Abstract
Perioperative chemotherapy has been shown to improve disease-free survival compared with surgery alone for resectable colorectal liver metastases (CLM). We examined our experience with systemic chemotherapy in this clinical setting. A prospectively collected liver surgery database identified 210 patients treated for resectable CLM from 1996 to 2010. Results were correlated to four treatment groups: posthepatectomy adjuvant only, prehepatectomy preoperative only, perioperative (preoperative and adjuvant), and surgery only. Seventy-nine (37.6%) patients received posthepatectomy adjuvant only treatment, 33 (15.7%) received prehepatectomy preoperative only treatment, 46 (21.9%) received perioperative (preoperative and adjuvant) treatment, whereas 52 (24.8%) received surgery alone. Preoperative and adjuvant systemic chemotherapy regimens were as follows: 23 (29.1%) and 18 (14.4%) received a 5-fluorouracil monotherapy regimen, 19 (24.1%) and 31 (24.8%) received an irinotecan-based regimen, and 28 (35.4%) and 37 (29.6%) received an oxaliplatin-based regimen. Nine (11.4%) and 12 (9.6%) received some other unknown combination. Treatment groups showed no difference in gender, mean tumor size, number of tumors, margin status, or postoperative complications with the only difference being a higher incidence of metachronous tumors in the preoperative only and perioperative groups (P = 0.01). Median follow-up and overall survival were 25 and 41 months, respectively. The adjuvant, preoperative, perioperative, and surgery only groups had a median survival time of 48, 35, 39, and 29 months, respectively (log-rank P = 0.04). Independent predictors of overall survival on multivariate analysis included treatment algorithm used and postoperative complication status. Adjuvant only systemic therapy was associated with an improved survival in resectable CLM. Prospective randomized trials are needed to confirm these findings.
Colorectal cancer is a commonly encountered malignancy in surgical oncology. Stage distribution at diagnosis for colorectal cancer as reported in the Surveillance, Epidemiology and End Results database is put at 39.7, 35.9, 18.5, and 5.9 per cent, respectively, for localized, regional, distant, and unstaged tumors, respectively.1 The liver is a common site of disseminated disease with synchronous colorectal liver metastases (CLM) accounting for approximately 23 to 51 per cent of all patients undergoing surgical resection for liver metastases.2
Surgical resection of isolated CLM still offers the most durable chance for long-term survival despite remarkable advances in systemic chemotherapy. Historical data put five-year survival after resection at 35 per cent and relapse rate associated with resection at approximately 75 per cent.3 Recent data on survival outcome after resection of colorectal liver metastases put the five-year survival at 45 to 60 per cent up from 30 to 40 per cent.4–9 This witnessed improvement over the past two decades in the survival of patients diagnosed with colorectal liver metastases is attributed in part to better patient selection for surgical resection of hepatic metastases, improved surgical technique, perioperative care, and improvements in systemic chemotherapy.10–13
Modern systemic chemotherapeutic agents can increase the resection rates and improve relapse-free rates when used as adjuncts to surgical resection for CLM.14, 15 Preoperative or perioperative chemotherapy has been shown to improve the outcome of hepatic resections. Nordlinger et al.16 in the EORTC 40983 trial showed that perioperative chemotherapy with FOLFOX4 is compatible with major liver surgery and improved progression-free survival in eligible and resected patients. The use and safety of preoperative chemotherapy in patients diagnosed with resectable liver metastases was also demonstrated.16, 17
As a result of the documented activity of systemic chemotherapy in the perioperative setting for CLM, patients have been managed with different combinations of surgical resection and adjunctive systemic therapy. However, the most appropriate and effective timing of institution of systemic chemotherapy in the management algorithm for resectable CLM has not been well defined.
We conducted a single-institutional review of patients who underwent surgical resection of CLM. The primary goal of our study was to assess whether the timing of institution of systemic therapy relative to surgical resection significantly impacted overall survival in this cohort of patients and secondarily to identify clinicopathologic factors that may have influenced when systemic therapy was administered.
Patient and Methods
Before commencement of this study, approval was obtained from the Protocol Review Committee of the Wake Forest Comprehensive Cancer Center and the Wake Forest University Health Sciences Institutional Review Board.
A prospectively collected liver surgery database identified 210 patients with assessable data treated for resectable CLM between 1996 and 2010. Resectability was assessed by the ability to obtain a complete resection (negative margins), preserve two contiguous hepatic segments, preservation of adequate vascular inflow and outflow as well as biliary drainage, and the ability to preserve adequate future liver remnant (greater than 20% in a healthy liver); all patients received a formal resection. Results were correlated to four treatment groups: posthepatectomy adjuvant only, prehepatectomy preoperative only, perioperative (preoperative and adjuvant), and surgery only. Synchronous disease was defined as liver metastases diagnosed within 3 months of the primary tumor. Patients receiving preoperative chemotherapy were considered resectable at the time of initial evaluation and no patients in this study were given preoperative therapy for downstaging purposes to make them resectable. Preoperative and adjuvant systemic therapy regimens included mainly a 5-fluorouracil (5-FU) monotherapy, irinotecan-based, or oxaliplatin-based regimen with some including the addition of biologic agents such as bevacizumab and cetuximab. Other regimens used mainly in the adjuvant setting include floxuridine and thalidomide.
The main outcome measures were: 1) whether the timing of institution of systemic therapy relative to surgical resection significantly impacted overall survival in this cohort of patients; 2) prognostic factors that may have affected administration of systemic therapy; and 3) effect of systemic therapy on postoperative complications. Clinicopathologic variables included in our analysis include: age at diagnosis, gender, primary tumor location, nodal status, tumor stage, size of largest hepatic metastases, number of liver tumors, margin status, postoperative complications, and preoperative carcinoembryonic antigen level.
Statistical Analyses
The frequency distributions among the groups were assessed using descriptive statistics. Univariate analysis using χ2 statistics was used to compare categorical variables between groups. One-way analysis of variance (ANOVA F test) was conducted to evaluate the significance of the difference in group means. Odds ratios using multinomial logistic regression were calculated to estimate the degree to which patient demographics and clinicopathologic tumor characteristics determined which treatment algorithm was chosen. Equality of survivorship for outcome measure overall survival as a function of the four treatment groups was assessed using the log-rank test of Kaplan-Meier. Overall survival is defined as time (measured in months) from initial diagnosis until death or censorship. The maximum duration of follow-up was 10 years, and the outcome measures were calculated based on observations during this period. To account for any confounding variable (characteristics of the individual patients in the four treatment groups that may be unevenly distributed), a concern in nonrandomized retrospective cohort studies, multivariate regression models (confounder models) were used to adjust for any differences in the distribution of the variables in the four treatment groups. We built confounder models using the Cox proportional hazards model, and the estimates from these models provided hazard ratios and 95 per cent confidence interval adjusted for all variables in the model. Life tables were used to calculate 3- and 5-year overall survival rates. Statistical analyses were performed using SPSS 18.
Results
Patient and Treatment Characteristics
From 1996 to 2010, 210 patients who underwent resection of CLM and had assessable data were included in this study. Median follow-up after diagnosis of liver metastases for living patients was 25 months. Seventy-nine (37.6%) patients received posthepatectomy adjuvant only treatment, 33 (15.7%) received prehepatectomy preoperative only treatment, 46 (21.9%) received perioperative (preoperative and adjuvant) treatment, whereas 52 (24.8%) received surgery alone. Preoperative and adjuvant systemic chemotherapy regimens were as follows: 23 (29.1%) and 18 (14.4%) received a 5-FU monotherapy regimen, 19 (24.1%) and 31 (24.8%) received an irinotecan-based regimen, and 28 (35.4%) and 37 (29.6%) received an oxaliplatin-based regimen. Nine (11.4%) and 12 (9.6%) received some other unknown combination. Biologic agents such as bevacizumab and cetuximab were given in 49.6 per cent of patients (Table 1).
Table 1.
Preoperative and Adjuvant Systemic Chemotherapy Regimen Delivered
| Treatment Group | 5-FU Monotherapy | Irinotecan-based Regimen | Oxaliplatin-based Regimen | FUDR | Thalidomide | Unknown | P Value |
|---|---|---|---|---|---|---|---|
| Preoperative Treatment | |||||||
| Prehepatectomy (n = 33) Preoperative only |
8 (24.2%) | 7 (21.2%) | 13 (39.4%) | — | — | 5 (15.2%) | 0.65 |
| Perioperative (n = 46) (Preoperative) | 15 (32.6%) | 12 (26.1%) | 15 (32.6%) | — | — | 4 (8.7%) | |
| Adjuvant Treatment | |||||||
| Posthepatectomy (n = 79) Adjuvant only |
10 (12.7%) | 21 (26.6%) | 23 (29.1%) | 16 (20.3%) | 2 (2.5%) | 7 (8.9%) | 0.96 |
| Perioperative (n = 46) (Postoperative) | 8 (17.4%) | 10 (21.7%) | 14 (30.4%) | 8 (17.4%) | 1 (2.2%) | 5 (10.9%) | |
5-FU, 5-fluorouracil; FUDR, floxuridine.
On the average, the cohort of patients in the pre-operative group received five cycles of preoperative neoadjuvant chemotherapy and the cohort of patients in the perioperative group received on the average 4.6 cycles of preoperative neoadjuvant chemotherapy (ANOVA F test, P = 0.71). In the posthepatectomy adjuvant only group, the average cycle of adjuvant chemotherapy was 6.8 cycles, whereas for the perioperative group, the average cycle of adjuvant chemotherapy was 5.3 cycles (ANOVA F test, P = 0.18).
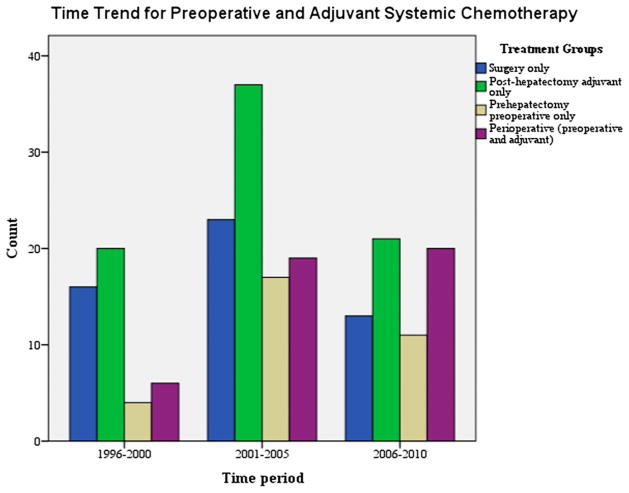
Over the course of the timeframe 1996 to 2010, within the time periods 1996 to 2000, 2001 to 2005, and 2006 to 2010, a significant change in the multiagent chemotherapy used for prehepatectomy preoperative (P < 0.000) or posthepatectomy adjuvant (P < 0.000) chemotherapy was noted (Fig. 1).
Fig. 1.
Time trend for preoperative and adjuvant systemic chemotherapy.
In comparing the four treatment groups in our study, significant differences were noted with regard to synchronous versus metachronous tumor status only (Table 2). The cohort of patients with metachronous tumors were more likely to be treated with prehepatectomy preoperative or perioperative chemotherapy (P = 0.01). Patients with synchronous tumors had a mean interval time to surgery from date of diagnosis of 2.3 months compared with 26.6 months of patients diagnosed with metachronous tumors (ANOVA t test P < 0.000).
Table 2.
Demographics, Clinicopathologic Tumor Characteristics, and Treatments of Patients Who Underwent Resection of CLM
| Characteristics | Posthepatectomy Adjuvant Only (n = 79) | Prehepatectomy Preoperative Only (n = 33) | Perioperative (preoperative and adjuvant) (n = 46) | Surgery Only (n = 52) | P Value |
|---|---|---|---|---|---|
| Age, years (mean/SD) | 59.9 (11.2) | 57.5 (10.0) | 57.7 (9.9) | 63.0 (11.8) | 0.06 |
| Age grouping | |||||
| 69 years or younger | 61 (78.2%) | 29 (90.6%) | 40 (88.9%) | 36 (69.2%) | 0.03 |
| 70 years or older | 17 (21.8%) | 3 (9.4%) | 5 (11.1%) | 16 (30.8%) | |
| Gender | |||||
| Female | 36 (45.6%) | 17 (51.5%) | 19 (41.3%) | 22 (42.3%) | 0.80 |
| Male | 43 (54.4%) | 16 (48.5%) | 27 (58.7%) | 30 (57.7%) | |
| Primary tumor location | |||||
| Colon cancer | 61 (78.2%) | 21 (70.0%) | 29 (67.4%) | 40 (83.3%) | 0.26 |
| Rectal cancer | 17 (21.8%) | 9 (30.0%) | 14 (32.6%) | 8 (16.7%) | |
| T3/T4 primary cancer | 52 (83.9%) | 22 (81.5%) | 31 (86.1%) | 30 (81.1%) | 0.93 |
| Nodal status primary cancer | |||||
| Positive | 39 (60.9%) | 19 (70.4%) | 25 (69.4%) | 26 (66.7%) | 0.76 |
| Mean tumor size (cm) | 3.3 (2.8) | 2.9 (2.6) | 3.1 (3.4) | 3.6 (3.4) | 0.75 |
| Synchronous | 18 (23.4%) | 2 (7.1%) | 4 (9.5%) | 15 (31.9%) | |
| Metachronous | 59 (76.6%) | 26 (92.9%) | 38 (90.5%) | 32 (68.1%) | 0.01 |
| Number of tumors resected | 1.3 (1.2) | 1.4 (1.3) | 1.6 (1.7) | 1.3 (1.2) | 0.77 |
| Margin status | |||||
| R0 | 53 (84.1%) | 22 (84.6%) | 38 (92.7%) | 36 (92.3%) | 0.43 |
| R1–2 | 10 (15.9%) | 4 (15.4%) | 3 (7.3%) | 3 (7.7%) | |
| Prehepatectomy CEA (ng/mL) | 25 (67.8) | 46 (99.1) | 32 (82.6) | 43 (157) | 0.21 |
| Mean cycles of chemotherapy | |||||
| Preoperative | — | 5.0 (3.6) | 4.4 (2.5) | — | 0.57 |
| Adjuvant | 6.8 (4.7) | — | 5.3 (3.0) | — | 0.18 |
| Posthepatectomy morbidity* | 27 (34.2%) | 13 (39.4%) | 21 (45.7%) | 29 (55.8%) | 0.09 |
| Year of treatment | |||||
| 1996–2000 | 20 (25.6%) | 4 (12.5%) | 6 (13.3%) | 16 (30.8%) | |
| 2001–2005 | 37 (47.4%) | 17 (53.1%) | 19 (42.2%) | 23 (44.2%) | 0.15 |
| 2006–2010 | 21 (26.9%) | 11 (34.4%) | 20 (44.4%) | 13 (25.0%) | |
Posthepatectomy morbidity is overall and includes both major and minor complications.
CLM, colorectal liver metastases; SD, standard deviation; CEA, carcinoembryonic antigen.
The variables age at diagnosis, posthepatectomy morbidity, synchronous versus metachronous tumor, and year of therapy that differed significantly or showed a trend toward this in univariate analysis among the four treatment groups were included in a multivariate analysis performed to determine significant independent predictors of which chemotherapeutic treatment algorithm that was chosen. Results from this analysis showed that older patients were less likely to receive preoperative or perioperative treatment; posthepatectomy complications resulted in the patients being more likely to be treated with surgery alone; and patients with metachronous tumors were less likely to be treated with surgery only (Table 3).
Table 3.
Odds Ratios Using Multinomial Logistic Regression to Determine Which Clinicopathologic Factor Predicted Which Treatment Algorithm That Was Chosen
| Characteristics | Prehepatectomy* Preoperative Only OR (95% CI), P Value | Perioperative* (preoperative/adjuvant) OR (95% CI), P Value | Posthepatectomy* Adjuvant Only OR (95% CI), P Value |
|---|---|---|---|
| Age at diagnosis, years | 0.9 (0.9–1.0), 0.04 | 0.9 (0.9–1.0), 0.02 | 0.9 (0.9–1.0), 0.11 |
| Complication status, positive versus negative | 2.0 (0.7–5.6), 0.18 | 1.5 (0.6–4.0), 0.32 | 2.8 (1.2–6.5), 0.01 |
| Metachronous versus synchronous | 0.1 (0.1–0.6), 0.01 | 0.1 (0.1–0.5), 0.005 | 0.6 (0.2–1.5), 0.36 |
| Year of treatment† | |||
| 1996–2000 | 0.2 (0.1–1.1), 0.07 | 0.1 (0.04–0.6), 0.008 | 0.5 (0.1–1.6), 0.30 |
| 2001–2005 | 1.0 (0.3–3.4), 0.96 | 0.4 (0.1–1.4), 0.20 | 1.1 (0.4–3.0), 0.79 |
Reference group surgery only.
Reference group year 2006–2010.
OR, odds ratio; CI, confidence interval.
Survival Analyses
Overall Survival
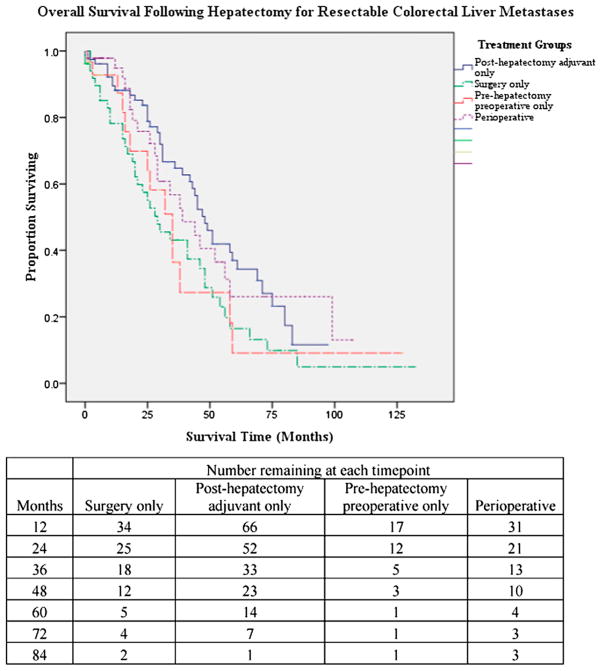
The three- and five-year overall survival (OS) rates were 54 and 26 per cent, respectively, with a median OS of 41 months. Median follow-up was 25 months. The adjuvant, preoperative, perioperative, and surgery only groups had a median survival time of 48, 35, 39, and 29 months, respectively (log-rank P = 0.04) (Fig. 2). The 5-year overall survival rates were 37.0, 9, 26.0, and 16.0 per cent, respectively, for the adjuvant, preoperative, perioperative, and surgery only groups. Other variables independently associated with overall survival in Kaplan-Meier univariate analysis include complication status (P = 0.02) and nodal status (P = 0.04). Constructing a confounder model using Cox regression analysis, and including all variables found to be statistically significant in univariate Kaplan-Meier analysis, independent predictors of OS on multivariate analysis included treatment algorithm used and postoperative complication status (Table 4).
Fig. 2.
Survival plots by Kaplan-Meier.
Table 4.
Multivariate Analysis for Overall Survival
| Characteristics | HR | 95% CI | P Value |
|---|---|---|---|
| Posthepatectomy morbidity | |||
| Complication versus none | 1.5 | 1.0–2.5 | 0.04 |
| Chemotherapy regimen surgery only* | |||
| Posthepatectomy/adjuvant only | 0.4 | 0.2–0.7 | 0.005 |
| Prehepatectomy/preoperative only | 0.6 | 0.3–1.4 | 0.31 |
| Perioperative | 0.6 | 0.3–1.1 | 0.14 |
Referent group.
HR, hazard ratio; CI, confidence interval.
Posthepatectomy Morbidity and Mortality
Overall morbidity rate for our study population was 42.9 per cent. Based on the Clavien-Dindo grading of postoperative complications, the distribution of the complications among the treatment groups were as follows: Grades I and II 19.2, 13.9, 12.1, and 17.4 per cent, respectively, for the surgery only, adjuvant only, preoperative only, and the perioperative groups. Grades III and IV were 36.5, 20.3, 27.3, and 28.3 per cent, respectively, for the surgery only, adjuvant only, preoperative only, and the perioperative groups. The era of treatment was the only independent predictor of postoperative complication in univariate analysis with patients treated within the time period 2001 to 2005 having a higher incidence of postoperative complications, 61.4 versus 13.6 and 25.0 per cent, respectively, for the time periods 1996 to 2000 and 2006 to 2010. A multivariate analysis showed independent predictors of postoperative complications to be 1) time period of treatment with patients treated within the years 2001 to 2005 6.5 times more likely to have an associated postoperative complication when compared with those treated in the time period 1996 to 2000 (95% confidence interval [CI], 1.7 to 24.8; P = 0.006); 2) treatment group with patients treated with the posthepatectomy adjuvant only option significantly less likely to have sustained a postoperative complication when compared with prehepatectomy preoperative only group (odds ratio, 0.2; 95% CI, 0.06 to 0.6; P = 0.05); and 3) number of tumors resected with patients undergoing resection of greater than three lesions 4.2 times more likely to sustain a postoperative complication when compared with those undergoing resection of less than three lesions (95% CI, 1.0 to 17.1; P = 0.04).
Overall survival for those patients who experienced a postoperative complication was 34 months versus 46 months (P = 0.02) for those who did not experience a postoperative complication.
Discussion
Surgical resection remains the standard of care for management of patients diagnosed with resectable CLM. Modern systemic chemotherapeutic agents have been shown to increase the resection rates and improve relapse-free rates when used as adjuncts to surgical resection.15, 16 However, the most appropriate and effective timing of institution of systemic chemotherapy relative to surgical resection in the management algorithm for resectable CLM still remains to be defined. The different treatment algorithms have included posthepatectomy adjuvant chemotherapy only, prehepatectomy preoperative chemotherapy only, and perioperative chemotherapy.
Results from our study show that posthepatectomy adjuvant only systemic chemotherapy is associated with an improved survival in resectable colorectal liver metastases. Nordlinger et al. in a randomized controlled trial comparing perioperative chemotherapy with FOLFOX and surgery versus surgery alone for resectable liver metastases from colorectal cancer showed that perioperative chemotherapy with FOLFOX is compatible with major liver surgery and improves progression-free survival in eligible and resected patients compared with surgery alone. The increase in progression-free survival at 3 years was 7.3 per cent (28.1 to 35.4%; P =0.058) in randomized patients; 8.1 per cent (28.1 to 36.2%; P =0.041) in eligible patients; and 9.2 per cent (33.2 to 42.4%; P = 0.025) in patients undergoing resection. Parks et al.,18 in a large study with patients stratified by risk of recurrence, demonstrated that systemic adjuvant chemotherapy such as a 5-FU-based regimen prolongs survival after hepatic resection for colorectal metastases. In their study, use of chemotherapy was associated with a 47-month median survival and a five-year survival rate of 37 per cent versus 36-month median survival and five-year survival of 31 per cent for patients not receiving chemotherapy (P = 0.007). Similarly, Portier et al. in a multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of CLM showed that despite a suboptimal regimen, adjuvant intravenous systemic chemotherapy provided a significant disease-free survival benefit with a five-year disease-free survival rate of 33.5 per cent for patients treated with chemotherapy versus 26.7 per cent for those not treated with chemotherapy (P = 0.028).
Wieser et al.19 in a meta-analysis aimed at evaluating the effectiveness of perioperative chemotherapy in patients with resected Stage IV colorectal cancer showed that of eight trials conducted on a total of 1174 patients, hazard ratio (HR) estimates suggested that perioperative chemotherapy yielded no survival advantage over surgery alone (HR, 0.94; 95% CI, 0.8 to 1.10; P = 0.43); in a subset analysis on intra-arterial chemotherapy alone, no survival benefit was evident (HR, 1.0; 95% CI, 0.84 to 1.21; P = 0.96); in the trials involving systemic chemotherapy, the difference between the groups approached statistical significance (HR, 0.74; 95% CI, 0.53 to 1.04; P = 0.08). However, both peri-operative treatment groups had a significant recurrence-free survival benefit (HR, 0.78; 95% CI, 0.65 to 0.95; P = 0.01 for hepatic arterial infusion; and HR, 0.75; 95% CI, 0.62 to 0.91; P = 0.003 for systemic therapy). In conducting a univariate and multivariate analysis using prehepatectomy chemotherapy only, posthepatectomy chemotherapy only, perioperative chemotherapy, and no chemotherapy as treatment variables, Reddy et al.20 showed that chemotherapy administered after and not before hepatic resection was associated with survival among patients with colorectal liver metastasis. Among these treatment variables, only posthepatectomy chemotherapy was associated with OS on multivariate analysis, although the perioperative treatment variable did show a trend toward statistical significance. A multivariate analysis in our study yielded a similar outcome (Table 4). Another significant independent predictor of OS in our study included postoperative complication status.
Part of the limitation of our study is its retrospective nature, which makes it difficult to control bias and confounders and also makes it difficult to establish cause and effect. Older patients in our series tended not to receive any type of systemic therapy and this could have skewed the results in favor of the other groups. Of note in our series is the fact that patients with synchronous disease tended not to receive prehepatectomy preoperative therapy and were more likely to have a shorter mean interval time to surgery of 2.3 months from date of diagnosis. Also of note, although not statistically significant, is the fact that the cohort of patients in the posthepatectomy adjuvant only and prehepatectomy preoperative only groups had a relatively higher incidence of R1–2 surgical resections. Poultsides et al.21 most recently documented after a more rigorous multi-institutional data analysis that the likelihood of local recurrence is independent of margin width. Rather than millimeters, tumor biology is a more important predictor of both intrahepatic any-site recurrence and worse OS.
As to why the cohort of patients in the preoperative group had a worse prognosis, we hypothesize that the associated postoperative complications, which also most likely precluded this cohort of patients from receiving adjuvant therapy, is responsible for this. Patients who had lower postoperative morbidities were more likely to receive adjuvant chemotherapy, whereas those with higher complication rates did not. Thus, patients receiving adjuvant chemotherapy could potentially be those with the best surgical outcomes who would have done well irrespective of when they received their chemotherapy. Ito et al.22 in their study showed that postoperative morbidity adversely affects long-term outcome after hepatic resection for CLM in patients at lower risk for recurrence; an overall morbidity rate of 42 per cent was noted in their study. On multivariate analysis by Ito et al., morbidity was not an independent predictor of either disease-specific survival (DSS) or disease-free survival (DFS); however, in a subgroup of patients with low clinical risk scores, morbidity was associated with a significant reduction in both DSS and DFS. Overall morbidity rate in our study was 42.9 per cent; we found postoperative complication to be an independent predictor of a shortened survival time in both univariate and multivariate analysis. Most deaths in the prehepatectomy preoperative group occurred in the time period 2001 to 2005 marked by an increased enthusiasm for use of prehepatectomy preoperative therapy.
Another possibility is if patients receiving preoperative therapy had some hepatic lesions and undergo complete radiologic response and these metastatic sites were not subsequently resected. Given the low incidence of a complete pathologic response, this could then lead to increased recurrence rates with poorer clinical outcomes. Benoist et al.23 in their study documented that in most patients receiving chemotherapy for colorectal liver metastasis, a complete response on computed tomography scan does not mean cure.
The time period for the data collected spans several era in systemic chemotherapy practice and surgical treatment paradigm, which thus limits the determination of any definitive conclusion about optimal timing of chemotherapy relative to hepatic resection. The time period 2001 to 2005 in our study witnessed an increase in the use of both pre- and posthepatectomy chemotherapy, coinciding with the evolution of more efficacious systemic chemotherapy. The use of the different chemotherapeutic agents over the time period of our study mirrors the timeline over the past decade of the evolution of different chemotherapeutic regimens used for management of metastatic colorectal cancer. Prehepatectomy preoperative chemotherapy may have benefit in certain cohorts of patients. Adam et al.24 demonstrated that liver resection is able to offer long-term survival to patients with multiple colorectal metastases provided that the metastatic disease is controlled by chemotherapy before surgery. They noted that tumor progression before surgery is associated with a poor outcome, even after potentially curative hepatectomy. Tumor response before surgery is important to offer a chance of surgical intervention in patients who would be considered to have borderline resectable disease. Thus, prehepatectomy preoperative chemotherapy is a viable tool for patient selection in this high-risk cohort. However, for patients with resectable CLM, it is unclear what is the most efficacious sequencing of systemic therapy. Given this fact and the potential benefits of posthepatectomy adjuvant chemotherapy only noted in this study and others (Reddy) as well as the results of the Nordlinger study, the need for prospective randomized trials to determine optimal timing for systemic therapy in these patients is of increasing importance. The National Surgical Adjuvant breast and Bowel project C-11 was a Phase III, multicenter, randomized trial that was designed to evaluate the difference in recurrence-free survival between patients with resectable CLM receiving perioperative (preoperative plus postoperative) chemotherapy versus those receiving only postoperative chemotherapy. Other goals of this clinical trial include OS, postoperative morbidity, and perioperative/postoperative toxicity of the chemotherapy regimens. Unfortunately, this trial recently closed as a result of low accrual. Further evaluation of perioperative chemotherapy for resectable CLM is needed, but this closure illustrates the challenges of prospective evaluation.
Footnotes
Presented at the Society of Surgical Oncology 64th Annual Meeting, San Antonio, Texas, March 2011.
References
- 1.Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD: National Cancer Institute; [Accessed: April 2012]. based on November 2009 SEER data submission, posted to the SEER web site. Available at: http://seer.cancer.gov/csr/1975_2007/.2010. [Google Scholar]
- 2.Minagawa M, Yamamoto Y, Miwa S, et al. Selection criteria for simultaneous resection in patients with synchronous liver metastasis. Arch Surg. 2006;141:1006–12. doi: 10.1001/archsurg.141.10.1006. [DOI] [PubMed] [Google Scholar]
- 3.Nordlinger B, Guiguet M, Vaillant J-C, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77:1254–62. [PubMed] [Google Scholar]
- 4.Abdalla EK, Adam R, Bilchik AJ, et al. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271–80. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of CLM defines cure. J Clin Oncol. 2007;25:4575–80. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 6.Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460–6. doi: 10.1001/archsurg.141.5.460. [DOI] [PubMed] [Google Scholar]
- 7.Zakaria S, Donohue JH, Que FG, et al. Hepatic resection for colorectal metastases: value of risk scoring systems. Ann Surg. 2007;246:183–91. doi: 10.1097/SLA.0b013e3180603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection of colorectal metastases. Ann Surg. 2005;241:715–22. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–66. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–83. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg RM, Sargent DJ, Morton RF, et al. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American intergroup trial. J Clin Oncol. 2006;24:3347–53. doi: 10.1200/JCO.2006.06.1317. [DOI] [PubMed] [Google Scholar]
- 12.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 13.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 14.Portier G, Elias D, Rougier P, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFDCD ACHBTHJ AURC 9002 trial. J Clin Oncol. 2006;24:4976–82. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 15.Nordlinger B, Sorbye H, Glimelius B, et al. Peri-operative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomized controlled trial. Lancet. 2008;371:1007–16. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruenberger B, Tamandl D, Schueller J, et al. Bevacizumab, capecitabine, and oxaliplatin as preoperative therapy for patients with potentially curable metastatic colorectal cancer. J Clin Oncol. 2008;26:1830–5. doi: 10.1200/JCO.2007.13.7679. [DOI] [PubMed] [Google Scholar]
- 17.Gruenberger B, Scheithauer W, Punzengruber R, et al. Importance of response to preoperative chemotherapy in potentially curable colorectal cancer liver metastases. BMC Cancer. 2008;8:120. doi: 10.1186/1471-2407-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parks R, Gonen M, Kemeny N, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg. 2007;204:753–61. doi: 10.1016/j.jamcollsurg.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 19.Wieser M, Sauerland S, Arnold D, et al. Peri-operative chemotherapy for the treatment of resectable liver metastases from colorectal cancer: a systematic review and meta-analysis of randomized trials. BMC Cancer. 2010;10:309. doi: 10.1186/1471-2407-10-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy SK, Zorzi D, Wei Lum Y, et al. Timing of multimodality therapy for resectable synchronous colorectal liver metastases: a retrospective multi-institutional analysis. Ann Surg Oncol. 2009;16:1809–19. doi: 10.1245/s10434-008-0181-y. [DOI] [PubMed] [Google Scholar]
- 21.Poultsides GA, Schulick RD, Pawlik TM. Hepatic resection for colorectal metastases: the impact of surgical margin status on outcome. HPB (Oxford) 2010;12:43–9. doi: 10.1111/j.1477-2574.2009.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito H, Are C, Gonen M, et al. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:994–1002. doi: 10.1097/SLA.0b013e31816c405f. [DOI] [PubMed] [Google Scholar]
- 23.Benoist S, Brouquet A, Penna C, et al. Complete response of colorectal liver metastases after chemotherapy: does it mean cure? J Clin Oncol. 2006;24:3939–45. doi: 10.1200/JCO.2006.05.8727. [DOI] [PubMed] [Google Scholar]
- 24.Adam R, Pascal G, Castaing D, et al. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–64. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]