Abstract
Objectives
High rates of household participation are critical to the success of door-to-door vector control campaigns. We used the Health Belief Model to assess determinants of participation, including neighbor participation as a cue to action, in a Chagas disease vector control campaign in Peru.
Methods
We evaluated clustering of participation among neighbors; estimated participation as a function of household infestation status, neighborhood type, and number of participating neighbors; and described reported reasons for refusal to participate in a district of 2911 households.
Results
We observed significant clustering of participation along city blocks (p< .0001). Participation was significantly higher for households in new vs. established neighborhoods, for infested households, and for households with more participating neighbors. The effect of neighbor participation was greater in new neighborhoods.
Conclusions
Results support a “contagion” model of participation, highlighting the possibility that one or two participating households can tip a block towards full participation. Future campaigns can leverage these findings by making participation more visible, by addressing stigma associated with spraying, and by employing group incentives to spray.
Keywords: Chagas disease, communicable diseases, control of disease, epidemiology, developing country, tropical health
INTRODUCTION
Community participation in health programs has been a core goal of health planners and practitioners since the 1978 Alma Ata Declaration.[1] Many health programs rely on household and community participation to achieve key outcomes. In the case of vector control, high rates of household participation are critical to the success of door-to-door campaigns that target mosquitoes,[2] triatomine bugs,[3] and other insects of medical importance.[4] Low participation rates decrease the effectiveness and efficiency of disease control efforts, and at the same time may signal that communities are not invested in the campaign aims or strategies.
The goal of this study is to describe patterns and predictors of participation in a Chagas disease vector control campaign in Peru. Chagas disease is a principal cause of morbidity and mortality in the Americas.[5] The economic burden of Chagas disease is estimated to exceed that of cervical cancer, rotavirus, or Lyme disease.[6] More than 8 million people are infected with Trypanosoma cruzi, the parasitic agent of the disease.[7] In the southern part of South America, T. cruzi is transmitted primarily by Triatoma infestans insect vectors. Since 1991, T. infestans has been the target of a widespread control program known as the Southern Cone Initiative. Through the efforts of this initiative, the disruption of T. cruzi transmission by T. infestans has been declared in Chile[8], Brazil[9] and Uruguay.[10] Vector control campaigns are ongoing in southern Peru, where in contrast to other areas, the vector is an urban rather than a rural problem.[11, 12]
The control of Chagas disease depends critically on successful indoor residual spraying campaigns paired with long-term surveillance to address vector return and reinfestation. [13, 14] Recent data from a vector control campaign in urban Arequipa, Peru indicate that participation was only 66%, which may be insufficient to control triatomine insects and to disrupt T. cruzi transmission. Declining participation in urban areas threatens the success of the Peru campaign, and ultimately that of the Southern Cone Initiative. Understanding the determinants of household participation is therefore an important step in designing new interventions to ensure campaign success.
To inform campaign improvements, we studied the correlates of participation in one district of Arequipa, Peru. Our analysis was guided by the Health Belief Model, which models health-related behaviors as a function of the perceived threat of the related health condition, perceived benefits and barriers, and cues to action.[15] We first hypothesized that the observable participation of neighbors would be an important cue to action—in other words, participation may be “contagious” along city blocks. Another obvious cue to action is vector infestation, which we hypothesized would also be associated with participation. We further hypothesized that the influence of neighbor participation may depend on infestation status: households that are already infested may be less motivated by neighbor participation than uninfested households. Finally, we assessed barriers to participation reported in a neighborhood with low participation rates.
METHODS
In 2003 the Ministry of Health (MOH) in Arequipa, Peru initiated a large-scale insecticide application campaign with the aim of eliminating T. cruzi transmission by T. infestans. The campaign proceeds district by district in three phases: In the preliminary survey phase, prior to application of insecticide, all houses are surveyed for the presence of T. infestans. In the attack phase, sensibilizadoras (health promoters) visit each household, explain the risks of Chagas disease and the role of the insect in transmission, and encourage residents to agree to insecticide application. Sensibilizadoras also explain how to prepare the home for insecticide spraying by moving furniture away from walls and stowing bed linens, food, and kitchenware. The following day an exterminator applies insecticide in the home and around peri-domestic animal enclosures. The process is repeated six months later. In the surveillance phase, health promoters and campaign staff monitor sprayed areas for vector reinfestation.
The Arequipa campaign stresses the specific objective of convincing community members to accept insecticide spraying; in the community participation literature, this approach is termed a “target-oriented” frame.[16, 17] In this context, we adopt a narrow definition of participation: household consent to and completing of insecticide spraying during the first spray of the attack phase. Non-participation may be due to several reasons: Households may refuse to participate, be away from home when the campaign visits, fail to adequately prepare the home for spraying, or not provide access to locked rooms (exterminators will only spray if all rooms can be accessed).
We used data from the preliminary and attack phases of the campaign in the Mariano Melgar (hereafter, MM) district of Arequipa collected between 2010 and 2012. The district is home to approximately 9500 households and three distinct neighborhood types: established, new, and land invasion. Land invasion neighborhoods emerge when recent migrants organize to “invade” undeveloped land and construct basic housing from cheap materials. If the settlers are not forcibly removed, housing stock is improved over time and residents may receive land title. At this point, a land invasion becomes a “new neighborhood” (pueblo joven). As new neighborhoods mature into established neighborhoods with wealthier residents, homes become larger and gain permanent utility connections. In our study, established neighborhoods were founded several decades ago, and tend to have wealthier residents. New neighborhoods were founded in the 1980s and 1990s during a time of mass rural to urban migration in Peru. The recent land invasions in our study setting originated around 2000, but did not have any triatomine bugs during the preliminary survey phase and were therefore not included in the spray campaign nor in these analyses.
Neighbor participation and infestation status as cues to action
We first looked for spatial patterns in participation using campaign data from the first round of the attack phase in Pueblo Tradicional (hereafter, PT), the largest neighborhood in MM (n=2911 households). We looked for statistically significant runs of participation along blocks, with a “run” defined as a series of similar responses (i.e, participation or non-participation). We used the Siegel and Castellan runs test, [18] which assesses the frequency of runs and compares the observed frequency to that expected by chance. The runs test requires that we linearize the city block; we followed the numbering assigned to households by the MOH to order the insecticide application. The first and last houses were therefore not considered neighbors even though they may have been contiguous.
Our second analysis exploited a transect sample (2 blocks wide by 2 km long) of MM that includes both established and new neighborhoods. We used detailed vector infestation data (collected by our study team during the preliminary survey in 2009-2010) and campaign participation data (collected during the first spray in 2011). In the preliminary survey, data was collected from 381 of the 443 households in the transect. Using a logistic regression model, we estimated participation as a function of: the number of immediate neighbors who participated in the campaign (0,1,2); a binary measure of infestation in the house (1=infested: any insects found during preliminary survey, 0=uninfested: no insects found during the preliminary survey); and the interaction of neighbor participation and infestation. We first fitted this model on the full transect sample controlling for neighborhood type (established vs. new). To test hypotheses based on campaign observations and our knowledge of neighborhood evolution, we then stratified the sample by neighborhood type to assess differences in the relationship between infestation status, neighbor participation, and household participation in established vs. new neighborhoods.
Perceived barriers to participation
Low participation in the first round of insecticide application motivated the Ministry of Health to record reported reasons for non-participation during the second round of insecticide application. We used data from 534 visits to 446 non-participating households in PT to assess perceived barriers to participation. Householders could provide more than one reason for refusal during any given visit, and 75 households (17%) were visited more than one time. All reasons mentioned by households at any visit were included in the analysis. Interviewers coded reasons for refusal according to a nine-category coding scheme developed from open-ended responses about non-participation collected previously in nearby neighborhoods of MM. We analyzed the distribution of reasons for refusal reported during the second spray in PT among this sample of households.
RESULTS
Participation in the first round of the attack phase in Mariano Melgar was 66% (6336 of 9579 total properties). We observed geographic clustering of participation (Figure 1), including significant runs of participation along blocks (p< .0001, Siegal-Castellan runs test). While this test of spatial auto-correlation does not imply that neighbor behavior influences participation decisions, it does suggest some social contagion.
Figure 1.
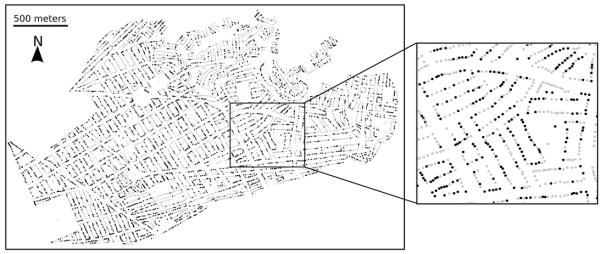
Participation in first round of attack phase of Chagas disease vector control campaign, Mariano Melgar District, Arequipa, Peru, 2011. Gray dots denote participation, black dots denote non-participation.
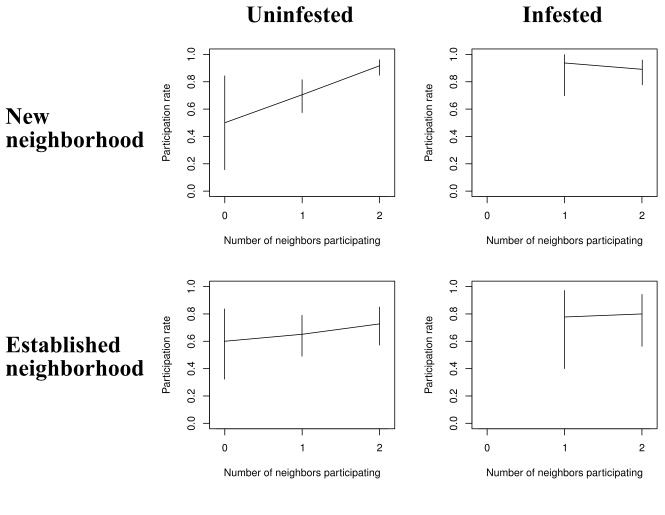
Participation in the transect sample was 77% (340 of 433 total properties), higher than in the MM overall. However, there were stark differences in participation rates along the transect. Most notably, participation was higher in new vs. established neighborhoods, and for infested vs. uninfested households (Figure 2). Furthermore, the upward slope for the uninfested households in new neighborhoods in Figure 2 indicates an association between the number of neighbors participating and household participation in this subgroup: The probability of participation was 50% for households with no neighbors participating vs. 92% for households with both adjacent neighbors participating. Within neighborhood type, infested households were more likely to participate than uninfested households, although the gap narrowed as more neighbors participated. Among infested households, those in new neighborhoods participated more than those in established neighborhoods.
Figure 2.
Participation in spray campaign by infestation status, neighborhood, and immediate neighbor participation, Mariano Melgar, 2011. Analysis excludes cells with fewer than five households (infested house, new neighborhood, 0 neighbors participating; and infested house, established neighborhood, 0 neighbors participating).
We confirmed the association of household and neighbor participation through regression models (Table 1). In Model 1, each additional participating neighbor more than doubled the odds of participating, while infestation increased the odds of participating more than tenfold. The negative interaction term indicates that the influence of neighbor participation was attenuated in infested households. The model also shows that households in established neighborhoods had, on average, half the odds of participating compared to households in new neighborhoods. In Models 2 and 3, we stratified by neighborhood type. Results confirmed that neighbor participation was a significant predictor of household participation only in new neighborhoods (OR = 3.79, p<.01); within new neighborhoods, neighbor participation was only a significant predictor of household participation among uninfested households (OR for interaction of neighbor participation and infestation = .14, p<.10). Infestation also significantly increased the odds of participating in new neighborhoods by almost 40 times. Our results suggest that neighbor participation and infestation are both important cues to action in new neighborhoods, but less so in established neighborhoods, thus motivating our analysis of perceived barriers to participation for these households.
Table 1.
Odds ratios from logistic models predicting participation in a Chagas disease vector control campaign as a function of neighbor participation, infestation status, and neighborhood type, Mariano Melgar District Transect Sample, Arequipa, Peru, 2010 (N= 381 households).
| (1) All households |
(2) Established neighborhood |
(3) New neighborhood |
|
|---|---|---|---|
| # of neighbors participating | 2.16** [0.74] |
1.35 [0.67] |
3.79*** [1.50] |
| Infested = 1 | 10.93*** [9.31] |
4.79 [5.32] |
39.13* [57.36] |
| Interaction: # of neighbors participating X infested | 0.32** [0.16] |
0.54 [0.38] |
0.14*** [0.10] |
| Established neighborhood = 1 | 0.52** [0.14] |
||
| Constant | 1.53 [0.80] |
1.43 [0.95] |
0.70 [0.36] |
| Observations | 381 | 133 | 248 |
Robust standard errors in brackets account for clustering at the block level.
p<0.01,
p<0.05,
p<0.1
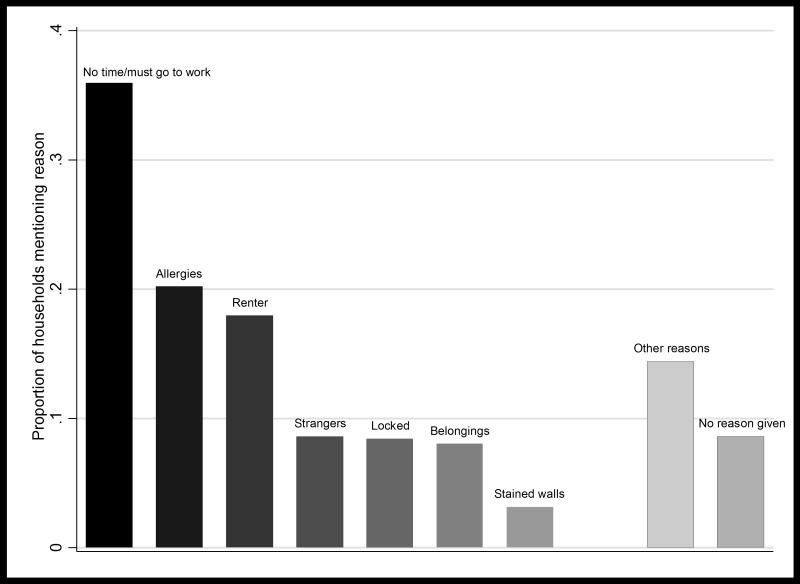
Figure 3 presents the distribution of reasons given for non-participation by 446 households in PT in 2012. The dominant reason was the inability to wait at home for the exterminators or having to go to work (36%). Two other commonly stated reasons were concerns about allergies (20%) and renters not willing to consent to spray on behalf of a landlord (18%). Less frequently mentioned were concerns about letting strangers into the house (9%), locked rooms with no key available (8%), not wanting to move furnishings to prepare for spraying (8%), and concerns about the insecticide staining walls (3%). An additional 14% of responses fell into the “other” category; the most frequently-mentioned reason among this group of responses was the lack of bugs in the home. In addition, 9% of responses indicated “no reason” for refusal, suggesting that householders were unwilling to state true reasons for refusal, were not the main decision-maker in the household, or were so little engaged with the vector control campaign that a clear reason for refusal could not be articulated.
Figure 3.
Reasons given for refusing indoor residual spray by households in the community of Pueblo tradicional, Mariano Melgar, Arequipa, Peru (N=446 households). Households could provide more than one reason per visit, and 17% of households provided reasons for refusal on more than one visit. See text for description of each refusal reason.
DISCUSSION
Our results suggest that participation in the campaign may have been influenced by neighbor participation. Household participation was clustered spatially, suggesting that neighbor participation may be an important cue to action. While the runs of participation we observed could have been due to spatial patterns of urban vector infestation [19] or other sociodemographic predictors of participation, the positive association between neighbor participation and each household’s participation decision was robust to the addition of vector infestation and neighborhood type controls in multivariate models. Our results highlight the potential to nudge others towards participation if just one or two households assent to insecticide application. Conversely, one or two refusals on a block may threaten the participation of many additional households.
Much of the previous work on vector control campaign participation has focused on surveillance or on prevention activities related to building materials and environmental hygiene;[20-23] our results contribute additional evidence on participation in indoor residual spraying activities, a less-studied topic. Several prior studies have also identified reasons for refusal that map to the Health Belief Model: lack of knowledge of the disease, low perceived risk, poor communication by control campaign staff, distrust of government services, stigma associated with vectors or with control activities, and perceived low efficacy and high cost of interventions.[24-26] Householders may be particularly wary when campaign activities require workers to enter the home.[24, 27]
Higher rates of participation have been observed in campaigns across several countries when the community is engaged early in the process; when control activities are integrated with primary health care and development activities; when campaigns include direct, face-to-face contact with public health officials and community health workers; and when householders are given choices about when and how to implement control activities.[20, 28] Positive engagement and incentives have also proved more effective than punitive measures such as fines or citations.[22] These findings are consistent with broader work on community participation showing that participatory approaches that empower communities to identify health problems and design solutions in partnership with government programs may be more successful.[29]
While the current Chagas vector control campaign in Arequipa already employs many of these lessons, participation remains low in urban areas, and a better understanding of participation determinants is needed. The present study contributes to that understanding in several important ways: First, we observed less participation among households in the wealthier established neighborhoods compared to poorer new communities. This is to be expected given the lower prevalence of vector infestation, an important cue to action. However, even when controlling for vector infestation, lower participation rates persisted in established neighborhoods. We propose both an economic and a social mechanism for this difference: Economically, campaign participation may impose a greater perceived burden on wealthier individuals, who have larger households with more belongings to move, and who may assign a higher opportunity cost to their time. Socially, wealthier households may perceive more social stigma associated with insecticide application, which may be construed as a public signal of vector infestation.
We make a second key contribution to the literature on the influence of social norms on health-related behavior (observed in many other contexts[30, 31]) by identifying two important effect modifiers in the relationship between descriptive norms and participation: First, we find that the influence of descriptive norms (i.e., observable neighbor participation) is weaker for households with stronger cues to action in the form of visible insect infestation. Second, social influence appears stronger in new vs. established neighborhoods. This may be due to the greater importance of social ties for managing risk and transmitting information in poor neighborhoods.[32] This finding is consistent with how neighborhoods evolved in the district as described above: In earlier stages of neighborhood evolution, settlers are well-organized. Social ties may then weaken in established neighborhoods as individuals become less interdependent. It is important to note here, however, that non-participating households do not report neighbor participation as a reason for refusal. We propose two reasons why neighbor participation was not raised by this sample of refuers. First, neighbor participation may be a stronger inducement to participate than neighbor non-participation is an inducement to not participate. The lack of responses about neighbor participation in this group of refusers may reflect that asymmetry. Second, people generally lack the ability to accurately report reasons for past behavior, unless those motivations are highly salient and plausible.[33] We therefore interpret the reported reasons for refusal as meaningful perceptions about barriers to participation, but do not interpret the failure to report neighbor participation as evidence against a contagion hypothesis.
Finally, our results inform interventions that may be effective in boosting participation in similar campaigns. Campaigns can first address some reasons for refusal through simple operational changes. For example, the most common stated reason for refusal in this study was the inability to wait at home for the spray brigades due to work commitments. Innovative scheduling schemes, such as guaranteed 2-hour windows, evening appointments, or priority scheduling for the first households to agree to spraying could address this concern. We caution, however, that such schemes could backfire if schedules are not feasible given available personnel and infrastructure.
Other stated reasons for refusal are less amenable to campaign changes. In Arequipa, concerns about allergies (either a purported allergy to the insecticide or the concern that the insecticide would exacerbate existing allergies or asthma, particularly among children) were common. Previous research has shown that beliefs about allergies and asthma triggers are strongly held and culturally-specific.[34, 35] These perceptions may therefore be difficult to change in the current campaign, even if a different insecticide were adopted or safety data were presented to concerned residents (both of which have been tried in the past with minimal results).
Another set of campaign innovations could leverage the observed importance of neighbor participation for household participation decisions, particularly in new neighborhoods where social influence appears strong. Making participation more visible and salient, for example, by giving participating households posters or t-shirts, could amplify the effect of neighbor participation. Participation symbols that frame participation as beneficial to the community or to child health may further combat stigma associated with insecticide spraying.
To increase the perceived benefits of participation, lottery-based incentives could be introduced.[36, 37] Lotteries, common in Peru, provide immediate and tangible benefits to participation that may overcome some of the perceived costs of participation (time, inconvenience, and stigma).[38] A lottery may also motivate participation by decoupling participation from the stigma of vector infestation, and linking it instead with interest in the lottery.[39] To leverage both social norms and the motivation provided by lotteries, group lotteries could be introduced in which groups of contiguous households must all participate to be eligible for a lottery prize.[40] As the response to lotteries may differ by socioeconomic status (with less wealthy households more likely to respond), rigorous trials evaluating the heterogeneous impact of these proposed interventions on participation are needed.
Our study leverages operational data from an ongoing vector control campaign to better understand patterns and determinants of participation. However, we note some important limitations. First, our conceptualization of community and household participation in this study is fairly narrow. This is driven primarily by the structure of the vector control campaign, which seeks to convince households to accept the recommended insecticide spraying. We recognize that a broader investigation into community perceptions about the campaign and strategies to better engage the community in the design and implementation of the campaign could be very fruitful; however, these approaches are not currently part of the vector control campaign in Arequipa and were beyond the scope of this study. Second, our data include spatial but not temporal aspects of household participation, and we are therefore not able to nail down the causal relationship between neighbor and household participation. Third, as refusing households are often visited by multiple health promoters, we are not able to control for the effect of individual health promoters on the participation decision. Fourth, there are certainly other covariates that are determinants or modifiers of participation that we were unable to measure, and our models can certainly be improved with additional studies. Particularly, we were unable to measure social networks, which may play an even more important role in the “contagiousness” of participation than strictly geographic neighborhood networks, and we were not able to assess within-neighborhood variation in socioeconomic status. Fifth, detecting vectors in households can be difficult, and our logistic regression models may suffer from misclassification of household infestation status, which would bias our estimates toward the null. Sixth, we had visited the households in the transect sample, which we used in our regression, many times and residents were much more cognizant of the dangers of Chagas disease and later participated more than residents outside the transect. This general increase in participation may also affect our regression analysis, most likely by biasing estimates towards the null if the increase in participation is uniform. Finally, our sample size for the analysis of refusal reasons is relatively small.
Household decisions about participation in vector control campaigns are multi-factorial. Using operational data from a door-to-door vector control campaign in Peru, we have shown significant “runs” of participation along city blocks. We have also demonstrated that household participation is associated with the participation of neighbors, and that this relationship varies by household and neighborhood characteristics. We have highlighted the diversity in stated reasons for non-participation, some of which can be addressed through changes in campaign operations. These results direct us to future interventions, which must decrease social and time costs to participation while increasing tangible and social benefits. Lessons learned can be extended to other urban public health campaigns, particularly vector control campaigns against bed bug infestations and against mosquitoes that carry dengue, West Nile virus, and malaria, where the social and spatial structure of city blocks link household decisions to community health outcomes.
What is already known on this subject?
Door-to-door vector control campaigns require high rates of household participation to succeed. To halt transmission of Chagas disease in an endemic area of South America, it is important to understand why households accept or refuse indoor residual spraying.
What this study adds?
The decision to participate in a vector control campaign is complex. This study of a Chagas disease vector control campaign in Arequipa, Peru shows that neighbor participation is association with household participation, and that this relationship is stronger for lower-income neighborhoods and for households that are not infested with insect vectors. The study also highlights the reasons given by households for non-participation, some of which can be addressed through changes in campaign operations. These results direct us to future interventions, which must decrease social and time costs of participation while increasing tangible and social benefits.
ACKNOWLEDGMENTS
The authors thank The Chagas Disease Working Group in Arequipa comprised of staff of the Gerencia Regional de Salud en Arequipa (GERSA), Ministerio de Salud del Perú (MINSA), Dirección General de Salud de las Personas (DGSP), Estrategia Sanitaria Nacional de Prevención y Control de Enfermedades Metaxénicas y Otras Transmitidas por Vectores (ESNPCEMOTVS), and the Dirección General de Salud Ambiental (DIGESA). This work could never have been completed without the support and efforts of Fernando Málaga Chávez, Karina Oppe Álvarez, Andy Catacora Rospigliossi, Dr. Juan Cornejo del Carpio, Dr. Carlos Palacios Rosado, Dra. Zeida Cáceres Cabana, Kate Levy, and Malwina Niemierko. We thank Yage Wu and Eileen Wang for manuscript and translation assistance.
In addition we wish to acknowledge the past and present support of the Chagas disease control program in Arequipa from the Gobierno Regional de Arequipa, the Organización Panamericana de la Salud (OPS) and the Canadian International Development Agency (CIDA). This work was supported by National Institutes of Health grant number P50AI074285 and grant number K01AI079162, and by the University Research Foundation and the Global Engagement Fund of the University of Pennsylvania.
Funding Statement: This work was supported by National Institutes of Health grant number P50AI074285 and grant number K01AI079162, and by the University Research Foundation and the Global Engagement Fund of the University of Pennsylvania.
Footnotes
Competing Interest: None to declare.
Contributorship Statement: AMB, MZL, CBa, VPS, CBi and CN conceived the study. Data collection and management was overseen by CBa, JQC, LMMR, amd JOC. Analyses were conducted by AMB, MZL, CBa, DS, and CS. Additional methodological consultation was provided by DS and CBi. AMB, MZL, CS, VPS, and CBa drafted the manuscript, tables, and figures. All authors reviewed, edited, and approved the final manuscript.
REFERENCES
- 1.Zakus JDL, Lysack CL. Revisiting community participation. Health Policy Plan. 1998;13:1–12. doi: 10.1093/heapol/13.1.1. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Indoor residual spraying: Use of indoor residual spraying for scaling up global malaria control and elimination. World Health Organization; Geneva: 2006. [Google Scholar]
- 3.Vazquez-Prokopec GM, Spillmann C, Zaidenberg M, et al. Cost-effectiveness of Chagas disease vector control strategies in northwestern Argentina. PLoS Negl Trop Dis. 2009;3:e363. doi: 10.1371/journal.pntd.0000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Environmental Protection Agency (EPA) Joint statement on bed bug control in the United States from the U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Environmental Protection Agency (EPA) U.S. Centers for Disease Control and Prevention and the U.S. Environmental Protection Agency; Atlanta, GA: 2010. [Google Scholar]
- 5.World Health Organization . The World Health Report 2003, Annex table 2, Deaths by cause, sex and mortality stratum in WHO regions, estimates for 2002. World Health Organization; Geneva: 2003. [Google Scholar]
- 6.Lee BY, Bacon KM, Bottazzi ME, et al. Global economic burden of Chagas disease: a computational simulation model. Lancet Infect Dis. 2013;13:342–8. doi: 10.1016/S1473-3099(13)70002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bern C, Martin DL, Gilman RH. Acute and congenital Chagas disease. Adv Parasitol. 2011;75:19–47. doi: 10.1016/B978-0-12-385863-4.00002-2. [DOI] [PubMed] [Google Scholar]
- 8.Lorca M, García A, Contreras MC, et al. Evaluation of a Triatoma infestans elimination program by the decrease of Trypanosoma cruzi infection frequency in children younger than 10 years, Chile, 1991-1998. Am J Trop Med Hyg. 2001;65:861–4. doi: 10.4269/ajtmh.2001.65.861. [DOI] [PubMed] [Google Scholar]
- 9.Silveira A, Vinhaes MC. Elimination of vector-borne transmission of Chagas disease. Mem Inst Oswaldo Cruz. 1999;94:405–11. doi: 10.1590/S0074-02761999000700080. [DOI] [PubMed] [Google Scholar]
- 10.Elimination of transmission of Chagas disease in southernmost Latin America. World Health Forum. 1994;15:299–300. [PubMed] [Google Scholar]
- 11.Levy MZ, Chavez FSM, del Carpio JGC, et al. Rational spatio-temporal strategies for controlling a Chagas disease vector in urban environments. J R Soc Interface. 2010;7:1061–70. doi: 10.1098/rsif.2009.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayer AM, Hunter GC, Gilman RH, et al. Chagas disease, migration and community settlement patterns in Arequipa, Peru. PLoS Negl Trop Dis. 2009;3:e567. doi: 10.1371/journal.pntd.0000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abad-Franch F, Vega MC, Rolon MS, et al. Community participation in Chagas disease vector surveillance: systematic review. PLoS Negl Trop Dis. 2011;5:e1207. doi: 10.1371/journal.pntd.0001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gürtler RE. Combining residual insecticide spraying campaigns with targeted detection and specific chemotherapy for Trypanosoma cruzi infection in children. PLoS Negl Trop Dis. 2007;1:e168. doi: 10.1371/journal.pntd.0000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the Health Belief Model. Health Educ Q. 1988;15:175–83. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 16.Rifkin SB. Lessons from community participation in health programmes: a review of the post Alma-Ata experience. International Health. 2009;1:31–6. doi: 10.1016/j.inhe.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Morgan LM. Community participation in health: perpetual allure, persistent challenge. Health Policy Plan. 2001;16:221–30. doi: 10.1093/heapol/16.3.221. [DOI] [PubMed] [Google Scholar]
- 18.Siegel S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences. McGraw-Hill; New York: 1988. [Google Scholar]
- 19.Barbu CMB, Hong A, Manne JM, et al. The effects of city streets on an urban disease vector. PLoS Comput Biol. doi: 10.1371/journal.pcbi.1002801. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryan RT, Balderrama F, Tonn RJ, et al. Community participation in vector control: lessons from Chagas’ disease. Am J Trop Med Hyg. 1994;50:61–71. doi: 10.4269/ajtmh.1994.50.61. [DOI] [PubMed] [Google Scholar]
- 21.Tapia-Conyer R, Mendez-Galvan J, Burciaga-Zuniga P. Community participation in the prevention and control of dengue: the patio limpio strategy in Mexico. Paediatr Int Child Health. 2012;32:10–3. doi: 10.1179/2046904712Z.00000000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaki PP, Dongus S, Fillinger U, et al. Community-owned resource persons for malaria vector control: enabling factors and challenges in an operational programme in Dar es Salaam, United Republic of Tanzania. Hum Resour Health. 2011;9:21. doi: 10.1186/1478-4491-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heintze C, Velsaco Garrido M, Kroeger A. What do community-based dengue control programmes achieve? A systematic review of published evaluations. Trans R Soc Trop Med Hyg. 2007;101:317–25. doi: 10.1016/j.trstmh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Winch PJ, Lloyd LS, Hoemeke L, et al. Vector control at the household level: an analysis of its impact on women. Acta Trop. 1994;56:327–39. doi: 10.1016/0001-706x(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 25.Atkinson JA, Vallely A, Fitzerald L, et al. The architecture and effect of participation: a systematic review of community participation for communicable disease control and elimination. Implications for malaria elimination. Malaria Journal. 2011;10:225. doi: 10.1186/1475-2875-10-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das P. Community participation in vector borne disease control: facts and fancies. Ann Soc Belg Med Trop. 1991;71:233–42. [PubMed] [Google Scholar]
- 27.Paz-Soldan VA, Plasai V, Morrison AC, et al. Initial assessment of the acceptability of a push-pull Aedes aegypti control strategy in Iquitos, Peru and Kanchanaburi, Thailand. Am J Trop Med Hyg. 2011;84:208–17. doi: 10.4269/ajtmh.2011.09-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adalja AA, Sell TK, Bouri N, et al. Lessons learned during dengue outbreaks in the United States, 2001-2011. Emerg Infect Dis. 2012;18:608–14. doi: 10.3201/eid1804.110968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosato M, Laverack G, Grabman LH, et al. Community participation: lessons for maternal, newborn, and child health. Lancet. 2008;372:962–71. doi: 10.1016/S0140-6736(08)61406-3. [DOI] [PubMed] [Google Scholar]
- 30.Reid AE, Cialdini RB, Aiken LS. Social norms and health behavior. Handbook of Behavioral Medicine: Methods and Applications. 2010;3:263. [Google Scholar]
- 31.Bicchieri C. The Grammar of Society: The Nature and Dynamics of Social Norms. Cambridge University Press; New York: 2006. [Google Scholar]
- 32.Woolcock M, Narayan D. Social capital: Implications for development theory, research, and policy. World Bank Res Obs. 2000;15:225–49. [Google Scholar]
- 33.Nisbett RE, Wilson TD. Telling more than we can know: Verbal reports on mental processes. Psychol Rev. 1977;84:231. [Google Scholar]
- 34.Pachter LM, Weller SC, Baer RD, et al. Variation in asthma beliefs and practices among mainland Puerto Ricans, Mexican-Americans, Mexicans, and Guatemalans. J Asthma. 2002;39:119–34. doi: 10.1081/jas-120002193. [DOI] [PubMed] [Google Scholar]
- 35.Koinis-Mitchell D, McQuaid EL, Friedman D, et al. Latino caregivers’ beliefs about asthma: causes, symptoms, and practices. J Asthma. 2008;45:205–10. doi: 10.1080/02770900801890422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volpp KG, John L, Troxel AB, et al. Financial incentive-based approaches for weight loss: A randomized trial. JAMA. 2008;300:2631–7. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yokley JM, Glenwick DS. Increasing the immunization of preschool children; an evaluation of applied community interventions. J Appl Behav Anal. 1984;17:313–25. doi: 10.1901/jaba.1984.17-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gneezy U, Meier S, Rey-Biel P. When and why incentives (don’t) work to modify behavior. J Econ Perspect. 2011;25:191–209. [Google Scholar]
- 39.Sabini J, Siepmann M, Stein J. The Really Fundamental Attribution Error in social psychological research. Psychological inquiry. 2001;12:1–15. [Google Scholar]
- 40.Tan JHW, Zizzo DJ. Groups, cooperation and conflict in games. Journal of Socio-Economics. 2008;37:1–17. [Google Scholar]