Abstract
Importance
Identification of steep corneal curvatures in a significant percentage of patients with posterior polymorphous corneal dystrophy (PPCD) confirms this previously reported association, and suggests a role for ZEB1 in keratocyte function.
Objective
To determine whether PPCD is characterized by significant corneal steepening.
Design
Cross-sectional study
Setting
University-based and private ophthalmology practices
Participants
Thirty-eight individuals (27 affected and 11 unaffected) from 23 families with PPCD
Interventions
Slit lamp examination and corneal topographic imaging were performed for individuals with PPCD and unaffected family members. Saliva or blood was collected from each individual for DNA isolation and ZEB1 sequencing. Corneal ZEB1 expression was measured using immunohistochemistry.
Main Outcome Measures
Percentage of individuals affected with PPCD and controls with average keratometry value >48.0D in each eye; mean keratometry value averaged for both eyes of individuals with PPCD and controls; correlation of ZEB1 mutation with keratometry value
Results
ZEB1 coding region mutations were identified in 7 of the 27 affected individuals. Ten of the 38 individuals (26.3%) had average keratometry values >48.0D OU: 10/27 (37.0%) individuals with PPCD (6/7 with ZEB1 mutations (85.7%) and 4/20 without ZEB1 mutations (20.0%)) and 0/11 unaffected individuals (p=0.037 for unaffected vs. affected; p=0.004 for PPCD with vs. without ZEB1 mutation). The mean keratometry value of each eye of affected individuals (48.2D) was significantly greater than unaffected family members (44.1D) (p value = 0.029). Affected individuals with ZEB1 mutations demonstrated a mean keratometry value of 53.3D, significantly greater than affected individuals without ZEB1 mutations (46.5D; p value = 0.004). Fluorescence immunohistochemistry demonstrated ZEB1 expression in keratocyte nuclei.
Conclusions and Relevance
Abnormally steep corneal curvatures are identified in 37% of all individuals with PPCD and 86% of affected individuals with PPCD secondary to ZEB1 mutations. ZEB1 is present in keratocyte nuclei, suggesting a role for ZEB1 in keratocyte function. Therefore, ZEB1 may play a role in both corneal stromal and endothelial development and function, and PPCD should be considered both an endothelial dystrophy and an ectatic disorder.
Keywords: posterior polymorphous corneal dystrophy, steep corneal curvature, ZEB1
INTRODUCTION
Posterior polymorphous corneal dystrophy (PPCD; MIM #122000) is an autosomal dominant corneal endothelial dystrophy characterized by well-described corneal endothelial abnormalities. Although the corneal endothelial dystrophies have traditionally been considered isolated disorders of the corneal endothelium, each has been associated with extraocular abnormalities: PPCD with abdominal hernias and Alport syndrome,1–3 and both Fuchs endothelial corneal dystrophy (FECD; MIM #613267) and congenital hereditary endothelial dystrophy (CHED; MIM #217700) with hearing loss.4–6 PPCD has also been associated with a number of other ocular abnormalities, including glaucoma, Terrien’s marginal degeneration and abnormalities of corneal curvature, including keratoconus.7–20 While the association of PPCD with keratoconus was initially reported in the English-language literature almost 40 years ago, the subsequent 9 reports of this association published between 1989 and 2010 consisted of either individual case reports (4) or small case series (3, 3, 4, 5 and 7 subjects).7, 8, 10–15, 18, 20 Given the relative frequency of keratoconus in the general population, estimated to have an incidence of 1 in 2000, as well as its reported association with a variety of ocular and non-ocular disorders, the significance of the reported association with PPCD has been questioned.21 However, mutations in the visual system homeobox gene 1 (VSX1; MIM #605020) have been implicated as playing a pathogenic role in both PPCD and keratoconus, thus providing support to the contention that the reported association between the disorders is more than just coincidental.22–30 In 2011, Raber and colleagues reported 18 patients from 10 families with PPCD who demonstrated steep corneal curvatures, with average keratometry values >48.0 D in each eye of 15 of the 18 patients.16 However, the authors acknowledge that the cohort that they reported included only individuals with PPCD and an average keratometry > 46.0 D and no clinical or topographic evidence of keratoconus. Thus, it is not possible to determine from their study what percentage of individuals with PPCD demonstrate abnormally steep corneal curvatures. Additionally, the authors did not screen the zinc finger E-box binding homeobox 1 gene (ZEB1; MIM 189909), previously known as the transcription factor 8 gene (TCF8), in which pathogenic mutations have been identified in approximately 1/3 of probands with PPCD. In fact, ZEB1 screening has been performed for only 4 individuals reported to date with PPCD associated with steep corneal curvatures.14, 20 Thus, it is not known whether abnormalities of corneal curvature are associated with PPCD that has been linked to chromosome 20 (the PPCD1 locus) or to PPCD associated with mutations in ZEB1 (located on chromosome 10, also known as the PPCD3 locus). Therefore, we performed corneal topographic imaging for all available affected probands and affected and unaffected family members from 45 families with PPCD. We also screened the ZEB1 coding and promoter regions in all 45 probands and determined segregation of identified presumed pathogenic variants in all available affected and unaffected family members.
MATERIALS AND METHODS
The authors followed the tenets of the Declaration of Helsinki in the treatment of the subjects reported herein. Study approval was obtained from the Institutional Review Board at The University of California, Los Angeles (UCLA IRB # 94-07-243-(14-33A), 02-10-092-(4,11), 10-001932).
Patient Identification/ZEB1 Coding and Promoter Region Screening
The diagnosis of PPCD was based on the presence of characteristic corneal endothelial changes in one or both eyes.1 Additionally, the diagnoses of clinical and topographic keratoconus were based on previously published criteria.31 After informed consent was obtained, either a peripheral blood sample, saliva sample (Oragene saliva collection kits; DNA Genotek, Inc., Ontario, Canada) or buccal epithelial sample (Cyto-SoftTM Cytology Brush; Medical Packaging Corporation, Camarillo, CA) was collected as a source of genomic DNA. After genomic DNA was prepared from the buccal epithelial cells and peripheral blood leukocytes using the QIAamp DNA Blood Mini Kits and FlexiGene DNA (Qiagen, Valencia, CA), respectively, each of the nine exons of ZEB1 and the 1 Kb upstream of the initiation methionine were amplified using previously described primers and conditions.1, 32 After purification of the PCR products, DNA sequencing was performed on an ABI-3100 Genetic Analyzer (Applied Biosystems). The coding region nucleotide sequence (including the donor and acceptor splice sites) was read manually by comparison to the ZEB1 cDNA sequences (GenBank accession numbers NM_001128128.2 and NM_030751), while promoter region sequences were compared to the ZEB1 RefSeqGene sequence (GenBank accession number NG_017048.1).
Corneal Topographic Imaging
A recruitment letter regarding the performance of corneal topography was sent to all individuals enrolled into the investigators’ ongoing study on PPCD for whom corneal topographic imaging had not been previously performed. Individuals who expressed interest in participating were encouraged to come to the Jules Stein Eye Institute for topographic imaging. For patients who were unable to travel to the Jules Stein Eye Institute, prior corneal topographies were obtained, when available, or patients were encouraged to return to their local ophthalmologist for corneal topographic imaging. For patients examined at the Jules Stein Eye Institute, corneal topographic imaging was performed using a commercially available corneal topographer (Marco OPD-Scan III, Jacksonville, FL). After a single topographic image was obtained of each cornea, the average keratometry value was determined using the mean of the simulated K readings from the keratometric map. Eyes in which penetrating keratoplasty was performed prior to topographic imaging or in which other pathology, such as bullous keratopathy, prevented an accurate assessment of the corneal curvature were excluded.
Immunohistochemical detection of ZEB1 in the Corneal Stroma
A donor cornea obtained from a commercial eye bank was fixed in 4% PFA and subsequently placed in 30% sucrose for cryoprotection. Immunodetection of ZEB1 (ab87280; Abcam, Inc., Cambridge, MA) and CD34 (keratocyte marker; 3569; Cell Signaling Technology, Beverly, MA) was performed using a standard immunohistochemistry protocol. Briefly, sections were rehydrated in PBS + 0.3% TritonX100, washed 2× in PBS and blocked in PBS + 0.05% Tween-20 supplemented with 1% BSA and 10% normal serum. The sections were then incubated overnight with each primary antibody diluted 1:100 in blocking buffer, washed 1× in PBS + 0.05% Tween-20 followed by 2× in PBS. The sections were subsequently incubated with the secondary antibody (Alexa Fluor 488 or 594; Life Technologies, Carlsbad CA), diluted 1:500 in blocking buffer, washed 1× in PBS + 0.05% Tween-20 followed by 2× in PBS and mounted with Vectashield (Vector Laboratories, Inc., Burlingame, CA) aqueous mounting medium containing 40, 6-diamidino-2-phenylindole (DAPI). A negative control consisting of a species-specific normal IgG (Jackson ImmunoResearch, West Grove, PA) was used at the same concentration as each primary antibody. Fluorescence imaging was performed on an epifluorescence Zeiss microscope (Axio Imager.A2, Carl Zeiss, Oberkochen, Germany).
Statistical Analyses
Fisher’s exact test was used to compare the percentage of affected and unaffected individuals with PPCD with average keratometry values greater than 48.0 D in each eye and the percentage of affected individuals with and without ZEB1 mutations with average keratometry values greater than 48.0 D in each eye. The student t-test was used to determine the significance of the difference between the mean keratometry value of eyes of affected individuals and unaffected family members as well as for eyes of affected individuals with and without ZEB1 mutations. P values less than 0.05 were considered significant.
RESULTS
Corneal Topographic and Slit Lamp Imaging
Corneal topographic imaging was performed for 38 individuals (27 affected and 11 unaffected) from 23 of the 45 families with PPCD recruited to date. Ten of the 38 subjects (26.3%) for whom corneal topographic imaging was performed demonstrated average keratometry values greater than 48.0 D in each eye, including a significantly greater percentage of individuals with PPCD (37.0%; 10/27) compared with unaffected individuals (0%; 0/11) (Fisher’s exact test: p=0.037). Corneal topographic imaging was performed for affected family members of two of these 10 subjects, demonstrating average keratometry values greater than 48.0 D in each eye of these individuals as well. The mean keratometry value of each eye of affected individuals measured 48.2 D (SD = 5.8 D), which was significantly greater than the mean keratometry value of 44.1 D (SD = 2.2 D) for unaffected family members (t-test p value = 0.029).
Slit lamp examination of the 10 individuals with PPCD who demonstrated average keratometry values greater than 48.0 D in each eye revealed characteristic clinical features of keratoconus in both eyes of two individuals (Table 1). In both of these patients, corneal topographic imaging demonstrated central corneal steepening, consistent with keratoconus. Additionally, imaging of the posterior corneal surface in one patient using slit scanning topography (Orbscan, Bausch & Lomb, Rochester, New York) demonstrated significant elevation of the posterior corneal profile compared to a best fit sphere (OD 0.172 mm; OS 0.078 mm), the apex of which corresponded in location to the thinnest portion of the cornea in each eye (OD 354 microns; OS 403 microns). A third patient demonstrated inferior nasal steeping on corneal topographic imaging, but did not demonstrate any clinical features of keratoconus on slit lamp biomicroscopy (Figures 1 and 2).
Table 1.
Clinical features of patients with PPCD and steep corneal curvature (> 48.0 D OU)
| Patient | Sex | Eye | Simulated Keratometry | Keratoconus | ZEB1 Mutation | |||
|---|---|---|---|---|---|---|---|---|
| Steep K | Flat K | Average K | Clinical Features | Topographic Features | ||||
| 1 | M | OD | 50.50 | 49.50 | 50.00 | No | No | p.(Gln12*) |
| OS | 50.75 | 48.25 | 49.50 | No | No | |||
| 2* | M | OD | 52.08 | 48.70 | 50.39 | No | Yes (Inferonasal steepening) | p.(Gln884fs) |
| OS | 49.34 | 47.87 | 48.61 | No | Yes (Inferonasal steepening) | |||
| 3 | M | OD | 51.50 | 54.00 | 52.75 | No | No | No |
| OS | 51.25 | 52.25 | 51.75 | No | No | |||
| 4 | M | OD | 51.25 | 55.75 | 53.50 | No | No | p.(Gln214*) |
| OS | 55.25 | 55.72 | 55.49 | No | No | |||
| 5 | M | OD | 55.00 | 56.00 | 55.50 | No | No | No |
| OS | 53.50 | 53.50 | 53.50 | No | No | |||
| 6 | F | OD | 57.00 | 57.00 | 57.00 | No | No | p.(Glu495fs) |
| OS | 55.50 | 58.00 | 56.75 | No | No | |||
| 7** | F | OD | 56.20 | 57.98 | 57.09 | No | No | p.(Arg325*) |
| OS | 58.02 | 59.32 | 58.67 | No | No | |||
| 8** | F | OD | 58.46 | 61.26 | 59.86 | No | No | p.(Arg325*) |
| OS | 56.02 | 59.21 | 57.62 | No | No | |||
| 9 | F | OD | 58.60 | 56.80 | 57.70 | Yes (conical corneal deformation; central and interior corneal thining; s/p hydrops) | Yes (Central corneal steepening; anterior protrusion of posterior corneal profile) | No |
| OS | 55.40 | 53.50 | 54.45 | Yes (conical corneal deformation; central and interior corneal thining; s/p hydrops) | Yes (Central corneal steepening; anterior protrusion of posterior corneal profile) | |||
| 10 | F | OD | 59.31 | 56.72 | 58.02 | Yes (conical corneal deformation) | Yes (Central corneal steepening) | No |
| OS | 64.90 | 59.52 | 62.21 | Yes (conical corneal deformation) | Yes (Central corneal steepening) | |||
Figure 1.
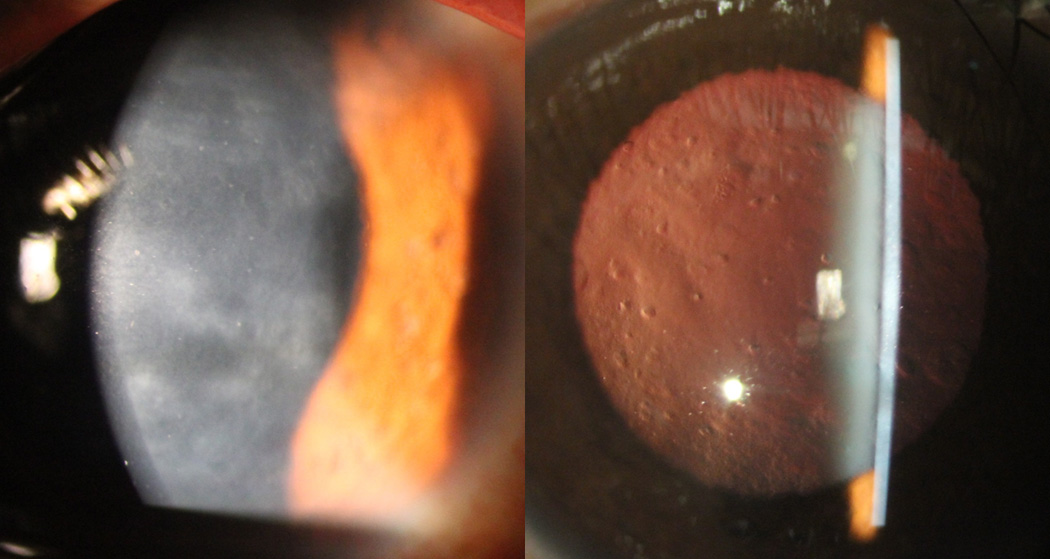
Slit lamp photomicrographs of individual with PPCD demonstrating confluent areas of grey-white opacification at the level of Descemet membrane (right). Scattered endothelial vesicles are seen with retroillumination against the red reflex (left). Screening of ZEB1 revealed the novel mutation p.(Gln884Argfs*37).
Figure 2.
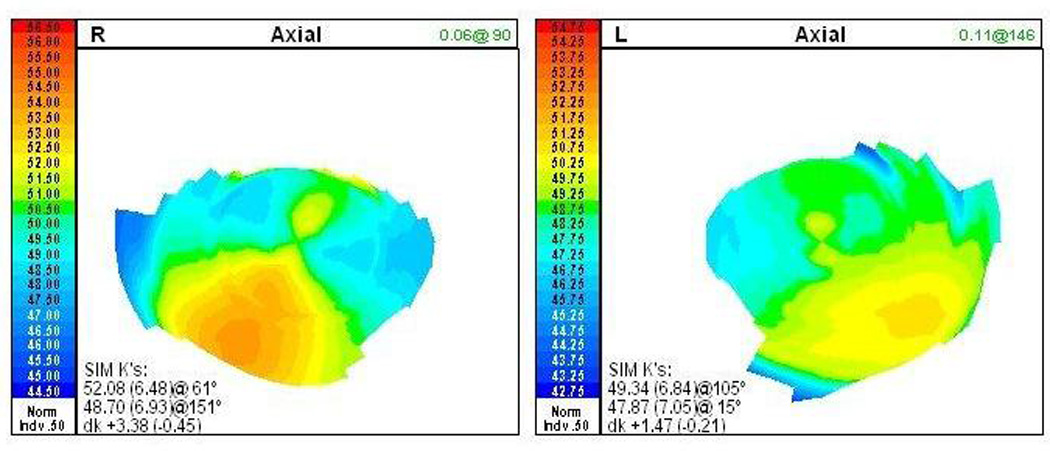
Corneal topographic imaging of the affected individual shown in Figure 1 demonstrating inferior temporal steepening in each cornea, with average keratometry values measuring 50.39 D in the right eye and 48.61 D in the left eye. No clinical features of keratoconus were noted on slit lamp biomicroscopic imaging of either cornea.
While corneal topographic imaging was not performed An insufficient number of individuals were recruited from each family in which an individual with PPCD and steep corneal curvature was identified to confirm that all affected relatives with PPCD had steep corneas as well. However, in both families in which two affected individuals underwent corneal topographic imaging, both individuals had steep corneas. Additionally, in the family in which an unaffected individual underwent corneal topographic imaging, the individual did not demonstrate a steep cornea in either eye.
ZEB1 Coding and Promoter Region Screening
Screening of the ZEB1 coding region demonstrated nonsense mutations in 7 of the 27 (26%) affected individuals, including 6 of the 23 (26%) probands. Screening of the ZEB1 promoter region in each affected individual did not reveal any presumed pathogenic sequence variants.32 A significantly greater percentage of affected individuals with a ZEB1 mutation (85.7%; 6/7) demonstrated average keratometry values greater than 48.0 D in each eye compared with affected individuals without a ZEB1 mutation (20.0%; 4/20) (Fisher’s exact test: p =0.004). Additionally, the mean keratometry value of each eye of affected individuals with a ZEB1 mutation (53.3 D; SD = 4.8 D) was significantly greater than the mean keratometry value of affected individuals without a ZEB1 mutation (46.5 D; SD = 5.0 D) (t-test: p value = 0.004).
Clinical Course and Management
The mean age of the 10 individuals with PPCD and average keratometry values > 48.0 D in each eye at the time of the initial slit lamp examination and corneal topographic imaging was 37.7 years (range, 12–66 years). Three of the individuals were less than 30 years old when first examined, but serial topographies are not available for any of the three patients to evaluate for progressive corneal steepening. Additionally, two of the three underwent penetrating keratoplasty in either one or both eyes, thus not permitting detection of changes in the native corneal curvature. Overall, seven of the 10 (70%) affected individuals with steep keratometry values underwent corneal transplantation in one or both eyes, a significantly higher percentage than the 15.9% (10/63) of affected individuals with either unknown keratometry or average keratometry values < 48.0D in each eye who required corneal transplantation (p = 0.0009). In six of the seven individuals, corneal transplantation was performed for visually significant corneal edema, while in the seventh, the indication was corneal ectasia and central corneal scarring.
Immunohistochemical detection of ZEB1 in the Corneal Stroma
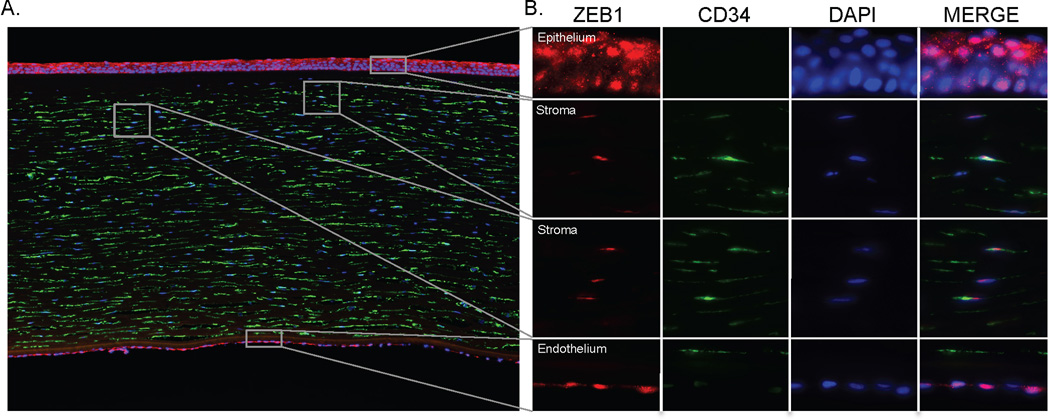
To determine whether ZEB1 is expressed in the corneal stroma, fluorescence immunodetection of keratocytes in an eye bank cornea was performed using an antibody to CD34, a type-I transmembrane glycophosphoprotein that is typically present in stromal fibroblasts (Figure 3). Antibodies directed against ZEB1 demonstrated that the protein is expressed by the stromal keratocytes, as well as by the corneal epithelial and endothelial cells. In each cell type, expression was localized to the nucleus, identified with the DAPI stain, as can clearly be seen in the merged images (Figure 3). Isotype and secondary only controls showed either no or low and diffuse background staining (data not shown).
Figure 3.
Immunodetection of ZEB1 in normal donor cornea. A. A cross-section of the cornea probed with anti-ZEB1 (Red, 594nm) and anti-CD34 (Green, 488nm) demonstrates ZEB1 expression in the epithelium, stroma and endothelium. Nuclei are stained with 40, 6-diamidino-2-phenylindole (DAPI) (10× objective). B. Higher magnification views of the indicated sections of the cornea demonstrate that ZEB1 is present in the nuclei of epithelial cells, stromal keratocytes and endothelial cells (100× oil objective).
DISCUSSION
While traditionally considered an isolated disorder of the corneal endothelium, PPCD has now been shown to be associated with significantly steeper mean keratometry values than unaffected individuals, as previous investigators have indicated in smaller, uncontrolled series. We defined abnormally steep corneal curvature as a mean keratometry value of >48.0 D in each eye, as this value is approximately three standard deviations (SD) above the mean keratometry value of 43.97 D described in normal individuals (SD = 1.54 D).16, 33 Thirty seven percent of individuals with PPCD and 86% of individuals with PPCD3 demonstrated corneal curvatures that were > 48.0 D in both eyes, as compared to none of the unaffected individuals. In addition, the mean keratometry value averaged for both eyes of individuals with PPCD (48.2 D) is approximately 3 SDs above the population mean and for individuals with PPCD3, the mean keratometry value (53.3 D) is 6 SDs above the population mean. Therefore, PPCD should be considered a corneal ectatic disorder, along with keratoconus, keratoglobus and pellucid marginal degeneration. Although the corneal ectasias are characterized by corneal stromal thinning, as corneal transplantation was performed for visually significant stromal edema in the majority of individuals with steep keratometry values, we are not able to correlate decreased corneal stromal thickness with steep corneal curvature. Additionally, as we had not performed corneal topographic imaging in patients with PPCD until recently, we do not have serial topographies in affected individuals to assess for progression. However, we plan to do so going forward to determine whether the abnormally steep corneal curvature associated with PPCD is progressive throughout life, or whether it stabilizes at a particular age in affected individuals, as is typically the case with the other corneal ectatic disorders.
An argument may be made that PPCD itself is not a corneal ectatic disorder, but instead presents in association with corneal steepening as it shares a common genetic basis with keratoconus. Supporters of this argument would point to the publications reporting mutations in VSX1 purported to play a pathogenic role in both PPCD and keratoconus.22–30 However, just as many publications have been published either not substantiating or refuting a role for VSX1 in both PPCD and keratoconus.27, 31, 34–44 Additionally, multiple genome-wide linkage and association studies for keratoconus have not identified involvement of either the PPCD1 locus on chromosome 20, containing VSX1, or the PPCD3 locus on chromosome 10, containing ZEB1.39, 45–55 Given this, and the association of PPCD with corneal steeping in the absence of clinical features of keratoconus described in this report as well as by Raber and others, significant evidence exists to suggest that PPCD is associated with corneal steepening independent of an association with keratoconus (Table 2).7, 11, 13, 16, 20
Table 2.
Previous reports of PPCD associated with abnormalities of corneal curvature
| Author | Year of Publication |
Subjects | Subjects with Average K value > 48 D OU |
Subjects with Average K value > 50 D OU |
Subjects Diagnosed with Keratoconus |
ZEB1 Screening |
|---|---|---|---|---|---|---|
| Gasset12 | 1974 | 2 | 2 | 2 | 2 | No |
| Weissman18 | 1989 | 4 | 3 | 2 | 4 | No |
| Bechara7 | 1991 | 1 | Not mentioned | Not mentioned | 0* | No |
| Blair8 | 1992 | 1 | 0 | 0 | 1 | No |
| Driver11 | 1994 | 3 | 2 | 2 | 2 | No |
| John13 | 1998 | 5 | 4 | 2 | 0 | No |
| Mazotta15 | 2008 | 1 | 0 | 0 | 1 | No |
| Cremona10 | 2009 | 7 | ≥1** | ≥1** | 7 | No |
| Lam14 | 2010 | 1 | 0 | 0 | 1 | Yes (negative) |
| Liskova20 | 2010 | 3 | 0† | 0† | 0†† | Yes (all positive) |
| Raber16 | 2011 | 18 | 15† | 9† | 0 | No |
Topographic and histopathologic features but no clinical features of keratoconus;
keratometry values provided for only 1 of the 7 patients;
One subject had undergone corneal transplantation in one eye, and pre-operative keratometry was not available;
One subject demonstrated topographic features but no clinical features of keratoconus
Clarification of the association of PPCD with steep corneal curvature will require further elucidation of the genetic factors that influence corneal curvature as well as the role that ZEB1 plays in both corneal endothelial and stromal development and function. To date, we have examined and collected DNA from 73 affected individuals with PPCD. Seventeen of these 73 individuals (23.3%) have required corneal transplantation, which includes 9 of the 26 individuals (34.6%) with ZEB1 mutations. The fact that this percentage is twice that of affected individuals without ZEB1 mutations (17.0%; 8/47) is suggestive that ZEB1 haploinsufficiency results in more several endothelial dysfunction than the yet to be identified protein dysfunction associated with non-ZEB1 PPCD, such as PPCD1. Similarly, the significantly greater mean keratometry value of affected individuals with a ZEB1 mutation compared to affected individuals without a ZEB1 mutation is additional evidence of a more severe clinical phenotype associated with PPCD3, and indicates an association between more severe endothelial dysfunction and more pronounced corneal steepening.
We acknowledge several limitations of this study, one of which is the use of the need for corneal transplantation as an indicator of the degree of endothelial dysfunction. As we have indicated that a significantly greater percentage of patients with average keratometry values > 48.0 D in each eye required corneal transplantation than those with average keratometry values < 48.0 D, this suggests that the difference is due the presence of corneal ectasia as the indication for transplantation in the former group. However, corneal transplantation was performed for visually significant corneal edema, not corneal ectasia, in all but one of these patients. The suggestion that the degree of endothelial dysfunction is greater in eyes with more pronounced corneal steepening is an association that will require more detailed clinical characterization in a larger number of affected individuals to definitively demonstrate. Another limitation of this study is the fact that keratometry and ZEB1 screening data were available and analyzed for 37% (27 of 73) of all recruited affected individuals from just over one-half (23 of 45) of the families recruited to date. Obviously, individuals with a more severely affected phenotype may be more likely to participate in research studies than those with more mild manifestations of a disease, a selection bias that may affect the study findings.
While PPCD is clearly a corneal endothelial dystrophy, we believe that this and the previous reports listed in Table 2 are sufficient to consider PPCD to be a corneal ectatic disorder as well. As truncating mutations in ZEB1 account for approximately 1/3 of PPCD, and the genetic basis of the other 2/3 remains to be elucidated, an understanding of the genetic basis of the endothelial dysfunction and corneal steepening that characterize PPCD begins with an analysis of ZEB1 expression and function. We have previously demonstrated ZEB1 expression in the corneal endothelium and present evidence of its expression in the corneal stroma in this report.56 In PPCD3, we have demonstrated decreased expression of ZEB1 and increased expression of collagen, type IV, alpha 3 (COL4A3; MIM 120070) in the corneal endothelium, leading to the proposed pathogenesis underlying the endothelial cell abnormalities observed in affected individuals and in Zeb1-heterozygous and -null mice.57 We are currently investigating whether ZEB1 and COL4A3 also demonstrate inversely related corneal stromal expression levels in PPCD3 and whether interaction of ZEB1 with other proteins expressed in the corneal stroma that contain E2-box motifs (to which ZEB1 binds) and/or are the product of genes implicated in the determination of corneal curvature (such as FRAP1 and PDGFRA) may be involved in the pathogenesis of the significant corneal steepening.58, 59
ACKNOWLEDGEMENT
Contributions
The authors thank Dr.Cosimo Mazzotta for providing unpublished keratometry data for the patient that he and his colleagues previously reported with posterior polymorphous corneal dystrophy and steep corneal curvature.
Funding
Support for the conduct of the study provided by National Eye Institute grants 1R01 EY022082 (A.J.A.), P30 EY000331 (core grant), an unrestricted grant from Research to Prevent Blindness and a grant from the Gerald Oppenheimer Family Foundation Center for the Prevention of Eye Disease (A.J.A.).
Footnotes
Disclosure
None of the authors has a conflict of interest, including financial interests, activities, relationships, and affiliations, relevant to the content of this article.
Data access and responsibility
The first author has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Aldave AJ, Yellore VS, Yu F, et al. Posterior polymorphous corneal dystrophy is associated with TCF8 gene mutations and abdominal hernia. Am J Med Genet A. 2007 Nov 1;143A(21):2549–2556. doi: 10.1002/ajmg.a.31978. [DOI] [PubMed] [Google Scholar]
- 2.Krafchak CM, Pawar H, Moroi SE, et al. Mutations in TCF8 cause posterior polymorphous corneal dystrophy and ectopic expression of COL4A3 by corneal endothelial cells. Am J Hum Genet. 2005 Nov;77(5):694–708. doi: 10.1086/497348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teekhasaenee C, Nimmanit S, Wutthiphan S, et al. Posterior polymorphous dystrophy and Alport syndrome. Ophthalmology. 1991 Aug;98(8):1207–1215. doi: 10.1016/s0161-6420(91)32152-3. [DOI] [PubMed] [Google Scholar]
- 4.Riazuddin SA, Parker DS, McGlumphy EJ, et al. Mutations in LOXHD1, a recessive-deafness locus, cause dominant late-onset Fuchs corneal dystrophy. Am J Hum Genet. 2012 Mar 9;90(3):533–539. doi: 10.1016/j.ajhg.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desir J, Abramowicz M. Congenital hereditary endothelial dystrophy with progressive sensorineural deafness (Harboyan syndrome) Orphanet J Rare Dis. 2008;3:28. doi: 10.1186/1750-1172-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desir J, Moya G, Reish O, et al. Borate transporter SLC4A11 mutations cause both Harboyan syndrome and non-syndromic corneal endothelial dystrophy. J Med Genet. 2007 May;44(5):322–326. doi: 10.1136/jmg.2006.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bechara SJ, Grossniklaus HE, Waring GO, 3rd, Wells JA., 3rd Keratoconus associated with posterior polymorphous dystrophy. Am J Ophthalmol. 1991 Dec 15;112(6):729–731. doi: 10.1016/s0002-9394(14)77284-8. [DOI] [PubMed] [Google Scholar]
- 8.Blair SD, Seabrooks D, Shields WJ, Pillai S, Cavanagh HD. Bilateral progressive essential iris atrophy and keratoconus with coincident features of posterior polymorphous dystrophy: a case report and proposed pathogenesis. Cornea. 1992 May;11(3):255–261. [PubMed] [Google Scholar]
- 9.Cibis GW, Krachmer JA, Phelps CD, Weingeist TA. The clinical spectrum of posterior polymorphous dystrophy. Arch Ophthalmol. 1977 Sep;95(9):1529–1537. doi: 10.1001/archopht.1977.04450090051002. [DOI] [PubMed] [Google Scholar]
- 10.Cremona FA, Ghosheh FR, Rapuano CJ, et al. Keratoconus associated with other corneal dystrophies. Cornea. 2009 Feb;28(2):127–135. doi: 10.1097/ICO.0b013e3181859935. [DOI] [PubMed] [Google Scholar]
- 11.Driver PJ, Reed JW, Davis RM. Familial cases of keratoconus associated with posterior polymorphous dystrophy. Am J Ophthalmol. 1994 Aug 15;118(2):256–257. doi: 10.1016/s0002-9394(14)72911-3. [DOI] [PubMed] [Google Scholar]
- 12.Gasset AR, Zimmerman TJ. Posterior polymorphous dystrophy associated with keratoconus. Am J Ophthalmol. 1974 Sep;78(3):535–537. doi: 10.1016/0002-9394(74)90249-9. [DOI] [PubMed] [Google Scholar]
- 13.John GR. Videokeratographic abnormalities in a family with posterior polymorphous dystrophy. Cornea. 1998 Jul;17(4):380–383. doi: 10.1097/00003226-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Lam HY, Wiggs JL, Jurkunas UV. Unusual presentation of presumed posterior polymorphous dystrophy associated with iris heterochromia, band keratopathy, and keratoconus. Cornea. 2010 Oct;29(10):1180–1185. doi: 10.1097/ICO.0b013e3181d007e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzotta C, Baiocchi S, Caporossi O, et al. Confocal microscopy identification of keratoconus associated with posterior polymorphous corneal dystrophy. J Cataract Refract Surg. 2008 Feb;34(2):318–321. doi: 10.1016/j.jcrs.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Raber IM, Fintelmann R, Chhabra S, Ribeiro MP, Eagle RC, Jr, Orlin SE. Posterior polymorphous dystrophy associated with nonkeratoconic steep corneal curvatures. Cornea. 2011 Oct;30(10):1120–1124. doi: 10.1097/ICO.0b013e3182114452. [DOI] [PubMed] [Google Scholar]
- 17.Wagoner MD, Teichmann KD. Terrien's marginal degeneration associated with posterior polymorphous dystrophy. Cornea. 1999 Sep;18(5):612–615. [PubMed] [Google Scholar]
- 18.Weissman BA, Ehrlich M, Levenson JE, Pettit TH. Four cases of keratoconus and posterior polymorphous corneal dystrophy. Optom Vis Sci. 1989 Apr;66(4):243–246. doi: 10.1097/00006324-198904000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Zarei-Ghanavati S, Javadi MA, Yazdani S. Bilateral Terrien's marginal degeneration and posterior polymorphous dystrophy in a patient with rheumatoid arthritis. J Ophthalmic Vis Res. 2012 Jan;7(1):60–63. [PMC free article] [PubMed] [Google Scholar]
- 20.Liskova P, Filipec M, Merjava S, Jirsova K, Tuft SJ. Variable ocular phenotypes of posterior polymorphous corneal dystrophy caused by mutations in the ZEB1 gene. Ophthalmic Genet. 2010 Dec;31(4):230–234. doi: 10.3109/13816810.2010.518577. [DOI] [PubMed] [Google Scholar]
- 21.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998 Jan-Feb;42(4):297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 22.Bisceglia L, Ciaschetti M, De Bonis P, et al. VSX1 mutational analysis in a series of Italian patients affected by keratoconus: detection of a novel mutation. Invest Ophthalmol Vis Sci. 2005 Jan;46(1):39–45. doi: 10.1167/iovs.04-0533. [DOI] [PubMed] [Google Scholar]
- 23.De Bonis P, Laborante A, Pizzicoli C, et al. Mutational screening of VSX1, SPARC, SOD1, LOX, and TIMP3 in keratoconus. Mol Vis. 2011;17:2482–2494. [PMC free article] [PubMed] [Google Scholar]
- 24.Eran P, Almogit A, David Z, et al. The D144E substitution in the VSX1 gene: a non-pathogenic variant or a disease causing mutation? Ophthalmic Genet. 2008 Jun;29(2):53–59. doi: 10.1080/13816810802008242. [DOI] [PubMed] [Google Scholar]
- 25.Heon E, Greenberg A, Kopp KK, et al. VSX1: a gene for posterior polymorphous dystrophy and keratoconus. Hum Mol Genet. 2002 May 1;11(9):1029–1036. doi: 10.1093/hmg/11.9.1029. [DOI] [PubMed] [Google Scholar]
- 26.Hosseini SM, Herd S, Vincent AL, Heon E. Genetic analysis of chromosome 20-related posterior polymorphous corneal dystrophy: genetic heterogeneity and exclusion of three candidate genes. Mol Vis. 2008;14:71–80. [PMC free article] [PubMed] [Google Scholar]
- 27.Mok JW, Baek SJ, Joo CK. VSX1 gene variants are associated with keratoconus in unrelated Korean patients. J Hum Genet. 2008;53(9):842–849. doi: 10.1007/s10038-008-0319-6. [DOI] [PubMed] [Google Scholar]
- 28.Paliwal P, Singh A, Tandon R, Titiyal JS, Sharma A. A novel VSX1 mutation identified in an individual with keratoconus in India. Mol Vis. 2009;15:2475–2479. [PMC free article] [PubMed] [Google Scholar]
- 29.Paliwal P, Tandon R, Dube D, Kaur P, Sharma A. Familial segregation of a VSX1 mutation adds a new dimension to its role in the causation of keratoconus. Mol Vis. 2011;17:481–485. [PMC free article] [PubMed] [Google Scholar]
- 30.Saee-Rad S, Hashemi H, Miraftab M, et al. Mutation analysis of VSX1 and SOD1 in Iranian patients with keratoconus. Mol Vis. 2011;17:3128–3136. [PMC free article] [PubMed] [Google Scholar]
- 31.Aldave AJ, Yellore VS, Salem AK, et al. No VSX1 gene mutations associated with keratoconus. Invest Ophthalmol Vis Sci. 2006 Jul;47(7):2820–2822. doi: 10.1167/iovs.05-1530. [DOI] [PubMed] [Google Scholar]
- 32.Bakhtiari P, Frausto RF, Roldan AN, Wang C, Yu F, Aldave AJ. Exclusion of pathogenic promoter region variants and identification of novel nonsense mutations in ZEB1 in posterior polymorphous corneal dystrophy. Mol Vis. Under Review. [PMC free article] [PubMed] [Google Scholar]
- 33.Bogan SJ, Waring GO, 3rd, Ibrahim O, Drews C, Curtis L. Classification of normal corneal topography based on computer-assisted videokeratography. Arch Ophthalmol. 1990 Jul;108(7):945–949. doi: 10.1001/archopht.1990.01070090047037. [DOI] [PubMed] [Google Scholar]
- 34.Aldave AJ, Yellore VS, Principe AH, et al. Candidate gene screening for posterior polymorphous dystrophy. Cornea. 2005 Mar;24(2):151–155. doi: 10.1097/01.ico.0000141235.26096.1d. [DOI] [PubMed] [Google Scholar]
- 35.Gwilliam R, Liskova P, Filipec M, et al. Posterior polymorphous corneal dystrophy in Czech families maps to chromosome 20 and excludes the VSX1 gene. Invest Ophthalmol Vis Sci. 2005 Dec;46(12):4480–4484. doi: 10.1167/iovs.05-0269. [DOI] [PubMed] [Google Scholar]
- 36.Aldave AJ. VSX1 mutation and corneal dystrophies. Ophthalmology. 2005 Jan;112(1):170–171. doi: 10.1016/j.ophtha.2004.10.017. author reply 171–172. [DOI] [PubMed] [Google Scholar]
- 37.Abu-Amero KK, Kalantan H, Al-Muammar AM. Analysis of the VSX1 gene in keratoconus patients from Saudi Arabia. Mol Vis. 2011;17:667–672. [PMC free article] [PubMed] [Google Scholar]
- 38.Dash DP, George S, O'Prey D, et al. Mutational screening of VSX1 in keratoconus patients from the European population. Eye (Lond) 2010 Jun;24(6):1085–1092. doi: 10.1038/eye.2009.217. [DOI] [PubMed] [Google Scholar]
- 39.Gajecka M, Radhakrishna U, Winters D, et al. Localization of a gene for keratoconus to a 5.6-Mb interval on 13q32. Invest Ophthalmol Vis Sci. 2009 Apr;50(4):1531–1539. doi: 10.1167/iovs.08-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeoung JW, Kim MK, Park SS, et al. VSX1 gene and keratoconus: genetic analysis in Korean patients. Cornea. 2012 Jul;31(7):746–750. doi: 10.1097/ICO.0b013e3181e16dd0. [DOI] [PubMed] [Google Scholar]
- 41.Liskova P, Ebenezer ND, Hysi PG, et al. Molecular analysis of the VSX1 gene in familial keratoconus. Mol Vis. 2007;13:1887–1891. [PMC free article] [PubMed] [Google Scholar]
- 42.Stabuc-Silih M, Strazisar M, Hawlina M, Glavac D. Absence of pathogenic mutations in VSX1 and SOD1 genes in patients with keratoconus. Cornea. 2010 Feb;29(2):172–176. doi: 10.1097/ICO.0b013e3181aebf7a. [DOI] [PubMed] [Google Scholar]
- 43.Tang YG, Picornell Y, Su X, Li X, Yang H, Rabinowitz YS. Three VSX1 gene mutations, L159M, R166W, and H244R, are not associated with keratoconus. Cornea. 2008 Feb;27(2):189–192. doi: 10.1097/ICO.0b013e31815a50e7. [DOI] [PubMed] [Google Scholar]
- 44.Tanwar M, Kumar M, Nayak B, et al. VSX1 gene analysis in keratoconus. Mol Vis. 2010;16:2395–2401. [PMC free article] [PubMed] [Google Scholar]
- 45.Bisceglia L, De Bonis P, Pizzicoli C, et al. Linkage analysis in keratoconus: replication of locus 5q21.2 and identification of other suggestive Loci. Invest Ophthalmol Vis Sci. 2009 Mar;50(3):1081–1086. doi: 10.1167/iovs.08-2382. [DOI] [PubMed] [Google Scholar]
- 46.Brancati F, Valente EM, Sarkozy A, et al. A locus for autosomal dominant keratoconus maps to human chromosome 3p14-q13. J Med Genet. 2004 Mar;41(3):188–192. doi: 10.1136/jmg.2003.012872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burdon KP, Coster DJ, Charlesworth JC, et al. Apparent autosomal dominant keratoconus in a large Australian pedigree accounted for by digenic inheritance of two novel loci. Hum Genet. 2008 Nov;124(4):379–386. doi: 10.1007/s00439-008-0555-z. [DOI] [PubMed] [Google Scholar]
- 48.Burdon KP, Macgregor S, Bykhovskaya Y, et al. Association of polymorphisms in the hepatocyte growth factor gene promoter with keratoconus. Invest Ophthalmol Vis Sci. 2011 Oct;52(11):8514–8519. doi: 10.1167/iovs.11-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hughes AE, Dash DP, Jackson AJ, Frazer DG, Silvestri G. Familial keratoconus with cataract: linkage to the long arm of chromosome 15 and exclusion of candidate genes. Invest Ophthalmol Vis Sci. 2003 Dec;44(12):5063–5066. doi: 10.1167/iovs.03-0399. [DOI] [PubMed] [Google Scholar]
- 50.Hutchings H, Ginisty H, Le Gallo M, et al. Identification of a new locus for isolated familial keratoconus at 2p24. J Med Genet. 2005 Jan;42(1):88–94. doi: 10.1136/jmg.2004.022103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Bykhovskaya Y, Haritunians T, et al. A genome-wide association study identifies a potential novel gene locus for keratoconus, one of the commonest causes for corneal transplantation in developed countries. Hum Mol Genet. 2012 Jan 15;21(2):421–429. doi: 10.1093/hmg/ddr460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Rabinowitz YS, Tang YG, et al. Two-stage genome-wide linkage scan in keratoconus sib pair families. Invest Ophthalmol Vis Sci. 2006 Sep;47(9):3791–3795. doi: 10.1167/iovs.06-0214. [DOI] [PubMed] [Google Scholar]
- 53.Liskova P, Hysi PG, Waseem N, Ebenezer ND, Bhattacharya SS, Tuft SJ. Evidence for keratoconus susceptibility locus on chromosome 14: a genome-wide linkage screen using single-nucleotide polymorphism markers. Arch Ophthalmol. 2010 Sep;128(9):1191–1195. doi: 10.1001/archophthalmol.2010.200. [DOI] [PubMed] [Google Scholar]
- 54.Tang YG, Rabinowitz YS, Taylor KD, et al. Genomewide linkage scan in a multigeneration Caucasian pedigree identifies a novel locus for keratoconus on chromosome 5q14.3-q21.1. Genet Med. 2005 Jul-Aug;7(6):397–405. doi: 10.1097/01.gim.0000170772.41860.54. [DOI] [PubMed] [Google Scholar]
- 55.Tyynismaa H, Sistonen P, Tuupanen S, et al. A locus for autosomal dominant keratoconus: linkage to 16q22.3-q23.1 in Finnish families. Invest Ophthalmol Vis Sci. 2002 Oct;43(10):3160–3164. [PubMed] [Google Scholar]
- 56.Yellore VS, Rayner SA, Nguyen CK, et al. Analysis of the role of ZEB1 in the pathogenesis of posterior polymorphous corneal dystrophy. Invest Ophthalmol Vis Sci. 2012 Jan;53(1):273–278. doi: 10.1167/iovs.11-8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Peng X, Tan J, Darling DS, Kaplan HJ, Dean DC. Zeb1 mutant mice as a model of posterior corneal dystrophy. Invest Ophthalmol Vis Sci. 2008 May;49(5):1843–1849. doi: 10.1167/iovs.07-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Han S, Chen P, Fan Q, et al. Association of variants in FRAP1 and PDGFRA with corneal curvature in Asian populations from Singapore. Hum Mol Genet. 2012 Sep 15;20(18):3693–3698. doi: 10.1093/hmg/ddr269. [DOI] [PubMed] [Google Scholar]
- 59.Mishra A, Yazar S, Hewitt AW, et al. Genetic variants near PDGFRA are associated with corneal curvature in Australians. Invest Ophthalmol Vis Sci. 2011 Oct;53(11):7131–7136. doi: 10.1167/iovs.12-10489. [DOI] [PMC free article] [PubMed] [Google Scholar]