Abstract
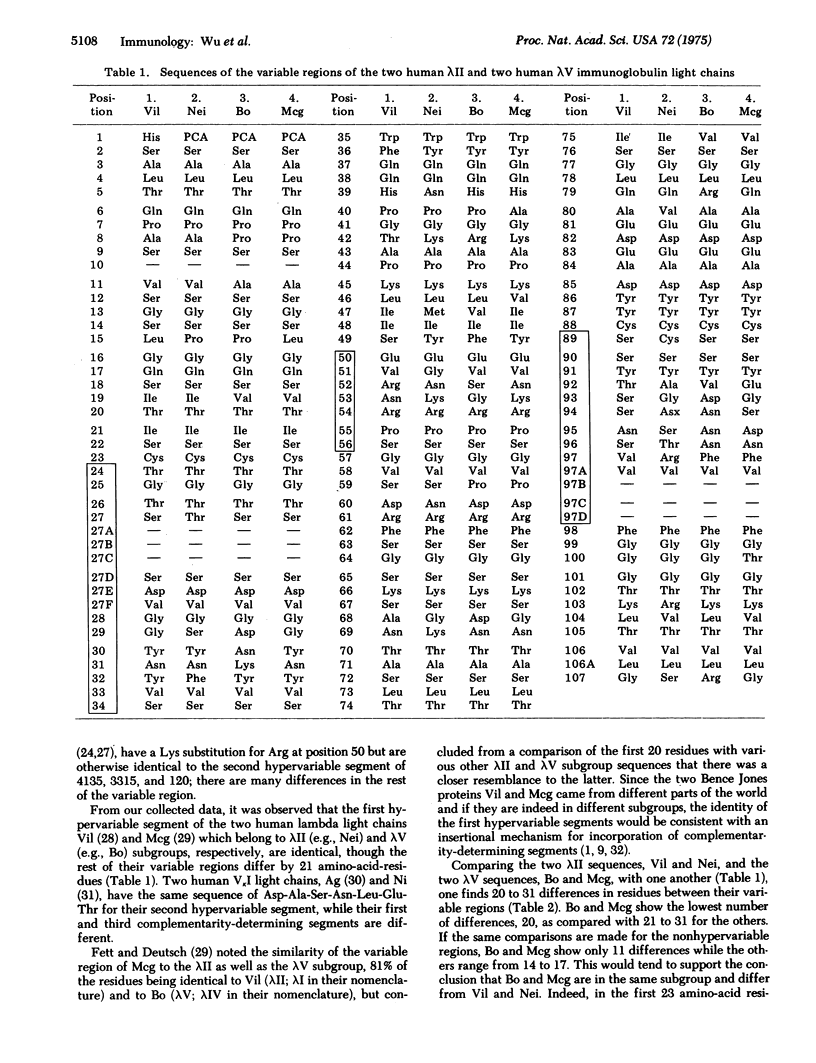
A human lambdaV (Mcg) and a human lambdaII (Vil) myeloma protein have identical sequences in their first hypervariable segments although they differ at 21 positions throughout the variable region. If a different structural gene is responsible for each subgroup, the findings favor insertion of information for the hypervariable or complementarity-determining segments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barstad P., Rudikoff S., Potter M., Cohn M., Konigsberg W., Hood L. Immunoglobulin structure: amino terminal sequences of mouse myeloma proteins that bind phosphorylcholine. Science. 1974 Mar 8;183(4128):962–966. doi: 10.1126/science.183.4128.962. [DOI] [PubMed] [Google Scholar]
- Capra J. D., Kehoe J. M. Hypervariable regions, idiotypy, and the antibody-combining site. Adv Immunol. 1975;20:1–40. doi: 10.1016/s0065-2776(08)60205-9. [DOI] [PubMed] [Google Scholar]
- Capra J. D., Kehoe J. M. Structure of antibodies with shared idiotypy: the complete sequence of the heavy chain variable regions of two immunoglobulin M anti-gamma globulins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4032–4036. doi: 10.1073/pnas.71.10.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra J. D., Kehoe J. M. Variable region sequences of five human immunoglobulin heavy chains of the VH3 subgroup: definitive identification of four heavy chain hypervariable regions. Proc Natl Acad Sci U S A. 1974 Mar;71(3):845–848. doi: 10.1073/pnas.71.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra J. D., Kunkel H. G. Amino acid sequence similarities in two human anti gamma globulin antibodies. Proc Natl Acad Sci U S A. 1970 Sep;67(1):87–92. doi: 10.1073/pnas.67.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra J. D., Tung A. S., Nisonoff A. Structural studies on induced antibodies with defined idiotypic specificities. I. The heavy chains of anti-p-azophenylarsonate antibodies from A/J mice bearing a cross-reactive idiotype. J Immunol. 1975 May;114(5):1548–1553. [PubMed] [Google Scholar]
- Chen K. C., Kindt T. J., Krause R. M. Primary structure of the L chain from a rabbit homogeneous antibody to streptococcal carbohydrate. II. Sequence determination of peptides from tryptic and peptic digests. J Biol Chem. 1975 May 10;250(9):3289–3296. [PubMed] [Google Scholar]
- Claflin J. L., Rudikoff S., Potter M., Davie J. M. Structural, functional, and idiotypic characteristics of a phosphorylcholine-binding IgA myeloma protein of C57BL/ka allotype. J Exp Med. 1975 Mar 1;141(3):608–619. doi: 10.1084/jem.141.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Segal D. M. Three-dimensional structure of immunoglobulins. Annu Rev Biochem. 1975;44:639–667. doi: 10.1146/annurev.bi.44.070175.003231. [DOI] [PubMed] [Google Scholar]
- Epp O., Colman P., Fehlhammer H., Bode W., Schiffer M., Huber R., Palm W. Crystal and molecular structure of a dimer composed of the variable portions of the Bence-Jones protein REI. Eur J Biochem. 1974 Jun 15;45(2):513–524. doi: 10.1111/j.1432-1033.1974.tb03576.x. [DOI] [PubMed] [Google Scholar]
- Fett J. W., Deutsch H. F. Primary structure of the Mcg lambda chain. Biochemistry. 1974 Sep 24;13(20):4102–4114. doi: 10.1021/bi00717a007. [DOI] [PubMed] [Google Scholar]
- Gergely J., Wang A. C., Fudenberg H. H. Chemical analyses of variable regions of heavy and light chains of cold agglutinins. Vox Sang. 1973 May;24(5):432–440. doi: 10.1111/j.1423-0410.1973.tb03484.x. [DOI] [PubMed] [Google Scholar]
- Givol D. Affinity labeling and topology of the antibody combining site. Essays Biochem. 1974;10:73–103. [PubMed] [Google Scholar]
- Jaton J. C. Comparison of the amino acid sequences of the variable domains of two homogeneous rabbit antibodies to type III pneumococcal polysaccharide. Biochem J. 1975 May;147(2):235–247. doi: 10.1042/bj1470235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat E. A., Wu T. T. Attempts to locate complementarity-determining residues in the variable positions of light and heavy chains. Ann N Y Acad Sci. 1971 Dec 31;190:382–393. doi: 10.1111/j.1749-6632.1971.tb13550.x. [DOI] [PubMed] [Google Scholar]
- Margolies M. N., Cannon L. E., 3rd, Strosberg A. D., Haber E. Diversity of light chain variable region sequences among rabbit antibodies elicited by the same antigens. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2180–2184. doi: 10.1073/pnas.72.6.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean D., Potter M., Hood L. Mouse immunoglobulin chains. Partial amino acid sequence of a kappa chain. Biochemistry. 1973 Feb;12(4):749–759. doi: 10.1021/bi00728a027. [DOI] [PubMed] [Google Scholar]
- McKean D., Potter M., Hood L. Mouse immunoglobulin chains. Pattern of sequence variation among kappa chains with limited sequence differences. Biochemistry. 1973 Feb;12(4):760–771. doi: 10.1021/bi00728a028. [DOI] [PubMed] [Google Scholar]
- Pink R., Wang A. C., Fudenberg H. H. Antibody variability. Annu Rev Med. 1971;22:145–170. doi: 10.1146/annurev.me.22.020171.001045. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Chen B. L., Phizackerley R. P., Saul F. The three-dimensional structure of the fab' fragment of a human myeloma immunoglobulin at 2.0-angstrom resolution. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3440–3444. doi: 10.1073/pnas.71.9.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponstingl H., Hilschmann N. Zur Strukturregel der Antikörper. Die vollständige Primärstruktur einer moniklonalen Immunglobulin-L-Kette von lambda-Typ, Subgruppe II (Bence-Jones-Protein VIL) Hoppe Seylers Z Physiol Chem. 1971 Jun;352(6):859–877. [PubMed] [Google Scholar]
- Raub W. F. The PROPHET system and resource sharing. Fed Proc. 1974 Dec;33(12):2390–2392. [PubMed] [Google Scholar]
- Rudikoff S., Potter M. Variable region sequence of the heavy chain from a phosphorylcholine binding myeloma protein. Biochemistry. 1974 Sep 10;13(19):4033–4038. doi: 10.1021/bi00716a034. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Girling R. L., Ely K. R., Edmundson A. B. Structure of a lambda-type Bence-Jones protein at 3.5-A resolution. Biochemistry. 1973 Nov 6;12(23):4620–4631. doi: 10.1021/bi00747a013. [DOI] [PubMed] [Google Scholar]
- Segal D. M., Padlan E. A., Cohen G. H., Rudikoff S., Potter M., Davies D. R. The three-dimensional structure of a phosphorylcholine-binding mouse immunoglobulin Fab and the nature of the antigen binding site. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4298–4302. doi: 10.1073/pnas.71.11.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda T. Amino acid sequence of a human kappa type Bence-Jones protein. II. Chymotryptic peptides and sequence of protein Ni. J Biochem. 1973 Feb;73(2):433–446. [PubMed] [Google Scholar]
- Titani K., Shinoda T., Putnam F. W. The amino acid sequence of a kappa type Bence-Jones protein. 3. The complete sequence and the location of the disulfide bridges. J Biol Chem. 1969 Jul 10;244(13):3550–3560. [PubMed] [Google Scholar]
- Wang A. C., Gergely J., Fudenberg H. H. Amino acid sequences at constant and variable regions of heavy chains of monotypic immunoglubulins G and M of a single patient. Biochemistry. 1973 Jan 30;12(3):528–534. doi: 10.1021/bi00727a027. [DOI] [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]


