Abstract
Accumulating evidence suggests that overexpression of the tyrosine kinase receptor EphB4, a mediator of vascular development, is a novel target for tumor diagnosis, prognosis and therapy. Noninvasive imaging of EphB4 expression could therefore be valuable for evaluating disease course and therapeutic efficacy at the earliest stages of anti-EphB4 treatment. In this study, we systematically investigated the use of anti-EphB4 antibody h131 (150 kD) and its fragments (h131-F(ab′)2, 110 kD; h131-Fab, 50 kD) for near-infrared fluorescence (NIRF) imaging of EphB4 expression in vivo. h131-F(ab′)2 and h131-Fab were produced through pepsin and papain digestion of h131 respectively, whose purity was confirmed by FPLC and SDS-PAGE. After conjugation with Cy5.5, in vivo characteristics of h131, h131-F(ab′)2 and h131-Fab were evaluated in EphB4-positive HT29 tumor model. Although h131-Cy5.5 demonstrated highest tumor uptake among these probes, its optimal tumor uptake level was obtained at 2 d post injection (p.i.). For h131-Fab-Cy5.5, maximum tumor uptake was achieved at 4 h p.i.. However, no significant difference was observed between h131-Fab-Cy5.5 and hIgG-Fab-Cy5.5, indicating the tumor accumulation was mainly caused by passive targeting. In contrast, h131-F(ab′)2-Cy5.5 demonstrated prominent tumor uptake at 6 h p.i. The target specificity was confirmed by hIgG-F(ab′)2-Cy5.5 control and immunofluorescent staining. Collectively, h131-F(ab′)2 exhibited prominent and specific tumor uptake at early time points, which suggests it is a promising agent for EphB4-targeted imaging.
Keywords: EphB4 receptor, near-infrared fluorescence (NIRF) imaging, antibody fragment, F(ab′)2, Fab
INTRODUCTION
The EphB4 receptor and its sole ligand EphrinB2 are essential regulators of vascular development under physiological conditions.1–3 EphB4 receptor is also overexpressed in various types of tumors and acts as a survival factor for tumor cells.4–9 Recent studies have shown that EphB4 knockdown could effectively inhibit tumor growth and invasion,5–9 and improve the treatment sensitization of tumor cells.5, 6, 9, 10 In several tumor types, EphB4 expression level is positively correlated with the degree of tumor malignancy8, 11–14 Furthermore, overexpression of EphB4 predicted poor survival8, 15, 16 or poor treatment response16 in tumor patients. Based on the extremely important function of EphB4, in vivo visualization and quantification of EphB4 expression would facilitate the early and sensitive tumor diagnosis, prognosis, and treatment monitoring.
Previously, two fully humanized monoclonal antibodies that specifically recognize the EphB4 extracellular region have been developed:17 antibody h47 targets the fibronectin-like domain 2; antibody h131 targets the fibronectin-like domain 1. Recently, we have demonstrated that near-infrared fluorescence (NIRF) dye conjugated h47 could be used as an EphB4-specific probe in preclinical studies.18 Although full antibodies may be promising ligands for EphB4-targeted imaging, their relatively large size (150 kD) and Fc domain would lead to slow accumulation at tumor site. Moreover, the probes would also be cleared from the blood slowly.19 For full antibody-based probes, it could take several days to obtain optimal tumor contrast, which would compromise imaging applications. Compared to intact antibodies, antibody fragments F(ab′)2 (110 kD) or Fab (50 kD), which lack the Fc domain, exhibit smaller molecular weight, faster clearance rate, and better tumor penetration capability. In our recent study, tumor accumulation of h131 was found to be significantly higher than that of h47,20 warranting comparison of the in vivo imaging characteristics of three different formats of anti-EphB4 antibody: h131, h131-F(ab′)2 and h131-Fab, in order to obtain an optimized EphB4-targeted imaging probe.
MATERIALS AND METHODS
Materials
h131 (monoclonal antibodies to EphB4, recognizes human EphB4) and EphB4-alkaline phosphatase (AP) were kindly provided by Vasgene Therapeutics Inc. (Los Angeles, CA). Cy5.5 monofunctional N-hydroxysuccinimide ester (Cy5.5-NHS) and PD-10 disposable columns were purchased from GE Healthcare Life Sciences (Piscataway, NJ), 5(6)-Carboxyfluorescein (FAM) from AnaSpec Inc. (San Jose, CA), human IgG (hIgG) from Rockland (Gilbertsville, PA), secondary antibodies goat anti-human Alexa Fluor 568 from Invitrogen (Paisley, Scotland).
Production of Antibody Fragments
F(ab′)2 and Fab fragments were produced according to the manufacturer’s protocol (ThermoScientific, Rockford, IL). F(ab′)2 fragments were produced by incubating 10 mg of h131 or hIgG with Sepharose-immobilized pepsin in digestion buffer (20 mM sodium acetate; pH 4.5) for 4 h at 37°C in an end-over-end mixer. Then the digest was separated from the immobilized pepsin by centrifugation and dialyzed against PBS (50K MWCO) to remove small Fc fragments. Fab fragments were produced by incubating 10 mg of h131 or hIgG with Sepharose-immobilized papain in digestion buffer (20 mM sodium phosphate, 10 mM EDTA, 20 mM cysteine•HCl; pH 7.0) for 4 h at 37°C in an end-over-end mixer. Then the digest was separated from the immobilized papain by centrifugation and the Fab fragments were separated from undigested IgG and Fc fragments using an immobilized Protein A column.
Fast Protein Liquid Chromatography (FPLC)
h131, h131-F(ab′)2 and h131-Fab were analyzed by fast protein liquid chromatography (FPLC) as reported previously.20 The mobile phase was 0.2 M sodium phosphate buffer (pH 7.0) and the flow rate was 0.1 ml/min.
SDS-PAGE
SDS-PAGE was performed as described previously.18, 20 Briefly, h131, h131-F(ab′)2 and h131-Fab were mixed with Laemmli buffer (BioRad, Hercules, CA) with or without dithiothreitol (50 mM), heated at 100 °C for 5 min and fractionated using SDS-PAGE. The gel was then stained with Coomassie blue and scanned with Odyssey Infrared Imager (LI-COR, Lincoln, NE).
Probe Synthesis
Probes were synthesized using literature reported procedure.18 The molar reaction ratio of h131, h131-F(ab′)2 or h131-Fab to Cy5.5-NHS was 1:1. hIgG-Cy5.5, hIgG-F(ab′)2-Cy5.5 and hIgG-Fab-Cy5.5 were synthesized as control probes accordingly. h131-FAM, h131-F(ab′)2-FAM, h131-Fab-FAM, hIgG-FAM, hIgG-F(ab′)2-FAM and hIgG-Fab-FAM were also synthesized using the same procedure. The fluorescence intensity of Cy5.5 and FAM probes was evaluated by measuring the OD 680 nm or OD 495 nm with Beckman DU 530 spectrophotometer (Beckman Instruments Inc., Fullerton, CA).
Cell Culture and Confocal Microscopy Analysis
The human colorectal cancer cell line HT29 was obtained from American Type Culture Collection (Manassas, VA) and maintained under standard conditions.18, 20 HT29 cells were planted on BD Falcon 4-well chamber slide at 5 × 104 cells/well. After 24 h, cells were incubated with 300 μl complete culture medium containing 60 nM h131-FAM, h131-F(ab′)2-FAM or h131-Fab-FAM for 30 min at 37°C. After incubation, cells were washed with PBS and fixed in 4% paraformaldehyde (ElectronMicroscopy Sciences, Hatfield, PA) for 20 minutes at room temperature. Subsequently, the slides were mounted and images were obtained with Zeiss LSM 510 confocal microscopy (Tokyo, Japan).
Tumor Xenografts
Animal procedures were performed according to protocol approved by the University of Southern California Institutional Animal Care and Use Committee. Tumor xenograft model was established by injecting 2×106 of HT29 cells subcutaneously in the right shoulder of nude mice as described previously. 18, 20
In Vivo and Ex Vivo Near-Infrared Fluorescence (NIRF) Imaging
In vivo and ex vivo fluorescence imaging was performed as reported previously.18 A Cy5.5 filter set (excitation: 675 nm, emission: 720 nm) was used for acquiring the fluorescence of Cy5.5-conjugated antibodies. The tumors of each group were size-matched (n=5 each group). The mice were put on 2018 Teklad Global 18% Protein Rodent Diet (Harlan, Indianapolis, IN) for 3 days before imaging. Each mouse was injected with 0.2 nmol of probe via tail vein and underwent optical imaging at various time points after injection. The tumor and major organs were dissected and subjected to ex vivo fluorescence imaging. The mean fluorescence for each sample was reported. The mean fluorescence of head region was used as the background value. Tumor to background ratio (TBR) was determined by dividing (mean fluorescence of tumor) with (mean fluorescence of background).
Immunofluorescence Staining
Antibody distribution was measured by using immunofluorescence staining as described previously.18, 20 Tumors were dissected at 6 h p.i for hIgG-F(ab′)2-Cy5.5 and h131-F(ab′)2-Cy5.5, or 4 h p.i. for hIgG-Fab-Cy5.5 and h131-Fab-Cy5.5. Secondary antibody goat anti-human Alexa Fluor 568 was used to detect antibodies.
Statistical Analysis
Quantitative data are expressed as mean ± SD. Means were compared using 1-way ANOVA and the Student t test. P values of < 0.05 were considered statistically significant.
RESULTS
Characterization of h131, h131-F(ab′)2 and h131-Fab
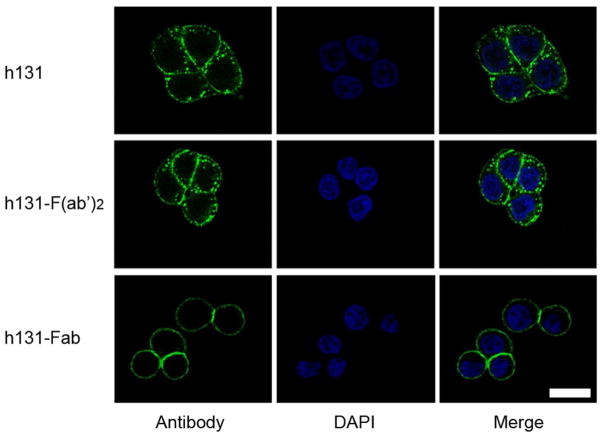
F(ab′)2 and Fab fragments of h131 were produced through pepsin and papain digestion, respectively. Figure 1A shows the schematic diagram of h131, h131-F(ab′)2 and h131-Fab. The chromatograms of h131, h131-F(ab′)2 and h131-Fab in fast protein liquid chromatography (FPLC) analysis had only one peak, which indicated the high purity of these antibody/fragments (Figure 1B). Because of their different molecular size, the retention time of h131, h131-F(ab′)2 and h131-Fab was 15.0 min, 15.8 min and 18.2 min, respectively. Molecular weight of these ligands was analyzed by SDS-PAGE (Figure 1C). Under non-reducing conditions, these ligands were kept intact. The molecular weight of h131, h131-F(ab′)2 and h131-Fab were 150 KD, 110 KD and 50 KD, respectively. Under reducing conditions, the ligands were broken into heavy chains and light chains. The heavy chains of h131, h131-F(ab′)2 and h131-Fab were 50 KD, 30 KD and 25 KD respectively while the light chains of these ligands kept the same (25 KD). The molar concentrations leading to 50% competition (IC50) of h131, h131-F(ab′)2 and h131-Fab were 0.24 nM, 0.26 nM and 2.54 nM, respectively (Table S1). This indicated that the binding affinity of h131 and h131-F(ab′)2 was comparable while that of h131-Fab was significantly lower. h131 is rapidly internalized when incubated with EphB4 expressing cells17 and this property may contribute to its high tumor targeting efficacy.20 Therefore, the endocytosis of two h131 derivatives h131-F(ab′)2 and h131-Fab was evaluated in EphB4 positive HT29 cells with confocal imaging. As shown in Figure 2, both h131 and h131-F(ab′)2 were internalized into the cytoplasm through EphB4-mediated endocytosis. In contrast, h131-Fab remained on cell membrane after binding. Additionally, hIgG, hIgG-F(ab′)2 and hIgG-Fab controls showed no binding to HT29 cells (Figure S1).
Figure 1.
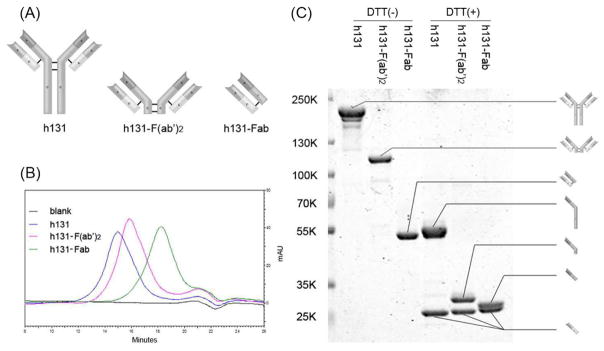
Characterization of h131-Cy5.5, h131-F(ab′)2-Cy5.5 and h131-Fab-Cy5.5. (A) Schematic diagram of h131, h131-F(ab′)2 and h131-Fab. (B) The chromatograms of h131, h131-F(ab′)2 and h131-Fab in fast protein liquid chromatography (FPLC) analysis. (C) SDS-PAGE of h131, h131-F(ab′)2 and h131-Fab under non-reducing or reducing conditions.
Figure 2.
Confocal analysis of the antibody distribution in EphB4 positive HT29 cells. HT29 cells were incubated with h131-FAM, h131-F(ab′)2-FAM or h131-Fab-FAM at 37°C for 30 min. Scale bar, 20 μm.
Synthesis and Characterization of h131-Cy5.5, h131-F(ab′)2-Cy5.5 and h131-Fab-Cy5.5
h131-Cy5.5, h131-F(ab′)2-Cy5.5 and h131-Fab-Cy5.5 were synthesized through the modification of amino groups. hIgG-Cy5.5, hIgG-F(ab′)2-Cy5.5 and hIgG-Fab-Cy5.5 were synthesized as the control. To study the impact of Cy5.5 conjugation on the EphB4 binding ability, binding activity assay was performed. As shown in Figure S2, binding activity of h131-Cy5.5, h131-F(ab′)2-Cy5.5 and h131-Fab-Cy5.5 was only reduced slightly compared to unmodified ligands. Whereas, hIgG-Cy5.5, hIgG-F(ab′)2-Cy5.5 and hIgG-Fab-Cy5.5 had minimal binding toward EphB4.
Comparison of h131-Cy5.5, h131-F(ab′)2-Cy5.5 and h131-Fab-Cy5.5 through In Vivo Near-Infrared Fluorescence (NIRF) Imaging
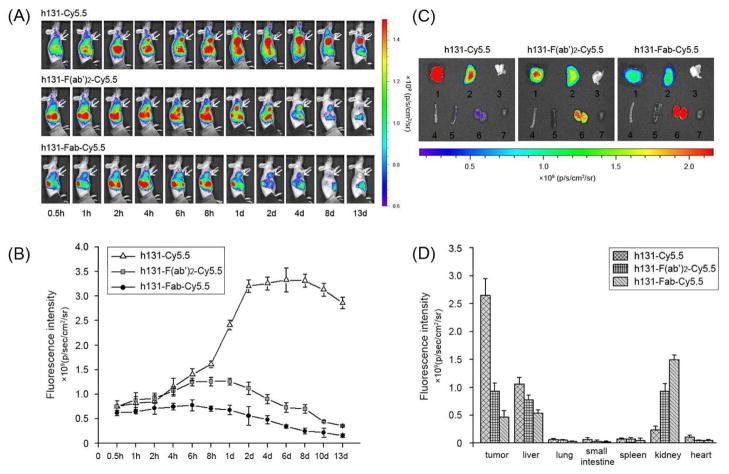
The tumor targeting efficacy of h131-Cy5.5, h131-F(ab′)2-Cy5.5 and h131-Fab-Cy5.5 was compared by multiple time-point NIRF imaging in EphB4-positive HT29 tumor bearing mice. Representative images and quantifications at different time points post injection (p.i.) are shown in Figure 3A, 3B. At early time points (0.5 h, 1 h, 2 h, 4 h and 6 h p.i.), the tumor uptake of h131-Cy5.5 and h131-F(ab′)2-Cy5.5 was comparable, while h131-Fab-Cy5.5 showed lowest tumor uptake. At late time points (after 8 h p.i.), the absolute tumor uptake value followed the order of h131-Cy5.5 > h131-F(ab′)2-Cy5.5 > h131-Fab-Cy5.5. The tumor to background ratio (TBR) of h131-Cy5.5, h131-F(ab′)2-Cy5.5 and h131-Fab-Cy5.5 were determined at all time points examined (Figure S3). Because the background values decreased faster than that of tumor, TBR kept increase with time even when the absolute tumor uptake values began to decrease. We would also like to point out that the obtained TBR information is semi-quantitative due to the tissue penetration limitation and light scattering of fluorescent signal. The time needed to reach maximum tumor uptake value correlated with molecular size and followed the order of h131-Cy5.5 > h131-F(ab′)2-Cy5.5 > h131-Fab-Cy5.5. To further characterize the probe distribution after injection, ex vivo imaging was performed at 2 d p.i.. As shown in Figure 3C, 3D, the tumor uptake of h131-Cy5.5 was 2.85 and 5.69 times of h131-F(ab′)2-Cy5.5 and h131-Fab-Cy5.5 respectively, which was consistent with in vivo NIRF imaging results. Probes accumulated mainly in liver and kidney. The liver uptake followed the order of h131-Cy5.5 > h131-F(ab′)2-Cy5.5 > h131-Fab-Cy5.5, while the kidney uptake followed the order of h131-Fab-Cy5.5 > h131-F(ab′)2-Cy5.5 > h131-Cy5.5.
Figure 3.
(A) In vivo near-infrared imaging of HT29 tumor-bearing mice after injection of h131-Cy5.5, h131-F(ab′)2-Cy5.5 or h131-Fab-Cy5.5. The tumors are indicated by white arrows. (B) Time-fluorescence intensity curves of HT29 tumor for h131-Cy5.5, h131-F(ab′)2-Cy5.5 and h131-Fab-Cy5.5. (C) Ex vivo near-infrared imaging of major organs harvested at 2 d p.i. of h131-Cy5.5, h131-F(ab′)2-Cy5.5 or h131-Fab-Cy5.5. 1 tumor, 2 liver, 3 lung, 4 small intestine, 5 spleen, 6 kidney, and 7 heart. (D) Fluorescence intensity quantification of tumors and major organs at 2 d p.i. of h131-Cy5.5, h131-F(ab′)2-Cy5.5 or h131-Fab-Cy5.5, respectively. All data in B and D are presented as the mean ± standard error of mean (SEM).
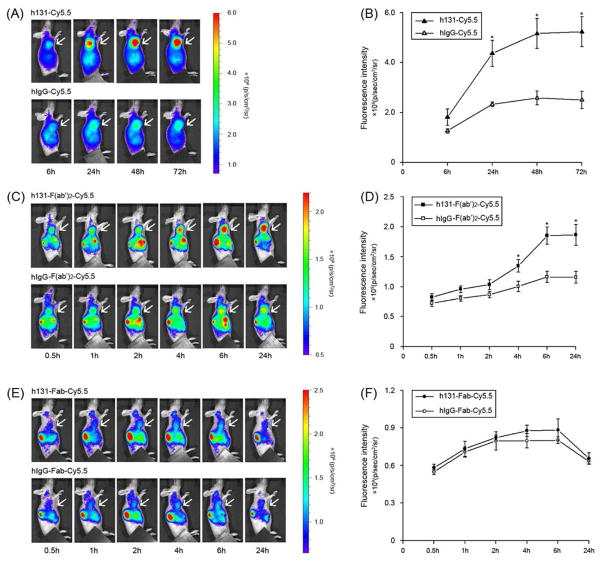
To confirm the receptor specificities of these probes, hIgG-Cy5.5, hIgG-F(ab′)2-Cy5.5 and hIgG-Fab-Cy5.5 (without binding affinity towards EphB4) were also tested. Figure 4A, 4B, S4A showed typical NIRF images, tumor uptake and TBR quantification of h131-Cy5.5 and hIgG-Cy5.5 in HT29 tumor bearing mice. At 6 h p.i., the tumor uptake of h131-Cy5.5 and hIgG-Cy5.5 had no significant difference (P > 0.05), indicating majority of the uptake was caused by passive targeting at this time point. At late time points (1 d, 2 d, 3 d), the tumor uptake of h131-Cy5.5 was significantly higher than that of hIgG-Cy5.5 (P < 0.05), which confirmed the target specificity of the tumor uptake. Representative NIRF images, tumor uptake and TBR quantification of h131-F(ab′)2-Cy5.5 and hIgG-F(ab′)2-Cy5.5 in HT29 tumor bearing mice were presented in Figure 4C, 4D, S4B. At 4 h p.i., tumor uptake of h131-F(ab′)2-Cy5.5 was significantly higher than that of hIgG-F(ab′)2-Cy5.5 (P < 0.05). The tumor uptake difference was further increased at 6 h and 24 h p.i.. Representative NIRF images, tumor uptake and TBR quantification h131-Fab-Cy5.5 of and hIgG-Fab-Cy5.5 in HT29 tumor bearing mice was shown in Figure 4E, 4F, S4C. The tumor uptake of h131-Fab-Cy5.5 and hIgG-Fab-Cy5.5 had no significant difference (P > 0.05) at all time points examined, indicating that the tumor uptake of h131-Fab-Cy5.5 was not EphB4 mediated.
Figure 4.
In vivo near-infrared imaging (A) and quantification (B) of HT29 tumor-bearing mice after injection of h131-Cy5.5 or hIgG-Cy5.5; in vivo near-infrared imaging (C) and quantification (D) of HT29 tumor-bearing mice after injection of h131-F(ab′)2-Cy5.5 or hIgG-F(ab′)2-Cy5.5; in vivo near-infrared imaging (E) and quantification (F) of HT29 tumor-bearing mice after injection of h131-Fab-Cy5.5 or hIgG-Fab-Cy5.5. The P value was calculated with Student’s t test. *, P < 0.05.
Table 1 summarized the in vivo behavior of these probes. Although h131-Cy5.5 has the highest tumor uptake, it needs longest time to reach maximum tumor uptake value. The target specificity was clearly demonstrated through the comparison with the hIgG-Cy5.5 control. h131-Fab-Cy5.5 demonstrated fastest but also lowest tumor uptake, most of which was non-specific targeting as confirmed through the comparison with hIgG-Fab-Cy5.5. Clearly, h131-Fab-Cy5.5 is not appropriate for EphB4-targeted imaging in this case. In contrast, h131-F(ab′)2-Cy5.5 can accumulate into tumor within reasonable time frame in a receptor-mediated manner. This property makes it suitable for the imaging of EphB4 expression at as early as 4 h p.i..
Table 1.
Comparison of Imaging characteristics of h131, h131-F(ab′)2 and h131-Fab.
| h131 | h131-F(ab′)2 | h131-Fab | |
|---|---|---|---|
| time-point reaching maximum tumor uptake | 48 h p.i. | 6 h p.i. | 4 h p.i. |
| maximum tumor uptake level | high | medium | low |
| tumor accumulation rate | low | medium | high |
| clearance rate from body | low | medium | high |
| Liver uptake | high | medium | low |
| Kidney uptake | low | medium | high |
| Specificity towards EphB4 receptor in in vivo imaging experiment | Yes, but at late time points | Yes, as early as 4 h p.i. | No |
Analysis of Probe Distribution in Tumor Tissues
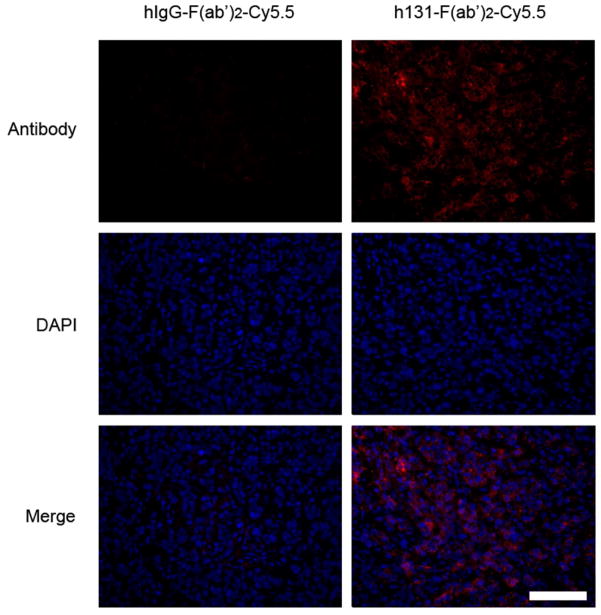
The distribution of probes in HT29 tumor tissues was further analyzed. Because the tumor uptake reached a plateau at 6 h p.i. for hIgG-F(ab′)2-Cy5.5 and h131-F(ab′)2-Cy5.5, or 4 h p.i. for hIgG-Fab-Cy5.5 or h131-Fab-Cy5.5, tumor tissues were collected at these time points for immunofluorescent assay. As shown in Figure 5, there was a large amount of h131-F(ab′)2 accumulated in the HT29 tumor tissues compared to hIgG-F(ab′)2. Whereas, there was minimal hIgG-Fab or h131-Fab accumulated in the tumor region (Figure S5). Both in vivo and in vitro data suggested that the high accumulation of h131-F(ab′)2-Cy5.5 over HT29 tumor region was due to the high expression of EphB4 within tumor regions. Therefore, h131-F(ab′)2-Cy5.5 could be applied for the imaging of EphB4 expression in vivo.
Figure 5.
Antibody distribution analysis on HT29 tumor sections 6 h p.i. of hIgG-F(ab′)2-Cy5.5 or h131-F(ab′)2-Cy5.5, respectively. Scale bar, 100 μm.
DISCUSSION
Converging evidence demonstrates that EphB4 is a promising target for tumor diagnosis, prognosis and therapy.4–9, 15, 16 Therefore, noninvasive imaging of EphB4 expression would significantly accelerate clinical application of EphB4 based tumor management. We have previously shown two anti-EphB4 antibodies, h47 and h131, could be applied for in vivo EphB4-targeted imaging after properly labeled.18, 20 Moreover, h131 showed significantly higher tumor accumulation than h47 based probes.20 Compared with peptide-based probes, antibody ligands have superior binding affinity and unmatched specificity towards the targets. However, the limitation of whole antibody (such as h131)-based probe lies on its high molecular weight and long circulatory time in vivo, which would lead to unfavorable imaging kinetics and high background at early time points. The main purpose of this study was to explore the relationship between ligand formats (whole antibody or antibody fragments) and in vivo characteristics of the corresponding imaging probes. We expect to identify an optimized probe for EphB4-targeted imaging through systematic comparison of h131, h131-F(ab′)2 and h131-Fab based probes.
In our initial study, h131-F(ab′)2 and h131-Fab were produced through pepsin and papain digestion of h131 respectively. The purity and molecular size of antibody and antibody fragments were confirmed by FPLC and SDS-PAGE (Figure 1B, 1C). Our previous studies showed that h131 is rapidly internalized after binding to EphB4 positive HT29 cells17 and this property may contribute to its high tumor targeting efficacy.20 Confocal imaging demonstrated that h131-F(ab′)2 was also partially internalized into the cytoplasm through EphB4-mediated endocytosis, while h131-Fab remained binding on cell membrane only (Figure 2). The internalization of h131 and h131-F(ab′)2 could be related to their two variable regions: each molecule of h131 or h131-F(ab′)2 can bind to two molecules of adjacent EphB4 receptors, which could trigger EphB4 clustering, phosphorylation, cytoskeletal assembly and finally endocytosis of receptor-antibody complexes.21 In contrast, h131-Fab has only one variable region per ligand (which can only bind to one molecule of EphB4 receptor), therefore may not trigger EphB4 clustering and afterward endocytosis of receptor-antibody complexes. Nonetheless, additional experiments are needed before a conclusion is drawn.
To acquire probes suitable for in vivo imaging application, h131, h131-F(ab′)2 and h131-Fab were labeled with NIRF dye Cy5.5 through amino groups. As demonstrated in Fig S2, the binding activity of h131-Cy5.5, h131-F(ab′)2-Cy5.5 and h131-Fab-Cy5.5 was only slightly reduced after modification. With most of the binding affinity maintained, the tumor targeting efficacy of h131-Cy5.5, h131-F(ab′)2-Cy5.5 and h131-Fab-Cy5.5 was evaluated in EphB4-positive HT29 tumor model using in vivo NIRF imaging (Figure 3A, 3B). By interacting with neonatal Fc receptor (FcRn), the Fc domain of intact antibodies can provide prolonged serum half life in vivo.22 Therefore, h131-Cy5.5 showed the slowest clearance rate. The maximum tumor uptake of h131-Cy5.5 was the highest but arrived at very late time point (2 d p.i.). h131-Fab-Cy5.5, which has the smallest molecular size, showed the fastest accumulation and clearance but lowest tumor uptake. In the case of h131-F(ab′)2-Cy5.5, the tumor uptake was modest and reached a plateau between 6 h to 1 d p.i.. Both h131-Cy5.5 and h131-F(ab′)2-Cy5.5 showed longer tumor retention compared with h131-Fab-Cy5.5, which could be caused by the efficient trapping of these two probes after binding to EphB4 receptor. Biodistribution pattern of these three probes was also studied by ex vivo NIRF imaging. As demonstrated in Figure 3C, 3D, h131-Cy5.5 cleared predominantly via liver, which is the major clearance route of large sized antibodies. h131-Fab-Cy5.5 cleared predominantly via kidney, which is the majour clearance route of small sized antibody fragments. The biodistribution behavior of h131-F(ab′)2-Cy5.5 was found to be of intermediate nature between h131-Cy5.5 and h131-Fab-Cy5.5. It showed modest retention in both liver and kidney, even though the size of F(ab′)2 fragment is above the threshold for glomerular filtration (< 60 kD). This renal uptake may be because of the dissociation of disuffide linkage by in vivo redox systems, which results in the generation of Fab′ fragments that can pass the glomerular membrane.23
Receptor specificity of these probe was confirmed by comparing to hIgG-Cy5.5, hIgG-F(ab′)2-Cy5.5 or hIgG-Fab-Cy5.5 respectively through in vivo NIRF imaging in HT29 tumor model (Figure 4, S4). At 6 h p.i., the tumor uptake of hIgG-Cy5.5 and h131-Cy5.5 had no significant difference (P > 0.05), indicating that the majority of h131-Cy5.5 tumor uptake was nonspecific at early time points. The enhanced permeability and retention (EPR) effect may contribute to the passive accumulation of antibodies in the tumor region at this time point. At late time points (1 d, 2 d, 3 d), the tumor uptake of h131-Cy5.5 was significantly higher than that of hIgG-Cy5.5 (P < 0.05). Clearly, the active tumor accumulation of h131-Cy5.5 was mainly caused by h131—EphB4 specific interaction at these time points. Although in vitro binding activity assay confirmed the specific binding ability of h131-Fab-Cy5.5 towards EphB4 (Figure S2C), no specific tumor targeting of h131-Fab-Cy5.5 was observed in in vivo imaging. Because h131-Fab fragment has only one antigen binding site (monovalent), its overall functional affinity towards EphB4 is reduced compared with h131 and h131-F(ab′)2 (Table S1).24 The faster clearance rate of h131-Fab could also contribute to this phenomenon. On the contrary, F(ab′)2 fragment contains two antigen binding sites (bivalent), which maintains comparable high binding affinity as the intact antibody (Table S1). After 4 h p.i., the tumor accumulation of h131-F(ab′)2-Cy5.5 was shown to be specific toward EphB4 as confirmed by hIgG-F(ab′)2-Cy5.5 control. Immunofluorescent staining also demonstrated that there was abundant h131-F(ab′)2 distributed in HT29 tumor tissues compared with hIgG-F(ab′)2 (Figure 5). In summary, h131-F(ab′)2-Cy5.5 exhibited good contrast, rapid and specific tumor targeting in an EphB4 positive model. Compared with h131-Cy5.5 and h131-Fab-Cy5.5, h131-F(ab′)2-Cy5.5 might be a more suitable lead candidate for EphB4-targeted imaging. It should be pointed out that these imaging probes need to be optimized further in our follow-up studies. The application of NIRF imaging is limited to the detection of superficial or endoscopy accessible tumors, as well as imaging guided surgery.25 Corresponding positron emission tomography (PET) or single photon emission computed tomography (SPECT) imaging agents might be preferred since they could be easily translated from preclinical evaluation into clinic practice. New forms of engineered antibody fragments, including minibodies (bivalent, 80 KD) and triabodies (trivalent, 75 KD),26 may have similar or even higher binding affinity than F(ab′)2 but with reduced molecular weight. A side by side comparison with these ligands could help us evaluate whether the smaller-sized antibody fragments would provide better imaging pharmacokinetics due to the further improved extravasation rate and tumor penetration. In addition, h131-F(ab′)2 showed appreciable kidney retention. This may cause problems in visualization of targets located in a close vicinity to kidneys. Strategies, such as, chemically stabilization of F(ab′)2 to resist in vivo cleavage,23 competitive inhibition of proximal tubular reabsorption,27 and PEGylation could be applied to reduce kidney uptake of F(ab′)2 fragments.
Supplementary Material
Acknowledgments
This work was supported by the NIBIB (R21 1 r21 eb012294-01a1), Early (Margaret E.) Medical Research Trust, the American Cancer Society (121991-MRSG-12-034-01-CCE), SC CTSI (12-2176-3135), Department of Defense (BC102678), Vasgene Therapeutics Inc. (NIH: CA168158-01; CA171538-01), National Natural Science Foundation of China (No. U1032002, 81071206, 81271621, 81301266), and Key Clinical Research Project of Public Health Ministry of China 2010–2012 (No. 164).
Footnotes
Notes
The authors declare the following competing financial interest(s): K.N. and V.K. is the employee of Vasgene Therapeutics Inc.
ASSOCIATED CONTENT
Additional experimental details. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93(5):741–53. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 2.Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4(3):403–14. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 3.Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13(3):295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia G, Kumar SR, Masood R, Zhu S, Reddy R, Krasnoperov V, Quinn DI, Henshall SM, Sutherland RL, Pinski JK, Daneshmand S, Buscarini M, Stein JP, Zhong C, Broek D, Roy-Burman P, Gill PS. EphB4 expression and biological significance in prostate cancer. Cancer Res. 2005;65(11):4623–32. doi: 10.1158/0008-5472.CAN-04-2667. [DOI] [PubMed] [Google Scholar]
- 5.Kumar SR, Singh J, Xia G, Krasnoperov V, Hassanieh L, Ley EJ, Scehnet J, Kumar NG, Hawes D, Press MF, Weaver FA, Gill PS. Receptor tyrosine kinase EphB4 is a survival factor in breast cancer. Am J Pathol. 2006;169(1):279–93. doi: 10.2353/ajpath.2006.050889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masood R, Kumar SR, Sinha UK, Crowe DL, Krasnoperov V, Reddy RK, Zozulya S, Singh J, Xia G, Broek D, Schonthal AH, Gill PS. EphB4 provides survival advantage to squamous cell carcinoma of the head and neck. Int J Cancer. 2006;119(6):1236–48. doi: 10.1002/ijc.21926. [DOI] [PubMed] [Google Scholar]
- 7.Xia G, Kumar SR, Stein JP, Singh J, Krasnoperov V, Zhu S, Hassanieh L, Smith DL, Buscarini M, Broek D, Quinn DI, Weaver FA, Gill PS. EphB4 receptor tyrosine kinase is expressed in bladder cancer and provides signals for cell survival. Oncogene. 2006;25(5):769–80. doi: 10.1038/sj.onc.1209108. [DOI] [PubMed] [Google Scholar]
- 8.Kumar SR, Masood R, Spannuth WA, Singh J, Scehnet J, Kleiber G, Jennings N, Deavers M, Krasnoperov V, Dubeau L, Weaver FA, Sood AK, Gill PS. The receptor tyrosine kinase EphB4 is overexpressed in ovarian cancer, provides survival signals and predicts poor outcome. Br J Cancer. 2007;96(7):1083–91. doi: 10.1038/sj.bjc.6603642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar SR, Scehnet JS, Ley EJ, Singh J, Krasnoperov V, Liu R, Manchanda PK, Ladner RD, Hawes D, Weaver FA, Beart RW, Singh G, Nguyen C, Kahn M, Gill PS. Preferential induction of EphB4 over EphB2 and its implication in colorectal cancer progression. Cancer Res. 2009;69(9):3736–45. doi: 10.1158/0008-5472.CAN-08-3232. [DOI] [PubMed] [Google Scholar]
- 10.Spannuth WA, Mangala LS, Stone RL, Carroll AR, Nishimura M, Shahzad MM, Lee SJ, Moreno-Smith M, Nick AM, Liu R, Jennings NB, Lin YG, Merritt WM, Coleman RL, Vivas-Mejia PE, Zhou Y, Krasnoperov V, Lopez-Berestein G, Gill PS, Sood AK. Converging evidence for efficacy from parallel EphB4-targeted approaches in ovarian carcinoma. Mol Cancer Ther. 2010;9(8):2377–88. doi: 10.1158/1535-7163.MCT-10-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berclaz G, Andres AC, Albrecht D, Dreher E, Ziemiecki A, Gusterson BA, Crompton MR. Expression of the receptor protein tyrosine kinase myk-1/htk in normal and malignant mammary epithelium. Biochem Biophys Res Commun. 1996;226(3):869–75. doi: 10.1006/bbrc.1996.1442. [DOI] [PubMed] [Google Scholar]
- 12.Wu Q, Suo Z, Risberg B, Karlsson MG, Villman K, Nesland JM. Expression of Ephb2 and Ephb4 in breast carcinoma. Pathol Oncol Res. 2004;10(1):26–33. doi: 10.1007/BF02893405. [DOI] [PubMed] [Google Scholar]
- 13.Takai N, Miyazaki T, Fujisawa K, Nasu K, Miyakawa I. Expression of receptor tyrosine kinase EphB4 and its ligand ephrin-B2 is associated with malignant potential in endometrial cancer. Oncol Rep. 2001;8(3):567–73. doi: 10.3892/or.8.3.567. [DOI] [PubMed] [Google Scholar]
- 14.Berclaz G, Karamitopoulou E, Mazzucchelli L, Rohrbach V, Dreher E, Ziemiecki A, Andres AC. Activation of the receptor protein tyrosine kinase EphB4 in endometrial hyperplasia and endometrial carcinoma. Ann Oncol. 2003;14(2):220–6. doi: 10.1093/annonc/mdg072. [DOI] [PubMed] [Google Scholar]
- 15.Alam SM, Fujimoto J, Jahan I, Sato E, Tamaya T. Coexpression of EphB4 and ephrinB2 in tumour advancement of ovarian cancers. Br J Cancer. 2008;98(4):845–51. doi: 10.1038/sj.bjc.6604216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guijarro-Munoz I, Sanchez A, Martinez-Martinez E, Garcia JM, Salas C, Provencio M, Alvarez-Vallina L, Sanz L. Gene expression profiling identifies EPHB4 as a potential predictive biomarker in colorectal cancer patients treated with bevacizumab. Med Oncol. 2013;30(2):572. doi: 10.1007/s12032-013-0572-1. [DOI] [PubMed] [Google Scholar]
- 17.Krasnoperov V, Kumar SR, Ley E, Li X, Scehnet J, Liu R, Zozulya S, Gill PS. Novel EphB4 monoclonal antibodies modulate angiogenesis and inhibit tumor growth. Am J Pathol. 2010;176(4):2029–38. doi: 10.2353/ajpath.2010.090755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li D, Liu S, Liu R, Park R, Hughes L, Krasnoperov V, Gill PS, Li Z, Shan H, Conti PS. Targeting the EphB4 receptor for cancer diagnosis and therapy monitoring. Mol Pharm. 2013;10(1):329–36. doi: 10.1021/mp300461b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin X, Xie J, Chen X. Protein-based tumor molecular imaging probes. Amino Acids. 2011;41(5):1013–36. doi: 10.1007/s00726-010-0545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Li D, Park R, Liu R, Xia Z, Guo J, Krasnoperov V, Gill PS, Li Z, Shan H, Conti PS. PET Imaging of Colorectal and Breast Cancer by Targeting EphB4 Receptor with 64Cu-Labeled hAb47 and hAb131 Antibodies. J Nucl Med. 2013;54(7):1094–100. doi: 10.2967/jnumed.112.116822. [DOI] [PubMed] [Google Scholar]
- 21.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6(6):462–75. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 22.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 23.Quadri SM, Lai J, Mohammadpour H, Vriesendorp HM, Williams JR. Assessment of radiolabeled stabilized F(ab′)2 fragments of monoclonal antiferritin in nude mouse model. J Nucl Med. 1993;34(12):2152–9. [PubMed] [Google Scholar]
- 24.Olafsen T, Wu AM. Antibody vectors for imaging. Semin Nucl Med. 2010;40(3):167–81. doi: 10.1053/j.semnuclmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, de Jong JS, Arts HJ, van der Zee AG, Bart J, Low PS, Ntziachristos V. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med. 2011;17(10):1315–9. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 26.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23(9):1126–36. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 27.Vegt E, de Jong M, Wetzels JF, Masereeuw R, Melis M, Oyen WJ, Gotthardt M, Boerman OC. Renal toxicity of radiolabeled peptides and antibody fragments: mechanisms, impact on radionuclide therapy, and strategies for prevention. J Nucl Med. 2010;51(7):1049–58. doi: 10.2967/jnumed.110.075101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.