Abstract
Background
Inflammation plays a fundamental role in atherothrombosis. Yet, whether direct inhibition of inflammation will reduce the occurrence of adverse cardiovascular outcomes is not known.
Design
The Cardiovascular Inflammation Reduction Trial (CIRT; ClinicalTrials.gov NCT01594333) will randomly allocate 7,000 patients with prior myocardial infarction and either type 2 diabetes or the metabolic syndrome to low dose methotrexate (target dose 15 to 20 mg per week) or placebo over an average follow-up period of 3 to 5 years. Low-dose methotrexate is a commonly used anti-inflammatory regimen for the treatment of rheumatoid arthritis, lacks significant effects on lipid levels, blood pressure, or platelet function. Both observational and mechanistic studies suggest that low-dose methotrexate has clinically relevant anti-atherothrombotic effects. The CIRT primary endpoint is a composite of nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death. Secondary endpoints are all-cause mortality, coronary revascularization plus the primary endpoint, hospitalization for congestive heart failure plus the primary endpoint, all-cause mortality plus coronary revascularization plus congestive heart failure plus the primary endpoint, incident type 2 diabetes, and net clinical benefit or harm. CIRT will employ standardized central methodology designed to ensure consistent performance of all dose adjustments and safety interventions at each clinical site in a manner that protects the blinding to treatment but maintains safety for enrolled participants.
Summary
CIRT aims to test the inflammatory hypothesis of atherothrombosis in patients with prior myocardial infarction and either type 2 diabetes or metabolic syndrome, conditions associated with persistent inflammation. If low-dose methotrexate reduces cardiovascular events, CIRT would provide a novel therapeutic approach for the secondary prevention of heart attack, stroke, and cardiovascular death. (Clinical Trial Registration Information - http://clinicaltrials.gov/; Identifier: NCT01594333)
Epidemiologic and Basic Evidence for Inflammation in Cardiovascular Disease
Inflammation plays a pivotal role in the development and progression of atherosclerosis.1 Both innate and acquired immunity play key roles in inflammatory cell adhesion and transmigration across the endothelium, fatty streak formation, smooth muscle migration, plaque progression, and ultimately lesion rupture and thrombosis. The clinical consequences of this process include myocardial infarction, stroke, and cardiovascular death.
Numerous epidemiologic studies support a key role for measures of subclinical vascular inflammation in the identification of patients at increased risk of myocardial infarction and stroke.2–4 In a comprehensive meta-analysis of more than 50 prospective studies, the magnitude of risk associated with a 1 standard deviation (SD) elevation in high-sensitivity C-reactive protein (hsCRP, a measure of subclinical vascular inflammation) was similar to that observed for a 1 SD elevation in blood pressure or total cholesterol.5 The addition of hsCRP to traditional risk factors improves cardiovascular risk stratification6, 7 and has led to its incorporation into cardiovascular risk prediction models and primary prevention screening guidelines.8
Clinical Evidence that Treating Inflammation Matters
To date, no clinical trial has directly addressed whether targeting inflammation alone will reduce cardiovascular risk. A number of clinical trials in both primary and secondary prevention, however, indicate that such an approach may be promising.
As first noted in the Cholesterol and Recurrent Events (CARE) trial more than a decade ago, the benefits of pravastatin were particularly pronounced among patients with evidence of ongoing vascular inflammation.9 The AFCAPS/TexCAPS trial of lovastatin yielded similar results.10 The Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial tested the hypothesis that markers of inflammation can identify a group of patients who do not qualify for statin therapy because of normal lipid levels but who might nonetheless benefit from treatment. JUPITER compared rosuvastatin to placebo among 17,802 individuals without preexisting cardiovascular disease or diabetes and a baseline LDL-C < 130 mg/dL, but with evidence of ongoing subclinical inflammation, as determined by an hsCRP ≥ 2.0 mg/L at baseline. Participants randomized to active rosuvastatin had a 44 percent lower risk of the primary endpoint, a composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, hospitalization for unstable angina, or arterial revascularization.11 Further analyses indicated that the reduction in cardiovascular events derived from both the observed reduction in vascular inflammation, as measured by hsCRP, and from reductions in LDL-C.12
In secondary prevention populations, hsCRP also has a strong relationship with recurrent events. Achieved levels of hsCRP and LDL-C independently relate to the risk of recurrent events in a number of randomized trials of patients post acute coronary syndrome, including the TIMI 22 PROVE-IT and A to Z studies of usual versus intensive statin therapy.13, 14 When viewed together with JUPITER, data from these three trials, conducted in either primary or secondary prevention, support the concept of using a dual target strategy of LDL-C and hsCRP reduction for cardiovascular disease prevention. As statins substantially lower both inflammation (as gauged by hsCRP) and LDL-C, none of these trials tested whether lowering inflammation alone – without lowering LDL-C – would lower vascular risk. The Cardiovascular Inflammation Reduction Trial’s primary aim tests this hypothesis, a matter of considerable clinical therapeutic as well as mechanistic biological interest (CIRT, ClinicaTrials.gov NCT01594333).
Type 2 Diabetes, Metabolic Syndrome, and Inflammation
Approximately 30 to 40 percent of patients with acute coronary syndromes have diabetes or metabolic syndrome, many diagnosed with these dysmetabolic states the time of their presentation.15, 16 Post-MI patients with type 2 diabetes or the metabolic syndrome have an increased risk of recurrent cardiovascular events, including myocardial infarction, stroke, and cardiovascular death. In the MIRACL, WIZARD, and TNT trials, patients with metabolic syndrome had a 33 to 44 percent increased risk of recurrent cardiovascular events.17–19 Patients with diabetes were at also at markedly elevated risk, with nearly double the event rate of those without diabetes in CARE (19.1% versus 10.5%),20 and had an adjusted relative risk that ranged from 1.4 (95% CI 1.3–1.5) in LIPID to 1.62 (1.29–2.03) in 4S.21, 22 Preventing macrovascular events in patients with diabetes and the metabolic syndrome remains challenging, and many recent trials of intensive lipid,23 blood pressure,24 glucose lowering,25, 26 or early coronary revascularization27 have failed to show reductions in cardiovascular events and mortality. Therefore, novel approaches to preventing the macrovascular complications of insulin resistance and diabetes are urgently needed. Patients with type 2 diabetes or the metabolic syndrome also have a high prevalence of subclinical vascular inflammation, as determined by high levels of circulating inflammatory biomarkers.28, 29 Indeed, some investigators have hypothesized that alterations in innate immunity underlie insulin resistance and diabetes.30
Methotrexate and Cardiovascular Disease: Epidemiology and Molecular Mechanisms
Directly testing the inflammatory hypothesis of atherothrombosis requires an agent that has systemic anti-inflammatory effects without substantive impact on lipids or blood pressure. The agent must also be well tolerated and have a reasonable safety profile. Low dose methotrexate (LDM) has these characteristics.
LDM (dose range 10 to 30 mg per week) is widely used as first-line treatment for patients with rheumatoid arthritis, psoriasis, and psoriatic arthritis. Due to the wide use of LDM as primary therapy for several rheumatologic conditions, the American College of Rheumatology has published safety guidelines regarding dosing regimens, drug monitoring, and the identification of high-risk patient subgroups.31, 32 LDM reduces several inflammatory biomarkers including CRP, IL-6, and TNF-alpha in patients with rheumatologic disease, without affecting lipid levels, blood pressure, platelet function, or measures of hemostasis.33 Moreover, among patients with rheumatoid arthritis and psoriasis, epidemiologic data suggest LDM reduces cardiovascular risk, even though patients taking LDM tended to have higher vascular risk profiles. Indeed, a recent meta-analysis found that patients with rheumatologic disease on methotrexate had a 21 percent lower risk of cardiovascular events (RR 0.79, 95% CI 0.73–0.87, P<0.001) compared to those on other disease-modifying anti-rheumatic therapy.34
A dihydrofolate reductase inhibitor, methotrexate given at high doses prevents the synthesis of both purine and pyrimidine nucleotides, and this mechanism of action forms the basis for its use in treating malignancy. However, at the far lower doses routinely used to treat rheumatoid arthritis and psoriasis, folic acid supplementation reduces methotrexate side effects without diminishing its efficacy.35 This observation raises the possibility that methotrexate’s antimetabolite properties are not responsible for its clinical benefit in these conditions, and alternative explanations for its anti-inflammatory effects have emerged. For example, LDM may reduce inflammation by inhibiting the production of cytokines known to be important in atherothrombosis, including interleukin (IL)-1β, IL-1 receptor antagonist, IL-6 and tumor necrosis factor (TNF)-alpha.36, 37 Alternatively, LDM may function by inducing inflammatory cell apoptosis, perhaps through the generation of reactive oxygen species.38 While lymphocytes display both of these mechanisms, monocyte macrophages, the predominant inflammatory cell type in atherosclerotic plaques, do not.37, 38 Emerging evidence raises the hypothesis that at least part of the anti-inflammatory and atheroprotective effects of LDM may result from increased adenosine release and subsequent agonism of the adenosine A2A receptor. Stimulation of this receptor induces the expression of key proteins in reverse cholesterol transport, including 27-hydroxylase and adenosine 5′-triphosphate-binding cassette transporter A1 (ABCA1), and prevents the formation of foam cells from human macrophages.39, 40 A2A stimulation also attenuates inflammation and neointima formation after arterial injury in mice, and reduces expression of adhesion molecules commonly associated with the development of atheroma, such as vascular cell adhesion molecule-1 and intercellular adhesion molecule-1.41 Direct anti-inflammatory effects at the level of the vascular endothelium also likely have relevance; in New Zealand rabbits consuming a high cholesterol diet, weekly intravenous methotrexate administration reduced new atheroma formation by 75%, and led to a 2-fold reduction in the intima to media ratio and a marked reduction of macrophages and apoptotic cells within the aorta intima.42
The Cardiovascular Inflammation Reduction Trial (CIRT): A Direct Test of the Inflammatory Hypothesis of Atherothrombosis
CIRT’s primary objective is to determine whether anti-inflammatory therapy with low dose methotrexate (target dose 15 to 20 mg per week) as compared with placebo will reduce the rate of recurrent cardiovascular events among patients with prior myocardial infarction and either type 2 diabetes or the metabolic syndrome. The primary trial endpoint, the composite of non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death, will be adjudicated by an independent Clinical Events Committee (CEC) blinded to study treatment allocation. Secondary trial endpoints are all-cause mortality; the primary endpoint plus coronary revascularization; hospitalization for congestive heart failure; the primary endpoint plus all-cause mortality plus coronary revascularization plus hospitalization for congestive heart failure; net clinical benefit or harm; and new onset type 2 diabetes among those with metabolic syndrome but not diabetes at study entry. Of these, events adjudicated by the CEC include all-cause mortality, all arterial revascularizations (including coronary revascularization), and hospitalization for congestive heart failure. The CEC will also adjudicate cases of unstable angina requiring unplanned revascularization. Tertiary endpoints are individual components of the primary endpoint; the primary endpoint plus unstable angina requiring unplanned coronary revascularization; coronary revascularization; peripheral artery disease; symptomatic deep vein thrombosis or pulmonary embolism; clinically significant aortic stenosis; atrial fibrillation; and standardized measure of quality of life and global health status. The effects of random allocation to LDM on a number of clinical events of interest thought to be related to subclinical inflammation will be assessed. These include age-related macular degeneration,43 sleep apnea,44 and microvascular disease (nephropathy45, 46 and retinopathy47).
CIRT will randomize 7,000 participants age 18 years or older who have had a documented myocardial infarction at least 60 days but no more than 5 years prior to screening. Eligible participants will also have either type 2 diabetes or the metabolic syndrome (defined by American Heart Association/National Heart Lung and Blood Institute criteria)48, will be medically stable, will have completed all planned coronary revascularization procedures, and will provide written informed consent. The inclusion and exclusion criteria for CIRT (Table) either match or exceed those published by the American College of Rheumatology’s guidelines for the use of low-dose methotrexate in patients with rheumatoid arthritis.31 Patients with a contraindication to methotrexate such as a history of chronic infection, tuberculosis, interstitial pneumonitis, pulmonary fibrosis, alcohol abuse, hepatic or renal dysfunction, or class IV heart failure will be excluded, as will women of child-bearing potential, and those requiring treatment with oral steroids or other immunosuppressive agents. Participants will continue to take indicated secondary prevention medications, including, for example, dual antiplatelet therapy, beta-blockers, statins, angiotensin converting enzyme inhibitors or receptor blockers, and anticoagulants.
Table 1.
Trial inclusion and exclusion criteria
| Inclusion Criteria |
| Age ≥ 18 years at screening |
| Documented myocardial infarction within the past five years, completed any planned coronary revascularization procedures associated with the qualifying event, and clinically stable for at least 60 days prior to screening; the qualifying prior myocardial infarction must be documented either by hospital records or by evidence on current ECG of Q waves in two contiguous leads and/or an imaging test demonstrating wall motion abnormality or scar |
| History of type 2 diabetes or metabolic syndrome* at time of study enrollment |
| Willing to participate as evidenced by signing the study informed consent |
| Exclusion Criteria |
| Prior history of chronic infectious disease, including tuberculosis, severe fungal disease or known HIV positive |
| Chronic hepatitis B or C infection |
| Interstitial pneumonitis, bronchiectasis, or pulmonary fibrosis. Chest X-ray evidence in the past 12 months of interstitial pneumonitis, bronchiectasis, or pulmonary fibrosis. |
| Prior history of non-basal cell malignancy or myeloproliferative or lymphoproliferative disease within the past 5 years |
| White blood cell count < 4,000/mm3, hematocrit < 32 percent, or platelet count < 75,000/mm3 |
| Liver transaminase levels (AST/ALT) >upper limit of normal (ULN) or albumin < the lower limit of normal (LLN) |
| Creatinine clearance < 40 ml/min/1.73m2 as estimated by the Cockcroft-Gault equation |
| History of alcohol abuse or unwillingness to limit alcohol consumption to < 4 drinks per week |
| Women of child bearing potential, even if currently using contraception, and women intending to breastfeed |
| Men who plan to father children during the study period or who are unwilling to use contraception |
| Requirement for use of drugs that alter folate metabolism (trimethoprim/sulfamethoxazol) or reduce tubular excretion (probenecid) or known allergies to antibiotics making avoidance of trimethoprim impossible |
| Current indication for methotrexate therapy |
| Chronic use of oral steroid therapy or other immunosuppressive or biologic response modifiers |
| Known chronic pericardial effusion, pleural effusion, or ascites |
| New York Heart Association Class IV congestive heart failure |
| Life expectancy of < 3 years |
The most recent AHA/NHLBI definition of metabolic syndrome48 will be used and requires evidence that any 3 of the following 5 diagnostic criteria are present: waist circumference ≥102 cm in men or≥ 88 cm in women; triglycerides ≥ 150 mg/dl (1.7 mmol/L) or on drug treatment for elevated triglycerides (fibrates, nicotinic acid, or omega 3 fatty acids ); HDL-C < 40 mg/dL in men or < 50 mg/dL in women or on drug treatment for reduced HDL-C (fibrates or nicotinic acid); systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥ 85 mm Hg or on drug treatment for hypertension; and elevated fasting glucose ≥ 100 mg/dL or on drug treatment for elevated glucose.
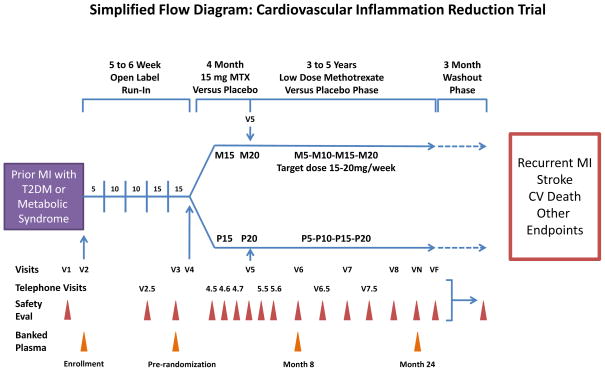
The overall study design of CIRT is shown in Figure 1. At the initial screening visit, informed consent will be obtained from eligible participants (Table) and blood samples for hepatitis B surface antigen and hepatitis C antibody, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, albumin, glucose, and a complete blood count (CBC) will be collected. If all laboratory values meet the pre-specified criteria, enrollment will be scheduled within 12 weeks. At the enrollment visit, lipids, glycated hemoglobin, hsCRP, and urinary albumin and creatinine will be ascertained and all participants entered into a 5- to 6-week (8-week maximum) open-label active run-in designed to exclude before randomization those initially intolerant of LDM; during this run-in, methotrexate dosing will increase from an initial weekly dose of 5 mg to the randomization target dose of 15 mg weekly. For those who clinically tolerate methotrexate during the run-in, show no signs of laboratory abnormality, and wish to continue, formal randomization will occur either to LDM or to placebo. All study drug will be dispensed in calendar packs with active LDM (or LDM placebo) to be taken once weekly on Saturday, and folic acid 1 mg, to be taken Sunday through Friday. Randomization will be stratified by time since qualifying myocardial infarction (< 6 months, ≥ 6 months), site, and the presence of either type 2 diabetes or the metabolic syndrome.
Figure 1.
Simplified flow diagram of the Cardiovascular Inflammation Reduction Trial (CIRT). Clinically stable patients with a history of myocardial infarction in the past 5 years and either type 2 diabetes or the metabolic syndrome will enter a 5 to 6 week active run-in period with escalating doses of methotrexate (advancing from 5 mg/week to 15 mg/week). Those who tolerate the run-in and have no new laboratory abnormalities will then be randomized to active methotrexate or placebo and followed for an average period of 3 to 4 years. The primary endpoint is the occurrence of myocardial recurrent infarction, stroke, of cardiovascular death. All safety evaluations except visit 2.5 consist of a safety laboratory evaluation and either a telephone or an in-person visit to ascertain medication side effects, adverse events, and any key clinical endpoints. Visit 2.5 is a telephone visit without laboratory assessment. M5-M10-M15-M20 indicates variable dose of active methotrexate with a target dose of 15–20 mg per week. P5-P10-P15-P20 indicates variable dosing of methotrexate placebo. The maximum dose of methotrexate is 15 mg/week until 4 months after randomization, when the dose can be increased to 20 mg/week. Abbreviations: Eval, evaluation; MI, myocardial infarction; MTX, methotrexate; T2DM, type 2 diabetes mellitus; VN, visit N (a representative follow-up visit); VF, final visit.
A member of the study staff will see participants every 4 months after randomization. Between the in-person visits, randomized participants will undergo monthly safety evaluations for the first 6 months of the trial, and bimonthly safety evaluations after 6 months. Safety evaluations include an assessment of adherence, side effects, safety laboratories, and existing medical conditions or planned procedures that might alter study drug dosing. These visits also include screening for the occurrence of primary, secondary, and other clinical events of interest.
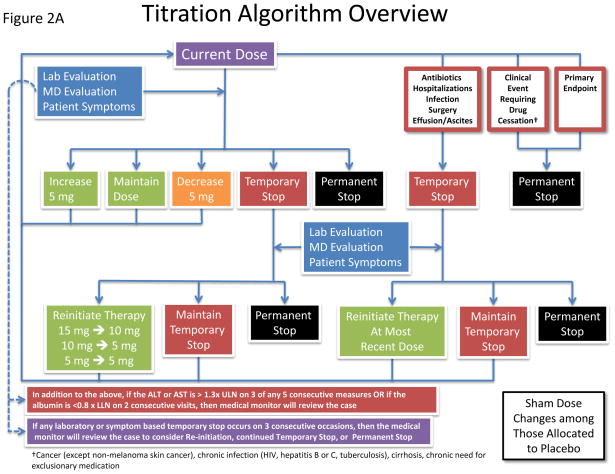
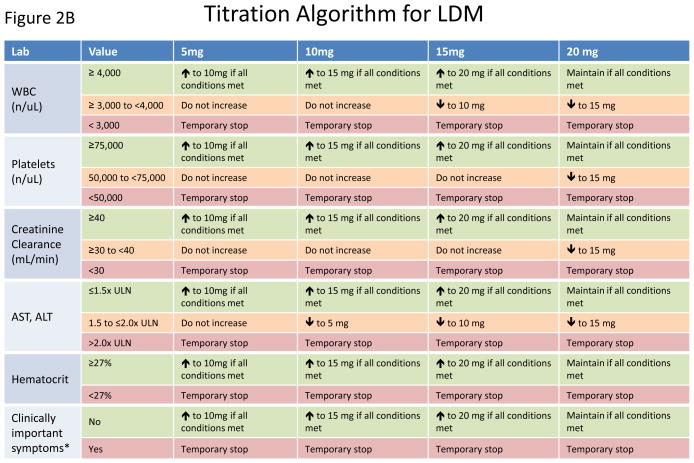
At randomization, participants will be assigned to active methotrexate 15 mg per week or to matching placebo. For those tolerant of study drug four months after randomization, up-titration to the maximal allowed dose of 20 mg weekly (or to matching placebo) will occur. To ensure consistency of methotrexate management at all study sites and to preserve the study blinding, dose changes will be made centrally using a formal titration algorithm specifically designed for CIRT (Figure 2A). At a given visit or safety evaluation, the algorithm will integrate the collected information to determine whether the current study medication dose should change. The dose can be increased by 5mg (if not already at the maximum allowed dose), maintained, decreased by 5 mg, stopped temporarily, or stopped permanently (Figure 2A). The algorithm allows dose adjustments for hospitalizations, infections, surgical procedures, and other clinical events (including the occurrence of the primary endpoint) and specifies repeat evaluations for possible resumption of study drug, continuation of a temporary stop, permanent stop, and sham changes in the placebo arm of the trial. This centralized titration algorithm also incorporates a matrix of current study drug dose and thresholds for the routine safety labs of white blood cell count, platelets, estimated creatinine clearance, AST, ALT, hematocrit, as well as patient symptoms (Figure 2B). Thus, as is common when using low-dose methotrexate in the setting of rheumatoid arthritis or psoriasis, dosing for individual participants will vary over time in response to transient medical conditions. However, the weekly dose will never exceed the maximum 4-month up-titration dose of 20 mg.
Figure 2.
Schematic representation of the study drug titration algorithm. A. Flow diagram for changes in study drug dose. Using information gathered from routine questionnaires and regular safety laboratory studies, the dose of study drug can be increased, maintained, decreased, and stopped (temporarily or permanently). Sham dose changes in the placebo arm maintain the study blind. B. Matrix of routine safety laboratory values and current study drug dose used in the drug titration algorithm.
A team of medical monitors with experience prescribing methotrexate will be available to address questions about study drug safety throughout the trial. The medical monitor will have access to individual participant laboratory results, clinical symptoms, and study drug dosage. Monitors can help sites determine whether the participant’s clinical scenario merits temporary or permanent interruption of study drug therapy, and whether study drug can be reintroduced.
The routine in-person and phone visits described above will be used to monitor the occurrence of adverse events. Adverse events will be categorized as serious or non-serious, and will be graded by investigators with respect to the possibility of being related to the study drug. Investigators will also be asked to determine if the event is expected or unexpected, based on what is known about the toxicities of low dose methotrexate.
CIRT is an event-driven trial designed to accrue 530 total confirmed major cardiovascular events over an estimated average follow up of 3 to 4 years. The trial has a 90 percent power to detect a 25 percent relative hazard reduction in the primary endpoint, based on a two-sided log-rank test with alpha=0.05. A formal pre-specified Statistical Analysis Plan is available in the online supplement to this paper. The formal DSMB Charter is available from the investigators.
After trial completion and cessation of study drug, an additional 3-month washout phase is incorporated into CIRT to address whether low-dose methotrexate results in any long-term benefits on hemoglobin A1c and other biomarkers of insulin resistance. At trial enrollment, randomization, 8 months and 24 months after randomization, participants will be given the opportunity to donate blood and DNA samples into a bio-bank being established as part of CIRT. These samples will be available after trial completion and used on a pre-specified basis to address issues of effect modification by genotype and inflammatory biomarker status as well as for a series of ancillary investigations.
Trial Sponsorship and Organization
CIRT is funded by the National Heart Lung and Blood Institute (NHLBI) through a U01 mechanism to Dr. Paul Ridker (HL101422), the trial Principal Investigator, and to Dr. Robert J. Glynn (HL101389) who is the Director of the Data Coordinating Center. Drs. Ridker and Glynn conduct all trial activities at Brigham and Women’s Hospital and Harvard Medical School in Boston, Massachusetts.
TEVA, Inc. is supplying active methotrexate (Trexall 5 mg) and Amneal Pharmaceuticals, Inc. is supplying active folic acid (Folvite 1 mg). Catalent, Inc. is supplying methotrexate placebo as well as drug packaging and distribution services. To improve compliance, safety, and drug accountability, 8-week child-resistant calendar packs are being used for all study drug allocation both during the trial run-in phase and the active treatment phase. The Investigational New Drug (IND) Application with the U.S. Federal Drug Administration and the Clinical Trial Application (CTA) with Health Canada will be held by the trial Principle Investigator.
CIRT is overseen by a fully independent Data and Safety Monitoring Board (DSMB), convened by the National Heart Lung and Blood Institute (NHLBI) and chaired by Robert Harrington, MD. The members of the DSMB are listed in Online Supplement Table 1. The DSMB will report directly to the funding entity (NHLBI) and will have responsibility for monitoring enrollment and adherence, biomarkers of safety, adverse events, serious adverse events, the occurrence of trial endpoints, and participant and site burden. The DSMB Charter prospectively establishes guidelines for possible early termination of the study (for efficacy or futility).
Current members of the CIRT Advisory Committees, including the Executive Committee, the Steering Committee, the Publications Committee, the Ancillary Study Committee, the Arthritis and Methotrexate Advisory Committee, the Clinical Endpoints Committee, the Data Coordinating Center Committee, the Operations Committee, and the NHLBI Project Officer and Staff are listed in Supplement Table 2.
Additional information regarding CIRT can be obtained at the CIRT.org website, by calling the Data coordinating Center at 855-437-9330 or at www.ClinTrials.gov (NCT01594333). The authors are solely responsible for the drafting and editing of this paper and its contents.
Conclusion
Inflammation mediates the effects of many risk factors on arterial biology and thus drives atherothrombosis. While considerable evidence suggests that reducing inflammation leads to improved cardiovascular outcomes, no study has yet tested whether directly targeting inflammation will reduce the occurrence of heart attack, stroke, and cardiovascular death. By using low dose methotrexate, an effective systemic anti-inflammatory medication without known effects on traditional determinants of cardiovascular risk, CIRT seeks to test whether directly treating inflammation can reduce the occurrence of heart attack, stroke, and cardiovascular death. If low-dose methotrexate reduces cardiovascular events, CIRT would both support the inflammatory hypothesis of atherothrombosis and serve as a catalyst for the development of novel therapeutic agents focused specifically on vascular inflammation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Libby P, Ridker PM, Hansson GK. Inflammation in Atherosclerosis: From Pathophysiology to Practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, Aspirin, and the Risk of Cardiovascular Disease in Apparently Healthy Men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Hennekens CH, Buring JE, et al. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 4.Koenig W, Lowel H, Baumert J, et al. C-Reactive Protein Modulates Risk Prediction Based on the Framingham Score: Implications for Future Risk Assessment: Results from a Large Cohort Study in Southern Germany. Circulation. 2004;109:1349–1353. doi: 10.1161/01.CIR.0000120707.98922.E3. [DOI] [PubMed] [Google Scholar]
- 5.Kaptoge S, Di Angelantonio E, Lowe G, et al. C-Reactive Protein Concentration and Risk of Coronary Heart Disease, Stroke, and Mortality: An Individual Participant Meta-Analysis. Lancet. 2010;375:132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ridker PM, Buring JE, Rifai N, et al. Development and Validation of Improved Algorithms for the Assessment of Global Cardiovascular Risk in Women: The Reynolds Risk Score. JAMA. 2007;297:611–9. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 7.Wilson PW, Pencina M, Jacques P, et al. C-Reactive Protein and Reclassification of Cardiovascular Risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes. 2008;1:92–7. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genest J, McPherson R, Frohlich J, et al. 2009 Canadian Cardiovascular Society/Canadian Guidelines for the Diagnosis and Treatment of Dyslipidemia and Prevention of Cardiovascular Disease in the Adult - 2009 Recommendations. Can J Cardiol. 2009;25:567–79. doi: 10.1016/s0828-282x(09)70715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridker PM, Rifai N, Pfeffer MA, et al. Inflammation, Pravastatin, and the Risk of Coronary Events after Myocardial Infarction in Patients with Average Cholesterol Levels. Cholesterol and Recurrent Events (Care) Investigators. Circulation. 1998;98:839–844. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Rifai N, Clearfield M, et al. Measurement of C-Reactive Protein for the Targeting of Statin Therapy in the Primary Prevention of Acute Coronary Events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 11.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Danielson E, Fonseca FA, et al. Reduction in C-Reactive Protein and Ldl Cholesterol and Cardiovascular Event Rates after Initiation of Rosuvastatin: A Prospective Study of the Jupiter Trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Morrow DA, Rose LM, et al. Relative Efficacy of Atorvastatin 80 Mg and Pravastatin 40 Mg in Achieving the Dual Goals of Low-Density Lipoprotein Cholesterol <70 Mg/Dl and C-Reactive Protein <2 Mg/L: An Analysis of the Prove-It Timi-22 Trial. J Am Coll Cardiol. 2005;45:1644–1648. doi: 10.1016/j.jacc.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 14.Morrow DA, de Lemos JA, Sabatine MS, et al. Clinical Relevance of C-Reactive Protein During Follow-up of Patients with Acute Coronary Syndromes in the Aggrastat-to-Zocor Trial. Circulation. 2006;114:281–8. doi: 10.1161/CIRCULATIONAHA.106.628909. [DOI] [PubMed] [Google Scholar]
- 15.Zeller M, Steg PG, Ravisy J, et al. Prevalence and Impact of Metabolic Syndrome on Hospital Outcomes in Acute Myocardial Infarction. Arch Intern Med. 2005;165:1192–8. doi: 10.1001/archinte.165.10.1192. [DOI] [PubMed] [Google Scholar]
- 16.Boyer NM, Laskey WK, Cox M, et al. Trends in Clinical, Demographic, and Biochemical Characteristics of Patients with Acute Myocardial Infarction from 2003 to 2008: A Report from the American Heart Association Get with the Guidelines Coronary Artery Disease Program. J Am Heart Assoc. 2012:1. doi: 10.1161/JAHA.112.001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz GG, Olsson AG, Szarek M, et al. Relation of Characteristics of Metabolic Syndrome to Short-Term Prognosis and Effects of Intensive Statin Therapy after Acute Coronary Syndrome: An Analysis of the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (Miracl) Trial. Diabetes Care. 2005;28:2508–13. doi: 10.2337/diacare.28.10.2508. [DOI] [PubMed] [Google Scholar]
- 18.Aguilar D, Fisher MR, O’Connor CM, et al. Metabolic Syndrome, C-Reactive Protein, and Prognosis in Patients with Established Coronary Artery Disease. Am Heart J. 2006;152:298–304. doi: 10.1016/j.ahj.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Deedwania P, Barter P, Carmena R, et al. Reduction of Low-Density Lipoprotein Cholesterol in Patients with Coronary Heart Disease and Metabolic Syndrome: Analysis of the Treating to New Targets Study. Lancet. 2006;368:919–28. doi: 10.1016/S0140-6736(06)69292-1. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular Events and Their Reduction with Pravastatin in Diabetic and Glucose-Intolerant Myocardial Infarction Survivors with Average Cholesterol Levels: Subgroup Analyses in the Cholesterol and Recurrent Events (Care) Trial. The Care Investigators. Circulation. 1998;98:2513–9. doi: 10.1161/01.cir.98.23.2513. [DOI] [PubMed] [Google Scholar]
- 21.Girman CJ, Rhodes T, Mercuri M, et al. The Metabolic Syndrome and Risk of Major Coronary Events in the Scandinavian Simvastatin Survival Study (4s) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (Afcaps/Texcaps) Am J Cardiol. 2004;93:136–41. doi: 10.1016/j.amjcard.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 22.Keech A, Colquhoun D, Best J, et al. Secondary Prevention of Cardiovascular Events with Long-Term Pravastatin in Patients with Diabetes or Impaired Fasting Glucose: Results from the Lipid Trial. Diabetes Care. 2003;26:2713–21. doi: 10.2337/diacare.26.10.2713. [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg HN, Elam MB, Lovato LC, et al. Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus. N Engl J Med. 2010;362:1563–74. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cushman WC, Evans GW, Byington RP, et al. Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus. N Engl J Med. 2010;362:1575–85. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerstein HC, Miller ME, Byington RP, et al. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N Engl J Med. 2008;358:2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duckworth W, Abraira C, Moritz T, et al. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 27.Frye RL, August P, Brooks MM, et al. A Randomized Trial of Therapies for Type 2 Diabetes and Coronary Artery Disease. N Engl J Med. 2009;360:2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pradhan AD, Manson JE, Rifai N, et al. C-Reactive Protein, Interleukin 6, and Risk of Developing Type 2 Diabetes Mellitus. JAMA. 2001;286:327–34. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Buring JE, Cook NR, et al. C-Reactive Protein, the Metabolic Syndrome, and Risk of Incident Cardiovascular Events: An 8-Year Follow-up of 14 719 Initially Healthy American Women. Circulation. 2003;107:391–7. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 30.Donath MY, Shoelson SE. Type 2 Diabetes as an Inflammatory Disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 31.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 Recommendations for the Use of Nonbiologic and Biologic Disease-Modifying Antirheumatic Drugs in Rheumatoid Arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 32.Singh JA, Furst DE, Bharat A, et al. 2012 Update of the 2008 American College of Rheumatology Recommendations for the Use of Disease-Modifying Antirheumatic Drugs and Biologic Agents in the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2012;64:625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rho YH, Oeser A, Chung CP, et al. Drugs Used in the Treatment of Rheumatoid Arthritis: Relationship between Current Use and Cardiovascular Risk Factors. Arch Drug Inf. 2009;2:34–40. doi: 10.1111/j.1753-5174.2009.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Micha R, Imamura F, Wyler von Ballmoos M, et al. Systematic Review and Meta-Analysis of Methotrexate Use and Risk of Cardiovascular Disease. Am J Cardiol. 2011;108:1362–70. doi: 10.1016/j.amjcard.2011.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgan SL, Baggott JE, Vaughn WH, et al. Supplementation with Folic Acid During Methotrexate Therapy for Rheumatoid Arthritis. A Double-Blind, Placebo-Controlled Trial. Ann Intern Med. 1994;121:833–41. doi: 10.7326/0003-4819-121-11-199412010-00002. [DOI] [PubMed] [Google Scholar]
- 36.Wessels JA, Huizinga TW, Guchelaar HJ. Recent Insights in the Pharmacological Actions of Methotrexate in the Treatment of Rheumatoid Arthritis. Rheumatology (Oxford) 2008;47:249–55. doi: 10.1093/rheumatology/kem279. [DOI] [PubMed] [Google Scholar]
- 37.Gerards AH, de Lathouder S, de Groot ER, et al. Inhibition of Cytokine Production by Methotrexate. Studies in Healthy Volunteers and Patients with Rheumatoid. Arthritis Rheumatology (Oxford, England) 2003;42:1189–1196. doi: 10.1093/rheumatology/keg323. [DOI] [PubMed] [Google Scholar]
- 38.Herman S, Zurgil N, Langevitz P, et al. Methotrexate Selectively Modulates Th1/Th2 Balance in Active Rheumatoid Arthritis Patients. Clin Exp Rheumatol. 2008;26:317–23. [PubMed] [Google Scholar]
- 39.Reiss AB, Rahman MM, Chan ES, et al. Adenosine A2a Receptor Occupancy Stimulates Expression of Proteins Involved in Reverse Cholesterol Transport and Inhibits Foam Cell Formation in Macrophages. J Leukoc Biol. 2004;76:727–34. doi: 10.1189/jlb.0204107. [DOI] [PubMed] [Google Scholar]
- 40.Reiss AB, Carsons SE, Anwar K, et al. Atheroprotective Effects of Methotrexate on Reverse Cholesterol Transport Proteins and Foam Cell Transformation in Human Thp-1 Monocyte/Macrophages. Arthritis Rheum. 2008;58:3675–83. doi: 10.1002/art.24040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McPherson JA, Barringhaus KG, Bishop GG, et al. Adenosine a(2a) Receptor Stimulation Reduces Inflammation and Neointimal Growth in a Murine Carotid Ligation Model. Arterioscler Thromb Vasc Biol. 2001;21:791–6. doi: 10.1161/01.atv.21.5.791. [DOI] [PubMed] [Google Scholar]
- 42.Bulgarelli A, Martins Dias AA, Caramelli B, et al. Treatment with Methotrexate Inhibits Atherogenesis in Cholesterol-Fed Rabbits. J Cardiovasc Pharmacol. 2012;59:308–14. doi: 10.1097/FJC.0b013e318241c385. [DOI] [PubMed] [Google Scholar]
- 43.Schaumberg DA, Christen WG, Buring JE, et al. High-Sensitivity C-Reactive Protein, Other Markers of Inflammation, and the Incidence of Macular Degeneration in Women. Arch Ophthalmol. 2007;125:300–5. doi: 10.1001/archopht.125.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shamsuzzaman AS, Winnicki M, Lanfranchi P, et al. Elevated C-Reactive Protein in Patients with Obstructive Sleep Apnea. Circulation. 2002;105:2462–4. doi: 10.1161/01.cir.0000018948.95175.03. [DOI] [PubMed] [Google Scholar]
- 45.Navarro-Gonzalez JF, Mora-Fernandez C. The Role of Inflammatory Cytokines in Diabetic Nephropathy. Journal of the American Society of Nephrology: JASN. 2008;19:433–42. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 46.Yozai K, Shikata K, Sasaki M, et al. Methotrexate Prevents Renal Injury in Experimental Diabetic Rats Via Anti-Inflammatory Actions. Journal of the American Society of Nephrology: JASN. 2005;16:3326–38. doi: 10.1681/ASN.2004111011. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen TT, Alibrahim E, Islam FM, et al. Inflammatory, Hemostatic, and Other Novel Biomarkers for Diabetic Retinopathy: The Multi-Ethnic Study of Atherosclerosis. Diabetes Care. 2009;32:1704–9. doi: 10.2337/dc09-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of Metabolic Syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]