Abstract
A key but poorly studied domain of sphingolipid functions encompasses endocytosis, exocytosis, cellular trafficking, and cell movement. Recently, the ezrin, radixin and moesin (ERM) family of proteins emerged as novel potent targets regulated by sphingolipids. ERMs are structural proteins linking the actin cytoskeleton to the plasma membrane, also forming a scaffold for signaling pathways that are used for cell proliferation, migration, and invasion, and cell division. Opposing functions of the bioactive sphingolipids ceramide and sphingosine-1-phosphate (S1P), contribute to ERM regulation. S1P robustly activates whereas ceramide potently deactivates ERM via phosphorylation/dephosphorylation, respectively. This recent dimension of cytoskeletal regulation by sphingolipids opens up new avenues to target cell dynamics, and provides further understanding of some of the unexplained biological effects mediated by sphingolipids. In addition, these studies are providing novel inroads into defining basic mechanisms of regulation and action of bioactive sphingolipids. This review describes the current understanding of sphingolipid regulation of the cytoskeleton, it also describes the biologies in which ERM proteins have been involved, and finally how these two large fields have started to converge.
Keywords: Sphingolipids, Ezrin, Radixin, Moesin, Ceramide, Sphingosine-1-phosphate, Cytoskeleton
1. Introduction
Long thought to be chiefly structural and metabolic molecules, sphingolipids have emerged as bioactive constituents of eukaryotic cells. They are involved in many vital cellular processes and functions and are generated in response to many extracellular inducers such as UV light, chemotherapy, growth factors, and cytokines [1]. The bioactive sphingolipid ceramide mediates cellular senescence, apoptosis and cell cycle arrest. Its metabolite, S1P, mediates cellular proliferation, mitogenesis, inflammation, angiogenesis, and cancer metastasis [1]. This model of sphingolipid-mediated biologies highlights the opposing roles that can be exerted by the different bioactive sphingolipids[2].
This review focuses on a special area in sphingolipid biology, specifically their pleiotropic effects on the actin cytoskeleton and their contribution to cell shape. Such effects are crucial for mediating cellular changes observed in endocytosis, exocytosis, cellular trafficking, and filopodia and lamellipodia formation, processes required for the maintenance of cell polarity and induction of cell migration [3]. Here we focus on the ERM family and the emerging intimate connections to sphingolipids.
ERM (ezrin, radixin, and moesin) proteins are a group of adaptor molecules linking the cortical actin cytoskeleton to the plasma membrane [4]. They are considered to be core proteins with which different molecules interact to facilitate signal transduction between the cell and the extracellular matrix [5]. ERMs are essential for dictating cellular morphology, a role made apparent by their ability to modulate formation of specialized cellular structures such as filopodia, lamellipodia, and other membrane protrusions in which ERMs exist in abundance. Also, ERMs have important roles in cell biological processes such as cell polarity, adhesion, migration, and invasion. Therefore, ERMs are emerging as critical regulators of cancer metastasis and progression [6-11]. ERM proteins exist in two conformational states, which dictate their function: an inactive or closed state in which the N-terminal domain (FERM) and the C-terminal domain (C-ERMAD) interact and an open or active state in which phosphorylation of the C-terminal threonine residue (ezrin Thr-567, radixin Thr-564, and moesin Thr-558) disrupts this interaction. When in the closed state, ERMs are cytosolic, and when in the open/active state, the FERM domain interacts with the plasma membrane and the C-ERMAD is linked to the actin cytoskeleton [4]. Other phosphorylation sites exist, but these have different functions as will be discussed later.
After providing a brief summary of sphingolipid metabolism and its regulation of cell morphology, this review will address two main areas. First, it will address ERM in-depth to educate the reader about the diverse functions of ERMs. Next, this review it will expand on the connections established between sphingolipid metabolic products and their regulation of ERM functions.
2. The sphingolipid pathway
Sphingolipid synthesis is a complex interconnected set of reactions that yield abundant bioactive intermediate products. De novo sphingolipid synthesis that occurs in the endoplasmic reticulum, begins with a condensation reaction between serine and palmitoyl CoA mediated by serine palmitoyl transferase (SPT) to form 3-keto-dihydrosphingosine, which is directly reduced to dihydrosphingosine [12]. This product is acted upon by dihydroceramide synthase (also known as Lass or CerS), to form dihydroceramide which is subsequently desaturated, generating ceramide [13]. Ceramide occupies a central position in this pathway, and it is a target for diverse enzymes to yield hundreds of biosynthetic pathway products. Multiple sugar residues can be added to ceramide to yield complex glycosphingolipids through the initial action of glucosyl- or galactosyl- ceramide synthases, the first steps in glycosphingolipid synthesis [14]. Alternately, sphingomyelin can be generated by the addition of a phosphocholine headgroup, a step catalyzed by sphingomyelin synthases [15]. Ceramide can also be phosphorylated by ceramide kinase to form ceramide-1-phosphate [16].
Sphingolipid catabolism is accomplished by multiple enzymes that drive the production of intermediate bioactive lipids and shift the balance among them. Sphingomyelin is acted upon by 4-6 sphingomyelinases (acid, 1-3 neutral, alkaline, and one that is mitochondrial), which differ in their cellular localization [17]. Complex glycosphingolipids are gradually degraded by hydrolases to form glucosylceramide and galactosylceramide, which serve as substrates for specific glucosidases and galactosidases [18, 19].
Finally, ceramide is metabolized to sphingosine through the action of five known ceramidases (acid, neutral, and three alkaline) that also differ in their cellular localization and tissue distribution [20]. Sphingosine, in turn, participates in the formation of ceramide through the salvage pathway via multiple ceramide synthases, or it can be phosphorylated by two known sphingosine kinase isoforms, SK1 and SK2, to form sphingosoine-1-phosphate (S1P), one of the most studied sphingolipid molecules [21]. S1P can either be dephosphorylated back to sphingosine by S1P phosphatases [22], or it can be cleaved irreversibly to ethanolamine phosphate and hexadecanal by S1P lyase, which represents the only exit point of the sphingolipid pathway [23]. Figure 1 provides a schematic illustration of the sphingolipid metabolic pathway described above.
Figure 1. Sphingolipid metabolic pathway.
This figure illustrates the sphingolipid metabolic pathway described in the text along with the structure of each product. It emphasizes the opposing roles played by two major bioactive products of sphingolipid metabolism, ceramide and sphingosine-1-phosphate. While ceramide is known to play a role in cell cycle arrest and apoptosis, S1P is known to be involved in promoting cell proliferation, inflammation, migration, and invasion. Glu (Glucose), Gal (Galactose), GCS (Glucosylceramide Synthase), GBA (Glucocerebrosidase), CK (ceramide kinase), SMS (sphingomyelin synthase), SMase (sphingomyelinase), CDase (Ceramidase), CerS (Ceramide Synthase), SK (sphingosine kinase), S1PPase (S1P Phosphatase).
3. Sphingolipids and cellular morphology
Sphingolipids are important for membrane and cell shape integrity because of their structural characteristics and their involvement in the activation/inhibition of signaling pathways that alter cellular morphology.
3.1. Sphingolipids in endocytosis and exocytosis
Endocytosis and exocytosis require major cytoskeletal and membranous rearrangements that are initiated by membrane-invagination and conclude with vesicle formation. Endocytosis is dramatically altered in yeast carrying mutations in lcb1p, a component of SPT, which catalyzes the first step of de novo lipid synthesis [24]. Such an effect is mediated by a deregulation of actin dynamics [25], an effect that can be reversed to the normal phenotype by overexpression of protein kinase C1 and PKH1 or PKH2 (orthologue of mammalian protein-phosphoinositide dependent protein kinase 1, PDK1) [26, 27]. These studies suggest a possible regulatory role for sphingolipids with these enzymes, modifying cellular structures. In contrast, membrane fusion between a donor vesicle and the plasma membrane during exocytosis requires the presence of membrane-associated proteins, SNAREs. These proteins are encoded by SNC genes, and are altered in the mutant yeast strain M42A snc2delta that has defective exocytosis. Interestingly, this defect is restored by overexpression of the sphingoid base phosphate lyase, which diminishes cellular dihydrosphingosine-1-phosphate [28]. In addition, a mutation in S1P lyase in Drosophila gives rise to cellular migration defects, specifically affecting F-actin distribution [29, 30]. Also, ceramidase has been reported to be essential in exocytosis in yeast and Drosophila. Yeast defective in YPC1, which encodes a ceramidase, have defective vesicular trafficking from the Golgi apparatus to the cell exterior [31]. Moreover, Drosophila with a mutated neutral ceramidase, also have defects in neuronal synaptic vesicular fusion and cellular trafficking [32]. Finally, neutral sphingomyelinase has been recently reported to play a crucial role in exosomes secretion [33, 34]. Exosomes are extracellular vesicles derived from the endocytic compartment and carry molecules involved in paracrine signaling. Overexpression of this enzyme increases the secretion of miRNA-loaded exosomes, which causes increased angiogenesis and metastasis [33].
3.2. Sphingolipids and plasma membrane protrusions
S1P is a potent bioactive sphingolipid that exerts its function either through intracellular interactions, or by being transported to cell exterior via the ABC transporter family [35] and/or the spinster-2-transporter [36], where it acts on G-protein coupled receptors (S1P receptors; S1PR). Five S1PRs have been identified (S1P1–5). S1P receptors differ with respect to tissue distribution and with respect to the G-proteins to which they are coupled [37]. S1P is a well-known chemoattractant and a modulator of cell migration, exerting its functions by acting on receptors that, in turn, couple to the Rho and Rac family of small G proteins [3]. Activation of the Rac pathway polarizes the cell and gives rise to the production of lamellipodia along with focal adhesion contacts with the extracellular matrix. Activation of the Rho pathway, in contrast, causes cell detachment and stress-fiber formation. Alternating and concerted activation of these two different pathways ultimately lead to cell migration and forward movement [38]. S1PR1 has been coupled to Rac activation and cell movement whereas S1PR2 has been linked to Rho activation and Rac inhibition, thereby inhibiting cell movement [37]. Detailed effects of S1P receptors on cell movement are beyond the scope of this review and have been adequately reviewed elsewhere [39].
4. Ezrin activation and role in biology
Ezrin activation is a multistep process requiring the phosphorylation of the threonine 567 residue. Multiple cellular stimuli are known to induce ezrin phosphorylation, which has a well-defined biological significance. Table 1 offers a current list of all kinases known to induce ERM phosphorylation with respect to the stimulus applied and the biology involved. The Table also contains details about the specific cell line in which the mechanism has been studied. As previously mentioned, ERM phosphorylation is not limited to the activating threonine 567 residue, but it involves several other residues key for cell signaling (also described in Table 1). The Table represents a reference for studying relationships between the ERM family of proteins and sphingolipids, which will be discussed in more detail later. A discussion of different functions affected by ERM phosphorylation is offered below.
Table 1.
The kinases known to phosphorylate ERM proteins and their functional consequences. (N.B. 1- The ERM column refers to the specific ERM member that is phosphorylated by the corresponding kinase, 2- the numbering of the phosphorylation sites reflects their position in the original ERM sequence; this might slightly differ in the original manuscripts as some authors use their tagged constructs for numbering).
| P-site | Kinase | ERM | Stimulus | Cell line | Function | Ref |
|---|---|---|---|---|---|---|
| EzrinThr567, RadixinThr564, MoesinThr558 | PKC | ERM | Amphetamine | PC12 cells | Synaptic plasticity | [50] |
| E | Dinitrosopiperazin | NPC cells | Motility, invasion, metastasis | [92] | ||
| E | N/A | Oteoscarcoma cells | Migration and invasion | [85, 93] | ||
| ERM | 2-methoxyestradiol | HPAEC cells | Hyperpermeability | [76] | ||
| ERM | Glutamate | Hippocampal cells | Filopodia and synaptic boutons formation | [51] | ||
| E | Latent membrane protein-1 expression | NPC cells | Motility and invasion | [6] | ||
| E | N/A | Intestinal epithelial cells | Epithelial differentiation | [124] | ||
| M | Hypotonicity | Collecting duct epithelial cells | Cytoskeletal remodeling | [125] | ||
| E | Androgen | LNCaP-FGC | Invasion | [126] | ||
| E | Parathyroid Hormone | Oposum Kidney cells | Endocytosis | [127] | ||
| E | N/A | 2C4 Fibrosarcoma | Migration | [128] | ||
| Rho/ROCK | ERM | Netrin | Cortical Neurones | Axonal growth | [49] | |
| E | Estrogen | T47-D Breast cancer cells | Migration and invasion | [91] | ||
| ERM | LPA | Ovarian Cancer | Migration and metastasis | [129] | ||
| E | N/A | Hepatoma cells | Invasion | [90] | ||
| E | Cisplatin | HT29 cells | Apoptosis | [100] | ||
| E | N/A | NPC cells | Metastasis | [130] | ||
| EM | Fas receptor activation | Jurkat T cells | Apoptosis | [131] | ||
| E | TNF | Endothelial cells | Inhibition of proliferation | [132] | ||
| E | Net and Dbl overexpression | NIH3T3 fibroblasts | Malignant transformation | [133] | ||
| P38 MAPK | ERM | TNF | Synoviocytes | Proliferation | [63] | |
| M | Advanced glycation end products | Endothelial cells | Hyperpermeability | [78] | ||
| GRK-2 | R | Wound closure | MDCK cells | Migration | [134] | |
| ERM | Angiotensin-II | Kidney Podocytes | Actin turnover and migration | [135] | ||
| E | Acetylcholine | Hep2 and HEK293 cells | Membrane ruffles formation and internalization | [136] | ||
| NIK | ERM | EGF, PDGF | MTLn3 cells | Lamellipodia formation | [137] | |
| AKT2 | E | Glucose | Caco-2BBe cells | Translocation of NHE-3 to plasma membrane | [138] | |
| LRRK2 | ERM | N/A | Primary Neurons | Retardation of growth and increased filopodia | [52] | |
| Mst-4 | E | N/A | W4 cell line | Apical brush border formation | [139] | |
| LOK | ERM | N/A | Lymphocytes | Decreased migration and polarization | [140] | |
| Slik | M | Mitosis initiation | Drosophila S2 cells | Cortical microtubular stability | [45] | |
| MRCK | ERM | Cdc42 overexpression | NIH3T3 fibroblasts | Filopodia Formation | [141] | |
| Tyr146 and/or 354 and/or 478 | Syk | E | CD81 engagement | B lymphocytes | Cell reorganization and signaling | [142] |
| Flt3 | E | Kit overexpression | Proerythroblasts | Apoptosis resistance and proliferation | [143] | |
| Src | E | EGF | A431 cells | Spreading and proliferation | [144] | |
| E | ICAM1 ligation | Endothelial cells | N/A | [145] | ||
| E | N/A | Hek239 | Association with KBTBD2 | [146] | ||
| Lck | E | N/A | Jurkat T cells | Signaling during T cell activation | [147] | |
| Thr235 | Cdk5 | E | Overexpression of Retinoblastoma | Osteosarcoma | Cell senescence and flat cell morphology | [148-150] |
| Ser66 | PKA | E | Histamine | Gastric Parietal cells | Acid secretion | [55, 56] |
4.1. Ezrin in normal physiology
ERM proteins are essential for normal physiologic human body functions. They have been studied in the nervous, digestive, endocrine, and genitourinary system, and they have been studied for their role in cell division.
First, the interaction between ezrin and the actin cytoskeleton is required to maintain a normal cell shape [40]. Phosphorylation of ezrin, or the expression of the phosphomimetic T567D, causes lamellipodia formation along with microvillar projections in LLC-PK1 epithelial cells [41]. Ezrin phosphorylation also produces an elongated cellular morphology and an enrichment of the actin cortical network in HeLa and NIH 3T3 cells [42]. Furthermore, cellular detachment and inhibition of cell adhesion also causes ezrin phosphorylation and induction of microvilli formation in HEK293 cells [43].
Next, ERM protein activation is needed to maintain a healthy reproductive system. Ezrin phosphorylation is needed for sperm capacitation, which is necessary for producing functional sperm [44]. After conception, ERM proteins guide cellular mitosis [45] required for proper blastocyst morphogenesis [46] and germ layer formation during gastrulation [47].
In the nervous system, phosphorylation of ERM proteins is essential for promotion of axonal outgrowth and regeneration. This effect has been related to the activation and recruitment of ERM proteins downstream of neural cell adhesion molecule L1, which creates a highly active cytoskeletal area and a new branch protrusion [48]. ERM have also been reported to cause this effect through interaction with the deleted-in-colorectal-cancer (DCC) receptor, which subsequently activates a growth cascade [49]. Furthermore, phosphorylation of ERMs has been shown to regulate dopamine transporter activity in the nucleus accumbens [50], and this may be involved in pre-synaptic trafficking [51]. Interestingly, creating an artificial milieu with excessive ERM phosphorylation caused neural growth retardation [52].
In the digestive system, ERM proteins are required for stomach acid secretion through their regulation of acid pump positioning. In fact, expression of the phosphomimetic mutant T567D changes the polarity of gastric parietal cells [53], causing internal cellular membranes to be inserted in the basal vesicles instead of into the apical ones [54]. Cargo proteins such as the H/K-ATPase transporter insert into the basolateral plasma membrane along with long, microvillar projections, effectively halting acid secretion [53]. PKA-mediated ezrin phosphorylation on the serine 66 residue is essential for positioning the acid pump on the apical membrane [55] through its interaction with an adaptor protein—the WW domain-containing oxido-reductase protein [56].
Ezrin activation has been linked to the sodium hydrogen exchanger isoforms 1 and 3 (NHE1, NHE3). In fact, ezrin has been reported to bind to NHE1, and, together, these two proteins form a scaffold for AKT activation and cellular proliferation [57]. This signaling role is essential after cardiovascular ischemia for the maintenance of left ventricular function, prompting its hypertrophy [58]. In addition, overexpression of ezrin in kidney cells increases the surface expression of NHE3, which is important for maintenance of body sodium homeostasis [59]. Moreover, ezrin affects phosphate balance through its interaction with the parathyroid hormone receptor and sodium hydrogen exchanger regulatory factor 1 (NHERF-1) that in turn regulates apical expression of the sodium-phosphate co-transporter 2a (NPT2a) [60].
Finally, ERM proteins are needed for a functional endocrine system. Ezrin phosphorylation is essential for insulin secretion from pancreatic cells. In fact, ezrin activation is necessary for insulin granule trafficking and membrane docking [61]. Ezrin deactivation is observed in diabetic mice [61], and in organs affected by diabetic complications such as the kidney [62]. Actually, advanced glycation end products bind the end terminal of ezrin in the kidney, subsequently inhibiting its activation and thus kidney tubule formation [62].
4.2. Ezrin in immunity and inflammation
Increased ezrin phosphorylation and activation is reported to occur in many inflammatory diseases such a rheumatoid arthritis [63, 64], systemic lupus erythematosus [65], pulmonary fibrosis [66] and traumatic brain injury [67]. Ezrin activation in the immune response is thought to be important for inflammation and immunity, either by influencing cellular movement, cell shape, and/or cell survival, which is discussed below.
4.2.1. Leukocyte recruitment
ERM proteins have been described to be needed for all phases of leukocyte recruitment to sites of inflammation. Capture of free circulating leukocytes is the first step in this process, and this is mediated by p-selectin glycoprotein ligand-1 (PSGL-1). The deletion of the ERM binding sequence in the cytoplasmic tail of PSGL-1 dramatically impairs leukocyte tethering, slow rolling on the activated endothelium, and promotes defective MAPK activation [68]. Furthermore, ERM proteins have been shown to link PSGL-1 to Syk kinase [69]. Generation of a moesin mutant that could not attach to Syk abrogated serum response element (SRE) transcriptional activity that is usually induced upon PSGL-1 stimulation [69]. In contrast, transgenic mice expressing ezrin phosphomimetic protein (T567E) had defective T lymphocyte migration across the endothelium but no disturbances with the slow rolling mechanism mentioned above. Such transgenic mice had decreased lymphocytes within lymph nodes along with increased cellular adhesion and decreased cellular migration rates [70]. White blood cells (WBC) undergo cytoskeletal polarization once outside the blood vessel for proper migration. In B cells, once phosphorylated, ERM proteins translocate to the front of the cell's lamellipodia to participate in forward movement [71]. In concordance with this activity, phosphorylation of ERM proteins in NK cells facilitates their polarization and their migration towards chemoattractants [72]. In T lymphocytes however, overexpression of the phosphomimetic T567D ezrin was reported to widen the uropod on the posterior aspect of the cell, enhancing its migration ability. ERM proteins are hypothesized to establish the “posteriority” of a cell [73]. Other groups reported that ERM proteins, specifically moesin, redistribute adhesion molecules to the posterior of the migrating lymphocytes to increase ligand accessibility [74]. Certainly, ERM proteins are involved in modulating WBC movement, but the mechanism by which this occurs remains unclear.
4.2.2. Endothelial permeability
ERM proteins have variable effects on endothelial permeability and this is dependent on the activation stimulus. While ERM depletion abolished S1P's protective effect on the endothelium that is usually manifested by increased adherens junctions and decreased endothelial permeability [75] (this point will be explained later), individual depletion of ERM proteins markedly attenuated membrane leakiness caused by 2-methoxyestradiol anti-proliferative treatment [76], TNF-alpha treatment [77] and the accumulation of advanced glycation end products in diabetes [78]. Therefore, the effect of ERM proteins on endothelial integrity may be related to different downstream targets with which they associate upon the application of different stimuli.
4.2.3. Infections
ERM phosphorylation is caused by diverse microorganisms such as chlamydia [79], HIV [80, 81], Listeria monocytogenes [82], and E. coli [83, 84]. This activation appears to be necessary for infectivity or pathogenesis via different mechanisms. Using a dominant -negative ezrin that cannot be phosphorylated, chlamydial infection was attenuated due to diminished entry into host cells [79]. Also, Listeria's ability to spread across cells without activating the immune system depends on induction of cellular protrusions, which are compromised upon ERM depletion. [82]. Enteropathogenic and enterohemorrhagic E. coli recruit and activate ezrin at their site of attachment on intestinal epithelial cells [84], disrupting the epithelial tight junctions which is manifested by diarrhea and gastric bleeding [83]. Interestingly, this effect is observed specifically in pathogenic strains of E. coli; non-pathogenic strains do not activate ezrin [83]. Finally, HIV's effect on ezrin activation depends on the component of HIV studied. Viral protein R (Vpr) decreases ezrin activity that is normally associated with AKT activation, and thus cell survival [80]. In contrast, the envelope glycoprotein gp120 combined with IL-2, causes a sustained ezrin phosphorylation that is associated with increased cell surface expression of CD95 (also named FAS receptor), which causes increased T lymphocyte susceptibility to apoptosis [81].
4.3. Ezrin in malignancies
4.3.1. ERM expression and phosphorylation status
ERM proteins have been widely implicated in tumor growth and metastasis; they affect cellular modeling and signaling pathways involved in cell survival and proliferation. Ezrin was found to increase migration and metastasis in dog models of osteosarcoma [85]. Ezrin overexpression in myxofibrosarcoma was associated with a higher histological grade of pathology and cancer stage [86]. In fact, ezrin predicted decreased metastasis-free survival [86]. Its overexpression was also correlated with worse outcomes in gastro-intestinal-stromal-tumors (GIST) [87], esophageal cancers [88] and ovarian epithelial carcinomas [89]. Whether ezrin overexpression alone drives a malignant phenotype, or whether over-activation is the culprit, studies show that overexpression of phospho-mimetic ezrin is sufficient to confer metastatic capacity in mouse xenografts of hepatocellular carcinoma [90]. Also, estrogen has been shown to increase cell migration and invasion of T47-D breast cancer cells by inducing ezrin phosphorylation [91]. This is also true for dinitrosopiperazine (a known carcinogen) which enhances cell motility, invasion, and metastasis of nasopharyngeal carcinoma through ezrin activation [92]. However other studies suggest that for successful metastatic progression, ezrin must undergo a dynamic recycling between a phosphorylated and dephosphorylated state. For example, ezrin is only found to be phosphorylated just after osteosarcoma seeding in the lung parenchyma and then later during metastatic progression [93]. Stable overexpression of either a phosphomimetic ezrin or a non-phosphorylatable form in osteosarcoma blocks primary tumor growth and metastasis [94]. In fact, cells expressing the T567A mutant undergo significant apoptosis once they arrive within the lung. This apoptosis arises from the cells’ inability to handle the metabolic stress of a different milieu. Evidence for this is provided by upregulation of genes involved in cellular metabolism and cellular energy [94]. Thus, a new perspective is emerging relating to the importance of ezrin as an essential molecule allowing cell metabolic flexibility in the face of environmental stressors. Another way by which cell manage these stressors is through ezrin dependent activation of the MAPK pathway [95]. Although an abundance of literature implicates ezrin phosphorylation as a driver of tumor progression and metastasis, other data contradict this. High-grade metastatic osteosarcoma was correlated with dephosphorylated cytoplasmic ezrin rather than an active isoform [96]. In addition, phosphatase of regenerating liver-3 (PRL-3) was shown to exert its function by promoting tumor development via ezrin dephosphorylation on thr567 [97]. Also, Raf-1 oncogene transfection into a mouse hepatic cell line deactivated ezrin [98]. Furthermore, knocking down ezrin in human colonic epithelial cells increased their matrix adhesion and their spreading, thus their invasive capabilities [99]. Finally, knocking down ezrin prevents cisplatin-induced apoptosis in human colon cancer cells [100]. In conclusion, further studies are needed to elucidate the exact role of ERM activation on cancer progression as it might vary depending on the cancer grade and stage.
4.3.2. Downstream effectors of ERM
ERM-promotion of cell invasion and metastasis is mediated by several downstream effectors, depending on which residue of ezrin is phosphorylated. Generally, phosphorylation of tyrosine residues activates a signaling cascade that subsequently activates the PI3K/AKT pathway as seen in breast cancer [101], whereas phosphorylation of threonine 567 promotes cell invasion by modulating cell shape (as seen in melanoma cells that form elongated microvilli) [102]. The tyrosine kinase src can phosphorylate ezrin, and thus promote several outcomes. An ezrin mutant that cannot be phosphorylated by this kinase abolishes src-induced anchorage-independent growth and cell invasion [103]. This has been confirmed in another study which described cooperatively between src and ezrin in destabilizing cell-cell contacts and promoting scattering of mammary carcinoma cells [104]. Furthermore, the pro-metastatic effects of androgen on prostate cancer cells occur through permissive effects on anchorage-independent growth also mediated by ezrin [105]. A possible explanation for how ezrin promotes this type of growth is postulated in a study in which shRNA was used against ezrin in breast cancer [106]. E-cadherin expression was dramatically increased upon ezrin knockdown, implicating this adhesion molecule as a downstream effector of ezrin. Matrix metalloproteinases (MMPs) have also been incriminated as an effector arm for ezrin. Podocalyxin, an anti-adhesive transmembrane protein involved in the development of aggressive forms of breast and prostate cancer, leads to increased MMP expression via interaction with ezrin [107]. Similarly, expression of a dominant-negative form of ezrin reduced MMP expression and invasiveness of human malignant glioma cells [108]. Another downstream effector of ezrin is the NF-KB pathway which is required for colorectal cancer metastasis to the liver [109]. Ezrin is essential for ikb phosphorylation in response to engagement of the L1 cell adhesion molecule (L1CAM) [109].
4.3.3. Upstream effectors of ERM
Many pathways have been shown to regulate ezrin expression and/or phosphorylation. The EGF signaling cascade can increase ezrin expression, and thus cellular invasion and metastasis in MDA-231 [110]. Similarly, fos oncogene-mediated malignant transformation of Rat-1 fibroblasts involves an increase in ezrin expression and ezrin hyperphosphorylation [7]. Inhibiting ezrin function in these transformed cells abolishes fos-mediated membrane ruffling and cell motility [7]. Furthermore, GIST harbors a mutation in the KIT oncogene that activates ezrin at different residues to promote tumor progression [111].
5. Ezrin de-activation
5.1. Deactivators
Little is known about ezrin threonine 567 dephosphorylation. As mentioned before, dephosphorylation of threonine 567 inactivates ezrin. Ezrin has a PIP2 binding site on its N-terminus that is required for its association with the plasma membrane. This site as well as other plasma membrane protein binding sites remain hidden by interacting with the c-terminus site. However, even when dephosphorylated, the two states of ezrin are in equilibrium: interacting with the plasma membrane and resting in the cytosol. Inactive ezrin remains either in a monomeric closed conformation or it forms dimers. Inactivation of ezrin is related to loss of cell attachment, loss of plasma membrane protrusions (filopodia, microvilli, etc) and enhancement of cell invasion, and cell migration. Few ezrin inactivators have been described, and these mainly affect PIP2 at the plasma membrane. Thus, when plasma membrane PIP2 is hydrolyzed, ezrin is quickly dephosphorylated. Another more recent mechanism offered to explain ezrin deactivation involves ceramide production at the plasma membrane, a concept that is expanded in the next section.
Ezrin is well-studied in lymphocytes, mainly T cells and NK cells. It plays an important role in lymphocyte polarization, immunological synapses, receptor signaling and T-cell activation [112]. In these cells, ezrin function is tightly regulated by tyrosine phosphorylation which is stimulated by ligation of CD3 and CD4 receptors. General activation of ezrin is also stimulated by the CD43 receptor. Other lymphocyte receptors have been reported to play an important role in ezrin biochemistry, such CD95, CD44, CD81 or I-CAMs. A mechanism of ezrin inactivation in lymphocytes is via ezrin cleavage by calpain [113]. The first described deactivator signal for ezrin was a cytokine, stroma derived factor 1-alpha (SDF-1 alpha, or CXCL12). Within seconds after SDF-1 treatment of human peripheral blood T cells, threonine 567 was dephosphorylated, and microvillie were lost. This effect was blocked by the phosphatase inhibitor calyculin A, although no phosphatase was defined in that study [114]. Next, the small GTPase Rac1 was suggested to mediate the SDF-1α effect on microvilli collapse and ezrin dephosphorylation. Accordingly, a constitutively active Rac1 mutant produced ezrin dephosphorylation, and a dominant-negative mutant blocked cytokine-induced ezrin dephosphorylation [115]. Moreover, the SDF-1α effect was blocked by PLC inhibitors, suggesting that hydrolysis of PIP2 was required for SDF-1 effects; the phosphomimetic mutant of ezrin (T567D) was released to the cytosol after PIP2 hydrolysis [116]. In naïve T cells, both chemokines CXCL12 and CCL21 triggered ezrin dephosphorylation, reducing cell attachment and enhancing cell migration.
In other cell lines, dephosphorylation of the ezrin threonine 567 occurred in response to confluence in endothelial cells[77], and in response to chemotherapeutics in breast cancer cell lines [117].
5.2. Phosphatases
Several phosphatases have been reported to be involved in dephosphorylation of ezrin at Threonine 567, and these include myosin phosphatase (PP1β-MYPT1) [118], PP2A, PP2C, PRL-3 (a tyrosine phosphatase reported to have dual activity) [97] and PP1α [119]. This diversity of phosphatase involvement suggests that regulation of ezrin dephosphorylation depends on the cell type and the nature of the stimulus applied.
Different strategies have been developed to study the functional and phenotypic consequences of ezrin dephosphorylation . The most common tool for this is expression of a phospho-incompetent mutant, in which threonine 567 has been substituted with an alanine residue. Knockdown of ezrin is another approach. Recently, two small molecule inhibitors have been reported to induce ezrin dephosphorylation, as well as inhibit cell migration and invasion of osteosarcoma cells [120].
6. Ezrin and sphingolipids
6.1. Ceramide
The first study to describe a connection between ezrin status and sphingolipids was from our group in 2008. We noted that cisplatin caused loss of plasma membrane protrusions in MCF-7 tumor breast cancer cells, which was accompanied by loss of ezrin association to the plasma membrane, ezrin dephosphorylation, and co-localization of ezrin with cortical actin. These cytoskeletal rearrangements coincided with the loss of sphingomyelin and the generation of ceramide. In fact, cisplatin activated the acid sphingomyelinase in a PKC delta-dependent mechanism, causing its translocation to the plasma membrane. Exogenous ceramide, or recombinant bacterial sphingomyelinase from Bacillus cereus (bSMase) treatment for 1 h produced the same phenotype as cisplatin treatment, suggesting that ceramide chiefly triggered ezrin inactivation. This study also involved a ceramide activated protein phosphatase (CAPP) as a downstream effector of ceramide that is required for ezrin dephosphorylation [117]. In a follow up study, HeLa cells were shown to undergo ezrin dephosphorylation after bSMase treatment, and this effect was more dramatic. As early as 2 min after treatment (25 mU bSMase), complete ERM dephosphorylation occurred [121]. To elucidate which sphingolipid was required for ezrin dephosphorylation, the sphingolipid pathway from ceramide was dissected using a combination of several recombinant enzymes and SK inhibitors. Thus, treatment with bSMase dephosphorylated ezrin in a dose-dependent manner, localizing ezrin to the cytoplasm. Purified recombinant Loxosceles sphingomyelinase D, which generates ceramide 1-phosphate (C1P) instead of ceramide, proved that ceramide, and not decreased sphingomyelin or C1P production, was required for ezrin deactivation [121]. Interestingly, regulation of ezrin dephosphorylation in HeLa cells after ceramide generation proved to be the result of another CAPP phosphatase; pretreatment with okadaic acid, and knockdown of PP2A did not block bSMase effects. In HeLa cells, the only serine/threonine phosphatase responsible for bSMase-induced ezrin dephosphorylation was PP1α [119]. This result was verified using pharmacologic inhibitors, siRNA technology, and PP1 catalytically inactive mutants [119]. Interestingly, bSMase treatment neither altered PIP2 levels nor localization as shown with PLCd-PH domain constructs, which specifically bind PIP2. Those data suggested that ceramide-induced ezrin dephosphorylation did not involve PIP2 loss. In contrast, PIP2 hydrolysis caused ezrin dephosphorylation in a PP1α-independent manner. Activation of PP1α by ceramide seemed necessary for ezrin dephosphorylation, and did not contribute to regulating plasma membrane attachment. This was confirmed by expressing the N-terminal fragment of ezrin, which could bind PIP2 but lacked the C-terminal region containing threonine 567, which could not be dephosphorylated in response to ceramide generation. This “ceramide-PP1α“ axis also suggested a novel mechanism for ERM protein regulation: it not only did not involve PIP2, but it was independent of PP1β-MYPT1, which has been shown to regulate basal levels of ERM phosphorylation [119].
6.2. S1P
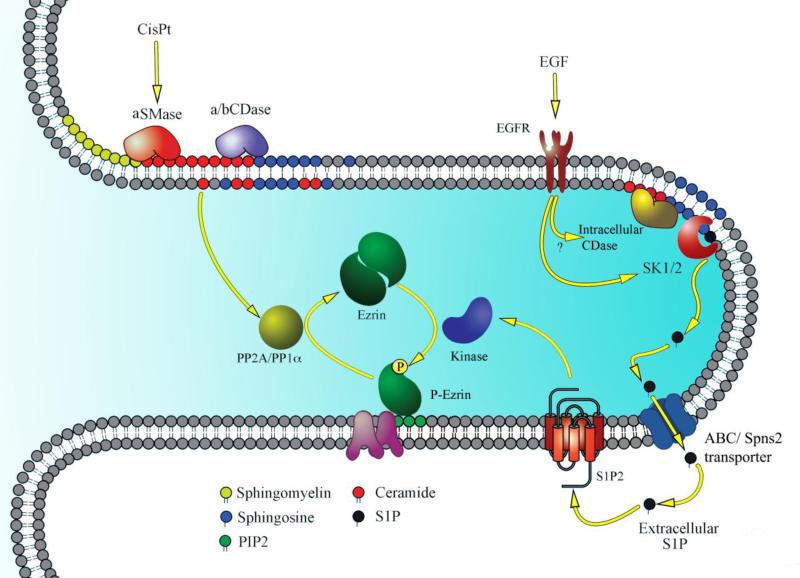
Recent experiments indicate that generation of high levels of S1P with the use of purified bacterial recombinant ceramidase (bCDase) after bSMase treatment was accompanied by ezrin hyperphosphorylation. In fact, we reported that S1P production and decreased ceramide was responsible for this dramatic effect. Thus, ceramide and S1P play opposing roles in ezrin regulation [121]. Subsequent studies by our group showed that exogenously added or endogenously produced S1P resulted in a time- and dose-dependent increase in ERM phosphorylation as early as 30 seconds after treatment. S1P induction of ezrin phosphorylation seemed to be a universal effect; it was observed in several cell lines including MDA, MCF7, A549, HEK, and MEF [122]. Dose-response experiments reveal that nanomolar concentrations could trigger the maximum S1P response on ezrin phosphorylation, suggesting a receptor-mediated effect. Indeed, it was shown that this phosphorylation required the activation of S1PR2 exclusively on HeLa cell surfaces, and blocking all other receptors maintained ezrin activation [122]. This was shown using pharmacologic inhibitors, and also siRNA and genetic knockout approaches. S1P-induced ezrin phosphorylation was required for filopodia formation; using dominant-negative nonphosphorylatable ezrin mutant abolished this S1P-mediated effect [122]. The fact that S1P mediated ezrin phosphorylation inhibited cell invasion is contradictory to what is currently known of this bioactive sphingolipid, probably because S1P works on difference receptors. How this system is coordinated is unclear. The significance of this pathway was further explored by Djanybek and colleagues in human pulmonary endothelial cells (EC) in studies of EC barrier function [75]. S1P protected EC barrier integrity by remodeling the actin cytoskeleton, increasing focal adhesion, and adherent junction formation. In this work, S1P was confirmed to activate ezrin and moesin at nanomolar concentrations within a few minutes of treatment. With pharmacological inhibitors, multiple PKC isoforms were shown to be responsible for ezrin and moesin (but not radixin) phosphorylation in response to S1P (in addition to P38 MAPK and Rac1). However, direct interactions with ERMs or other approaches involving these protein kinases were not proposed [75]. Radixin was not phosphorylated after S1P stimulation, but it was shown to be required for S1P's effect on ezrin and moesin activation [75]. A summary of sphingolipids modulation of ERM activity is illustrated in figure 2.
Figure 2. Sphingolipid regulation of ERM activation.
This figure illustrates the mechanism of ERM regulation by sphingolipids on the tip of a cellular extension. Cisplatin causes activation of the acid sphingomyelinase (aSMase), which causes ceramide production. Ceramide activates protein phosphatase 2A (PP2A) to cause ezrin dephosphorylation and detachment from the cell membrane. Alternatively, the use of bacterial sphingomyelinase causes activation of protein phosphatase 1 alpha (PP1a) to cause ezrin dephosphorylation. In contrast, S1P, generated after bacterial ceramidase use (bCDase) or after EGF treatment translocates to the extracellular space using one of the described transporters such as the ABC transporter to activate sphingosine-1-phosphate receptor 2 (S1P2). S1P2 signaling activates a yet unknown kinase to cause ezrin phosphorylation and binding to the plasma membrane using phosphatidylinositol biphosphate (PIP2).
7. Conclusion
This review describes novel mechanisms by which different bioactive sphingolipids can differentially modulate the cell cytoskeleton and cell behavior. They can mediate acute changes via activating or deactivating ERM proteins. On one end, ceramide causes acute ERM de-activation by recruiting protein phosphatase 1 alpha; and at the other end, S1P causes acute and potent ERM activation by a yet un-identified kinase that is under investigation in our laboratory.
Until now, the scientific literature reviewed herein described experiments that used sphingolipids exogenously applied to the cell. Although this approach is limited, it does offer discovery of the basic regulation of this novel pathway. Recently, using a known activator of the endogenous SK/S1P pathway, epidermal growth factor (EGF), we found that EGF induced ERM phosphorylation in an SK/S1P-dependent manner. Furthermore, the extracellular S1P generated upon EGF treatment acted on the same previously described receptor (S1PR2) to mediate this activation. This has been confirmed with siRNA technology as well as pharmacologic inhibitors. Interestingly, EGF induced lamellipodia formation and such invasive behavior was partly dependent on ERM activation through the SK/S1P/S1PR2 pathway [123]. This discovery will open new avenues for novel cancer treatments; EGF-dependent cancers can now be targeted from different angles. Because SK, S1PR2, and ezrin inhibitors are commercially available, they can be tested for cancer prevention or for slowing the progression of established cancers and metastases.
Table 1 lists ERM activators with well-defined biologies. Possibly, these stimuli act through the SK/S1P pathway to mediate this activation and the corresponding biology. A number of these functions have already been described to be sphingolipid-related. These data warrant additional investigations.
Highlights.
- Sphingolipids are major regulators of the ERM family of Proteins
- ERM family of proteins play essential roles in modulating the cell cytoskeleton
- While ceramide is a major de-activator of these proteins, S1P is a potent inducer
- This regulation reveals a new understanding of many sphingolipid-mediated functions
Abbreviations
- UV
Ultraviolet light
- S1P
sphingosine-1-phosphate
- SPT
Serine Palmitoyl Transferase
- CerS
Ceramide synthase
- SK
Sphingosine Kinase
- PKH
Phosphoinositide-dependent protein kinase (PDK) Kinase Homolog
- SNARE
Soluble NSF attachment protein receptor
- S1PR
S1P receptor
- PKA
Protein Kinase A
- NHE
Sodium Hydrogen Exchanger
- NHERF
Sodium Hydrogen Exchanger Regulatory Factor
- NPT2a
Sodium Phosphate co-transporter 2a
- PSGL-1
P-Selectin Glycoprotein Ligand-1
- MAPK
Mitogen Activated Protein Kinase
- SRE
Serum Response Element
- WBC
White blood cells
- NK
Natural Killer Cells
- TNF
Tumor Necrosis Factor
- HIV
Human Immunodeficiency Virus
- GIST
Gastro-Intestinal Stromal Tumors
- PI3K
Phospho-Inositide-3-Kinase
- MMP
Matrix Metallo-Proteinase
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- IκB
inhibitor of NF-κB
- L1CAM
L1 Cell adhesion molecule
- PRL-3
Phosphatase of Regenerating Liver-3
- PIP2
Phosphatidylinositol 4,5-bisphosphate
- CD
Cluster of Differentiation
- SDF-1
Stroma Derived Factor 1 alpha
- PP
Protein Phosphatase
- bSMase
Bacterial Sphingomyelinase
- CAPP
Ceramide Activated Protein Phosphatase
- C1P
Ceramide-1-Phosphate
- PLC
Phospholipase C
- MYPT
Myosin Phosphatase Targeting Protein
- bCDase
Bacterial Ceramidase
- EC
Endothelial Cells
- PKC
Protein Kinase C
- EGF
Epithelial Growth Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This article is part of a Special Issue entitled New Frontiers in Sphingolipid Biology
Funding: This manuscript is based upon work supported in part by a MERIT Award, [BX000156] (LMO) by the Office of Research and Development, Department of Veterans Affairs, Northport VA Medical Center, Northport, NY. The content of this material does not represent the views of the Department of Veterans Affairs or the United States Government; and National Cancer Institute (PO1-CA97132 LMO]) and National Institutes of Health National Institute of General Medical Sciences (R01-GM062887 [LMO]).
References
- 1.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature reviews. Molecular cell biology. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 2.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Advances in experimental medicine and biology. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao RP, Acharya JK. Sphingolipids and membrane biology as determined from genetic models. Prostaglandins & other lipid mediators. 2008;85:1–16. doi: 10.1016/j.prostaglandins.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nature reviews. Molecular cell biology. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 5.Tsukita S, Yonemura S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Current opinion in cell biology. 1997;9:70–75. doi: 10.1016/s0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]
- 6.Endo K, Kondo S, Shackleford J, Horikawa T, Kitagawa N, Yoshizaki T, Furukawa M, Zen Y, Pagano JS. Phosphorylated ezrin is associated with EBV latent membrane protein 1 in nasopharyngeal carcinoma and induces cell migration. Oncogene. 2009;28:1725–1735. doi: 10.1038/onc.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamb RF, Ozanne BW, Roy C, McGarry L, Stipp C, Mangeat P, Jay DG. Essential functions of ezrin in maintenance of cell shape and lamellipodial extension in normal and transformed fibroblasts. Current biology : CB. 1997;7:682–688. doi: 10.1016/s0960-9822(06)00295-8. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Nance MR, Kulikauskas R, Nyberg K, Fehon R, Karplus PA, Bretscher A, Tesmer JJ. Self-masking in an intact ERM-merlin protein: an active role for the central alpha-helical domain. Journal of molecular biology. 2007;365:1446–1459. doi: 10.1016/j.jmb.2006.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prag S, Parsons M, Keppler MD, Ameer-Beg SM, Barber P, Hunt J, Beavil AJ, Calvert R, Arpin M, Vojnovic B, Ng T. Activated ezrin promotes cell migration through recruitment of the GEF Dbl to lipid rafts and preferential downstream activation of Cdc42. Molecular biology of the cell. 2007;18:2935–2948. doi: 10.1091/mbc.E06-11-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serrador JM, Alonso-Lebrero JL, del Pozo MA, Furthmayr H, Schwartz-Albiez R, Calvo J, Lozano F, Sanchez-Madrid F. Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization. The Journal of cell biology. 1997;138:1409–1423. doi: 10.1083/jcb.138.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zohar R, Suzuki N, Suzuki K, Arora P, Glogauer M, McCulloch CA, Sodek J. Intracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migration. Journal of cellular physiology. 2000;184:118–130. doi: 10.1002/(SICI)1097-4652(200007)184:1<118::AID-JCP13>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 12.Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. The Journal of biological chemistry. 2002;277:25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 13.Causeret C, Geeraert L, Van der Hoeven G, Mannaerts GP, Van Veldhoven PP. Further characterization of rat dihydroceramide desaturase: tissue distribution, subcellular localization, and substrate specificity. Lipids. 2000;35:1117–1125. doi: 10.1007/s11745-000-0627-6. [DOI] [PubMed] [Google Scholar]
- 14.Raas-Rothschild A, Pankova-Kholmyansky I, Kacher Y, Futerman AH. Glycosphingolipidoses: beyond the enzymatic defect. Glycoconjugate journal. 2004;21:295–304. doi: 10.1023/B:GLYC.0000046272.38480.ef. [DOI] [PubMed] [Google Scholar]
- 15.Tafesse FG, Ternes P, Holthuis JC. The multigenic sphingomyelin synthase family. The Journal of biological chemistry. 2006;281:29421–29425. doi: 10.1074/jbc.R600021200. [DOI] [PubMed] [Google Scholar]
- 16.Wijesinghe DS, Massiello A, Subramanian P, Szulc Z, Bielawska A, Chalfant CE. Substrate specificity of human ceramide kinase. Journal of lipid research. 2005;46:2706–2716. doi: 10.1194/jlr.M500313-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Marchesini N, Hannun YA. Acid and neutral sphingomyelinases: roles and mechanisms of regulation. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2004;82:27–44. doi: 10.1139/o03-091. [DOI] [PubMed] [Google Scholar]
- 18.Hakomori S. Traveling for the glycosphingolipid path. Glycoconjugate journal. 2000;17:627–647. doi: 10.1023/a:1011086929064. [DOI] [PubMed] [Google Scholar]
- 19.Ichikawa S, Hirabayashi Y. Glucosylceramide synthase and glycosphingolipid synthesis. Trends in cell biology. 1998;8:198–202. doi: 10.1016/s0962-8924(98)01249-5. [DOI] [PubMed] [Google Scholar]
- 20.Xu R, Jin J, Hu W, Sun W, Bielawski J, Szulc Z, Taha T, Obeid LM, Mao C. Golgi alkaline ceramidase regulates cell proliferation and survival by controlling levels of sphingosine and S1P. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:1813–1825. doi: 10.1096/fj.05-5689com. [DOI] [PubMed] [Google Scholar]
- 21.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochimica et biophysica acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Johnson KR, Johnson KY, Becker KP, Bielawski J, Mao C, Obeid LM. Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. The Journal of biological chemistry. 2003;278:34541–34547. doi: 10.1074/jbc.M301741200. [DOI] [PubMed] [Google Scholar]
- 23.Bandhuvula P, Saba JD. Sphingosine-1-phosphate lyase in immunity and cancer: silencing the siren. Trends in molecular medicine. 2007;13:210–217. doi: 10.1016/j.molmed.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Zanolari B, Friant S, Funato K, Sutterlin C, Stevenson BJ, Riezman H. Sphingoid base synthesis requirement for endocytosis in Saccharomyces cerevisiae. The EMBO journal. 2000;19:2824–2833. doi: 10.1093/emboj/19.12.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubler E, Riezman H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. The EMBO journal. 1993;12:2855–2862. doi: 10.1002/j.1460-2075.1993.tb05947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friant S, Lombardi R, Schmelzle T, Hall MN, Riezman H. Sphingoid base signaling via Pkh kinases is required for endocytosis in yeast. The EMBO journal. 2001;20:6783–6792. doi: 10.1093/emboj/20.23.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friant S, Zanolari B, Riezman H. Increased protein kinase or decreased PP2A activity bypasses sphingoid base requirement in endocytosis. The EMBO journal. 2000;19:2834–2844. doi: 10.1093/emboj/19.12.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grote E, Vlacich G, Pypaert M, Novick PJ. A snc1 endocytosis mutant: phenotypic analysis and suppression by overproduction of dihydrosphingosine phosphate lyase. Molecular biology of the cell. 2000;11:4051–4065. doi: 10.1091/mbc.11.12.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Wessels D, Daniels KJ, Alexander H, Alexander S, Soll DR. Sphingosine-1-phosphate plays a role in the suppression of lateral pseudopod formation during Dictyostelium discoideum cell migration and chemotaxis. Cell motility and the cytoskeleton. 2004;59:227–241. doi: 10.1002/cm.20035. [DOI] [PubMed] [Google Scholar]
- 30.Li G, Foote C, Alexander S, Alexander H. Sphingosine-1-phosphate lyase has a central role in the development of Dictyostelium discoideum. Development. 2001;128:3473–3483. doi: 10.1242/dev.128.18.3473. [DOI] [PubMed] [Google Scholar]
- 31.Proszynski TJ, Klemm RW, Gravert M, Hsu PP, Gloor Y, Wagner J, Kozak K, Grabner H, Walzer K, Bagnat M, Simons K, Walch-Solimena C. A genome-wide visual screen reveals a role for sphingolipids and ergosterol in cell surface delivery in yeast. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17981–17986. doi: 10.1073/pnas.0509107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohrbough J, Rushton E, Palanker L, Woodruff E, Matthies HJ, Acharya U, Acharya JK, Broadie K. Ceramidase regulates synaptic vesicle exocytosis and trafficking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:7789–7803. doi: 10.1523/JNEUROSCI.1146-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosaka N, Iguchi H, Hagiwara K, Yoshioka Y, Takeshita F, Ochiya T. Neutral Sphingomyelinase 2 (nSMase2)-dependent Exosomal Transfer of Angiogenic MicroRNAs Regulate Cancer Cell Metastasis. The Journal of biological chemistry. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. The Journal of biological chemistry. 2012;287:10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gandy KA, Obeid LM. Regulation of the sphingosine kinase/sphingosine 1-phosphate pathway. Handbook of experimental pharmacology. 2013;216:275–303. doi: 10.1007/978-3-7091-1511-4_14. [DOI] [PubMed] [Google Scholar]
- 36.Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PloS one. 2012;7:e38941. doi: 10.1371/journal.pone.0038941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nature reviews. Molecular cell biology. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 38.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 39.Aarthi JJ, Darendeliler MA, Pushparaj PN. Dissecting the role of the S1P/S1PR axis in health and disease. Journal of dental research. 2011;90:841–854. doi: 10.1177/0022034510389178. [DOI] [PubMed] [Google Scholar]
- 40.Woodward AM, Crouch DH. Cellular distributions of the ERM proteins in MDCK epithelial cells: regulation by growth and cytoskeletal integrity. Cell biology international. 2001;25:205–213. doi: 10.1006/cbir.2000.0635. [DOI] [PubMed] [Google Scholar]
- 41.Gautreau A, Louvard D, Arpin M. Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane. The Journal of cell biology. 2000;150:193–203. doi: 10.1083/jcb.150.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hatzoglou A, Ader I, Splingard A, Flanders J, Saade E, Leroy I, Traver S, Aresta S, de Gunzburg J. Gem associates with Ezrin and acts via the Rho-GAP protein Gmip to down-regulate the Rho pathway. Molecular biology of the cell. 2007;18:1242–1252. doi: 10.1091/mbc.E06-06-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamane J, Ohnishi H, Sasaki H, Narimatsu H, Ohgushi H, Tachibana K. Formation of microvilli and phosphorylation of ERM family proteins by CD43, a potent inhibitor for cell adhesion: cell detachment is a potential cue for ERM phosphorylation and organization of cell morphology. Cell adhesion & migration. 2011;5:119–132. doi: 10.4161/cam.5.2.13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Chen W, Zhao C, Huo R, Guo XJ, Lin M, Huang XY, Mao YD, Zhou ZM, Sha JH. The role of ezrin-associated protein network in human sperm capacitation. Asian journal of andrology. 2010;12:667–676. doi: 10.1038/aja.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carreno S, Kouranti I, Glusman ES, Fuller MT, Echard A, Payre F. Moesin and its activating kinase Slik are required for cortical stability and microtubule organization in mitotic cells. The Journal of cell biology. 2008;180:739–746. doi: 10.1083/jcb.200709161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dard N, Louvet-Vallee S, Santa-Maria A, Maro B. Phosphorylation of ezrin on threonine T567 plays a crucial role during compaction in the mouse early embryo. Developmental biology. 2004;271:87–97. doi: 10.1016/j.ydbio.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 47.Link V, Carvalho L, Castanon I, Stockinger P, Shevchenko A, Heisenberg CP. Identification of regulators of germ layer morphogenesis using proteomics in zebrafish. Journal of cell science. 2006;119:2073–2083. doi: 10.1242/jcs.02928. [DOI] [PubMed] [Google Scholar]
- 48.Diez-Revuelta N, Velasco S, Andre S, Kaltner H, Kubler D, Gabius HJ, Abad-Rodriguez J. Phosphorylation of adhesion- and growth-regulatory human galectin-3 leads to the induction of axonal branching by local membrane L1 and ERM redistribution. Journal of cell science. 2010;123:671–681. doi: 10.1242/jcs.058198. [DOI] [PubMed] [Google Scholar]
- 49.Antoine-Bertrand J, Ghogha A, Luangrath V, Bedford FK, Lamarche-Vane N. The activation of ezrin-radixin-moesin proteins is regulated by netrin-1 through Src kinase and RhoA/Rho kinase activities and mediates netrin-1-induced axon outgrowth. Molecular biology of the cell. 2011;22:3734–3746. doi: 10.1091/mbc.E10-11-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeong HJ, Kim JH, Jeon S. Amphetamine-induced ERM Proteins Phosphorylation Is through PKCbeta Activation in PC12 Cells. The Korean journal of physiology & pharmacology : official journal of the Korean Physiological Society and the Korean Society of Pharmacology. 2011;15:245–249. doi: 10.4196/kjpp.2011.15.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim HS, Bae CD, Park J. Glutamate receptor-mediated phosphorylation of ezrin/radixin/moesin proteins is implicated in filopodial protrusion of primary cultured hippocampal neuronal cells. Journal of neurochemistry. 2010;113:1565–1576. doi: 10.1111/j.1471-4159.2010.06713.x. [DOI] [PubMed] [Google Scholar]
- 52.Parisiadou L, Xie C, Cho HJ, Lin X, Gu XL, Long CX, Lobbestael E, Baekelandt V, Taymans JM, Sun L, Cai H. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:13971–13980. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou R, Zhu L, Kodani A, Hauser P, Yao X, Forte JG. Phosphorylation of ezrin on threonine 567 produces a change in secretory phenotype and repolarizes the gastric parietal cell. Journal of cell science. 2005;118:4381–4391. doi: 10.1242/jcs.02559. [DOI] [PubMed] [Google Scholar]
- 54.Zhu L, Crothers J, Jr., Zhou R, Forte JG. A possible mechanism for ezrin to establish epithelial cell polarity. American journal of physiology. Cell physiology. 2010;299:C431–443. doi: 10.1152/ajpcell.00090.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou R, Cao X, Watson C, Miao Y, Guo Z, Forte JG, Yao X. Characterization of protein kinase A-mediated phosphorylation of ezrin in gastric parietal cell activation. The Journal of biological chemistry. 2003;278:35651–35659. doi: 10.1074/jbc.M303416200. [DOI] [PubMed] [Google Scholar]
- 56.Jin C, Ge L, Ding X, Chen Y, Zhu H, Ward T, Wu F, Cao X, Wang Q, Yao X. PKA-mediated protein phosphorylation regulates ezrin-WWOX interaction. Biochemical and biophysical research communications. 2006;341:784–791. doi: 10.1016/j.bbrc.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 57.Wu KL, Khan S, Lakhe-Reddy S, Jarad G, Mukherjee A, Obejero-Paz CA, Konieczkowski M, Sedor JR, Schelling JR. The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. The Journal of biological chemistry. 2004;279:26280–26286. doi: 10.1074/jbc.M400814200. [DOI] [PubMed] [Google Scholar]
- 58.Darmellah A, Rucker-Martin C, Feuvray D. ERM proteins mediate the effects of Na+/H+ exchanger (NHE1) activation in cardiac myocytes. Cardiovascular research. 2009;81:294–300. doi: 10.1093/cvr/cvn320. [DOI] [PubMed] [Google Scholar]
- 59.Di Sole F, Babich V, Moe OW. The calcineurin homologous protein-1 increases Na(+)/H(+) -exchanger 3 trafficking via ezrin phosphorylation. Journal of the American Society of Nephrology : JASN. 2009;20:1776–1786. doi: 10.1681/ASN.2008121255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahon MJ. Ezrin promotes functional expression and parathyroid hormone-mediated regulation of the sodium-phosphate cotransporter 2a in LLC-PK1 cells. American journal of physiology. Renal physiology. 2008;294:F667–675. doi: 10.1152/ajprenal.00276.2007. [DOI] [PubMed] [Google Scholar]
- 61.Lopez JP, Turner JR, Philipson LH. Glucose-induced ERM protein activation and translocation regulates insulin secretion. American journal of physiology. Endocrinology and metabolism. 2010;299:E772–785. doi: 10.1152/ajpendo.00199.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McRobert EA, Gallicchio M, Jerums G, Cooper ME, Bach LA. The amino-terminal domains of the ezrin, radixin, and moesin (ERM) proteins bind advanced glycation end products, an interaction that may play a role in the development of diabetic complications. The Journal of biological chemistry. 2003;278:25783–25789. doi: 10.1074/jbc.M210433200. [DOI] [PubMed] [Google Scholar]
- 63.Huang H, Xiao Y, Lin H, Fu D, Zhan Z, Liang L, Yang X, Fan J, Ye Y, Sun L, Xu H. Increased phosphorylation of ezrin/radixin/moesin proteins contributes to proliferation of rheumatoid fibroblast-like synoviocytes. Rheumatology. 2011;50:1045–1053. doi: 10.1093/rheumatology/keq440. [DOI] [PubMed] [Google Scholar]
- 64.Singh K, Colmegna I, He X, Weyand CM, Goronzy JJ. Synoviocyte stimulation by the LFA-1-intercellular adhesion molecule-2-Ezrin-Akt pathway in rheumatoid arthritis. Journal of immunology. 2008;180:1971–1978. doi: 10.4049/jimmunol.180.3.1971. [DOI] [PubMed] [Google Scholar]
- 65.Li Y, Harada T, Juang YT, Kyttaris VC, Wang Y, Zidanic M, Tung K, Tsokos GC. Phosphorylated ERM is responsible for increased T cell polarization, adhesion, and migration in patients with systemic lupus erythematosus. Journal of immunology. 2007;178:1938–1947. doi: 10.4049/jimmunol.178.3.1938. [DOI] [PubMed] [Google Scholar]
- 66.Buckley ST, Medina C, Kasper M, Ehrhardt C. Interplay between RAGE, CD44, and focal adhesion molecules in epithelial-mesenchymal transition of alveolar epithelial cells. American journal of physiology. Lung cellular and molecular physiology. 2011;300:L548–559. doi: 10.1152/ajplung.00230.2010. [DOI] [PubMed] [Google Scholar]
- 67.Moon Y, Kim JY, Choi SY, Kim K, Kim H, Sun W. Induction of ezrin-radixin-moesin molecules after cryogenic traumatic brain injury of the mouse cortex. Neuroreport. 2011;22:304–308. doi: 10.1097/WNR.0b013e3283460265. [DOI] [PubMed] [Google Scholar]
- 68.Spertini C, Baisse B, Spertini O. Ezrin-radixin-moesin-binding sequence of PSGL-1 glycoprotein regulates leukocyte rolling on selectins and activation of extracellular signal-regulated kinases. The Journal of biological chemistry. 2012;287:10693–10702. doi: 10.1074/jbc.M111.318022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Urzainqui A, Serrador JM, Viedma F, Yanez-Mo M, Rodriguez A, Corbi AL, Alonso-Lebrero JL, Luque A, Deckert M, Vazquez J, Sanchez-Madrid F. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity. 2002;17:401–412. doi: 10.1016/s1074-7613(02)00420-x. [DOI] [PubMed] [Google Scholar]
- 70.Liu Y, Belkina NV, Park C, Nambiar R, Loughhead SM, Patino-Lopez G, Ben-Aissa K, Hao JJ, Kruhlak MJ, Qi H, von Andrian UH, Kehrl JH, Tyska MJ, Shaw S. Constitutively active ezrin increases membrane tension, slows migration, and impedes endothelial transmigration of lymphocytes in vivo in mice. Blood. 2012;119:445–453. doi: 10.1182/blood-2011-07-368860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parameswaran N, Matsui K, Gupta N. Conformational switching in ezrin regulates morphological and cytoskeletal changes required for B cell chemotaxis. Journal of immunology. 2011;186:4088–4097. doi: 10.4049/jimmunol.1001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kramer B, Schulte D, Korner C, Zwank C, Hartmann A, Michalk M, Sohne J, Langhans B, Nischalke HD, Coenen M, Mohl C, Vogt A, Hennenberg M, Sauerbruch T, Spengler U, Nattermann J. Regulation of NK cell trafficking by CD81. European journal of immunology. 2009;39:3447–3458. doi: 10.1002/eji.200939234. [DOI] [PubMed] [Google Scholar]
- 73.Lee JH, Katakai T, Hara T, Gonda H, Sugai M, Shimizu A. Roles of p-ERM and Rho-ROCK signaling in lymphocyte polarity and uropod formation. The Journal of cell biology. 2004;167:327–337. doi: 10.1083/jcb.200403091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Serrador JM, Vicente-Manzanares M, Calvo J, Barreiro O, Montoya MC, Schwartz-Albiez R, Furthmayr H, Lozano F, Sanchez-Madrid F. A novel serine-rich motif in the intercellular adhesion molecule 3 is critical for its ezrin/radixin/moesin-directed subcellular targeting. The Journal of biological chemistry. 2002;277:10400–10409. doi: 10.1074/jbc.M110694200. [DOI] [PubMed] [Google Scholar]
- 75.Adyshev DM, Moldobaeva NK, Elangovan VR, Garcia JG, Dudek SM. Differential involvement of ezrin/radixin/moesin proteins in sphingosine 1-phosphate-induced human pulmonary endothelial cell barrier enhancement. Cellular signalling. 2011;23:2086–2096. doi: 10.1016/j.cellsig.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bogatcheva NV, Zemskova MA, Gorshkov BA, Kim KM, Daglis GA, Poirier C, Verin AD. Ezrin, radixin, and moesin are phosphorylated in response to 2-methoxyestradiol and modulate endothelial hyperpermeability. American journal of respiratory cell and molecular biology. 2011;45:1185–1194. doi: 10.1165/rcmb.2011-0092OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koss M, Pfeiffer GR, 2nd, Wang Y, Thomas ST, Yerukhimovich M, Gaarde WA, Doerschuk CM, Wang Q. Ezrin/radixin/moesin proteins are phosphorylated by TNF-alpha and modulate permeability increases in human pulmonary microvascular endothelial cells. Journal of immunology. 2006;176:1218–1227. doi: 10.4049/jimmunol.176.2.1218. [DOI] [PubMed] [Google Scholar]
- 78.Guo X, Wang L, Chen B, Li Q, Wang J, Zhao M, Wu W, Zhu P, Huang X, Huang Q. ERM protein moesin is phosphorylated by advanced glycation end products and modulates endothelial permeability. American journal of physiology. Heart and circulatory physiology. 2009;297:H238–246. doi: 10.1152/ajpheart.00196.2009. [DOI] [PubMed] [Google Scholar]
- 79.Swanson KA, Crane DD, Caldwell HD. Chlamydia trachomatis species-specific induction of ezrin tyrosine phosphorylation functions in pathogen entry. Infection and immunity. 2007;75:5669–5677. doi: 10.1128/IAI.01096-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janket ML, DeRicco JS, Borowski L, Ayyavoo V. Human immunodeficiency virus (HIV-1) Vpr induced downregulation of NHE1 induces alteration in intracellular pH and loss of ERM complex in target cells. Virus research. 2007;126:76–85. doi: 10.1016/j.virusres.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luciani F, Matarrese P, Giammarioli AM, Lugini L, Lozupone F, Federici C, Iessi E, Malorni W, Fais S. CD95/phosphorylated ezrin association underlies HIV-1 GP120/IL-2-induced susceptibility to CD95(APO-1/Fas)-mediated apoptosis of human resting CD4(+)T lymphocytes. Cell death and differentiation. 2004;11:574–582. doi: 10.1038/sj.cdd.4401374. [DOI] [PubMed] [Google Scholar]
- 82.Pust S, Morrison H, Wehland J, Sechi AS, Herrlich P. Listeria monocytogenes exploits ERM protein functions to efficiently spread from cell to cell. The EMBO journal. 2005;24:1287–1300. doi: 10.1038/sj.emboj.7600595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Simonovic I, Arpin M, Koutsouris A, Falk-Krzesinski HJ, Hecht G. Enteropathogenic Escherichia coli activates ezrin, which participates in disruption of tight junction barrier function. Infection and immunity. 2001;69:5679–5688. doi: 10.1128/IAI.69.9.5679-5688.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goosney DL, DeVinney R, Finlay BB. Recruitment of cytoskeletal and signaling proteins to enteropathogenic and enterohemorrhagic Escherichia coli pedestals. Infection and immunity. 2001;69:3315–3322. doi: 10.1128/IAI.69.5.3315-3322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hong SH, Osborne T, Ren L, Briggs J, Mazcko C, Burkett SS, Khanna C. Protein kinase C regulates ezrin-radixin-moesin phosphorylation in canine osteosarcoma cells. Veterinary and comparative oncology. 2011;9:207–218. doi: 10.1111/j.1476-5829.2010.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang HY, Li CF, Fang FM, Tsai JW, Li SH, Lee YT, Wei HM. Prognostic implication of ezrin overexpression in myxofibrosarcomas. Annals of surgical oncology. 2010;17:3212–3219. doi: 10.1245/s10434-010-1185-y. [DOI] [PubMed] [Google Scholar]
- 87.Wei YC, Li CF, Yu SC, Chou FF, Fang FM, Eng HL, Uen YH, Tian YF, Wu JM, Li SH, Huang WW, Li WM, Huang HY. Ezrin overexpression in gastrointestinal stromal tumors: an independent adverse prognosticator associated with the non-gastric location. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2009;22:1351–1360. doi: 10.1038/modpathol.2009.107. [DOI] [PubMed] [Google Scholar]
- 88.Gao SY, Li EM, Cui L, Lu XF, Meng LY, Yuan HM, Xie JJ, Du ZP, Pang JX, Xu LY. Sp1 and AP-1 regulate expression of the human gene VIL2 in esophageal carcinoma cells. The Journal of biological chemistry. 2009;284:7995–8004. doi: 10.1074/jbc.M809734200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Z, Fadiel A, Feng Y, Ohtani K, Rutherford T, Naftolin F. Ovarian epithelial carcinoma tyrosine phosphorylation, cell proliferation, and ezrin translocation are stimulated by interleukin 1alpha and epidermal growth factor. Cancer. 2001;92:3068–3075. doi: 10.1002/1097-0142(20011215)92:12<3068::aid-cncr10149>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 90.Chen Y, Wang D, Guo Z, Zhao J, Wu B, Deng H, Zhou T, Xiang H, Gao F, Yu X, Liao J, Ward T, Xia P, Emenari C, Ding X, Thompson W, Ma K, Zhu J, Aikhionbare F, Dou K, Cheng SY, Yao X. Rho kinase phosphorylation promotes ezrin-mediated metastasis in hepatocellular carcinoma. Cancer research. 2011;71:1721–1729. doi: 10.1158/0008-5472.CAN-09-4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng S, Huang J, Zhou K, Zhang C, Xiang Q, Tan Z, Wang T, Fu X. 17beta-Estradiol enhances breast cancer cell motility and invasion via extra-nuclear activation of actin-binding protein ezrin. PloS one. 2011;6:e22439. doi: 10.1371/journal.pone.0022439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang F, Zou F, Peng Z, Huang D, Wu Y, Chen Y, Duan C, Cao Y, Mei W, Tang X, Dong Z. N,N'-dinitrosopiperazine-mediated ezrin protein phosphorylation via activation of Rho kinase and protein kinase C is involved in metastasis of nasopharyngeal carcinoma 6-10B cells. The Journal of biological chemistry. 2011;286:36956–36967. doi: 10.1074/jbc.M111.259234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ren L, Hong SH, Cassavaugh J, Osborne T, Chou AJ, Kim SY, Gorlick R, Hewitt SM, Khanna C. The actin-cytoskeleton linker protein ezrin is regulated during osteosarcoma metastasis by PKC. Oncogene. 2009;28:792–802. doi: 10.1038/onc.2008.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ren L, Hong SH, Chen QR, Briggs J, Cassavaugh J, Srinivasan S, Lizardo MM, Mendoza A, Xia AY, Avadhani N, Khan J, Khanna C. Dysregulation of ezrin phosphorylation prevents metastasis and alters cellular metabolism in osteosarcoma. Cancer research. 2012;72:1001–1012. doi: 10.1158/0008-5472.CAN-11-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, Yeung C, Gorlick R, Hewitt SM, Helman LJ. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nature medicine. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- 96.Di Cristofano C, Leopizzi M, Miraglia A, Sardella B, Moretti V, Ferrara A, Petrozza V, Della Rocca C. Phosphorylated ezrin is located in the nucleus of the osteosarcoma cell. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2010;23:1012–1020. doi: 10.1038/modpathol.2010.77. [DOI] [PubMed] [Google Scholar]
- 97.Forte E, Orsatti L, Talamo F, Barbato G, De Francesco R, Tomei L. Ezrin is a specific and direct target of protein tyrosine phosphatase PRL-3. Biochimica et biophysica acta. 2008;1783:334–344. doi: 10.1016/j.bbamcr.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 98.Lan M, Kojima T, Murata M, Osanai M, Takano K, Chiba H, Sawada N. Phosphorylation of ezrin enhances microvillus length via a p38 MAP-kinase pathway in an immortalized mouse hepatic cell line. Experimental cell research. 2006;312:111–120. doi: 10.1016/j.yexcr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 99.Hiscox S, Jiang WG. Ezrin regulates cell-cell and cell-matrix adhesion, a possible role with E-cadherin/beta-catenin. Journal of cell science. 1999;112(Pt 18):3081–3090. doi: 10.1242/jcs.112.18.3081. [DOI] [PubMed] [Google Scholar]
- 100.Rebillard A, Jouan-Lanhouet S, Jouan E, Legembre P, Pizon M, Sergent O, Gilot D, Tekpli X, Lagadic-Gossmann D, Dimanche-Boitrel MT. Cisplatin-induced apoptosis involves a Fas-ROCK-ezrin-dependent actin remodelling in human colon cancer cells. European journal of cancer. 2010;46:1445–1455. doi: 10.1016/j.ejca.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 101.Elliott BE, Meens JA, SenGupta SK, Louvard D, Arpin M. The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast cancer research : BCR. 2005;7:R365–373. doi: 10.1186/bcr1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luo Y, Zheng C, Zhang J, Lu D, Zhuang J, Xing S, Feng J, Yang D, Yan X. Recognition of CD146 as an ERM-binding protein offers novel mechanisms for melanoma cell migration. Oncogene. 2012;31:306–321. doi: 10.1038/onc.2011.244. [DOI] [PubMed] [Google Scholar]
- 103.Heiska L, Melikova M, Zhao F, Saotome I, McClatchey AI, Carpen O. Ezrin is key regulator of Src-induced malignant phenotype in three-dimensional environment. Oncogene. 2011;30:4953–4962. doi: 10.1038/onc.2011.207. [DOI] [PubMed] [Google Scholar]
- 104.Elliott BE, Qiao H, Louvard D, Arpin M. Co-operative effect of c-Src and ezrin in deregulation of cell-cell contacts and scattering of mammary carcinoma cells. Journal of cellular biochemistry. 2004;92:16–28. doi: 10.1002/jcb.20033. [DOI] [PubMed] [Google Scholar]
- 105.Chuan YC, Iglesias-Gato D, Fernandez-Perez L, Cedazo-Minguez A, Pang ST, Norstedt G, Pousette A, Flores-Morales A. Ezrin mediates c-Myc actions in prostate cancer cell invasion. Oncogene. 2010;29:1531–1542. doi: 10.1038/onc.2009.442. [DOI] [PubMed] [Google Scholar]
- 106.Li Q, Wu M, Wang H, Xu G, Zhu T, Zhang Y, Liu P, Song A, Gang C, Han Z, Zhou J, Meng L, Lu Y, Wang S, Ma D. Ezrin silencing by small hairpin RNA reverses metastatic behaviors of human breast cancer cells. Cancer letters. 2008;261:55–63. doi: 10.1016/j.canlet.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 107.Sizemore S, Cicek M, Sizemore N, Ng KP, Casey G. Podocalyxin increases the aggressive phenotype of breast and prostate cancer cells in vitro through its interaction with ezrin. Cancer research. 2007;67:6183–6191. doi: 10.1158/0008-5472.CAN-06-3575. [DOI] [PubMed] [Google Scholar]
- 108.Wick W, Grimmel C, Wild-Bode C, Platten M, Arpin M, Weller M. Ezrin-dependent promotion of glioma cell clonogenicity, motility, and invasion mediated by BCL-2 and transforming growth factor-beta2. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:3360–3368. doi: 10.1523/JNEUROSCI.21-10-03360.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gavert N, Ben-Shmuel A, Lemmon V, Brabletz T, Ben-Ze'ev A. Nuclear factor-kappaB signaling and ezrin are essential for L1-mediated metastasis of colon cancer cells. Journal of cell science. 2010;123:2135–2143. doi: 10.1242/jcs.069542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma L, Liu YP, Zhang XH, Xing LX, Wang JL, Geng CZ. Effect of RhoA signaling transduction on expression of Ezrin in breast cancer cell lines. Ai zheng = Aizheng = Chinese journal of cancer. 2009;28:108–111. [PubMed] [Google Scholar]
- 111.Weng WH, Yeh CN, Cheng YF, Lenka G, Liaw YW. Phosphorylated T567 ezrin is associated with merlin expression in KIT-mutant gastrointestinal stromal tumors. Molecular medicine reports. 2012;5:17–21. doi: 10.3892/mmr.2011.609. [DOI] [PubMed] [Google Scholar]
- 112.Charrin S, Alcover A. Role of ERM (ezrin-radixin-moesin) proteins in T lymphocyte polarization, immune synapse formation and in T cell receptor-mediated signaling. Front Biosci. 2006;11:1987–1997. doi: 10.2741/1940. [DOI] [PubMed] [Google Scholar]
- 113.Shcherbina A, Bretscher A, Kenney DM, Remold-O'Donnell E. Moesin, the major ERM protein of lymphocytes and platelets, differs from ezrin in its insensitivity to calpain. FEBS Lett. 1999;443:31–36. doi: 10.1016/s0014-5793(98)01674-3. [DOI] [PubMed] [Google Scholar]
- 114.Brown MJ, Nijhara R, Hallam JA, Gignac M, Yamada KM, Erlandsen SL, Delon J, Kruhlak M, Shaw S. Chemokine stimulation of human peripheral blood T lymphocytes induces rapid dephosphorylation of ERM proteins, which facilitates loss of microvilli and polarization. Blood. 2003;102:3890–3899. doi: 10.1182/blood-2002-12-3807. [DOI] [PubMed] [Google Scholar]
- 115.Nijhara R, van Hennik PB, Gignac ML, Kruhlak MJ, Hordijk PL, Delon J, Shaw S. Rac1 mediates collapse of microvilli on chemokine-activated T lymphocytes. J Immunol. 2004;173:4985–4993. doi: 10.4049/jimmunol.173.8.4985. [DOI] [PubMed] [Google Scholar]
- 116.Hao JJ, Liu Y, Kruhlak M, Debell KE, Rellahan BL, Shaw S. Phospholipase C-mediated hydrolysis of PIP2 releases ERM proteins from lymphocyte membrane. J Cell Biol. 2009;184:451–462. doi: 10.1083/jcb.200807047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeidan YH, Jenkins RW, Hannun YA. Remodeling of cellular cytoskeleton by the acid sphingomyelinase/ceramide pathway. The Journal of cell biology. 2008;181:335–350. doi: 10.1083/jcb.200705060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fukata Y, Kimura K, Oshiro N, Saya H, Matsuura Y, Kaibuchi K. Association of the myosin-binding subunit of myosin phosphatase and moesin: dual regulation of moesin phosphorylation by Rho-associated kinase and myosin phosphatase. J Cell Biol. 1998;141:409–418. doi: 10.1083/jcb.141.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Canals D, Roddy P, Hannun YA. Protein phosphatase 1alpha mediates ceramide-induced ERM protein dephosphorylation: a novel mechanism independent of phosphatidylinositol 4, 5-biphosphate (PIP2) and myosin/ERM phosphatase. The Journal of biological chemistry. 2012;287:10145–10155. doi: 10.1074/jbc.M111.306456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bulut G, Hong SH, Chen K, Beauchamp EM, Rahim S, Kosturko GW, Glasgow E, Dakshanamurthy S, Lee HS, Daar I, Toretsky JA, Khanna C, Uren A. Small molecule inhibitors of ezrin inhibit the invasive phenotype of osteosarcoma cells. Oncogene. 2012;31:269–281. doi: 10.1038/onc.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Canals D, Jenkins RW, Roddy P, Hernandez-Corbacho MJ, Obeid LM, Hannun YA. Differential effects of ceramide and sphingosine 1-phosphate on ERM phosphorylation: probing sphingolipid signaling at the outer plasma membrane. The Journal of biological chemistry. 2010;285:32476–32485. doi: 10.1074/jbc.M110.141028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gandy KA, Canals D, Adada M, Wada M, Roddy P, Snider AJ, Hannun YA, Obeid LM. Sphingosine 1-phosphate induces filopodia formation through S1PR2 activation of ERM proteins. The Biochemical journal. 2013;449:661–672. doi: 10.1042/BJ20120213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Orr Gandy KA, Adada M, Canals D, Carroll B, Roddy P, Hannun YA, Obeid LM. Epidermal growth factor-induced cellular invasion requires sphingosine-1-phosphate/sphingosine-1-phosphate 2 receptor-mediated ezrin activation. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013 doi: 10.1096/fj.13-228460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wald FA, Oriolo AS, Mashukova A, Fregien NL, Langshaw AH, Salas PJ. Atypical protein kinase C (iota) activates ezrin in the apical domain of intestinal epithelial cells. Journal of cell science. 2008;121:644–654. doi: 10.1242/jcs.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tamma G, Procino G, Svelto M, Valenti G. Hypotonicity causes actin reorganization and recruitment of the actin-binding ERM protein moesin in membrane protrusions in collecting duct principal cells. American journal of physiology. Cell physiology. 2007;292:C1476–1484. doi: 10.1152/ajpcell.00375.2006. [DOI] [PubMed] [Google Scholar]
- 126.Chuan YC, Pang ST, Cedazo-Minguez A, Norstedt G, Pousette A, Flores-Morales A. Androgen induction of prostate cancer cell invasion is mediated by ezrin. The Journal of biological chemistry. 2006;281:29938–29948. doi: 10.1074/jbc.M602237200. [DOI] [PubMed] [Google Scholar]
- 127.Nashiki K, Taketani Y, Takeichi T, Sawada N, Yamamoto H, Ichikawa M, Arai H, Miyamoto K, Takeda E. Role of membrane microdomains in PTH-mediated down-regulation of NaPi-IIa in opossum kidney cells. Kidney international. 2005;68:1137–1147. doi: 10.1111/j.1523-1755.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 128.Ng T, Parsons M, Hughes WE, Monypenny J, Zicha D, Gautreau A, Arpin M, Gschmeissner S, Verveer PJ, Bastiaens PI, Parker PJ. Ezrin is a downstream effector of trafficking PKC-integrin complexes involved in the control of cell motility. The EMBO journal. 2001;20:2723–2741. doi: 10.1093/emboj/20.11.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kim EK, Park JM, Lim S, Choi JW, Kim HS, Seok H, Seo JK, Oh K, Lee DS, Kim KT, Ryu SH, Suh PG. Activation of AMP-activated protein kinase is essential for lysophosphatidic acid-induced cell migration in ovarian cancer cells. The Journal of biological chemistry. 2011;286:24036–24045. doi: 10.1074/jbc.M110.209908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tang F, Wang D, Duan C, Huang D, Wu Y, Chen Y, Wang W, Xie C, Meng J, Wang L, Wu B, Liu S, Tian D, Zhu F, He Z, Deng F, Cao Y. Berberine inhibits metastasis of nasopharyngeal carcinoma 5-8F cells by targeting Rho kinase-mediated Ezrin phosphorylation at threonine 567. The Journal of biological chemistry. 2009;284:27456–27466. doi: 10.1074/jbc.M109.033795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hebert M, Potin S, Sebbagh M, Bertoglio J, Breard J, Hamelin J. Rho-ROCK-dependent ezrin-radixin-moesin phosphorylation regulates Fas-mediated apoptosis in Jurkat cells. Journal of immunology. 2008;181:5963–5973. doi: 10.4049/jimmunol.181.9.5963. [DOI] [PubMed] [Google Scholar]
- 132.Kishore R, Qin G, Luedemann C, Bord E, Hanley A, Silver M, Gavin M, Yoon YS, Goukassian D, Losordo DW. The cytoskeletal protein ezrin regulates EC proliferation and angiogenesis via TNF-alpha-induced transcriptional repression of cyclin A. The Journal of clinical investigation. 2005;115:1785–1796. doi: 10.1172/JCI22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tran Quang C, Gautreau A, Arpin M, Treisman R. Ezrin function is required for ROCK-mediated fibroblast transformation by the Net and Dbl oncogenes. The EMBO journal. 2000;19:4565–4576. doi: 10.1093/emboj/19.17.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kahsai AW, Zhu S, Fenteany G. G protein-coupled receptor kinase 2 activates radixin, regulating membrane protrusion and motility in epithelial cells. Biochimica et biophysica acta. 2010;1803:300–310. doi: 10.1016/j.bbamcr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hsu HH, Hoffmann S, Endlich N, Velic A, Schwab A, Weide T, Schlatter E, Pavenstadt H. Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. Journal of molecular medicine. 2008;86:1379–1394. doi: 10.1007/s00109-008-0399-y. [DOI] [PubMed] [Google Scholar]
- 136.Cant SH, Pitcher JA. G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Molecular biology of the cell. 2005;16:3088–3099. doi: 10.1091/mbc.E04-10-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baumgartner M, Sillman AL, Blackwood EM, Srivastava J, Madson N, Schilling JW, Wright JH, Barber DL. The Nck-interacting kinase phosphorylates ERM proteins for formation of lamellipodium by growth factors. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13391–13396. doi: 10.1073/pnas.0605950103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hu Z, Wang Y, Graham WV, Su L, Musch MW, Turner JR. MAPKAPK-2 is a critical signaling intermediate in NHE3 activation following Na+-glucose cotransport. The Journal of biological chemistry. 2006;281:24247–24253. doi: 10.1074/jbc.M602898200. [DOI] [PubMed] [Google Scholar]
- 139.ten Klooster JP, Jansen M, Yuan J, Oorschot V, Begthel H, Di Giacomo V, Colland F, de Koning J, Maurice MM, Hornbeck P, Clevers H. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Developmental cell. 2009;16:551–562. doi: 10.1016/j.devcel.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 140.Belkina NV, Liu Y, Hao JJ, Karasuyama H, Shaw S. LOK is a major ERM kinase in resting lymphocytes and regulates cytoskeletal rearrangement through ERM phosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4707–4712. doi: 10.1073/pnas.0805963106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakamura N, Oshiro N, Fukata Y, Amano M, Fukata M, Kuroda S, Matsuura Y, Leung T, Lim L, Kaibuchi K. Phosphorylation of ERM proteins at filopodia induced by Cdc42. Genes to cells : devoted to molecular & cellular mechanisms. 2000;5:571–581. doi: 10.1046/j.1365-2443.2000.00348.x. [DOI] [PubMed] [Google Scholar]
- 142.Coffey GP, Rajapaksa R, Liu R, Sharpe O, Kuo CC, Krauss SW, Sagi Y, Davis RE, Staudt LM, Sharman JP, Robinson WH, Levy S. Engagement of CD81 induces ezrin tyrosine phosphorylation and its cellular redistribution with filamentous actin. Journal of cell science. 2009;122:3137–3144. doi: 10.1242/jcs.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Monni R, Haddaoui L, Naba A, Gallais I, Arpin M, Mayeux P, Moreau-Gachelin F. Ezrin is a target for oncogenic Kit mutants in murine erythroleukemia. Blood. 2008;111:3163–3172. doi: 10.1182/blood-2007-09-110510. [DOI] [PubMed] [Google Scholar]
- 144.Srivastava J, Elliott BE, Louvard D, Arpin M. Src-dependent ezrin phosphorylation in adhesion-mediated signaling. Molecular biology of the cell. 2005;16:1481–1490. doi: 10.1091/mbc.E04-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang Q, Pfeiffer GR, 2nd, Gaarde WA. Activation of SRC tyrosine kinases in response to ICAM-1 ligation in pulmonary microvascular endothelial cells. The Journal of biological chemistry. 2003;278:47731–47743. doi: 10.1074/jbc.M308466200. [DOI] [PubMed] [Google Scholar]
- 146.Heiska L, Carpen O. Src phosphorylates ezrin at tyrosine 477 and induces a phosphospecific association between ezrin and a kelch-repeat protein family member. The Journal of biological chemistry. 2005;280:10244–10252. doi: 10.1074/jbc.M411353200. [DOI] [PubMed] [Google Scholar]
- 147.Autero M, Heiska L, Ronnstrand L, Vaheri A, Gahmberg CG, Carpen O. Ezrin is a substrate for Lck in T cells. FEBS letters. 2003;535:82–86. doi: 10.1016/s0014-5793(02)03861-9. [DOI] [PubMed] [Google Scholar]
- 148.Yang HS, Alexander K, Santiago P, Hinds PW. ERM proteins and Cdk5 in cellular senescence. Cell cycle. 2003;2:517–520. doi: 10.4161/cc.2.6.582. [DOI] [PubMed] [Google Scholar]
- 149.Yang HS, Hinds PW. Increased ezrin expression and activation by CDK5 coincident with acquisition of the senescent phenotype. Molecular cell. 2003;11:1163–1176. doi: 10.1016/s1097-2765(03)00135-7. [DOI] [PubMed] [Google Scholar]
- 150.Yang HS, Hinds PW. Phosphorylation of ezrin by cyclin-dependent kinase 5 induces the release of Rho GDP dissociation inhibitor to inhibit Rac1 activity in senescent cells. Cancer research. 2006;66:2708–2715. doi: 10.1158/0008-5472.CAN-05-3141. [DOI] [PubMed] [Google Scholar]