preclinical models, we have previously demonstrated that transfection of T cells with messenger RNA (mRNA) coding for a CAR is an alternative strategy that has antitumor efficacy and the potential to evaluate the on-target off-tumor toxicity of new CAR targets safely due to transient mRNA CAR expression. Here, we report the safety observed in four patients treated with autologous T cells that had been electroporated with mRNA coding for a CAR derived from a murine antibody to human mesothelin. Due to the transient nature of CAR expression on the T cells, subjects in the clinical study were given repeated infusions of the CAR-T cells in order to assess their safety. One subject developed anaphylaxis and cardiac arrest within minutes of completing the 3rd infusion. Although human anti-mouse IgG antibodies have been known to develop with CAR-transduced T cells, they have been thought to have no adverse clinical consequences. This is the first description of clinical anaphylaxis resulting from CAR-modified T cells, most likely through IgE antibodies specific to the CAR. These results indicate that the potential immunogenicity of CARs derived from murine antibodies may be a safety issue for mRNA CARs, especially when administered using an intermittent dosing schedule.
Introduction
T cells engineered with chimeric antigen receptors (CARs) represent a promising novel form of adoptive immunotherapy (1). The CAR tumor binding function is usually accomplished by the inclusion of a single chain antibody variable fragment (scFv), often of murine origin. Although there are several reports where CAR T cells containing an scFv with murine sequences have been given to cancer patients, and antibodies (IgG’s) to the CAR have been detected (2, 3), to date adverse effects of these antibodies have not been reported in human studies. Similarly, human subjects given infusions of T cells engineered to express murine T cell receptors have developed antibodies to the T cell receptors without adverse effects (4).
Mesothelin is a tumor-associated antigen that is overexpressed in a variety of malignancies including malignant pleural mesothelioma, pancreatic, ovarian, and lung cancer (5, 6). We developed an investigational agent consisting of autologous T cells expressing an anti-mesothelin CAR using lentiviral vector engineering (7). Mesothelin has relatively limited and low level expression in normal tissues, including the mesothelial cells that line the peritoneal, pleural, and pericardial cavities (6). It is a target of a natural immune response in mesothelioma and ovarian cancer (8), and has been proposed as a target for cancer immunotherapy (9). In studies testing a mesothelin specific antibody drug conjugate, the reagent was well tolerated with dose-limiting toxicity consisting of pleuritis (8). Because we have observed persistent B cell aplasia following anti-CD19 CAR T cell infusions (9, 10), an on-target, off-tumor toxicity, we developed an approach to transiently express the anti-mesothelin CAR on T cells by using electroporation of anti-mesothelin CAR mRNA. This approach offers the opportunity to test the safety and potential antitumor effects of mesothelin directed CAR T cells (meso-RNA-CAR-T) (11). In preclinical models, we demonstrated that multiple infusions of anti-mesothelin and anti-CD19 RNA CAR T cells have potent anti-tumor effects (11, 12).
Based on the above, we have been conducting a first-in-human study to test the safety of meso-RNA-CAR-T (clinicaltrials.gov NCT01355965). Our approach is to test multiple infusions of T-cells electroporated with mesothelin CAR mRNA, maximizing safety by allowing CAR expression for only a limited period. The intent of our study was that if adverse events were noted, we could terminate T-cell infusions with the expectation that toxicity would rapidly abate because mRNA CAR expression is limited to a few days, thus rendering adverse effects self- limiting. Here we report the first incidence, to our knowledge, of anaphylactic shock following CAR T cell infusion, a toxicity that could not be managed by terminating T-cell infusions.
Methods
RNA CAR T cell manufacturing
Autologous T cells were engineered to express an extracellular single chain antibody (scFv) with specificity for mesothelin (13, 14), along with a transmembrane domain and an intracellular signaling molecule comprised of the 4-1BB and TCRζ signaling modules (7, 15). The scFv is derived from the murine monoclonal antibody SS1, and thus contains murine sequences, while the cytoplasmic T cell transgene signaling domains are entirely native human sequences. These studies used the same antibody region used in previous studies (8, 16), but the antibody sequences were in the form of an scFv displayed on T cells rather than a soluble antibody-toxin conjugate. The CAR T cells were stimulated with bead immobilized anti-CD3 and anti-CD28 antibodies and cultured for 10 days in cell culture medium supplemented with human serum, electroporated with mRNA encoding the mesothelin CAR and then cryopreserved in human serum albumin (11).
Clinical protocols
Subjects were enrolled on two clinical protocols using T cells transduced with the mRNA encoding the mesothelin CAR (NCT01355965=UPCC 17510 and UPCC 21211 is a compassionate use protocol for a single patient). All subjects had serum collected at multiple pre-determined time points after infusion for monitoring of cytokine production resulting from immune modulation and T cell activation.
Soluble factor analysis
Whole blood was collected in red top (no additive) BD vacutainer tubes (Becton Dickinson), processed to obtain serum using established laboratory standard operating procedure (SOP), aliquoted for single use and stored at −80°C. Quantification of soluble cytokine factors was performed using Luminex bead array technology and kits purchased from Life Technologies. Assays were performed as per the manufacturer protocol with a 9-point standard curve generated using a 3-fold dilution series as previously described (10).
Tryptase was measured retrospectively from serum samples collected as scheduled and cryopreserved after the SAE. Serum samples were sent to ARUP laboratories (Salt Lake City, Utah) for measurement of tryptase levels. Cryopreserved or fresh serum samples were sent to Quest Diagnostics (Horsham, PA) or LabCorp (Raritan, NJ) for measurement of human anti-mouse antibodies (HAMA) using an ELISA assay specific for human IgG and IgE antibodies.
Results and Discussion
Twenty-one infusions of meso-RNA-CAR-T cells have been given to four patients to date, and with the exception of one infusion, all have been well tolerated. One subject with pancreatic adenocarcinoma was treated with 8 intravenous infusions over 20 days (data not shown). Three subjects with malignant pleural mesothelioma were treated with single infusions of meso-RNA CAR-T cells spaced one week apart as shown (Figure 1). Subjects were then eligible to enroll in an extended cohort with repeated infusions of meso-RNA-CAR-T cells (Figure 1). Of the three subjects, two (101 and 105) were enrolled into the extended cohort; subject 102 developed progressive disease and died prior to enrollment on the extended cohort. Subject 101 tolerated an additional six infusions of meso-mRNA CAR-T cells well with minimal arthralgias and fatigue. However, subject 105 experienced a serious adverse event (SAE) within minutes of the first infusion on the extended cohort; this was his 3rd infusion overall.
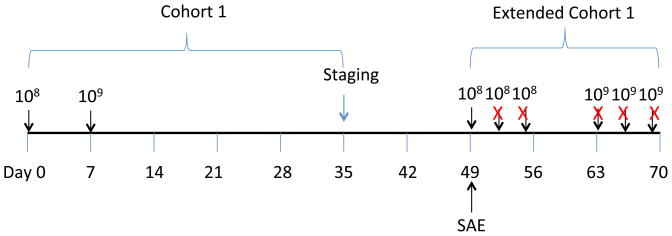
Figure 1. Clinical trial schema.
The subject was originally enrolled onto cohort 1, where 108 meso-RNA-CART cells were administered on Day 0 and 109 meso RNA CART cells were administered on Day 7. Safety assessments were performed between days 0 and 7, and repeat staging was performed by CT scan on day 35. The subject was then enrolled into an extended cohort to receive an additional 6 doses of T cells that were scheduled for Monday/Wednesday/Friday for two weeks. However, the subject developed anaphylaxis immediately after the 3rd infusion (i.e. the first of Extended Cohort 1), and therefore, did not receive any further infusions (marked with an ‘X’).
Case report
Subject UPCC17510-105 is an 81-year-old lifelong non-smoking man with a past medical history notable for asthma and asbestos exposure. He was diagnosed with Stage IV mesothelioma with bilateral pleural disease and mediastinal and peritoneal nodal involvement three years prior to enrollment on this protocol. He was treated with pemetrexed and carboplatin for 10 cycles followed by single-agent pemetrexed maintenance therapy. Six months prior to this event, he was enrolled in a different study where he received gemcitabine and intrapleural adenovirus vector expressing interferon alpha (17).
He was enrolled in the current study and underwent leukapheresis for T cell collection. As part of cohort 1, he received two infusions of meso-RNA CAR-T cells one week apart; the first dose was 1×108 T cells, and the second dose was 1×109 T cells (Figure 1). Each infusion was tolerated well with no side effects. Because of the short-lived nature of the study product, and the fact that he had tolerated prior infusions well, he was given the opportunity for a series of infusions to be given over the course of two weeks according to the amended protocol “Extended cohort 1.”
Forty-nine days after the first infusion, the patient received 1×108 meso-RNA CAR-T cells over 15 minutes (Figure 1). The infused cells were an aliquot of cryopreserved cells from the same lot of cryopreserved cells that were used for the original infusions. Within 1 minute of completing the infusion, he developed plethora, tingling hands, shortness of breath, hypoxia, and then underwent cardiac arrest manifested as pulseless electrical activity. He was intubated, CPR was performed, and he was treated with epinephrine along with aggressive volume resuscitation, high dose steroids, and vasopressors. All microbiology cultures were negative. The patient experienced a rapid recovery; pressors were withdrawn within 2 days and the patient was extubated on day 3. He was discharged to home ten days after the SAE, on room air and a short prednisone taper. Overall, the patient experienced a complete clinical recovery and repeat imaging revealed a transient partial response of mesothelioma.
An Anaphylaxis Event
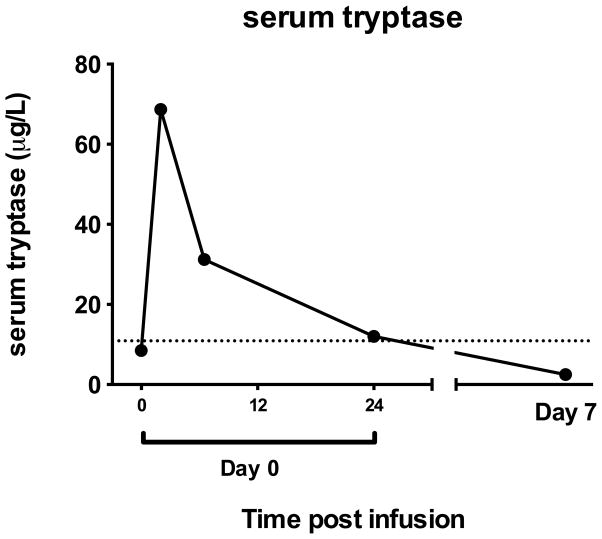
This infusion was the only infusion that was poorly tolerated of 21 infusions of meso-mRNA CAR-T cells in 4 patients. Moreover, the aliquot of meso-mRNA CAR T cells was an aliquot of the same product lot administered in the previous two infusions. Therefore, we hypothesized that the SAE observed in subject 105 was a result of an acquired immune response to a component of the cell product, either related to the CAR itself or a carrier protein in the cell product, such as IgA or albumin. The clinical scenario was most consistent with an IgE-mediated anaphylaxis event triggered by systemic degranulation of mast cells (18) and basophils. To confirm the clinically suspected anaphylaxis event, tryptase levels were measured in cryopreserved sera in samples collected before and after the SAE (tryptase is stable in frozen serum for at least one year). Serum tryptase is the best clinically measurable indicator of recent mast cell degranulation, with levels typically peaking 15–60 minutes after symptom onset and declining with a half-life of about two hours (19). We found that tryptase levels were markedly elevated in the first few hours after the SAE, confirming that the event was anaphylaxis (Figure 2).
Figure 2.
Serum tryptase in blood. Peripheral blood serum samples at baseline and several time points after the SAE were analyzed for levels of serum tryptase. The dashed line indicates the upper limit of normal range (0.4 – 10.9 μg/L).
Cytokine patterns
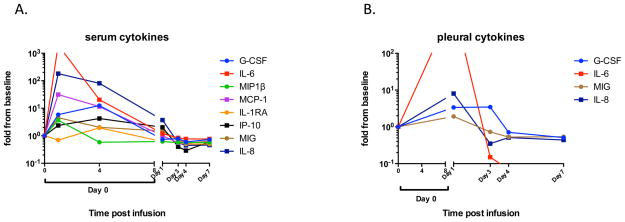
The serum cytokines in all subjects infused were consistent with transient T cell activation, including mild elevations in MIP-1 beta, G-CSF, IL-6, and IL-17 for several days after each infusion (data not shown). In stark contrast, there was a rapid and significant elevation of several cytokines both in serum and in pleural fluid immediately after the 3rd infusion (SAE event) of subject 105. Specifically, IL-6, G-CSF, MIP-1beta, MCP-1, IP-10, MIG, and IL-8 were all elevated up to 1000-fold in serum within the first 24 hours of the event (Figure 3A). Immediately after the event, IL-6 was elevated above the assay range. All cytokine levels returned to baseline by day 4 and remained at baseline through day 35. Cytokine analysis of pleural fluid obtained from the indwelling pleural catheter one day after the event revealed increased levels of IL-6 that were out of range for our assay, and increased levels of IL-8, G-CSF, and MIG (Figure 3B).
Figure 3.
(A) Serum cytokines. Peripheral blood serum samples obtained at baseline and from several time points after the event were analyzed for levels of 30 cytokines. Only cytokines elevated at least 1.5-fold over baseline are shown. IL-6 levels at 1 hour after infusion were above the limit of quantification of the assay. (B) Pleural fluid cytokines. The subject had a pleurex catheter in place and pleural fluid was collected 24 hours after the event and periodically thereafter. The levels of 30 cytokines were measured but only the four that were elevated at least 1.5-fold above baseline are shown. IL-6 levels 24 hours after the event were above the limit of quantification of the assay.
Although the tryptase levels are pathognomonic of mast cell activation, anaphylaxis also produces significant elevations in other cytokines, particularly IL-6, IL-10, and IL-2 (20). In this case both the allergen (CAR-T cells) and the mast cells were potentially immunologically active and capable of secreting cytokines, which confounds determination of the source of cytokines observed in the serum, particularly in the first 24 hours after the infusion.
Detection of human-anti-mouse antibodies
Serum samples were analyzed by ELISA testing for both IgG and IgE specific for mouse serum proteins. Results are shown for each patient in Table 1. Interestingly, subject 105 had a minimally positive IgG HAMA prior to enrollment (day -3), which increased after completing infusions in the first cohort (day 14, day 21). Surprisingly, it returned to normal levels at day 107 (2 months after the SAE event), but was again elevated at day 219 (~7 months). Another IgG HAMA test sent to a different facility (LabCorp) using a fresh clinical specimen obtained at day 141 (3 months after the SAE) returned positive and out-of-range high for the assay (>600 ng/ml). Frozen serum samples were assayed and found to be negative for IgE specific for mouse serum proteins at all the same time points. This is not unexpected because of the low levels of IgE in normal serum. Of note, subject 102 also developed a positive IgG HAMA test at day 21; he did not receive any further CAR-T cell infusions, and therefore the HAMA remained clinically silent in this patient.
Table 1.
Human anti-mouse antibodies (HAMA) levels in serum of three subjects with mesothelioma.
| Day-3 | Day 14 | Day 21 | Day 107 | Day 141 | Day 219 | |
|---|---|---|---|---|---|---|
| 101* | 36 ng/ml | 30 ng/ml | 40 ng/ml | |||
| 102* | 48 ng/ml | 88 ng/ml | ||||
| 105* | 80 ng/ml | 87 ng/ml | 119 ng/ml | 63 ng/ml | 79 ng/ml | |
| 105** | >600 ng/ml |
Normal range for (Quest) HAMA: <75 ng/ml
Normal range for (LabCorp) HAMA: <188 ng/ml
The above results establish that subject 105 had classical clinical and laboratory findings of anaphylaxis. It was possible, though, that the immune response was directed to the activated autologous T cells in addition to or instead of the CAR moiety. We have not previously observed anaphylaxis during our considerable experience with more than 400 patients infused with T cells cultured with immobilized mouse antibodies to CD3 and CD28 on beads, making it unlikely that the response was directed to antibody carry over, especially considering that the monoclonal antibodies are covalently attached to the beads (21). Furthermore, using the same T cell manufacturing process with the exception of RNA electroporation, we found that CD4zeta CARs were not immunogenic, as 41 of 43 patients had decade-long survival of the CARs (22). Another potential source of anaphylaxis induction relates to the in vitro T cell production process; specifically, the CAR T cells were cultured in media containing pooled human serum, and it is well established that patients deficient in IgA can develop anaphylaxis upon exposure to human serum containing IgA. Quantitative immunoglobulins in subject 105 were normal, excluding IgA deficiency as the cause of anaphylaxis. In addition, total IgE levels were also normal, indicating no generalized pre-existing atopy in subject 105. Thus, we have concluded that meso-RNA-CAR-T cells triggered anaphylaxis most likely by inducing an IgE antibody specific for the murine-based antibody sequences present in the CAR-modified T cell product. The elevated levels of human anti-mouse IgG, the finding that these levels increased with exposure to meso-RNA CAR-T cells, and the clinical scenario of anaphylaxis with highly elevated tryptase levels, are all supportive of an IgE-mediated anaphylactic event.
Our data are consistent with the scenario that the IgE antibodies developed as a result of the dosing schedule consisting of 3 infusions given over a period of 49 days. Based on this event, in cases where multiple infusions are expected, we have modified the infusion schedule of RNA-CAR-T cells, such that infusions may not be separated by more than 10 days, and must be completed within 21 days. This schedule creates a window that would be too short for the time required for isotype switching from IgG to IgE. While we have also considered using an ELISA-based HAMA assay as a screening tool, at present this is not feasible because 1) the turnaround time of such an assay produces a long gap between screening and infusion, 2) findings would not be predictive of allergic reactions (23), and 3) many of the positive HAMA antibodies are directed to mouse albumin or the Fc portions of mouse immunoglobulins (24), which are not present in the CAR-T cell product. Alternatively, if meso-RNA CAR-T cells are found to be safe from the standpoint of on-target off-tumor toxicity, a single infusion of stably transduced, long-lived CAR-T cells may be sufficient for efficacy. In such a case, CAR-T cell infusion would not be expected to generate IgE antibodies because of the continuous, persistent exposure to the product, which is one principle of desensitization. Ultimately, constructs based on fully humanized antibodies (25) are expected to have minimal antigenic potential as allergens.
Acknowledgments
Supported by grants from the National Institutes of Health (2R01CA120409 and P01CA0667261). MVM received support from the Conquer Cancer Foundation and NCI K08CA166039. We thank Erica Suppa and Tim Macatee for excellent technical assistance. We thank Mariana Castells and Arnie Levinson for insightful discussions on allergy and anaphylaxis.
Footnotes
Authorship Contributions
MVM directed research and wrote the manuscript, ARH was the principal investigator on the mesothelioma clinical protocol and edited the manuscript, GLB was the principal investigator on the pancreas cancer clinical protocol and edited the manuscript, SMA performed research and edited the manuscript, BLL manufactured the clinical-grade cell product and edited the manuscript, XL and YZ performed research, MK directed research and edited the manuscript and CHJ sponsored the clinical trial and wrote the manuscript.
Disclosure of Conflicts of Interest
Some authors have intellectual property for chimeric antigen receptor technology (BLL, YZ, CHJ and MK). The other authors have no conflict.
References
- 1.Pinthus JH, Waks T, Malina V, Kaufman-Francis K, Harmelin A, Aizenberg I, et al. Adoptive immunotherapy of prostate cancer bone lesions using redirected effector lymphocytes. J Clin Invest. 2004;114:1774–81. doi: 10.1172/JCI22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:6106–15. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–71. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis JL, Theoret MR, Zheng Z, Lamers CH, Rosenberg SA, Morgan RA. Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:5852–61. doi: 10.1158/1078-0432.CCR-10-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang K, Pai LH, Batra JK, Pastan I, Willingham MC. Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer research. 1992;52:181–6. [PubMed] [Google Scholar]
- 6.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:136–40. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:5274–9. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. The New England journal of medicine. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science translational medicine. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan A, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9062–72. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett DM, Zhao Y, Liu X, Jiang S, Carpenito C, Kalos M, et al. Treatment of advanced leukemia in mice with mRNA engineered T cells. Human gene therapy. 2011;22:1575–86. doi: 10.1089/hum.2011.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassan R, Lerner MR, Benbrook D, Lightfoot SA, Brackett DJ, Wang QC, et al. Antitumor activity of SS(dsFv)PE38 and SS1(dsFv)PE38, recombinant antimesothelin immunotoxins against human gynecologic cancers grown in organotypic culture in vitro. Clin Cancer Res. 2002;8:3520–6. [PubMed] [Google Scholar]
- 14.Fan D, Yano S, Shinohara H, Solorzano C, Van Arsdall M, Bucana CD, et al. Targeted therapy against human lung cancer in nude mice by high-affinity recombinant antimesothelin single-chain Fv immunotoxin. Mol Cancer Ther. 2002;1:595–600. [PubMed] [Google Scholar]
- 15.Finney HM, Akbar AN, Lawson ADG. Activation of resting human primary T cells with chimeric receptors: Costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. Journal of Immunology. 2004;172:104–13. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 16.Hassan R, Ebel W, Routhier EL, Patel R, Kline JB, Zhang J, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- 17.Sterman DH, Haas A, Moon E, Recio A, Schwed D, Vachani A, et al. A trial of intrapleural adenoviral-mediated Interferon-alpha2b gene transfer for malignant pleural mesothelioma. American journal of respiratory and critical care medicine. 2011;184:1395–9. doi: 10.1164/rccm.201103-0554CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castells MC, Irani AM, Schwartz LB. Evaluation of human peripheral blood leukocytes for mast cell tryptase. Journal of immunology. 1987;138:2184–9. [PubMed] [Google Scholar]
- 19.Schwartz LB, Yunginger JW, Miller J, Bokhari R, Dull D. Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. The Journal of clinical investigation. 1989;83:1551–5. doi: 10.1172/JCI114051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone SF, Cotterell C, Isbister GK, Holdgate A, Brown SG Emergency Department Anaphylaxis I. Elevated serum cytokines during human anaphylaxis: Identification of potential mediators of acute allergic reactions. The Journal of allergy and clinical immunology. 2009;124:786–92. e4. doi: 10.1016/j.jaci.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 21.Levine BL, Cotte J, Small CC, Carroll RG, Riley JL, Bernstein WB, et al. Large-scale production of CD4+ T cells from HIV-1-infected donors after CD3/CD28 costimulation. Journal of hematotherapy. 1998;7:437–48. doi: 10.1089/scd.1.1998.7.437. [DOI] [PubMed] [Google Scholar]
- 22.Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Science translational medicine. 2012;4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frodin JE, Lefvert AK, Mellstedt H. The clinical significance of HAMA in patients treated with mouse monoclonal antibodies. Cell biophysics. 1992;21:153–65. doi: 10.1007/BF02789485. [DOI] [PubMed] [Google Scholar]
- 24.Hosono M, Endo K, Sakahara H, Watanabe Y, Saga T, Nakai T, et al. Human/mouse chimeric antibodies show low reactivity with human anti-murine antibodies (HAMA) British journal of cancer. 1992;65:197–200. doi: 10.1038/bjc.1992.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanitis E, Poussin M, Hagemann IS, Coukos G, Sandaltzopoulos R, Scholler N, et al. Redirected antitumor activity of primary human lymphocytes transduced with a fully human anti-mesothelin chimeric receptor. Molecular therapy: the journal of the American Society of Gene Therapy. 2012;20:633–43. doi: 10.1038/mt.2011.256. [DOI] [PMC free article] [PubMed] [Google Scholar]