Abstract
Cholangiocarcinomas are a diverse group of tumors that are presumed to originate from the biliary tract epithelium either within the liver or the biliary tract. These cancers are often difficult to diagnose, their pathogenesis is poorly understood, and their dismal prognosis has resulted in a nihilistic approach to their management. The two major clinical phenotypes are intrahepatic, mass-forming tumors and large ductal tumors. Among the ductal cancers, lesions at the liver hilum are most prevalent. The risk factors, clinical presentation, natural history and management of these two types of cholangiocarcinoma are distinct. Efforts to improve outcomes for patients with these diseases are affected by several challenges to effective management. For example, designations based on anatomical characteristics have been inconsistently applied, which has confounded analysis of epidemiological trends and assessment of risk factors. The evaluation of therapeutic options, particularly systemic therapies, has been limited by a lack of appreciation of the different phenotypes. Controversies exist regarding the appropriate workup and choice of management approach. However, new and emerging tools for improved diagnosis, expanded indications for surgical approaches, an emerging role for locoregional and intrabiliary therapies and improved systemic therapies provide optimism and hope for improved outcomes in the future.
Introduction
Cholangiocarcinomas, broadly described as malignancies that arise from the biliary tract epithelia, are enigmatic and challenging cancers. These cancers are rare in many parts of the world such as Europe and the United States, accounting for <3% of all malignant tumors. However, there is a dramatic geographic variation in their incidence, which reflects regional differences in risk factors and epidemiology.1,2 Cholangiocarcinomas can be divided into two major clinical phenotypes: intrahepatic and ductal. However, the underdiagnosis of intrahepatic cancers and variable classifications of hilar tumors as intrahepatic or extrahepatic tumors have confounded analyses of the true incidence rates of these cancers.3 Although it seems that the age-adjusted incidence of intrahepatic cholangiocarcinoma has increased in several parts of the world such as the United States and Europe, the true picture is unclear and might be obscured by diagnostic misclassification.4,5
Treatment outcomes and survival for patients with these cancers have improved little over the past three decades—a period in which successful new treatments have increased patient survival for many other cancers. Patients with cholangiocarcinoma usually present at late stages of the disease, and symptoms might be nonspecific, such as painless jaundice, weight loss or cholangitis.6 Therefore, these cancers remain difficult to diagnose and treat and their prognosis is generally poor. Approximately half of untreated patients die within 3–4 months of presentation from the indirect effects of local tumor progression, bile duct obstruction, liver failure or sepsis from cholangitis and abscesses. The management of these patients is palliative, aimed at reducing obstructive cholestasis, pruritus and cholangitis.
Few patients are candidates for potentially curative surgical resection at the time of presentation. Moreover, the outcomes after resection with curative intent are poor, with 5-year survival of ∼30–40% for intrahepatic cancers and up to ∼50% for ductal cancers.7–9 Many patients are not well enough to undergo aggressive chemotherapy or radiation therapy. Furthermore, cholangiocarcinomas respond poorly to these therapies. In carefully selected patients, aggressive multimodality treatment approaches that combine liver transplantation, systemic chemotherapy and radiation therapy have resulted in 5-year survival exceeding 70%.10 In a study published in 2010, a median survival of 11.7 months was noted when systemic therapy with cisplatin and gemcitabine was used to treat patients with unresectable biliary tract cancers.11 Although treatment with this combination has being touted as the standard of care for all biliary tract cancers, comparable survival has been reported in randomized trials in patients with ductal cancers who were treated with palliative photodynamic therapy and stenting.12–15
Understanding of risk factors and pathogenesis, and thus optimal management, is hampered by the inconsistent use of nomenclature. Progress in defining optimal systemic therapies for patients with cholangiocarcinomas is hindered by the lack of stratification and consideration of phenotypic heterogeneity in clinical trials. Approaches for palliative care that have improved effectiveness are emerging; for example, local ablation or radiation therapy, or systemic therapy with medical chemotherapy. The adoption of multimodality approaches might expand the role of surgical resection and improve its outcomes. Thus, there remains reason for optimism. In this Review we outline some of the current challenges (Table 1) and offer recommendations for the management of patients with cholangiocarcinomas.
Classification
The need to adopt a consistent classification for cholangiocarcinoma has become a critical issue. Classifications of cholangiocarcinoma based on anatomical location (inside or outside the liver) have been inconsistently applied, particularly with respect to hilar ductal cancers that extend into the liver.3 Findings from diagnostic or preoperative evaluations do not correlate well with pathological findings. . In addition, a molecular classification is lacking. Although attempts have been made to define genetic changes in cholangiocarcinomas, their use for classifying these cancers is premature.16–20
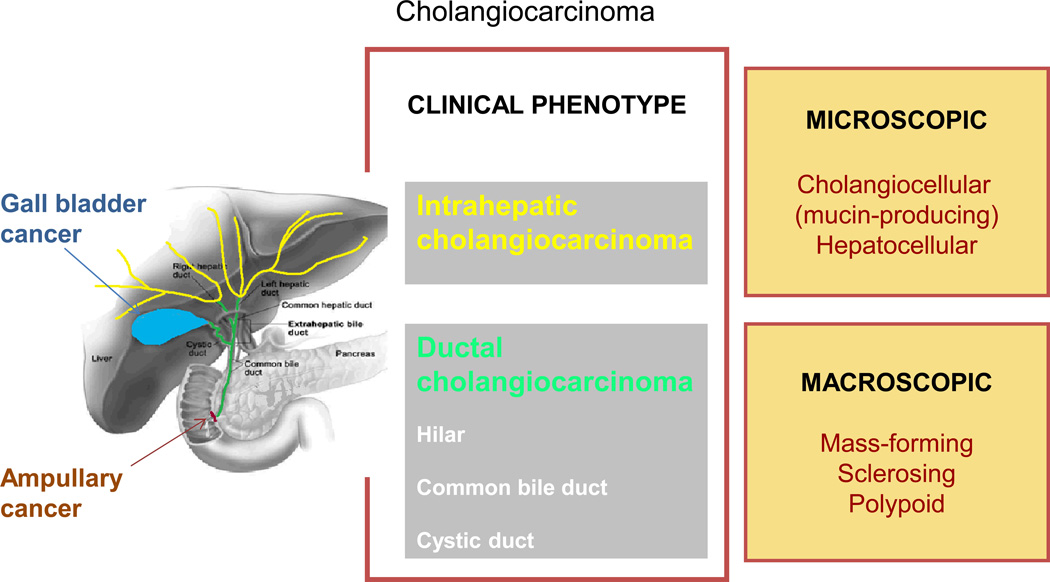
A classification based on the clinical phenotype of the tumor is appropriate (Figure 1). Cholangiocarcinomas typically present in one of two ways: either as mass lesions within the liver (intrahepatic cholangiocarcinoma) or with biliary tract obstruction attributable to large duct obstruction (ductal cholangiocarcinoma). Other than in their mode of presentation, these two distinct phenotypes differ in their etiology, risk factors, natural history, clinical behavior and response to therapies. Consequently, the management and outcomes of patients with these cancers are different. Intrahepatic cholangiocarcinoma presumably arise from small ducts within the liver, and can grow to a large size before the patient becomes symptomatic. By contrast, ductal cholangiocarcinomas arise from large ducts, up to the second order of branching, and the patients present with biliary tract obstruction.
Figure 1. Classification of biliary tract cancers.
Biliary tract cancers can be classified into clinically distinct types: gall bladder cancers, ampullary cancers and cholangiocarcinoma. Cholangiocarcinoma are phenotypically classified into intrahepatic or ductal cholangiocarcinoma to emphasize the distinctions between these clinically distinct cancers. Ductal cholangiocarcinoma are further classified as hilar or nonhilar (cystic duct or common bile duct). Hilar cancers that arise from extrahepatic large duct epithelium at the hilum can extend into the liver, and have been misclassified as intrahepatic in some schema and in epidemiological and clinical reports. Further subclassification on the basis of macroscopic or microscopic characteristics can provide additional distinction that might correlate with clinical outcomes. Cancers that arise from the gall bladder or the ampulla of Vater are biliary tract cancers with unique clinical presentations, natural history, etiology and patterns of growth or spread and are considered separately from cholangiocarcinoma.
The clinical phenotype of ductal cholangiocarcinoma encompasses cancers that arise from within the large ducts at the hilum, even when they present with mass lesions or extend into the liver. These perihilar cancers comprise a large proportion of the cholangiocarcinomas included in surgical series from tertiary referral centers and have been considered as a separate type of cholangiocarcinoma because their surgical management differs from that of other ductal cancers.20a–20c The biological behavior and therapeutic outcomes of perihilar cancers are distinct from those of intrahepatic cholangiocarcinoma. A classic description of perihilar cancers by Gerald Klatskin included both ductal cancers that extend into the liver and intrahepatic cancers with hilar involvement.20d The eponymous classification of these cancers as Klatskin tumors is not helpful as it does not adequately differentiate between the different clinical phenotypes. These major phenotypes, intrahepatic or ductal,can often be distinguished based on their presentation or with the diagnostic modalities that are currently available. Similarly, the clinical phenotype of intrahepatic cholangiocarcinoma encompasses cancers that might share varying degrees of hepatocytic or ductular differentiation. These cancers require distinction as they might behave differently. Thus, anatomical or pathological subclassifications can be used to define the major subtypes of cancers in this phenotype-based classification.
Consistent use of terminology is necessary to understand disease pathogenesis, to enhance communication among clinicians, researchers and patients, and to enable the use of effective staging and management approaches. The adoption of a consistent designation in clinical, pathological and epidemiological reporting will avoid those difficulties inherent in the current terminologies. A uniform classification will also facilitate the design and implementation of phase II and III clinical trials of therapeutic approaches. These trials have been hampered by the lack of validated staging systems to stratify populations of patients. Until stratification schemes that incorporate phenotypic heterogeneity are applied, the results from these trials will be difficult, if not impossible, to interpret.
Intrahepatic cholangiocarcinoma
These cancers arise from intrahepatic ducts, which extend from the periphery of the liver to the second-order bile ducts within the liver.25
Epidemiology
Patients with intrahepatic cholangiocarcinoma comprise a small proportion of all patients with cholangiocarcinoma referred to surgical centers in the USA.7 Their true incidence is probably greater than appreciated because of underdiagnosis owing to a lack of differentiation from other primary or secondary tumors in the liver. Risk factors for intrahepatic cholangiocarcinoma include chronic biliary tract diseases such as primary sclerosing cholangitis (PSC), hepatolithiasis, choledochal cysts and liver fluke infections (Table 1).26–28 Nonbiliary diseases such as heavy alcohol use, obesity, nonalcoholic fatty liver disease, chronic hepatitis C and cirrhosis are also more prevalent in patients with these cancers compared to the general1,2,29
Pathology
The tumors typically form masses with a well-demarcated nodule that grows in a radial pattern, or spreads longitudinally along the bile duct: tumors can also show both patterns of growth.30 A classification based on macroscopic type (mass-forming, sclerosing or polypoid) has been proposed by the Liver Cancer Study Group, Japan.31 Microscopically, a proportion of intrahepatic tumors might have features of ductular morphology with mucin production, or features of hepatocellular differentiation to a varying extent.31 These distinct macroscopic and microscopic subtypes might vary in their clinical or biological behavior and thus deserve inclusion in classifications of intrahepatic cholangiocarcinoma.
Presentation and diagnosis
The typical presentation of an intrahepatic cholangiocarcinoma is an incidental hepatic mass lesion.32 Contrast enhanced CT or MRI imaging will be necessary to determine the size, number and location of the lesions, vascular invasion and the extent to which the tumor has spread.33 The diagnosis is often made during evaluation of solitary masses within the liver. A finding of adenocarcinoma from a biopsy sample of a hepatic mass lesion should indicate to the clinician that a diagnosis of cholangiocarcinoma is possible, particularly in the absence of another obvious primary lesion outside the liver. Levels of tumor markers, such as carbohydrate antigen 19–9 (CA19-9) or carcinoembryonic antigen (CEA), might be raised, but these markers lack sensitivity for diagnostic use. Concomitant increases of the levels of CA19-9 and α-fetoprotein should suggest a mixed hepatocellular–cholangiocarcinoma. Distinguishing these mixed tumors from hepatocellular carcinoma is important, as they respond differently to therapy and the outcomes following transplantation are poorer in patients with mixed hepatocellular–cholangiocarcinoma than for those with hepatocellular carcinoma.
Staging
Staging systems for all intrahepatic tumors were previously based on the TNM classification and stage grouping criteria used for hepatocellular carcinoma. However, these criteria were not predictive of prognosis in patients with intrahepatic cholangiocarcinoma and are unnecessarily complex.34 A specific staging system for intrahepatic cholangiocarcinoma has now been proposed in the American Joint Committee on Cancer (AJCC) 7th edition staging manual 87a . This system is simpler and a correlation with prognosis has been validated 87b. , The AJCC system is based on the number of intrahepatic lesions, presence or absence of vascular invasion, and lymph node and distal metastases. In data from the Surveillance, Epidemiology, and End Results (SEER) database, overall median, 3-year and 5-year survival were 21 months, 31% and 18%, respectively; however, in the absence of nodal involvement or distant intrahepatic metastases, the overall median, 3-year and 5-year survival were 29 months, 40% and 25%, respectively.35,36
Treatment of intrahepatic cholangiocarcinoma
Surgery
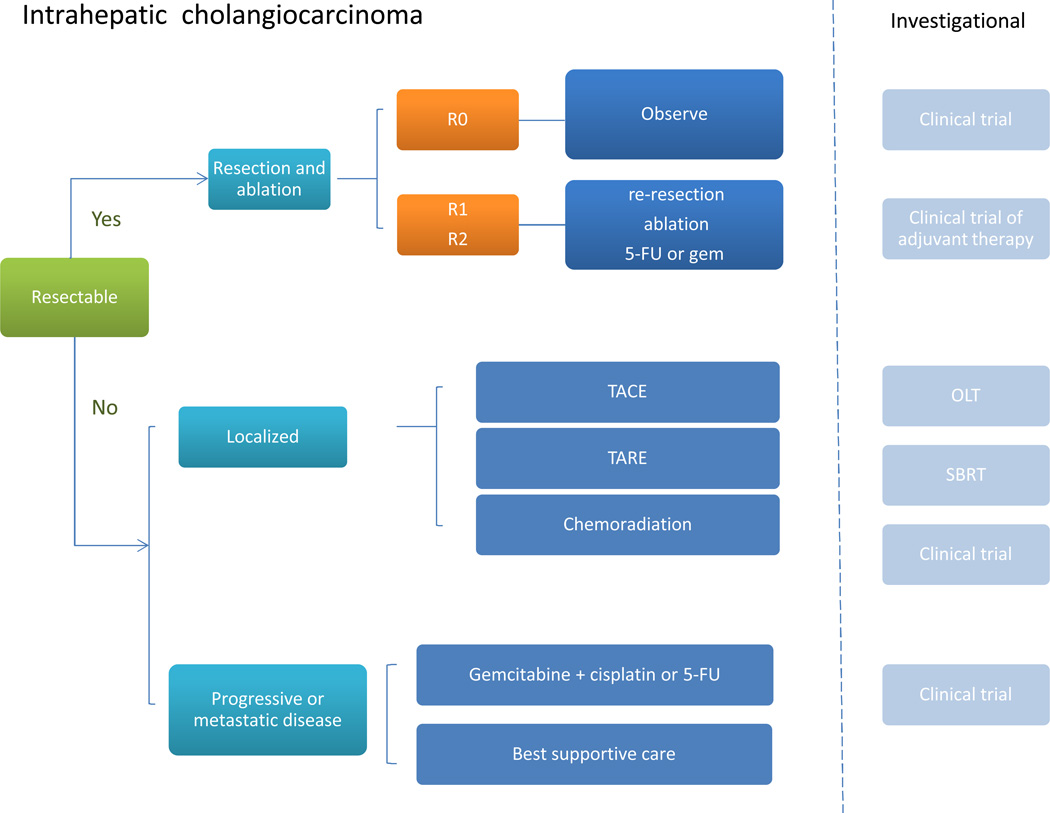
Surgical resection should be considered for patients with localized disease, as this is the only strategy with the potential for cure (Figure 2).37,38 A critical factor is the extent of hepatic resection that is necessary and also compatible with a functionally adequate remnant liver. Advances in techniques to predict residual tumor volume and function, and portal embolization enable more extensive resections than were possible previously.38a,38b For patients with an R0 resection (those with negative surgical margins and a lack of regional lymph node involvement), imaging every 6 months for up to 3 years is reasonable to monitor for recurrence. Enrollment of patients with an R0 resection in a clinical trial of adjuvant therapy should be considered, if available, to evaluate approaches to improve outcomes of R0 resections.39 For resections with either positive margins (R1) or residual tumor or positive lymph nodes (R2), re-resection or ablation should be considered if feasible. Otherwise, these patients could be managed with systemic therapy with 5-fluorouracil (5FU) or gemcitabine. Liver transplantation has poor outcomes for patients with intrahepatic cholangiocarcinoma and is generally not recommended unless in the context of an approved trial protocol.
Figure 2. Approach to management of intrahepatic cholangiocarcinoma.
For resectable tumors, consider surgery or ablation. The extent of resection is prognostically important and should form the basis for subsequent management. For R0 resections, with negative surgical margins and lack of regional lymph node involvement, observation for recurrence, with imaging every 6 months for 3 years, or enrollment in a clinical trial of adjuvant therapy should be considered. For resections with either microscopically positive margins (R1) or residual tumor or positive lymph nodes (R2), consider re-resection or ablation. There are no data to guide optimal therapy for patients with unresectable disease and the choice of therapy will be based on the available local expertise and resources. For nonresectable but localized tumors, potential options include locoregional approaches such as chemoembolization, and radioembolization, or systemic chemotherapy. For metastatic or progressive tumors, systemic therapy with gemcitabine and cisplatin, or 5FU could be considered. Abbreviations: 5FU, 5-fluorouracil; OLT, orthotopic liver transplant; SBRT, stereotactic body radiotherapy; TACE, transarterial chemoembolization; TARE, transarterial radioembolization.
Locoregional therapy
Locoregional approaches, such as ablation, transarterial chemoembolization (TACE) and transarterial radioembolization, have been used in patients with cholangiocarcinoma.40,41 However, the experience has been limited and these modalities have not been systematically evaluated.42 Local disease control with TACE is possible in up to 76% of patients, and the outcomes might be improved with systemic chemotherapy.43 Although small, localized lesions are seldomly encountered, local ablation might be considered if complete tumor necrosis can be achieved and there is no evidence that the tumor has spread.44,45 Ablation might be considered in patients with local recurrence or residual tumor after surgery with curative intent.46
Radiation therapy
Although the response to external beam radiation therapy in patients with intrahepatic cholangiocarcinoma has been poor, anecdotal evidence indicates that a reduction in tumor burden can be achieved with stereotactic body radiotherapy treatment (SBRT).47,48,49 In a recent phase I study of SBRT in 10 patients with unresectable intrahepatic cholangiocarcinoma, a median survival of 15 months was noted 87c. Two patients developed transient biliary obstruction and two patients progressed from Child–Pugh class A to Child–Pugh class B. Although there is no current role for radiation therapy in the treatment of patients with intrahepatic cholangiocarcinoma, approaches such as SBRT could be considered as part of future multimodality proposals.
Systemic therapy
For patients with advanced cancers that are unresectable or those with metastatic disease, systemic therapy with gemcitabine plus cisplatin is a first-line approach (Figure 2) A benefit has been demonstrated for gemcitabine plus cisplatin compared to gemcitabine alone, and for chemotherapy based on 5FU compared to the best supportive care.11,50 Alternative choices, particularly in individuals who might not tolerate the combination of gemcitabine and cisplatin would include gemcitabine monotherapy, 5FU-based regimens, or supportive care.51,52 Where available, enrollment in a clinical trial with appropriate stratification that would enable an evaluation of the responses of patients with intrahepatic cholangiocarcinoma who undergo these treatments should be considered.
Outcomes (level 3 subheading)
Untreated, survival of patients with advanced intrahepatic cancers is short: survival of 3.0 ± 5.3 months was reported in a large cohort study.53 By contrast, the median survival of patients with intrahepatic cancer who are treated with systemic chemotherapy ranges from 8 to 12 months. After surgical resection with curative intent, 1-year and 5-year survival of 68% and 32%, respectively, have been reported.54
Ductal cholangiocarcinoma
Ductal cholangiocarcinomas arise from the epithelia of large ducts of the biliary tract and include cancers of the common bile duct, common hepatic duct and the right and/or left hepatic ducts including their secondary bifurcation. They include cancers that occur at the hilum, which have been termed Klatskin tumors, but not tumors that arise at the ampulla of Vater.
Epidemiology
Unlike the trends reported for intrahepatic cholangiocarcinoma, studies indicate that the incidence of ductal cholangiocarcinomas might not be increasing.4,5 However, precise data are not available because this group includes cancers that have been variably classified as both extrahepatic and intrahepatic cholangiocarcinomas.3 Thus, misclassification of ductal cancers that arise at the hilum as intrahepatic lesions might have resulted in under-recognition of the true prevalence and incidence of these cancers.
Presentation
Ductal cancers characteristically present with signs and symptoms of biliary obstruction, such as jaundice, or cholangitis. Imaging studies that show biliary obstruction and laboratory tests that indicate cholestasis such as hyperbilirubinemia and bilirubinuria might be present. Tumor markers such as CEA and CA19-9 lack specificity as levels of these markers can be increased by extrahepatic obstruction from any cause. Untreated, death occurs within a few months of presentation, mostly attributable to biliary sepsis, liver failure or hemorrhage. These causes of death are mostly sequelae of local tumor effects, and metastatic spread is rare at the time of presentation.
Diagnosis
For patients with ductal cancers, biliary tract obstruction can be evaluated with ultrasonography or CT scanning, which will enable the level of obstruction to be ascertained. Imaging might indicate intrahepatic duct dilation with variable dilation of extrahepatic ducts that depends on the location of the cancer. Macroscopically, these cancers can present as sclerosing or polypoid lesions. These subtypes can sometimes be differentiated on either cholangiography or cholangioscopy. The diagnosis of malignancy is challenging, particularly for sclerosing lesions or in the setting of underlying stricturing disease, and there are many diseases that mimic malignancy.55 The sparse nature of tumor cells within a fibrotic matrix (as seen in ductal strictures), and the poor accessibility of the lesions makes it difficult to obtain a tissue diagnosis. Cholangiography can also be useful to determine the extent of the disease, which is important to plan treatment. Although the cholangiographic appearance can be suggestive, microscopic confirmation is needed to confirm the diagnosis. Brush cytology is recommended, but is often not diagnostic even with repeated brushings as the tumor grows underneath the mucosa, or reactive cells might be obtained in patients with inflammatory conditions such as PSC. The diagnosis of dysplastic changes within the biliary tract epithelia is extremely challenging. Combining cytology with image analysis of DNA content or fluorescence in situ hybridization using specific chromosomal probes to detect polysomy might improve diagnosis without a reduction in specificity. Aspirations or biopsy samples might be useful, and can be obtained under direct visualization at cholangioscopy. The use of chromoendoscopy, confocal endoscopy, or narrow band imaging within the biliary tract coupled with cholangioscopy are emerging techniques that might be promising methods to aid the identification of epithelial regions for targeted biopsy samples. However, these techniques are not widely available and require advanced endoscopic equipment.
The selection of patients for resection or transplantation depends on the available expertise, demonstration of resectability and absence of tumor spread. The extent of intrabiliary spread can be assessed by determining luminal changes on cholangiography. Duplex ultrasound, CT and magnetic resonance cholangiopancreatography or surgical exploration and laparotomy might all be needed to adequately stage disease and assess locoregional extension or distant metastases. Selection for surgery is also dependent on adequate localized lobar involvement. Thus, the lobar bile duct involvement (for example, right hepatic duct) with contralateral vascular encasement (such as left portal vein encasement) precludes surgical resection, the only established, potentially curative therapy for patients with ductal cancers. Staging laparoscopy might be needed in patients with TNM stage T2 or T3 hilar cholangiocarcinoma who do not show evidence of unresectable or metastatic disease as determined by preoperative imaging.
Staging
The AJCC–TNM staging system for ductal cholangiocarcinoma is based on pathological data that is useful to identify the patient prognosis, but has little applicability for assessing the feasibility of surgical treatment. The Japanese Society of Biliary Surgery staging system might be more valuable for determining patient survival than the TNM system.56 Hilar tumors that extend within the liver can be staged using these criteria. The Bismuth–Corlette classification for hilar lesions describes tumor location and its spread within the biliary tract but is not predictive of resectability 87d,87e. In addition, this classification was described to help plan surgery and is not a true staging system. Similarly, the Memorial Sloan Kettering Cancer Center (MSKCC) staging systems for hilar lesions pertain to selection of patients for surgery 87f. The MSKCC T-stage criteria are based on the location and extent of ductal involvement, presence or absence of portal vein invasion, and presence or absence of hepatic lobar atrophy irrespective of metastases or lymph node status. This system correlates with resectability and survival, as 59% of T1 lesions are resectable with a median survival of 20 months compared to 0% resectability for T3 lesions with a median survival of only 8 months. However, these systems were not developed to correlate with survival and do not pertain to patients with advanced disease.
Factors that can affect outcomes for patients with ductal cancers include the size of the primary lesion; extrahepatic spread; the extent of ductal and vascular obstruction and their functional consequences for hepatic function and selection for surgery; presence or absence of lobar atrophy; the performance status of the patient; and the availability and effectiveness of therapy. A validated staging system that incorporates these factors is needed.57 The radial rather than longitudinal diameter of a mass lesion might have prognostic value, and the presence of a mass lesion of ≥3 cm has been proposed as a cut-off value for surgery although validation of this strategy is needed. The use of size criteria is limited by the inability to accurately determine tumor diameter during radiological assessments, particularly in the presence of local tissue inflammation.
Treatment of ductal cholangiocarcinoma
Surgery
Complete surgical resection of the ductal cholangiocarcinoma offers the best chance of long-term survival. For tumors that seem resectable, the surgical procedure that is used will depend on the location of the tumor. For hilar lesions, resection with excision of extrahepatic bile duct, regional lymphadenectomy and hepatectomy of the right or left lobe, with or without caudate lobe removal might be needed. For nonhilar lesions, a pancreatico-duodenectomy might be appropriate. If preoperative assessments do not indicate distal spread, lymphadenopathy or extension to second-order ducts, laparoscopy for staging or surgical exploration might be needed.58 Preoperative stenting does not seem to confer any advantages, but might be considered in patients with long-standing jaundice and cholangitis to enhance bile flow.59–61 Where possible, bile duct margins should be examined to assess for the presence of tumor, given the propensity of cholangiocarcinoma to spread along the bile ducts. Transplantation should be considered as an option for patients with PSC with localized disease, if available.
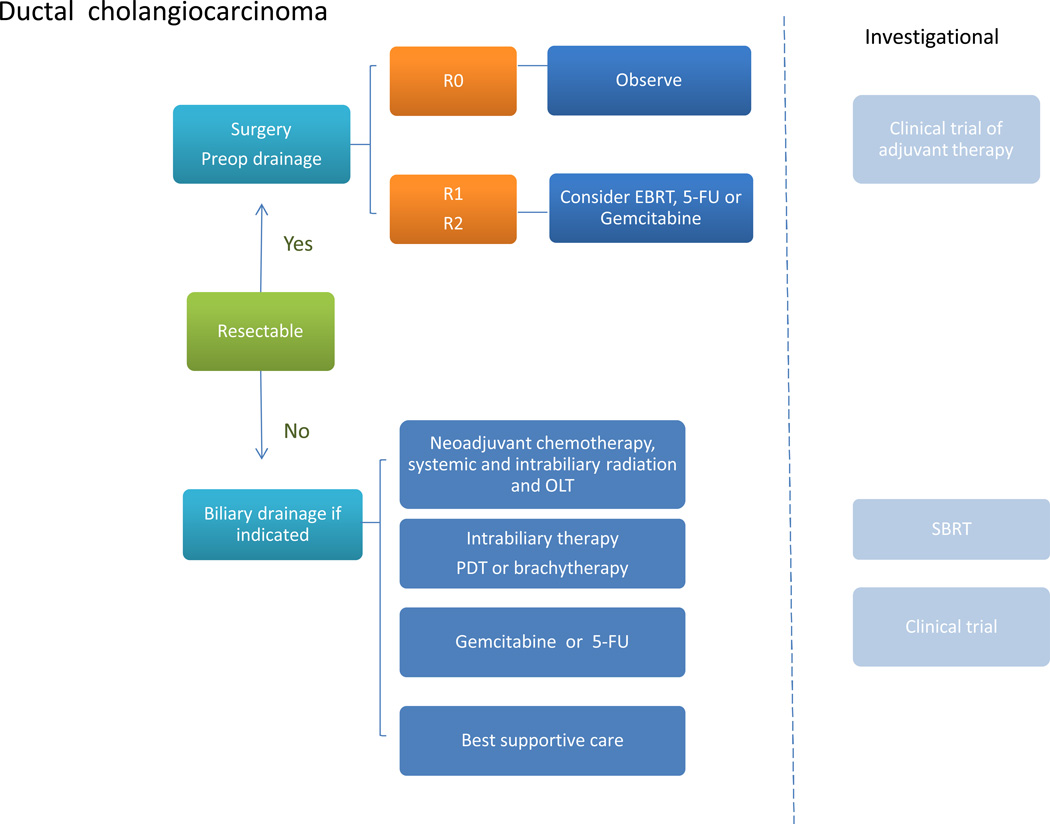
For patients with ductal cancers that arise at the hilum, the outcomes following resection have improved over the past two decades with the adoption of more aggressive surgical approaches, such as extended hepatectomy, portal vein embolization and portal vein resections.61a–61l Other contributing factors might include improvements in perioperative and postoperative management. Following surgical resections with complete tumor removal, negative surgical margins and a lack of regional lymph node involvement, appropriate options include either imaging every 6 months for up to 3 years to monitor for recurrence, or enrollment in a clinical trial of adjuvant therapy if available.62 Local or regional relapse can occur, even after curative resections, but the role of postoperative radiation remains unproven. Retrospective studies or database analyses of adjuvant therapy have not shown a survival benefit with postoperative chemoradiation.63,64 In addition, biliary complications can occur with radiation therapy.65 For resections with either positive margins (R1) or residual tumor, adjuvant chemoradiation should be considered. If distant spread or positive lymph nodes are encountered, systemic therapy with gemcitabine plus cisplatin, or 5FU can be considered (Figure 3).
Figure 3. Approach to management of ductal cholangiocarcinoma.
For resectable tumors, consider surgery. The choice of surgical approach will depend on the location of the tumor. For R0 resections, with negative surgical margins and lack of regional lymph node involvement, observation for recurrence, with imaging every 6 months for 3 years, or enrollment in a clinical trial of adjuvant systemic therapy should be considered. For resections with either positive margins (R1) or residual tumor or positive lymph nodes (R2), consider EBRT or systemic chemotherapy. For nonresectable or metastatic tumors, consider palliative biliary drainage if indicated, followed by intrabiliary PDT or brachytherapy, systemic chemotherapy with gemcitabine, or enrollment in a clinical trial evaluating new treatment modalities. Multimodality management based on liver transplantation might be appropriate for selected individuals with localized tumors and without any extrahepatic spread, but is available only at a few centers. Abbreviations: EBRT, external beam radiation therapy; 5FU, 5-fluorouracil; OLT, orthotopic liver transplant; PDT, photodynamic therapy; SBRT, stereotactic body radiotherapy.
If the tumor is not resectable, patients might be candidates for transplantation provided there is no evidence of extrahepatic spread and a multimodality approach is used with preoperative chemoradiation and operative staging. This option is available in very few centers, and can benefit very highly selected patients. In the experience of clinicians at the Mayo Clinic, USA, with such a protocol, routine preoperative staging precludes about a fifth of all patients undergoing this protocol from transplantation, and thus there is morbidity to be considered with this approach. A retrospective analysis reported that for patients with localized hilar cancers, liver transplantation with neoadjuvant chemoradiation was more effective than resection.66 Extending the option of liver transplantation for resectable tumors might be feasible with living donor programs but is unlikely to be widely used. Similarly, excision of all biliary tract epithelia with en bloc hepatectomy-Whipple, followed by liver transplantation might be an option for patients with early stage hilar cancers and PSC.
Biliary drainage
Cholestasis and cholangitis related to biliary obstruction contribute to the morbidity associated with ductal cholangiocarcinoma. Relief of biliary obstruction is helpful for palliative care and might delay death by a few weeks or months. Biliary drainage can be achieved by endoscopic or percutaneous stenting, which are preferable to surgical drainage as anastomotic leakage and mortality can occur with the latter.67,68 Self-expanding metal stents have longer patency rates and lower rates of cholangitis compared to plastic stents. Stent placement also enables subsequent intrabiliary treatment with brachytherapy or photodynamic therapy.
Intrabiliary therapies
Locally ablative intrabiliary therapies, such as photodynamic therapy and brachytherapy, have roles in the palliative treatment of patients with ductal cancers.12,13,69–71 Results from two small, randomized studies indicate that biliary stenting with photodynamic therapy can prolong survival, compared to stent placement alone.13,15,72 An increase in median survival from 3–6 months to 16–21 months was noted when photodynamic therapy was performed in addition to stenting. In view of these findings, photodynamic therapy with stenting could be considered for patients with unresectable ductal cancers, where available. In two studies, palliative photodynamic therapy has comparable survival in patients who underwent resection with curative intent, but had residual tumor (R1 or R2).73,74 Although these approaches do not detract from an aggressive surgical approach, they further emphasize the need and value of accurate preoperative staging.
Systemic therapies
Histological or cytopathological confirmation should be obtained if experimental or aggressive systemic therapies are being considered. For patients with advanced cancers that are unresectable or in those with metastatic disease, gemcitabine is a first-line approach. In contrast to patients with intrahepatic cholangiocarcinoma, the combination of gemcitabine with cisplatin does not seem to offer an advantage for those with ductal cancers. Alternative choices include 5FU or supportive care.51,52 As with intrahepatic cancers, enrollment in a clinical trial with appropriate stratification for ductal cancers should be considered if available.
Outcomes
For patients with hilar ductal cancers, a median survival of ∼3 years and a 5-year survival of 20–40% can be expected with resection. With complete resections without residual tumor (R0), and in the absence of nodal involvement or vascular invasion, a 45% 5-year survival is possible. Survival in patients with R1 or R2 resections is much lower, and is comparable to palliative stenting with photodynamic therapy. In carefully selected patients with PSC and early-stage unresectable ductal cancers, a multimodality approach of neoadjuvant chemoradiation with liver transplantation can provide excellent results, with 5-year survival of up to 72%.
Management considerations
Goals of treatment
The goals of therapy in patients with cholangiocarcinoma are to treat the cancer, relieve symptoms and provide supportive care to the patient. Where the intent is to cure the cancer, therapeutic options include surgical resection or liver transplantation and possibly ablation for small tumors . Approaches for palliative care include biliary stenting, intrabiliary therapies such as PDT, locoregional therapies such as TACE, chemotherapy and radiation therapy. Prevention or management of cholangitis and the effects of cholestasis might be needed.
Multidisciplinary care
The diagnosis of cholangiocarcinoma can be very challenging to establish and might require skilled and experienced interventional endoscopists, radiologists and cytopathologists. Where possible, the feasibility of curative therapy should be considered, whether by surgery or liver transplantation. The use of this strategy will depend on the availability of the appropriate surgical expertise. Although liver transplantation for cholangiocarcinoma is only offered at a few centers in the United States, carefully selected patients with localized disease can benefit from good long-term outcomes after transplantation. The use of adjuvant therapies to treat patients with cholangiocarcinoma is not currently supported by robust evidence from clinical trials. As the long-term outcomes from surgical resection with curative intent are currently suboptimal, further studies to evaluate and refine adjuvant therapies should be a high priority. Most patients with ductal cancers will require palliative biliary drainage, which can be combined with photodynamic therapy or brachytherapy, external beam radiation therapy or systemic chemotherapy. The optimal management of patients with these cancers thus requires a coordinated effort that involves hepatologists, therapeutic endoscopists, transplant surgeons, diagnostic and interventional radiologists, and radiation, surgical and medical oncologists as appropriate. Evaluation and management by a multidisciplinary team is ideal to ensure that the most appropriate options are considered.
Screening and prevention
Risk factors for cholangiocarcinoma include both infectious and noninfectious causes (Table 1). Currently, there are no established screening systems for cholangiocarcinoma. Approaches for prevention involve strategies to screen and treat for hepatolithiasis or infestations with liver flukes to decrease the subsequent tumor formation that is associated with these conditions. However, these risk factors are geographically limited to certain parts of Asia. Other prevention strategies include surgery for choledochal cysts, as individuals with these cysts are presumed to have a high risk of cancer. PSC is a well characterized risk factor for cholangiocarcinoma in Western societies; however, there are no validated screening strategies for PSC. Liver transplantation as a preventive strategy in patients with PSC is controversial. Although the existence of a dysplasia to carcinoma sequence has been postulated, technologies that can recognize preneoplastic changes are lacking.
A grading system for mild, moderate and severe biliary dysplasia, termed biliary intraepithelial neoplasia 1, 2 and 3, respectively was proposed and further refined in a multiobserver study.21,87g An increased prevalence of bile duct dysplasia was noted in patients who had an increased risk of cancer. Similarly, biliary intraepithelial neoplasia lesions with multiple microinvasive foci of cholangiocarcinoma were reported in liver explants from a patient with an HCV infection and alcohol-related cirrhosis.22 However, demonstration of the premalignant potential of biliary dysplasia, clear distinction from reactive inflammatory changes, and a detailed molecular and genetic characterization will be necessary to define the role and understand the importance of dysplasia in cholangiocarcinoma.
Diagnosis and staging
Tumor biomarkers are of limited value in the diagnosis of cholangiocarcinoma.23 Although CA19-9 is used, it has low sensitivity, and is prone to both false negatives and false positives.24 Abdominal imaging by ultrasound, CT or MRI, and cholangiography by direct contrast injection or MRI might be needed for an accurate diagnosis and staging of both intrahepatic and ductal cancers. An MRI with cholangiography will enable evaluation of hepatic parenchyma as well as ductal and vascular anatomy. However, endoscopic cholangiography or cholangioscopy is needed for tissue sampling by biopsy samples or brushings for pathological or cytological analysis. Additional information might be obtained by endoscopic ultrasound, which is a useful tool for evaluation of biliary tract obstruction and for the detection and sampling of regional nodes.
Cytology has low sensitivity for the diagnosis of ductal cancers, as the desmoplastic nature of these tumors limits the ability to obtain adequate tissue samples for cytological analysis. In addition, interpretation of the samples is difficult because of reactive changes in the cells, particularly in the presence of inflammation. The yield can be increased by taking more samples and using techniques such as digital image analysis or fluorescent in situ hybridization, but sensitivity remains low even with these approaches. In some patients, the diagnosis of cholangiocarcinoma cannot be established definitively, and laparoscopy or surgical intervention might be necessary when suspicion is high.
Imaging studies can provide information about the extent of disease and the resectability that is needed for staging. Most cholangiocarcinomas are not resected unless they are localized, and thus staging data is currently based on clinical, and not pathological, information. Intrahepatic and ductal cancers require different staging systems, as they have distinct clinical behaviors. Staging data is needed to assess prognosis, to plan the optimal treatment and to evaluate the results of treatments. Particularly for ductal cancers, the lack of a well-validated staging system that recapitulates the natural history and prognosis and can guide the choice of therapy is a major challenge that has been identified as being of high priority.
Multimodality therapies
Several ongoing studies listed on the clinical trials.gov website and management protocols are using multimodality approaches with careful selection of patients with localized disease, therapy to reduce the tumor burden and surgery with curative intent. As an example, the outcomes when liver transplantation alone was used to treat patients with cholangiocarcinoma were dismal with 5-year survival of 25% in one series 87h . However, a multimodality approach to treat patients and limit the spread of cancer prior to transplantation has improved outcomes for patients with hilar ductal cancers, with a 5-year survival rate exceeding 70% 87i . The adoption of similar multimodality approaches should be considered to improve the poor outcomes currently reported following surgical resections performed for cure, or for patients with unresectable cancers.
Evaluation of new therapies
Studies of new therapies have been limited by the lack of well-designed, appropriately stratified randomized or controlled studies in carefully defined groups of patients. However, several studies are ongoing in which chemotherapeutic drugs and biologic agents, alone or in various combinations are being assessed (Table 1) [. The proliferation of small, unstratified trials that lack randomization, are inadequately powered and include a mix of several different types of cancer remain challenges to the definition of effective treatment regimens for patients with cholangiocarcinoma. Single-center studies to rapidly evaluate new therapies or combinations of therapies might be economical and enable an adequate accrual of patients if heterogeneous groups are enrolled. However, such studies have contributed little to the definition of optimal therapies or improvements in outcomes. The rarity of cholangiocarcinoma has been a challenge to the enrollment of patients in large, multicenter studies. The low incidence and heterogeneous nature of these cancers will require multicenter collaborative efforts of appropriately stratified groups in adequately powered studies if progress is to be made. Thus, the development and implementation of such studies should be of the highest priority.
Conclusions
There are several challenges and needs for the future (Box 1). An urgent need is to adopt a consistent and useful nomenclature and classification of cholangiocarcinoma. This change is essential to improve the evaluation of risk factors, to understand pathogenesis, define appropriate therapies and evaluate outcomes. The definition of risk factors and the natural history of cholangiocarcinoma might eventually identify those who could benefit from directed preventive efforts. Strategies to prevent disease are critical in low-resource regions with a high prevalence of cholangiocarcinoma, such as areas of Southeast Asia. Concerted efforts to improve diagnostic techniques are needed to identify individuals who could benefit from surgical resection and improve preoperative staging. Multimodality approaches need to be evaluated with a systematic approach. The need to address these issues for this rare disease requires well-coordinated and collaborative efforts to make progress.
Box 1 Cholangiocarcinoma: challenges and future needs.
Adopt a consistent nomenclature and clinical classification of disease
Define risk factors specific to phenotypic classification
Develop appropriate preventive strategies in areas of high prevalence
Establish and validate an accurate prognostic staging system for both hilar and nonhilar ductal cancers
Identify effective markers to distinguish intrahepatic cholangiocarcinoma from secondary adenocarcinoma and hepatocellular cancer
Develop diagnostic modalities with improved sensitivity and specificity for ductal cancers
Identify effective multimodality treatment strategies to improve outcomes
Evaluate benefits of adjuvant therapies following resection in prospective, controlled trials
Identify palliative therapies that are safe, effective and well tolerated
Establish and validate disease appropriate quality of life outcomes and measures for use as end points of treatment
Encourage meaningful clinical trial designs for biliary cancers with appropriate stratification into each distinct type of cancer, ductal, intrahepatic, gall bladder and ampullary
Evaluate new therapeutics with respect to ductal or intrahepatic phenotype, geographical region, performance status, prior therapy, and extent (locally advanced versus metastatic). Where possible, incorporate any relevant molecular or genetic data.
Box 1 Risk factors for cholangiocarcinoma.
General1
Age >65 years
Obesity
Diabetes
Inflammatory diseases75–77
Primary sclerosing cholangitis
Hepatolithiasis (oriental cholangiohepatitis)
Biliary tract stone disease
Biliary-enteric anastomosis
Liver cirrhosis
Infectious diseases1,29,78–81
Opisthorchis viverrini (liver flukes)
Clonorchis sinensis (liver flukes)
Hepatitis C
Hepatitis B
HIV
Drugs, toxins or chemicals1,29,78,82–86
Alcohol
Smoking
Thorotrast
Dioxin
Vinyl chloride
Nitrosamines
Asbestos
Oral contraceptive pills
Isoniazid
Congenital87
Choledochal cysts
Caroli’s disease
Congenital hepatic fibrosis
Variations exist in the risk factors for intrahepatic and ductal cancers, but the extent is unknown as distinctions between these clinical phenotypes have not usually been reported. Hepatitis C virus infection, smoking, obesity, for example, are risk factors for intrahepatic but not for ductal cancers.
Box 2: Current investigational approaches.
Chemoprevention
Erlotinib
Diagnosis
Biliary forceps
Cytology brushes
Confocal endomicroscopy
Endoscopic ultrasound
Surgery
Preoperative staging laparoscopy
Preoperative portal vein embolization
Neoadjuvant chemotherapy
Portal vein resection
Adjuvant therapy
Gemcitabine
Gemcitabine plus cisplatin
Capecitabine or gemcitabine plus radiation therapy
Local therapies
Intrahepatic arterial 90Y microspheres
Photodynamic therapy
WST-11-vascular targeted photodynamic therapy Radiation therapy
Proton beam therapy
External beam radiation therapy or cyberknife
Stereotactic radiotherapy
Chemotherapy and biologic agents (single or in various combinations)
Gemcitabine, cisplatin, sorafenib, docetaxal, 5-fluorouracil, capecitabine
Dolastatin, pemetrexed, everolimus, oxaliplatin, irinotecan, ixabepilone
S1, taxotere, vandetanib, celecoxib, selumetinib cetuximab
Cediranib, panitumumab, bevacizumab, erlotininb
Multimodality
External beam radiation with cyberknife boost plus capecitabine
Chemoradiation: gemcitabine or docetaxal plus radiation therapy
Photodynamic therapy plus S1 (tegafur/ 5-chloro-2,4-dihydropyrimidine/potassium oxonate)
Neoadjuvant chemoradiation plus orthotopic liver transplantation
A selection of investigational approaches and agents currently under evaluation in observational, phase I or phase II studies for the prevention, diagnosis or treatment of cholangiocarcinoma.
Key points.
Cholangiocarcinomas are a diverse group of tumors that occur within the liver and biliary tract and are thought to originate from the epithelial lining of the biliary tract
The two major clinical phenotypes of cholangiocarcinoma are intrahepatic and ductal tumors (further divided into lesions that arise at the liver hilum or the extrahepatic ductal system)
Macroscopic (mass-forming, sclerosing or polypoid) and microscopic (cholangiocellular or hepatocellular) are additional subclassifications that might correlate with biological behavior and therapeutic responses
Inconsistent use of designations based on anatomical characteristics such as intrahepatic/extrahepatic or hilar has confounded analysis of epidemiological trends
A lack of appreciation of the different phenotypes has limited the evaluation of systemic therapies
Appropriate evaluation and choice of management approach requires a multidisciplinary approach; a multimodality approach to the management of patients with these cancers should be considered
Emerging new tools for improved diagnosis, expanded indications for surgical approaches, an emerging role for locoregional and intrabiliary therapies and improved systemic therapies might improve outcomes in the future
Review criteria.
Literature searches were performed using the PubMed database and the following search terms: “cholangiocarcinoma”, “biliary tract cancers” and “liver cancers” in combination with “incidence”, “therapy”, “prevention”, “classification”, “diagnosis” and “treatment”. Data from full-text papers and abstracts published in English were reviewed. Relevant articles were also identified from the reference lists of review articles. A search was performed of the Clinical Trials.gov database using the search terms: “cholangiocarcinoma” and “biliary tract cancers” to identify active or planned studies.
Acknowledgments
We apologize to the many excellent contributors to this field whose work is not acknowledged in the reference list owing to space limitations. This work was supported by NIH grant DK069370.
Biography
Tushar Patel is a Senior Associate Consultant at the Mayo Clinic, Jacksonville, FL, USA. His clinical interests include the management of liver cancers and hepatobiliary disease, and liver transplantation. The focus of his research is the molecular pathogenesis of cholangiocarcinoma and other liver cancers.
Footnotes
Competing interests
The author declares no competing interests.
Reference List
- 1.Welzel TM, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol. Hepatol. 2007;5:1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaib YH, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J. Gastroenterol. 2007;102:1016–1021. doi: 10.1111/j.1572-0241.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 3.Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J. Natl. Cancer Inst. 2006;98:873–875. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 4.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC. Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 6.Patel T. Cholangiocarcinoma. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006;3:33–42. doi: 10.1038/ncpgasthep0389. [DOI] [PubMed] [Google Scholar]
- 7.DeOliveira ML, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagino M, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann. Surg. 2006;243:364–372. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann. Surg. 2005;241:693–699. doi: 10.1097/01.sla.0000160701.38945.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rea DJ, Rosen CB, Nagorney DM, Heimbach JK, Gores GJ. Transplantation for cholangiocarcinoma: when and for whom? Surg. Oncol. Clin. N. Am. 2009;18:325–37. doi: 10.1016/j.soc.2008.12.008. ix. [DOI] [PubMed] [Google Scholar]
- 11.Valle J, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 12.Gao F, Bai Y, Ma SR, Liu F, Li ZS. Systematic review: photodynamic therapy for unresectable cholangiocarcinoma. J. Hepatobiliary. Pancreat. Sci. 2010;17:125–131. doi: 10.1007/s00534-009-0109-3. [DOI] [PubMed] [Google Scholar]
- 13.Ortner MA. Photodynamic therapy for cholangiocarcinoma: overview and new developments. Curr. Opin. Gastroenterol. 2009;25:472–476. doi: 10.1097/MOG.0b013e32832e6e1f. [DOI] [PubMed] [Google Scholar]
- 14.Quyn AJ, Ziyaie D, Polignano FM, Tait IS. Photodynamic therapy is associated with an improvement in survival in patients with irresectable hilar cholangiocarcinoma. HPB (Oxford) 2009;11:570–577. doi: 10.1111/j.1477-2574.2009.00102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortner ME, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Jinawath N, et al. Comparison of gene expression profiles between Opisthorchis viverrini and non-Opisthorchis viverrini associated human intrahepatic cholangiocarcinoma. Hepatology. 2006;44:1025–1038. doi: 10.1002/hep.21330. [DOI] [PubMed] [Google Scholar]
- 17.Yang B, House MG, Guo M, Herman JG, Clark DP. Promoter methylation profiles of tumor suppressor genes in intrahepatic and extrahepatic cholangiocarcinoma. Mod. Pathol. 2005;18:412–420. doi: 10.1038/modpathol.3800287. [DOI] [PubMed] [Google Scholar]
- 18.Xu MJ, et al. Identification and characterization of microRNAs in Clonorchis sinensis of human health significance. BMC. Genomics. 2010;11:521. doi: 10.1186/1471-2164-11-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawahigashi Y, et al. MicroRNA profiling of human intrahepatic cholangiocarcinoma cell lines reveals biliary epithelial cell-specific microRNAs. J. Nippon Med. Sch. 2009;76:188–197. doi: 10.1272/jnms.76.188. [DOI] [PubMed] [Google Scholar]
- 20.Meng F, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–2129. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 20a.Nakeeb A, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224(4):463–473. doi: 10.1097/00000658-199610000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20b.Sano T, et al. Prognosis of perihilar cholangiocarcinoma: hilar bile duct cancer versus intrahepatic cholangiocarcinoma involving the hepatic hilus. Ann Surg Oncol. 2008;15(2):590–599. doi: 10.1245/s10434-007-9687-y. [DOI] [PubMed] [Google Scholar]
- 20c.Ebata T, et al. The concept of perihilar cholangiocarcinoma is valid. Br J Surg. 2009;96(8):926–934. doi: 10.1002/bjs.6655. [DOI] [PubMed] [Google Scholar]
- 20d.Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis. An unusual tumor with distinctive clinical and pathological features. Am J Med. 1965;38:241–256. doi: 10.1016/0002-9343(65)90178-6. [DOI] [PubMed] [Google Scholar]
- 21.Zen Y, et al. Proposal of histological criteria for intraepithelial atypical/proliferative biliary epithelial lesions of the bile duct in hepatolithiasis with respect to cholangiocarcinoma: preliminary report based on interobserver agreement. Pathol. Int. 2005;55:180–188. doi: 10.1111/j.1440-1827.2005.01816.x. [DOI] [PubMed] [Google Scholar]
- 22.Wu TT, Levy M, Correa AM, Rosen CB, Abraham SC. Biliary intraepithelial neoplasia in patients without chronic biliary disease: analysis of liver explants with alcoholic cirrhosis, hepatitis C infection, and noncirrhotic liver diseases. Cancer. 2009;115:4564–4575. doi: 10.1002/cncr.24471. [DOI] [PubMed] [Google Scholar]
- 23.Gatto M, et al. Cholangiocarcinoma: update and future perspectives. Dig. Liver Dis. 2010;42:253–260. doi: 10.1016/j.dld.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Levy C, et al. The value of serum CA 19–9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig. Dis. Sci. 2005;50:1734–1740. doi: 10.1007/s10620-005-2927-8. [DOI] [PubMed] [Google Scholar]
- 25.Farges O, Fuks D. Clinical presentation and management of intrahepatic cholangiocarcinoma. Gastroenterol. Clin. Biol. 2010;34:191–199. doi: 10.1016/j.gcb.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Gatto M, Alvaro D. Cholangiocarcinoma: risk factors and clinical presentation. Eur. Rev. Med. Pharmacol. Sci. 2010;14:363–367. [PubMed] [Google Scholar]
- 27.Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford) 2008;10:77–82. doi: 10.1080/13651820801992641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh P, Patel T. Advances in the diagnosis, evaluation and management of cholangiocarcinoma. Curr. Opin. Gastroenterol. 2006;22:294–299. doi: 10.1097/01.mog.0000218967.60633.64. [DOI] [PubMed] [Google Scholar]
- 29.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620–626. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 30.Chung YE, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics. 2009;29:683–700. doi: 10.1148/rg.293085729. [DOI] [PubMed] [Google Scholar]
- 31.Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J. Hepatobiliary. Pancreat. Surg. 2003;10:288–291. doi: 10.1007/s00534-002-0732-8. [DOI] [PubMed] [Google Scholar]
- 32.Slattery JM, Sahani DV. What is the current state-of-the-art imaging for detection and staging of cholangiocarcinoma? Oncologist. 2006;11:913–922. doi: 10.1634/theoncologist.11-8-913. [DOI] [PubMed] [Google Scholar]
- 33.Chung YE, et al. Varying appearances of cholangiocarcinoma: radiologic-pathologic correlation. Radiographics. 2009;29:683–700. doi: 10.1148/rg.293085729. [DOI] [PubMed] [Google Scholar]
- 34.Nathan H, Pawlik TM. Staging of intrahepatic cholangiocarcinoma. Curr. Opin. Gastroenterol. 2010;26:269–273. doi: 10.1097/MOG.0b013e328337c899. [DOI] [PubMed] [Google Scholar]
- 35.Kokudo N, Arita J. Staging for intrahepatic cholangiocarcinoma. Liver Int. 2010;30:931–933. doi: 10.1111/j.1478-3231.2010.02242.x. [DOI] [PubMed] [Google Scholar]
- 36.Nathan H, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2009;16:14–22. doi: 10.1245/s10434-008-0180-z. [DOI] [PubMed] [Google Scholar]
- 37.Carpizo DR, D'Angelica M. Management and extent of resection for intrahepatic cholangiocarcinoma. Surg. Oncol. Clin. N. Am. 2009;18 doi: 10.1016/j.soc.2008.12.010. 289-2ix. [DOI] [PubMed] [Google Scholar]
- 38.Giuliante F, et al. Liver resection for intrahepatic cholangiocarcinoma. Tumori. 2005;91:487–492. doi: 10.1177/030089160509100608. [DOI] [PubMed] [Google Scholar]
- 38a.Poultsides GA, Zhu AX, Choti MA, Pawlik TM. Intrahepatic cholangiocarcinoma. Surg Clin North Am. 2010;90(4):817–37. doi: 10.1016/j.suc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 38b.Yokoyama Y, et al. Value of indocyanine green clearance of the future liver remnant in predicting outcome after resection for biliary cancer. Br J Surg. 2010;97(8):1260–8. doi: 10.1002/bjs.7084. [DOI] [PubMed] [Google Scholar]
- 39.Murakami Y, et al. Prognostic Factors After Surgical Resection for Intrahepatic, Hilar, and Distal Cholangiocarcinoma. Ann. Surg. Oncol. 2010 doi: 10.1245/s10434-010-1325-4. [DOI] [PubMed] [Google Scholar]
- 40.Burger I, et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J. Vasc. Interv. Radiol. 2005;16:353–361. doi: 10.1097/01.RVI.0000143768.60751.78. [DOI] [PubMed] [Google Scholar]
- 41.Ahmadzadehfar H, Biersack HJ, Ezziddin S. Radioembolization of liver tumors with yttrium-90 microspheres. Semin. Nucl. Med. 2010;40:105–121. doi: 10.1053/j.semnuclmed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Hong K, Geschwind JF. Locoregional intra-arterial therapies for unresectable intrahepatic cholangiocarcinoma. Semin. Oncol. 2010;37:110–117. doi: 10.1053/j.seminoncol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Kiefer MV, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer. 2010 doi: 10.1002/cncr.25625. [DOI] [PubMed] [Google Scholar]
- 44.Carrafiello G, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc. Intervent. Radiol. 2010;33:835–839. doi: 10.1007/s00270-010-9849-3. [DOI] [PubMed] [Google Scholar]
- 45.Chiou YY, et al. Percutaneous ultrasound-guided radiofrequency ablation of intrahepatic cholangiocarcinoma. Kaohsiung. J. Med. Sci. 2005;21:304–309. doi: 10.1016/S1607-551X(09)70125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JH, et al. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur. J. Radiol. 2010 doi: 10.1016/j.ejrad.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 47.Kopek N, Holt MI, Hansen AT, Hoyer M. Stereotactic body radiotherapy for unresectable cholangiocarcinoma. Radiother. Oncol. 2010;94:47–52. doi: 10.1016/j.radonc.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Czito BG, Anscher MS, Willett CG. Radiation therapy in the treatment of cholangiocarcinoma. Oncology (Williston. Park) 2006;20:873–884. [PubMed] [Google Scholar]
- 49.Chen YX, et al. Determining the role of external beam radiotherapy in unresectable intrahepatic cholangiocarcinoma: a retrospective analysis of 84 patients. BMC. Cancer. 2010;10:492. doi: 10.1186/1471-2407-10-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glimelius B, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann. Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 51.Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008;13:415–423. doi: 10.1634/theoncologist.2007-0252. [DOI] [PubMed] [Google Scholar]
- 52.Mazhar D, Stebbing J, Bower M. Chemotherapy for advanced cholangiocarcinoma: what is standard treatment? Future. Oncol. 2006;2:509–514. doi: 10.2217/14796694.2.4.509. [DOI] [PubMed] [Google Scholar]
- 53.Park J, et al. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver. 2009;3:298–305. doi: 10.5009/gnl.2009.3.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cho SY, et al. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann. Surg. Oncol. 2010;17:1823–1830. doi: 10.1245/s10434-010-0938-y. [DOI] [PubMed] [Google Scholar]
- 55.Kassahun WT, Jonas S. Spectrum of benign lesions mimicking a malignant stricture at the liver hilum. Rev. Recent Clin. Trials. 2009;4:185–194. doi: 10.2174/157488709789957547. [DOI] [PubMed] [Google Scholar]
- 56.Sasaki R, et al. Evaluation of UICC-TNM and JSBS staging systems for surgical patients with extrahepatic cholangiocarcinoma. Langenbecks Arch. Surg. 2010;395:615–623. doi: 10.1007/s00423-010-0640-3. [DOI] [PubMed] [Google Scholar]
- 57.Blechacz BR, Sanchez W, Gores GJ. A conceptual proposal for staging ductal cholangiocarcinoma. Curr. Opin. Gastroenterol. 2009;25:238–239. doi: 10.1097/MOG.0b013e3283292383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Connor S, et al. The utility of laparoscopic assessment in the preoperative staging of suspected hilar cholangiocarcinoma. J. Gastrointest. Surg. 2005;9:476–480. doi: 10.1016/j.gassur.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 59.El-Hanafy E. Pre-operative biliary drainage in hilar cholangiocarcinoma, benefits and risks, single center experience. Hepatogastroenterology. 2010;57:414–419. [PubMed] [Google Scholar]
- 60.Liu F, Li Y, Wei Y, Li B. Preoperative Biliary Drainage Before Resection for Hilar Cholangiocarcinoma: Whether or Not? A Systematic Review. Dig. Dis. Sci. 2010 doi: 10.1007/s10620-010-1338-7. [DOI] [PubMed] [Google Scholar]
- 61.Nagino M, et al. Preoperative biliary drainage for biliary tract and ampullary carcinomas. J. Hepatobiliary. Pancreat. Surg. 2008;15:25–30. doi: 10.1007/s00534-007-1277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61a.Regimbeau JM, et al. Surgery for Hilar Cholangiocarcinoma: A Multi-institutional Update on Practice and Outcome by the AFC-HC Study Group. J Gastrointest Surg. 2011 doi: 10.1007/s11605-011-1414-0. E-pub. [DOI] [PubMed] [Google Scholar]
- 61b.Shimizu H, et al. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left-sided hepatectomy. Ann Surg. 2010;251(2):281–286. doi: 10.1097/SLA.0b013e3181be0085. [DOI] [PubMed] [Google Scholar]
- 61c.Lee SG, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci. 2010;17(4):476–489. doi: 10.1007/s00534-009-0204-5. [DOI] [PubMed] [Google Scholar]
- 61d.Neuhaus P, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230(6):808–18. doi: 10.1097/00000658-199912000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61e.Jarnagin WR, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4) doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61f.Capussotti L, Muratore A, Polastri R, Ferrero A, Massucco P. Liver resection for hilar cholangiocarcinoma: in-hospital mortality and longterm survival. J Am Coll Surg. 2002;195(5):641–647. doi: 10.1016/s1072-7515(02)01481-3. [DOI] [PubMed] [Google Scholar]
- 61g.Seyama Y, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238(1):73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61h.Kawasaki S, et al. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238(1):84–92. doi: 10.1097/01.SLA.0000074984.83031.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61i.Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241(5):693–699. doi: 10.1097/01.sla.0000160701.38945.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61j.Sano T, et al. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244(2):240–247. doi: 10.1097/01.sla.0000217605.66519.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61k.Shimizu H, et al. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left-sided hepatectomy. Ann Surg. 2010;251(2):281–286. doi: 10.1097/SLA.0b013e3181be0085. [DOI] [PubMed] [Google Scholar]
- 61l.Nagino M, et al. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Ann Surg. 2010;252(1):115–123. doi: 10.1097/SLA.0b013e3181e463a7. [DOI] [PubMed] [Google Scholar]
- 62.Anderson C, Kim R. Adjuvant therapy for resected extrahepatic cholangiocarcinoma: a review of the literature and future directions. Cancer Treat. Rev. 2009;35:322–327. doi: 10.1016/j.ctrv.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 63.Sagawa N, Kondo S, Morikawa T, Okushiba S, Katoh H. Effectiveness of radiation therapy after surgery for hilar cholangiocarcinoma. Surg. Today. 2005;35:548–552. doi: 10.1007/s00595-005-2989-4. [DOI] [PubMed] [Google Scholar]
- 64.Vern-Gross TZ, et al. Survival Outcomes in Resected Extrahepatic Cholangiocarcinoma: Effect of Adjuvant Radiotherapy in a Surveillance, Epidemiology, and End Results Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2010 doi: 10.1016/j.ijrobp.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Schoenthaler R, et al. Carcinoma of the extrahepatic bile ducts. The University of California at San Francisco experience. Ann. Surg. 1994;219:267–274. doi: 10.1097/00000658-199403000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rea DJ, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann. Surg. 2005;242:451–458. doi: 10.1097/01.sla.0000179678.13285.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soderlund C, Linder S. Covered metal versus plastic stents for malignant common bile duct stenosis: a prospective, randomized, controlled trial. Gastrointest. Endosc. 2006;63:986–995. doi: 10.1016/j.gie.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 68.Brugge WR. Endoscopic techniques to diagnose and manage biliary tumors. J. Clin. Oncol. 2005;23:4561–4565. doi: 10.1200/JCO.2005.19.729. [DOI] [PubMed] [Google Scholar]
- 69.Simmons DT, et al. A novel endoscopic approach to brachytherapy in the management of Hilar cholangiocarcinoma. Am. J. Gastroenterol. 2006;101:1792–1796. doi: 10.1111/j.1572-0241.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 70.Qian XJ, Zhai RY, Dai DK, Yu P, Gao L. Treatment of malignant biliary obstruction by combined percutaneous transhepatic biliary drainage with local tumor treatment. World J. Gastroenterol. 2006;12:331–335. doi: 10.3748/wjg.v12.i2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nag S, DeHaan M, Scruggs G, Mayr N, Martin EW. Long-term follow-up of patients of intrahepatic malignancies treated with iodine-125 brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006;64:736–744. doi: 10.1016/j.ijrobp.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 72.Shim CS, et al. Prospective study of the effectiveness of percutaneous transhepatic photodynamic therapy for advanced bile duct cancer and the role of intraductal ultrasonography in response assessment. Endoscopy. 2005;37:425–433. doi: 10.1055/s-2005-861294. [DOI] [PubMed] [Google Scholar]
- 73.Matull WR, et al. R0 but not R1/R2 resection is associated with better survival than palliative photodynamic therapy in biliary tract cancer. Liver Int. 2010 doi: 10.1111/j.1478-3231.2010.02345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Witzigmann H, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann. Surg. 2006;244:230–239. doi: 10.1097/01.sla.0000217639.10331.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sorensen HT, et al. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998;28:921–925. doi: 10.1002/hep.510280404. [DOI] [PubMed] [Google Scholar]
- 76.Hakamada K, et al. Late development of bile duct cancer after sphincteroplasty: a ten- to twenty-two-year follow-up study. Surgery. 1997;121:488–492. doi: 10.1016/s0039-6060(97)90101-x. [DOI] [PubMed] [Google Scholar]
- 77.Su CH, Shyr YM, Lui WY, P'Eng FK. Hepatolithiasis associated with cholangiocarcinoma. Br. J. Surg. 1997;84:969–973. doi: 10.1002/bjs.1800840717. [DOI] [PubMed] [Google Scholar]
- 78.Donato F, et al. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12:959–964. doi: 10.1023/a:1013747228572. [DOI] [PubMed] [Google Scholar]
- 79.Lee TY, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J. Gastroenterol. 2008;103:1716–1720. doi: 10.1111/j.1572-0241.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 80.Kobayashi M, et al. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88:2471–2477. doi: 10.1002/1097-0142(20000601)88:11<2471::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 81.Charlier C, et al. Cholangiocarcinoma in HIV-Infected patients with a history of Cholangitis. J. Acquir. Immune. Defic. Syndr. 2005;39:253–255. [PubMed] [Google Scholar]
- 82.Lowenfels AB, Norman J. Isoniazid and bile duct cancer. JAMA. 1978;240:434–435. doi: 10.1001/jama.1978.03290050024007. [DOI] [PubMed] [Google Scholar]
- 83.Yen S, Hsieh CC, MacMahon B. Extrahepatic bile duct cancer and smoking, beverage consumption, past medical history, and oral-contraceptive use. Cancer. 1987;59:2112–2116. doi: 10.1002/1097-0142(19870615)59:12<2112::aid-cncr2820591226>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 84.Rubel LR, Ishak KG. Thorotrast-associated cholangiocarcinoma: an epidemiologic and clinicopathologic study. Cancer. 1982;50:1408–1415. doi: 10.1002/1097-0142(19821001)50:7<1408::aid-cncr2820500728>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 85.Wong O, Whorton MD, Foliart DE, Ragland D. An industry-wide epidemiologic study of vinyl chloride workers, 1942–1982. Am. J. Ind. Med. 1991;20:317–334. doi: 10.1002/ajim.4700200305. [DOI] [PubMed] [Google Scholar]
- 86.Szendroi M, Nemeth L, Vajta G. Asbestos bodies in a bile duct cancer after occupational exposure. Environ. Res. 1983;30:270–280. doi: 10.1016/0013-9351(83)90213-x. [DOI] [PubMed] [Google Scholar]
- 87.Lipsett PA, Pitt HA, Colombani PM, Boitnott JK, Cameron JL. Choledochal cyst disease. A changing pattern of presentation. Ann. Surg. 1994;220:644–652. doi: 10.1097/00000658-199411000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87a.S Edge, D Byrd, CC Compton, AG Fritz, F Greene, Trotti A, editors. AJCC Cancer Staging Manual. 7th Edition. Springer; 2009. ISBN: 978-0-387-88440-0. [Google Scholar]
- 87b.Farges O, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: by the AFC-IHCC-2009 study group. Cancer. 2010 Nov 29; doi: 10.1002/cncr.25712. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 87c.Tse RV, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26(4):657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 87d.Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet. 1975;140(2):170–178. [PubMed] [Google Scholar]
- 87e.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215(1):31–38. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87f.Jarnagin WR, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234(4):507–17. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87g.Zen Y, et al. Biliary intraepithelial neoplasia: an international interobserver agreement study and proposal for diagnostic criteria. Mod Pathol. 2007;20(6):701–709. doi: 10.1038/modpathol.3800788. [DOI] [PubMed] [Google Scholar]
- 87h.Iwatsuki S, et al. Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J Am Coll Surg. 1988;187(4):358–364. doi: 10.1016/s1072-7515(98)00207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87i.Rosen CB, Heimbach JK, Gores GJ. Liver transplantation for cholangiocarcinoma. Transpl Int. 2010;23(7):692–697. doi: 10.1111/j.1432-2277.2010.01108.x. [DOI] [PubMed] [Google Scholar]