Many attempts have been made at developing biomarkers for stroke. Though successful to some degree1–3, none have been sufficiently robust to be used in clinical practice. Thus, there is still a great need for more in depth studies of the biology of human stroke in order to better understand its pathogenesis. This should make it possible to develop “blood tests” for stroke and transient ischemic attacks (TIAs) to guide treatment and ultimately improve outcomes.
The traditional approach to developing stroke biomarkers has been to select candidate markers based upon known pathobiology. The majority of markers that have been evaluated are proteins that are measured in patients at various times before or after stroke. The rationale for this approach is that brain injury releases molecules into blood that can be measured as evidence of brain injury; or that other cells and organs release molecules that either cause or contribute to a stroke, or are a response to the stroke. These approaches have been handicapped because they require a “guess” at the most reliable biomarker.
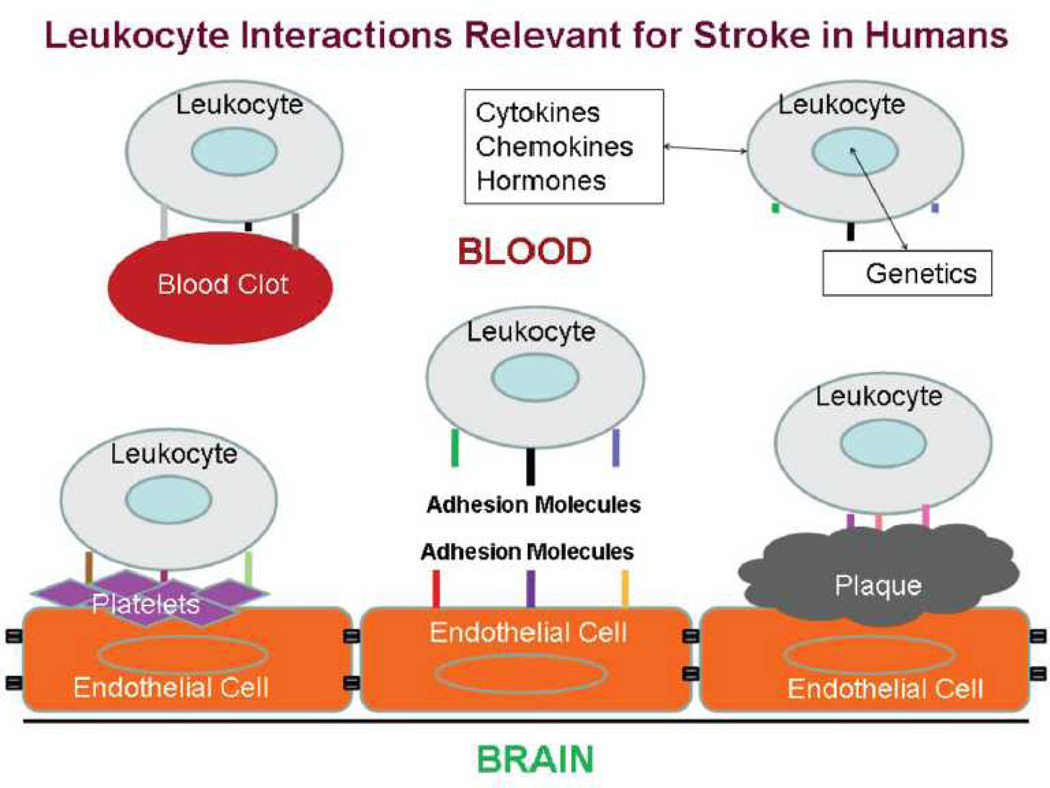
Our group took a different approach to the problem by assessing the immune system following stroke4, 5. The rationale for this approach is shown in Figure 1. The expression of genes in leukocytes in influenced by many factors associated with ischemic stroke. Leukocytes interact with blood clots, platelets, atherosclerotic plaque, and injured brain endothelial cells via adhesion molecules5. In addition, leukocytes detect circulating cytokines, chemokines and hormones. Each has the potential to modulate RNA expression in leukocytes.
Figure 1.
Schematic of interactions between circulating leukocytes and factors involved in ischemic stroke, including blood clots, platelets, atherosclerotic plaque and damaged endothelial cells. Each factor can influence expression of leukocyte RNA. This figure is reproduced from an article published in Journal of Cerebral Blood Flow and Metabolism by Sharp et al.5
To provide proof of principle that circulating leukocytes could provide insight into the pathogenesis of stroke, we performed a series of animal studies. For the first study blood of rats was obtained 24 hours following ischemic stroke, intracerebral hemorrhage, status epilepticus, hypoxia and hypoglycemia6. RNA was isolated from the blood and processed on whole genome microarrays. Whole genome microarrays were used because they permit an unbiased selection of molecular markers for each type of brain injury, rather than making a guess at what those markers might be. The results demonstrated several key principles that have guided our subsequent studies 6. (1) A large number of RNAs changed expression in leukocytes following brain injuries. (2) No single RNA was sufficient to distinguish different types of brain injury . (3) A panel of RNAs (or so called “gene profile”) did characterize a given type of injury, and different gene profiles could distinguish each brain injury . This led us to hypothesize there would be a distinct gene profile for different brain injuries in humans, and that these would be useful for diagnosis and prognosis. We have gone on to show that brief global cerebral ischemia produces unique gene profiles in blood of rats depending upon whether there is neuronal cell death in hippocampus or not7. In addition, brief periods of focal ischemia that mimic TIAs in humans produce unique gene profiles that differ based upon the duration of focal ischemia8, 9.
The first proof of principle studies in humans were reported by Moore et al in 2005 10. They showed an RNA expression profile from peripheral blood mononuclear cells could distinguish ischemic stroke from control patients11. We confirmed these studies in humans and showed that genes specifically expressed in whole blood before 3 hours, at 5 hours and at 24 hours after ischemic stroke could distinguish ischemic stroke from controls with >85% sensitivity and specificity 12. One of the surprises that came from these first whole genome studies of human stroke was that the genes expressed in human blood following ischemic stroke were very different than those expressed in rodent blood. For these reasons, almost all of our subsequent studies have been in humans.
With this promising human data we then evaluated these results in a second validation cohort. This is a crucial, but often under appreciated, because biomarkers often fail to replicate in a second population. The reasons for this are many but can include small samples upon which the predictor was identified, non-representative samples for derivation or validation cohort, and lack of robust biological effects that are swamped by inter- individual and technical variability. Stamova et al.13 and Barr et al. 14 were able to confirm our initial study. Profiles from our original study predicted ischemic stroke with >85% sensitivity and specificity, and new profiles based upon a larger cohort were able to distinguish ischemic stroke from disease controls with >85% sensitivity and specificity13. Further study however is required to compare stroke to diseases that mimic stroke.
Studies evaluating RNA expression differences in stroke etiology have also been performed. In the first study, large vessel stroke was compared to cardioembolic stroke. A total of 77 genes differed between the two; and 23 genes could distinguish the two types of stroke 15. A subsequent larger study by Jickling et al., evaluated 194 samples from 76 acute ischemic stroke patients 16. RNA was isolated from blood and run on Affymetrix microarrays. Genes that distinguished large-vessel from cardioembolic stroke were determined at 3, 5, and 24 hours following stroke. A 40-gene profile differentiated cardioembolic stroke from large-vessel stroke with >95% sensitivity and specificity. A separate 37-gene profile distinguished cardioembolic stroke due to atrial fibrillation from non-atrial fibrillation causes with >90% sensitivity and specificity16. Finally, our most recent study shows specific profiles for lacunar stroke compared to large vessel and cardioembolic ischemic strokes17.
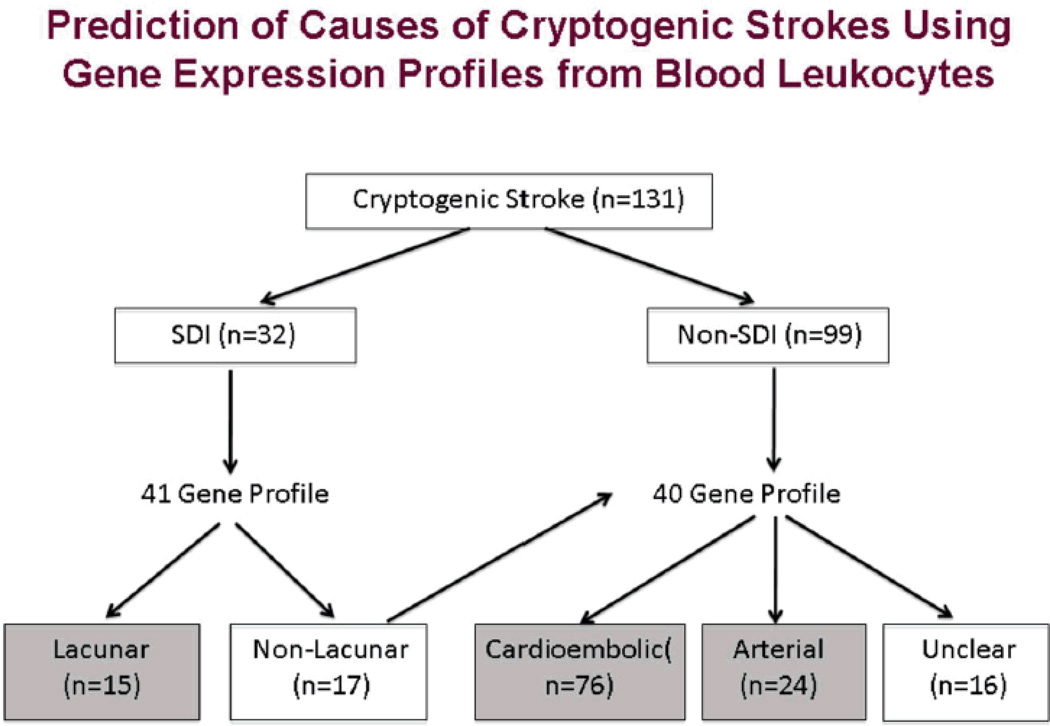
Having developed cardioembolic, large vessel and lacunar profiles for cause of stroke, we applied them to cryptogenic stroke18. RNA was isolated from peripheral blood of 131 cryptogenic strokes and compared with profiles derived from 149 strokes of known cause. Each sample was run on Affymetrix microarrays. Cause of cryptogenic stroke was predicted using gene expression in blood and infarct location. Cryptogenic strokes were predicted to be 58% cardioembolic, 18% arterial, 12% lacunar, and 12% unclear etiology (Figure 2)18. Cryptogenic stroke of predicted cardioembolic etiology had more prior myocardial infarction and higher CHA(2)DS(2)-VASc scores compared with stroke of predicted arterial etiology. Predicted lacunar strokes had higher systolic and diastolic blood pressures and lower National Institutes of Health Stroke Scale compared with predicted arterial and cardioembolic strokes. Cryptogenic strokes of unclear predicted etiology were less likely to have a prior transient ischemic attack or ischemic stroke18. These data provide the first proof-of-principle that gene expression profiles in blood could be used to predict a probable cause in cryptogenic strokes.
Figure 2.
Algorithm for predicting causes of cryptogenic strokes using gene expression profiles from circulating leukocytes. MRI is used to classify a stroke as a Small Deep Infarct (SDI) or non-SDI infarct (cortical, cerebellar, etc). A 41 gene profile is used to distinguish lacunar and non-lacunar SDI. A 40 gene profile is then used to distinguish cardioembolic and large vessel arterial causes of non-SDI causes of stroke. Approximately 58% of cryptogenic strokes are predicted to be cardioembolic. This Figure is reproduced from an article published in Stroke by Jickling et al. 18
Given these promising results, the more difficult problem of TIAs has been tackled. A challenge in TIA is the lack of a gold standard for a transient neurological event to due to cerebral ischemia. We first performed an animal study showing that 5 and 10 minutes of focal ischemia that might “simulate” TIAs seen in humans produced characteristic gene expression profiles in 9. This work was translated to humans, where RNA expression in blood of TIA patients (n = 26) was compared to vascular risk factor control subjects without symptomatic cardiovascular disease (n = 26)19. There were 449 genes differentially expressed between TIA and controls. Hierarchical cluster analysis of the identified genes suggested the presence of 2 patterns of RNA expression in patients with TIA, with one group possibly being associated with those with high risk of stroke given positive DWI-MRI in two patients and another going on to have a stroke19.
On further study, 74 genes expressed in TIA were found to be common to those in ischemic stroke20. Functional pathways common to TIA and stroke involved granulocytes and B cells. A prediction model using 26 of the 74 ischemia genes distinguished TIA and stroke subjects from control subjects with 89% sensitivity and specificity. In the validation cohort, 17 of 17 TIA diffusion-weighted imaging-positive/minor strokes were predicted to be ischemic, and 10 of 13 non-ischemic transient neurological events (TNE) were predicted to be non-ischemic. In TNE of unclear etiology, 71% were predicted to be ischemic. Thus, this study identified a common molecular response to ischemia in TIA and stroke20. We have used a similar approach to show that genes associated with White Matter Hyperintensities in brain are not associated with those found in ischemic stroke or TIAs21.
In spite of the successes, there remain several hurdles to translate these proof-of- concept studies to practice. For example, for prospective prediction problems with data normalization need to be solved in order to eliminate batch effects that occur over time. If accomplished, large studies will be needed to confirm the results.
Acknowledgements
The authors thank recent collaborators.
Sources of Funding: These studies were supported by grants from the National Institutes of Health and the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/ Disclosures. The authors have no conflicts of interest or disclosures to report.
References
- 1.Whiteley W, Chong WL, Sengupta A, Sandercock P. Blood markers for the prognosis of ischemic stroke: A systematic review. Stroke. 2009;40:e380–e389. doi: 10.1161/STROKEAHA.108.528752. [DOI] [PubMed] [Google Scholar]
- 2.Montaner J. Blood biomarkers to guide stroke thrombolysis. Front Biosci. 2009;1:200–208. doi: 10.2741/E19. [DOI] [PubMed] [Google Scholar]
- 3.Jensen MB, Chacon MR, Sattin JA, Levine RL, Vemuganti R. Potential biomarkers for the diagnosis of stroke. Expert Rev Cardiovasc Ther. 2009;7:389–393. doi: 10.1586/erc.09.9. [DOI] [PubMed] [Google Scholar]
- 4.Jickling GC, Sharp FR. Blood biomarkers of ischemic stroke. Neurotherapeutics. 2011;8:349–360. doi: 10.1007/s13311-011-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharp FR, Jickling GC, Stamova B, Tian Y, Zhan X, Liu D, et al. Molecular markers and mechanisms of stroke: RNA studies of blood in animals and humans. J Cereb Blood Flow Metab. 2011;31:1513–1531. doi: 10.1038/jcbfm.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Y, Lu A, Aronow BJ, Sharp FR. Blood genomic responses differ after stroke, seizures, hypoglycemia, and hypoxia: Blood genomic fingerprints of disease. Ann Neurol. 2001;50:699–707. doi: 10.1002/ana.10042. [DOI] [PubMed] [Google Scholar]
- 7.Tang Y, Nee AC, Lu A, Ran R, Sharp FR. Blood genomic expression profile for neuronal injury. J Cereb Blood Flow Metab. 2003;23:310–319. doi: 10.1097/01.WCB.0000048518.34839.DE. [DOI] [PubMed] [Google Scholar]
- 8.Zhan X, Kim C, Sharp FR. Very brief focal ischemia simulating transient ischemic attacks (TIAs) can injure brain and induce hsp70 protein. Brain Res. 2008;1234:183–197. doi: 10.1016/j.brainres.2008.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan X, Ander BP, Jickling G, Turner R, Stamova B, Xu H, et al. Brief focal cerebral ischemia that simulates transient ischemic attacks in humans regulates gene expression in rat peripheral blood. J Cereb Blood Flow Metab. 2010;30:110–118. doi: 10.1038/jcbfm.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore DF, Li H, Jeffries N, Wright V, Cooper RA, Jr, Elkahloun A, et al. Using peripheral blood mononuclear cells to determine a gene expression profile of acute ischemic stroke: A pilot investigation. Circulation. 2005;111:212–221. doi: 10.1161/01.CIR.0000152105.79665.C6. [DOI] [PubMed] [Google Scholar]
- 11.Baird AE. Blood genomics in human stroke. Stroke. 2007;38:694–698. doi: 10.1161/01.STR.0000250431.99687.7b. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y, Xu H, Du X, Lit L, Walker W, Lu A, et al. Gene expression in blood changes rapidly in neutrophils and monocytes after ischemic stroke in humans: A microarray study. J Cereb Blood Flow Metab. 2006;26:1089–1102. doi: 10.1038/sj.jcbfm.9600264. [DOI] [PubMed] [Google Scholar]
- 13.Stamova B, Xu H, Jickling G, Bushnell C, Tian Y, Ander BP, et al. Gene expression profiling of blood for the prediction of ischemic stroke. Stroke. 2010;41:2171–2177. doi: 10.1161/STROKEAHA.110.588335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr TL, Conley Y, Ding J, Dillman A, Warach S, Singleton A, et al. Genomic biomarkers and cellular pathways of ischemic stroke by rna gene expression profiling. Neurology. 2010;75:1009–1014. doi: 10.1212/WNL.0b013e3181f2b37f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu H, Tang Y, Liu DZ, Ran R, Ander BP, Apperson M, et al. Gene expression in peripheral blood differs after cardioembolic compared with large-vessel atherosclerotic stroke: Biomarkers for the etiology of ischemic stroke. J Cereb Blood Flow Metab. 2008;28:1320–1328. doi: 10.1038/jcbfm.2008.22. [DOI] [PubMed] [Google Scholar]
- 16.Jickling GC, Xu H, Stamova B, Ander BP, Zhan X, Tian Y, et al. Signatures of cardioembolic and large-vessel ischemic stroke. Ann Neurol. 2010;68:681–692. doi: 10.1002/ana.22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jickling GC, Stamova B, Ander BP, Zhan X, Tian Y, Liu D, et al. Profiles of lacunar and nonlacunar stroke. Ann Neurol. 2011;70:477–485. doi: 10.1002/ana.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jickling GC, Stamova BS, Ander BP, Zhan X, Liu DZ, Sison SM, et al. Prediction of cardioembolic, arterial and lacunar causes of cryptogenic stroke by gene expression and infarct location. Stroke. 2012;43:2036–2041. doi: 10.1161/STROKEAHA.111.648725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhan X, Jickling GC, Tian Y, Stamova B, Xu H, Ander BP, et al. Transient ischemic attacks characterized by RNA profiles in blood. Neurology. 2011;77:1718–1724. doi: 10.1212/WNL.0b013e318236eee6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jickling GC, Stamova BS, Zhan X, Ander BP, Tian Y, Liu DZ, et al. Ischemic transient neurological events identified by immune response to cerebral ischemia. Stroke. 2012;43:1006–1012. doi: 10.1161/STROKEAHA.111.638577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Stamova B, Jickling G, Tian Y, Zhan X, Ander BP, et al. Distinctive RNA expression profiles in blood associated with white matter hyperintensities in brain. Stroke. 2010;41:2744–2749. doi: 10.1161/STROKEAHA.110.591875. [DOI] [PMC free article] [PubMed] [Google Scholar]