Abstract
Introduction
An open-label trial suggested that valproic acid (VPA) improved strength in adults with spinal muscular atrophy (SMA). We report a 12-month, double-blind, cross-over study of VPA in ambulatory SMA adults.
Methods
There were 33 subjects, aged 20–55 years, included in this investigation. After baseline assessment, subjects were randomized to receive VPA (10–20 mg/kg/day) or placebo. At 6 months, patients were switched to the other group. Assessments were performed at 3, 6, and 12 months. The primary outcome was the 6-month change in maximum voluntary isometric contraction testing with pulmonary, electrophysiological, and functional secondary outcomes.
Results
Thirty subjects completed the study. VPA was well tolerated, and compliance was good. There was no change in primary or secondary outcomes at 6 or 12 months.
Conclusions
VPA did not improve strength or function in SMA adults. The outcomes used are feasible and reliable and can be employed in future trials in SMA adults.
Spinal muscular atrophy (SMA) is unique among human genetic disorders in that a genomic duplication at the causative gene locus (the survival of motor neuron or SMN locus) has resulted in a nearly identical gene, SMN2, which lies centromeric to the SMN1 gene.1–3 This gene differs from SMN1 mainly by a single C-to-T nucleotide substitution at the splice junction of exon 7. Although this mutation does not affect the amino acid sequence, it does alter mRNA splicing in favor of transcripts lacking exon 7. Due to alternative splicing, however, a small amount of full-length SMN transcript is produced by the SMN2 gene so that SMN2 copy number is a major determinant of phenotype.4–11 Murine models of SMA reflect the protective effect of SMN2 copy number on phenotypic severity,12 suggesting that pharmacologic or genetic strategies to increase production of full-length transcript from SMN2 might be an effective therapeutic strategy.1–3,13–16
Valproic acid (VPA) is a histone deacetylase (HDAC) inhibitor that increases SMN expression in SMA patient-derived cell lines as well as in SMA patients.17–23 SMA mice treated with VPA have improved gross motor function and survival compared with controls. These positive effects are also reflected in larger compound muscle action potentials (CMAPs) on electrophysiological studies and less degeneration of spinal motor neurons and improved neuromuscular junction innervation.23 Four open-label trials of VPA in humans with SMA all suggested a benefit in strength, motor function, or both.24–26 One study of 7 ambulatory adults in particular found a 49% improvement in baseline strength tested with hand-held myometry after treatment with VPA for a mean of 8 months.24 These encouraging results led the Project Cure SMA Group to perform a series of controlled clinical trials with VPA in various SMA populations.27–29 We report here the results of a double-blind clinical trial of a 6-month treatment course of VPA versus placebo in a cohort of ambulatory SMA adults.
METHODS
Trial Design
The study was a single-center, prospective, randomized, placebo controlled, doubleblind, phase 3 cross-over trial of VPA in ambulatory, genetically confirmed SMA adults (>18 years of age) (Clinicaltrials.gov ID NCT00481013). The cross-over design was chosen primarily to reduce the number of patients needed to address efficacy, because the population of ambulatory adults with SMA is relatively small. Another major consideration was that VPA was generally prescribed by subjects’ treating physicians independent of this study, so recruitment would have been extremely difficult without a guarantee that all patients would receive the drug at some time during the study. Wheelchair-mobile patients were excluded. Patients were also excluded for the following reasons:
Coexisting medical conditions that precluded travel, testing, or study medications.
Participation in a treatment trial for SMA in the 3 months prior to this trial, or plan to enroll in any other treatment trial during this study.
Requirement for any mechanical respiratory support >12 hours per day.
Inability to meet visit requirements or cooperate reliably with functional testing.
Mental or legal incapacitation from giving informed consent, or inability to read and understand written material including in the consent form.
Abnormalities in baseline blood testing beyond established values.
Use of medications or supplements which interfere with VPA metabolism, or are hypothesized to have a beneficial effect in SMA animal models or human neuromuscular disorders within 3 months of study enrollment, including riluzole, creatine, butyrate derivatives, growth hormone, anabolic steroids, albuterol, anticonvulsants, or other HDAC inhibitors.
The study was approved by the institutional review board at the Ohio State University, Wexner Medical Center, and written informed consent was obtained from all subjects.
Study Procedures
All subjects completed 2 baseline visits within a 6-week period to assure that the methodologies were reliable and that subjects enrolled in the study exhibited test–retest stability. After the second visit, subjects were randomized to receive either VPA or an identical placebo. VPA and placebo were provided by Abbott Pharmaceuticals as 250-mg divalproex sodium capsules (Depakote ER) and administered in divided doses 2 or 3 times daily at a starting dose of 10–20 mg/kg orally to maintain trough levels of 50–100 mg/dl. Study compliance was assessed through pill counts and VPA levels at each visit with dosing adjusted for both VPA and placebo groups by an unblinded investigator (M.F.). After the 6-month visit, subjects were switched to the other treatment group (i.e., VPA or placebo) in a blinded fashion.
Treatment assessments were performed at 3 (V1), 6 (V2), and 12 (V3) months. Safety laboratory studies were performed at baseline, 2–3 weeks after initiation, at each treatment visit, and midway between V2 and V3. They included a basic chemistry profile, complete blood and platelet count, transaminases, carnitine profile, amylase, lipase, and trough VPA levels. A non-blinded medical monitor reviewed all subject blood tests and adverse events and performed dosing adjustments or additional testing when necessary. Adverse events were graded using Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE v3.0). An independent data and safety monitoring committee provided oversight for the study, performed interim safety data analyses, and had the ability to stop the study if there was a safety concern.
Outcome Measures
The primary outcome measure was the 6-month change in strength as assessed by maximum voluntary isometric contraction testing (MVICT). Muscles tested included bilateral elbow flexors, elbow extensors, knee flexors, knee extensors, and grip. The results from the 4 upper and 4 lower extremity muscles were also combined into upper and lower extremity composite group scores. Secondary outcomes included 6-month changes in:
Function measured using the modified SMA Functional Rating Scale (SMAFRS).11
Muscle strength measured by hand-held dynamometer of elbow flexors/extensors and knee flexors/extensors.
Ulnar CMAP as a surrogate for assessing disease severity and capacity for reinnervation.
Assays of SMN2 copy number, mRNA levels, and SMN protein levels.
Pulmonary function tests (PFTs), including forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and maximum inspiratory pressure (MIP).
Muscle mass as measured by dual-energy X-ray absorptiometry (DEXA) scanning.
Endurance assessed through 6-minute walk test.
Function assessed in time to climb 4 standard stairs.
Quality-of-life (QOL) assessment using the mini–Sickness Illness Profile.30
Details of testing methodologies and protocols are available at http://smaoutcomes.org and in prior published trials.27–29 For statistical purposes, each component of the testing was analyzed individually as discussed below.
Statistical Analysis
Two baseline visits were performed, and the visit closest to the start of treatment was used as the baseline evaluation. Changes from baseline comparisons were assessed using t-tests to compare groups. Mixed effects models were used to test the data out to 12 months; these models included the data from the second 6 months of the study for both the primary and secondary outcomes. Period and sequence were included as possible fixed effects with patient as the random effect. Least-squares means, which are the average values after accounting for factors in the model, are provided along with 95% confidence intervals. Missing values were not imputed; all available data were used in the analyses.
The study was designed to recruit 36 patients with an anticipated 20% dropout rate to yield at least 28 analyzable patients, a number sufficient to provide 90% power to detect a 9.0-N change in MVICT. Because of a higher retention rate than anticipated we elected to curtail recruitment after 33 patients were enrolled.
RESULTS
Study Population
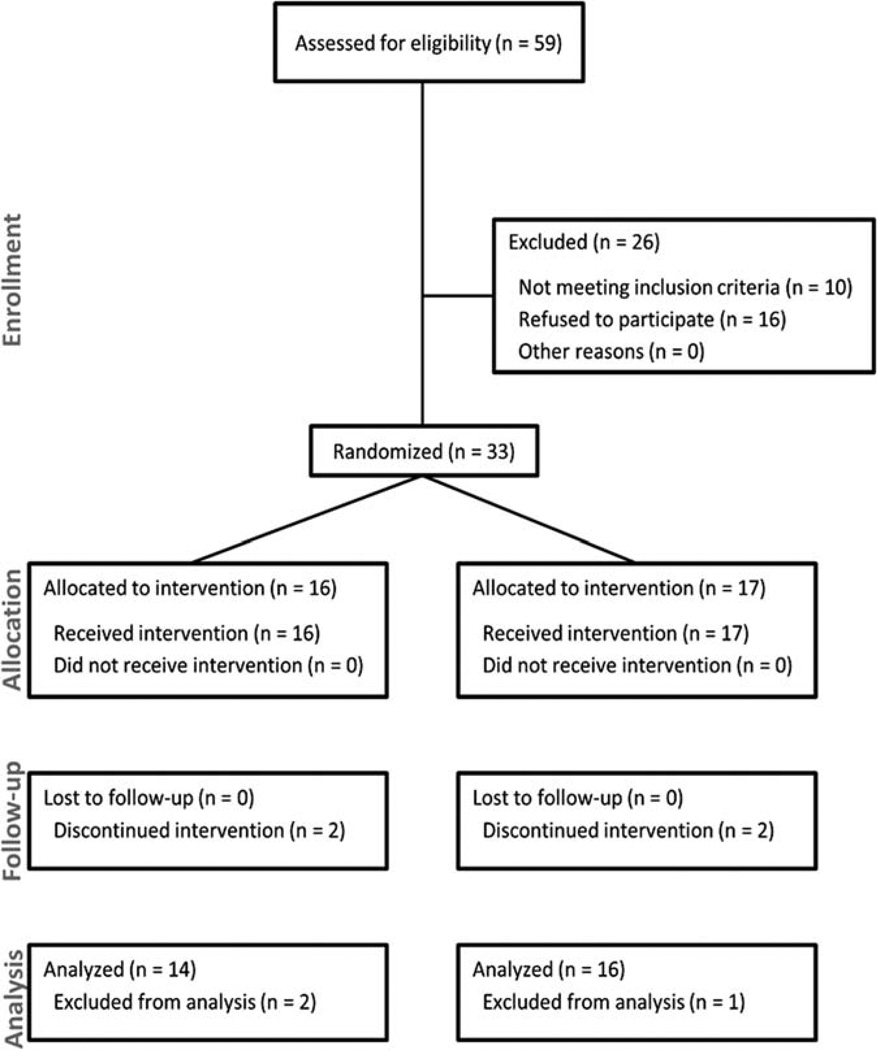
Fifty-nine patients were screened to enroll 33 patients. There were 20 men and 13 women, with a mean age of 37.2 ± 9.1 years (range 19.9–55.3 years) (Fig. 1). Sixteen patients were initially randomized to VPA and 17 to placebo. Four patients had 5 copies of SMN2, 19 patients had 4 copies, 5 patients had 3 copies, 3 patients had 2 copies, and for 2 patients SMN2 copy number was not available. There was no significant difference between these groups in any demographic or baseline assessment (Table 1). Four patients did not complete the entire study—3 prior to reaching the primary endpoint and 1 after the crossover; the latter patient missed only the final visit and was included in the data analysis because she had passed the primary endpoint (at cross-over). Of the 3 patients who did not complete the study, 2 did so because of adverse events/side effects and 1 did so after moving out of state and being unable to attend his study visits.
FIGURE 1.
Consort flow sheet.
Table 1.
Patients’ demographics.
| Treatment group | ||||
|---|---|---|---|---|
| Pretreatment characteristic |
Summary statistic |
Valproic acid (n = 16) |
Placebo (n = 17) |
Total (n = 33) |
| Age | N | 16 | 17 | 33 |
| Mean | 35.93 | 38.30 | 37.15 | |
| SD | 8.72 | 9.48 | 9.06 | |
| Gender | Men | 11 (68.8%) | 9 (52.9%) | 20 (60.6%) |
| Women | 5 (31.3%) | 8 (47.1%) | 13 (39.4%) | |
| SMN2 | 2 | 1 (6.7%) | 2 (12.5%) | 3 (9.7%) |
| 3 | 3 (20.0%) | 2 (12.5%) | 5 (16.1%) | |
| 4 | 8 (53.3%) | 11 (68.8%) | 19 (61.3%) | |
| 5 | 3 (20.0%) | 1 (6.3%) | 4 (12.9%) | |
| Weight | N | 16 | 17 | 33 |
| Mean | 85.70 | 82.03 | 83.81 | |
| SD | 25.33 | 27.25 | 25.99 | |
| Height | N | 16 | 16 | 32 |
| Mean | 171.55 | 163.46 | 167.50 | |
| SD | 10.41 | 32.73 | 24.24 | |
| BMI | N | 16 | 16 | 32 |
| Mean | 28.76 | 26.07 | 27.41 | |
| SD | 6.90 | 5.50 | 6.29 | |
| SIP score | N | 16 | 17 | 33 |
| Mean | 1.26 | 3.98 | 2.66 | |
| SD | 2.31 | 4.74 | 3.96 | |
SMN2, survival motor neuron gene; BMI, body mass index; SIP, Sickness Impact Profile.
Primary Outcome
Table 2 shows the initial 6-month change in MVICT for each muscle group in the placebo and VPA groups. There were no statistically significant differences in the change in MVICT for any muscle group at 6 months and essentially no change in baseline in either group. Table 3 presents the least-squares means from the mixed effects models for each MVICT summary endpoint for the entire 12 months of the study. There was no difference between the treatment groups over 1 year.
Table 2.
Maximum voluntary isometric contraction testing (MVICT) change from baseline at 6 months.
| Valproic acid (n = 16) | Placebo (n = 17) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Change from base | N | Mean | SD | Median | N | Mean | SD | Median | P-value |
| Elbow extensors | 12 | −0.01 | 0.49 | −0.01 | 14 | −0.09 | 1.04 | 0.21 | 0.8100 |
| Elbow flexors | 14 | −0.20 | 1.31 | 0.24 | 16 | 0.09 | 1.21 | −0.04 | 0.5396 |
| Upper extremity | 14 | −0.24 | 1.17 | −0.20 | 16 | −0.01 | 1.05 | 0.05 | 0.5704 |
| Knee extensors | 11 | 0.12 | 0.77 | 0.06 | 13 | 0.01 | 0.64 | −0.18 | 0.7114 |
| Knee flexors | 12 | −0.09 | 1.34 | −0.43 | 16 | −0.19 | 1.58 | 0.06 | 0.8508 |
| Lower extremity | 13 | −0.02 | 0.65 | −0.04 | 16 | 0.35 | 1.30 | 0.10 | 0.3674 |
| Grip | 14 | 1.45 | 4.77 | 0.17 | 16 | −0.34 | 3.47 | 0.01 | 0.2462 |
| Total MVICT | 14 | −0.46 | 2.99 | 0.10 | 16 | 0.03 | 1.55 | 0.01 | 0.5708 |
Table 3.
Least-squares means for MVICT over 12 months.
| Upper extremity |
Arm | Estimate | SE | Lower CI | Upper CI |
|---|---|---|---|---|---|
| VAL | 8.3836 | 0.1944 | 7.9860 | 8.7813 | |
| Placebo | 8.8093 | 0.1976 | 8.4050 | 9.2135 | |
| Lower extremity | Arm | ||||
| VAL | 4.8155 | 0.2532 | 4.2968 | 5.3343 | |
| Placebo | 5.0016 | 0.2576 | 4.4740 | 5.5292 | |
| Grip | Arm | ||||
| VAL | 16.3168 | 0.8080 | 14.6643 | 17.9693 | |
| Placebo | 16.8072 | 0.8109 | 15.1488 | 18.4656 | |
| Total MVICT | Arm | ||||
| VAL | 8.7514 | 0.4066 | 7.9198 | 9.5831 | |
| Placebo | 8.9608 | 0.4082 | 8.1260 | 9.7956 |
CI, confidence interval; MVICT, maximum voluntary isometric contraction testing; VAL, valproic acid; SE, standard error.
Secondary Outcomes
There was no significant difference at either 6 or 12 months for any of the secondary clinical or electrophysiological outcome measures by either the primary analysis or the least-squares means analysis. Body composition analysis through DEXA testing showed that all body parameters (bone density, lean body mass, fat mass) increased with VPA therapy, but there were no significant differences from the changes noted in the placebo group. Unlike the pediatric SMA population, in which adiposity and weight gain are problematic, especially with VPA administration,27–29,31,32 weight gain was not a major complication in this study. This may be due in part to the relatively low average VPA levels achieved in the study.
Outcomes by SMN2 Copy Number
The mean and standard deviation data for myometry, 6-minute walk, Functional Rating Scale, and CMAP by SMN2 copy number are presented in the Supplementary Material [Tables S1 (baseline) and S2 (6-month data)] available online. Only summary data are presented, because the large variations and the fact that only the 4-SMN2 copy group had >5 subjects precluded a meaningful statistical analysis. The baseline data did not show a consistent trend, but the numbers in each group were small. At 6 months, the values were all highest in the subjects with 5 copies, but the changes are mixed, with some areas improving and others declining.
Adverse Events
At least 1 adverse event (AE) occurred at some time during the study in 88% of subjects, with a total of 96 AEs reported. Two AEs led to study withdrawal—1 patient developed fatigue and 1 noted increased arm weakness, which the subject attributed to VPA. There were 2 serious AEs (cardiac failure related to chronic aortic valve disease and an episode of recreational drug overdose), neither related to VPA use. Headache (21%), fatigue (12%), and nausea (12%) were the most common AEs.
VPA Trough Levels
Subject compliance was good, as determined by pill counts and patient history. The mean overnight trough VPA level of 58.5 (±16.7) was within the targeted range (>50) and was similar to that achieved in the CARNI-VAL II/III study. All subjects appeared to be taking VPA during the appropriate phase of the study; that is, no levels were detected in patients during the placebo phase of the study, providing strong evidence of compliance with the regimen. There were no differences in the VPA levels as a function of sequence, whether patients were on VPA during the first or second 6 months of the study.
DISCUSSION
In this study, VPA given at standard doses did not result in improvement in any outcome variable in an ambulatory adult cohort of SMA patients. Although the drug was well tolerated in this population, and weight gain was not a major problem as in prior studies in pediatric SMA cohorts, the robust improvement found in previous open-label studies was not confirmed in this blinded, prospective study. In contrast to studies in younger patients, there were no encouraging findings in the secondary outcome measures.
There are several possible reasons for these negative findings. The serum VPA levels attained in this study were relatively low, although they were within our targeted range. It is therefore possible that higher doses or more prolonged treatment may have had more of a therapeutic effect. On the other hand, the levels achieved in this study were within the general therapeutic range in terms of the biological effects shown for VPA use in other FDA-approved indications, including migraine headaches, bipolar disorder, and seizures, and the dosing employed was similar to that used in the previous open-label studies. Alternatively, VPA may simply not be a sufficiently potent HDAC inhibitor to achieve the desired increase in SMN2 transcription. Multiple other agents that increase SMN2 output through HDAC inhibition or other mechanisms are currently in various stages of development.
Another obvious issue relates to the timing of therapy in the adult population. Prior work has also demonstrated that motor unit loss in SMA tends to occur relatively early in the clinical course and is often followed by long periods of relative clinical and electrophysiological stability. Multiple studies in murine SMA models have confirmed that early treatment is necessary to prevent the devastating effects of SMN deficiency on motor neurons. In this regard, treating adults with agents like VPA over such a relatively short time in light of the longstanding nature of their disease and related secondary changes that accompany such a prolonged disease course may be a flawed strategy, even though their increased number of SMN2 genes would theoretically make them more responsive to a therapeutic strategy aimed at increasing SMN2 expression.
A major aspect of this issue relates to the determination of SMN protein and transcript levels in this cohort. These analyses are ongoing. The data will be particularly germane, as neither the peripheral SMN protein nor transcript levels in prior VPA studies achieved the targeted increase of 1.3–1.7-fold over baseline. This suggests that VPA did not have the expected biochemical effect in the peripheral blood in these cohorts, so its effect at the spinal cord/anterior-horn-cell level is even more uncertain. Of course, it is presently unclear whether peripheral blood transcript or protein levels are adequate surrogate biomarkers for what is happening at the motor neuron level; such a determination will await a clinical trial with an effective therapeutic agent. Additional efforts to identify reliable biomarkers in SMA are currently underway.33,34
Taken with the previously reported negative CARNI-VAL cohort 1 and 2 studies in SMA children, these data suggest that VPA at the doses used in these studies is not effective in SMA, at least when provided over a relatively short time period. However, the response observed in the CARNI-VAL II/III clinical trial in the youngest cohort of subjects who received treatment over 12 months lends some support to the idea that future studies should concentrate on treating patients earlier in the disease process or, if we must count on reinnervation as the primary means for recovery, over a longer treatment duration. Additional information on VPA in SMA should be forthcoming from the recently completed Project Cure trial of VPA in type 1 infants, for which the data are being analyzed.
Supplementary Material
Acknowledgments
J.K. received drugs from Abbott Pharmaceuticals for a clinical trial in SMA, is a paid consultant for Alexion Pharmaceuticals and Cytokinetics, and is funded by the National Institutes of Health (NIH U10 NS77382-2 for NeuroNEXT). B.E. received drugs from Abbott Pharmaceuticals for a clinical trial in SMA. S.R. has received grants from Families of SMA. K.K has received grant funding from the Families of SMA. G.A. receives funding from NIH/NINDS. B.L. received grants from Families of SMA and the National Center for Research Resources (UL1RR025764 to the University of Utah Center for Clinical and Translational Science). K.S. has contracts with ISIS Pharmaceuticals, Inc., Orphamed, and Biomarin for clinical trials and receives grant support from the NIH (R01-HD69045 from NICHD and U10 NS077305 from NINDS), the Muscular Dystrophy Association, and the Alternating Hemiplegia of Childhood Foundation.
This work was funded by Families of Spinal Muscular Atrophy and also by grants from the Center for Clinical and Translational Sciences, University of Utah (UL1RR025764), and the Center for Clinical and Translational Sciences, Ohio State University (UL1RR025755).
The authors gratefully acknowledge all the site clinical coordinators, research nurses, and evaluators, who were instrumental in developing the protocol and critical to the success of this study, and also to the patients who participated in this study.
Abbreviations
- AE
adverse event
- CMAP
compound muscle action potential
- DEXA
dual-energy X-ray absorptiometry
- FEV1
forced expiratory volume in 1 second
- HDAC
histone deacetylase
- MIP
maximum inspiratory pressure
- MVICT
maximum voluntary isometric contraction testing
- PFT
pulmonary function test
- QOL
quality of life
- SMA
spinal muscle atrophy
- VPA
valproic acid.
Footnotes
Additional Supporting Information may be found in the online version of this article.
This article includes Supplementary Material available via the internet at http://www.mrw.interscience.wiley.com/suppmat/0148–639X/suppmat
Disclosures: The remaining authors have no disclosures to report.
REFERENCES
- 1.Kolb S, Kissel JT. Spinal muscular atrophy: a timely review. Arch Neurol. 2011;68:978–984. doi: 10.1001/archneurol.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burghes AHM, Beatty CE. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat Rev Neurosci. 2009;10:597–609. doi: 10.1038/nrn2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorson CL, Rindt H, Shababi M. Spinal muscular atrophy: mechanisms and therapeutic strategies. Hum Mol Genet. 2010;19:R111–R118. doi: 10.1093/hmg/ddq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightcycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70:358–368. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefebvre S, Burlet P, Liu Q, Bertrandy S, Clermont O, Munnich A, et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet. 1997;16:265–269. doi: 10.1038/ng0797-265. [DOI] [PubMed] [Google Scholar]
- 6.Mailman MD, Heinz JW, Papp AC, Snyder PJ, Sedra MS, Wirth B, et al. Molecular analysis of spinal muscular atrophy and modification of the phenotype by SMN2. Genet Med. 2002;4:20–26. doi: 10.1097/00125817-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Prior TW, Swoboda KJ, Scott HD, Hejmanowski AQ. Homozygous SMN1 deletions in unaffected family members and modification of the phenotype by SMN2. Am J Med Genet A. 2004;130:307–310. doi: 10.1002/ajmg.a.30251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swoboda KJ, Prior TW, Scott CB, McNaught TP, Wride MC, Reyna SB, et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol. 2005;57:704–712. doi: 10.1002/ana.20473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wirth B, Brichta L, Schrank B, Lochmüller H, Blick S, Baasner A, et al. Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum Genet. 2006;119:422–428. doi: 10.1007/s00439-006-0156-7. [DOI] [PubMed] [Google Scholar]
- 10.Prior TW, Krainer AR, Hua Y, Swoboda KJ, Snyder PC, Bridgeman SJ, et al. A positive modifier of spinal muscular atrophy in the SMN2 gene. Am J Hum Genet. 2009;85:408–413. doi: 10.1016/j.ajhg.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsheikh B, Prior T, Zhang X, et al. An analysis of disease severity based on SMN2 copy number in adults with spinal muscular atrophy. Muscle Nerve. 2009;40:652–656. doi: 10.1002/mus.21350. [DOI] [PubMed] [Google Scholar]
- 12.Bebee TW, Doninguez CE, Chandler DS. Mouse models of SMA: tools for disease characterization and therapeutic development. Hum Genet. 2012;131:1277–1293. doi: 10.1007/s00439-012-1171-5. [DOI] [PubMed] [Google Scholar]
- 13.Burghes AH, McGovern VL. Antisense oligonucleotides and spinal muscular atrophy: skipping along. Genes Dev. 2010;24:1574–1579. doi: 10.1101/gad.1961710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacKenzie A. Sense in antisense therapy for spinal muscular atrophy. N Engl J Med. 2012;366:761–763. doi: 10.1056/NEJMcibr1114629. [DOI] [PubMed] [Google Scholar]
- 15.Markowitz JA, Singh P, Darras BT. Spinal muscular atrophy: a clinical and research update. Pediatr Neurol. 2012;46:1–12. doi: 10.1016/j.pediatrneurol.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Mercuri E, Bertini E, Iannaccone ST. Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurol. 2012;11:443–452. doi: 10.1016/S1474-4422(12)70061-3. [DOI] [PubMed] [Google Scholar]
- 17.Leng Y, Chuang DM. Endogenous α-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J Neurosci. 2006;26:7502–7512. doi: 10.1523/JNEUROSCI.0096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Bergeijk J, Haastert K, Grothe C, Claus P. Valproic acid promotes neurite outgrowth in PC12 cells independent from regulation of the survival of motoneuron protein. Chem Biol Drug Des. 2006;67:244–247. doi: 10.1111/j.1747-0285.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 19.Brichta L, Hofmann Y, Hahnen E, Siebzehnrubl FA, Raschke H, Blumcke I, et al. Valproic acid increases the SMN2 protein level: a well-known drug as a potential therapy for spinal muscular atrophy. Hum Mol Genet. 2003;12:2481–2489. doi: 10.1093/hmg/ddg256. [DOI] [PubMed] [Google Scholar]
- 20.Sumner CJ, Huynh TN, Markowitz JA, et al. Valproic acid increases SMN levels in spinal muscular atrophy patient cells. Ann Neurol. 2003;54:647–654. doi: 10.1002/ana.10743. [DOI] [PubMed] [Google Scholar]
- 21.Brichta L, Holker I, Haug K, Klockgether T, Wirth B. In vivo activation of SMN in spinal muscular atrophy carriers and patients treated with valproate. Ann Neurol. 2006;59:970–975. doi: 10.1002/ana.20836. [DOI] [PubMed] [Google Scholar]
- 22.Kernochan LE, Russo ML, Woodling NS, Huynh TN, Avila AM, Fischbeck KH, et al. The role of histone acetylation in SMN gene expression. Hum Mol Genet. 2005;14:1171–1182. doi: 10.1093/hmg/ddi130. [DOI] [PubMed] [Google Scholar]
- 23.Tsai LK, Tsai MS, Ting CH, Li H. Multiple therapeutic effects of valproic acid in spinal muscular atrophy model mice. J Mol Med. 2008;86:1243–1254. doi: 10.1007/s00109-008-0388-1. [DOI] [PubMed] [Google Scholar]
- 24.Weihl CC, Connolly AM, Pestronk A. Valproate may improve strength and function in patients with type III/IV spinal muscular atrophy. Neurology. 2006;67:500–501. doi: 10.1212/01.wnl.0000231139.26253.d0. [DOI] [PubMed] [Google Scholar]
- 25.Tsai LK, Yang CC, Hwu WL, Li H. Valproic acid treatment in six patients with spinal muscular atrophy. Eur J Neurol. 2007;14:e8–e9. doi: 10.1111/j.1468-1331.2007.01992.x. [DOI] [PubMed] [Google Scholar]
- 26.Darbar IA, Plaggert PG, Zanoteli E, Resende MBD, Reed UC. Evaluation of muscle strength and motor abilities in children with type II and III spinal muscular atrophy treated with valproic acid. BMC Neurol. 2011;11:36. doi: 10.1186/1471-2377-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swoboda KJ, Scott CB, Reyna SP, Prior TW, LaSalle B, Sorenson SL, et al. Phase II open label study of valproic acid in spinal muscular atrophy. PLoS ONE. 2009;4:e5268. doi: 10.1371/journal.pone.0005268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swoboda KJ, Scott CB, Crawford TO, Simard LR, Reyna SP, Krosschell KJ, et al. SMA CARNI-VAL Trial Part 1: double-blind, randomized, placebo-controlled trial of L-carnitine and valproic acid in spinal muscular atrophy. PLoS ONE. 2010;5:e12140. doi: 10.1371/journal.pone.0012140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kissel JT, Scott CB, Reyna SP, et al. SMA CARNI-VAL Trial Part II: a prospective, single-armed trial of L-carnitine and valproic acid in ambulatory children with spinal muscular atrophy. PLoS One. 2011;6:e21296. doi: 10.1371/journal.pone.0021296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGuire D, Garrison L, Miller RG. Relationship of the Tufts Quantitative Neuromuscular Exam (TQNE) and the Sickness Impact Profile (SIP) in measuring progression of ALS. Neurology. 1996;46:1442–1444. doi: 10.1212/wnl.46.5.1442. [DOI] [PubMed] [Google Scholar]
- 31.Sproule DM, Montes J, Montgomery M, Battista V, Koenigsberger D, Shen W, et al. Increased fat mass and high incidence of overweight despite low body mass index in patients with spinal muscular atrophy. Neuromuscul Disord. 2009;19:391–396. doi: 10.1016/j.nmd.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sproule DM, Montes, Dunaway S, Montgomery M, Battista V, Koenigsberger D, et al. Adiposity is increased among high-functioning, non-ambulatory patients with spinal muscular atrophy. Neuromuscul Disord. 2010;20:448–452. doi: 10.1016/j.nmd.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crawford TO, Paushkin SV, Kobayashi DT, Forrest SJ, Joyce CL, Finkel RS, et al. Evaluation of SMN protein, transcript and copy number in the biomarkers for spinal muscular atrophy (BforSMA) clinical study. PLoS ONE. 2012;7:e33572. doi: 10.1371/journal.pone.0033572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkel RS, Crawford TO, Swoboda KJ, Kaufmann P, Juhasz P, Li X, et al. Candidate proteins, metabolites, and transcripts in the biomarkers for spinal muscular atrophy (BforSMA) clinical study. PLoS ONE. 2012;7:e35462. doi: 10.1371/journal.pone.0035462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.