Abstract
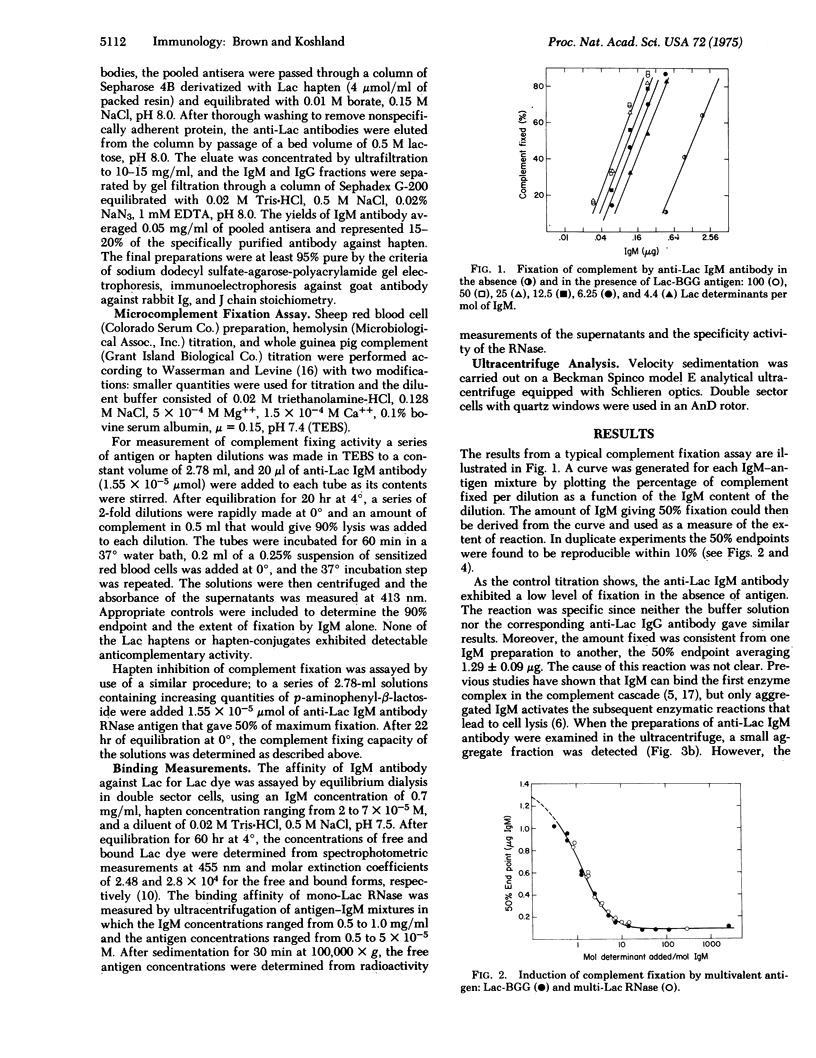
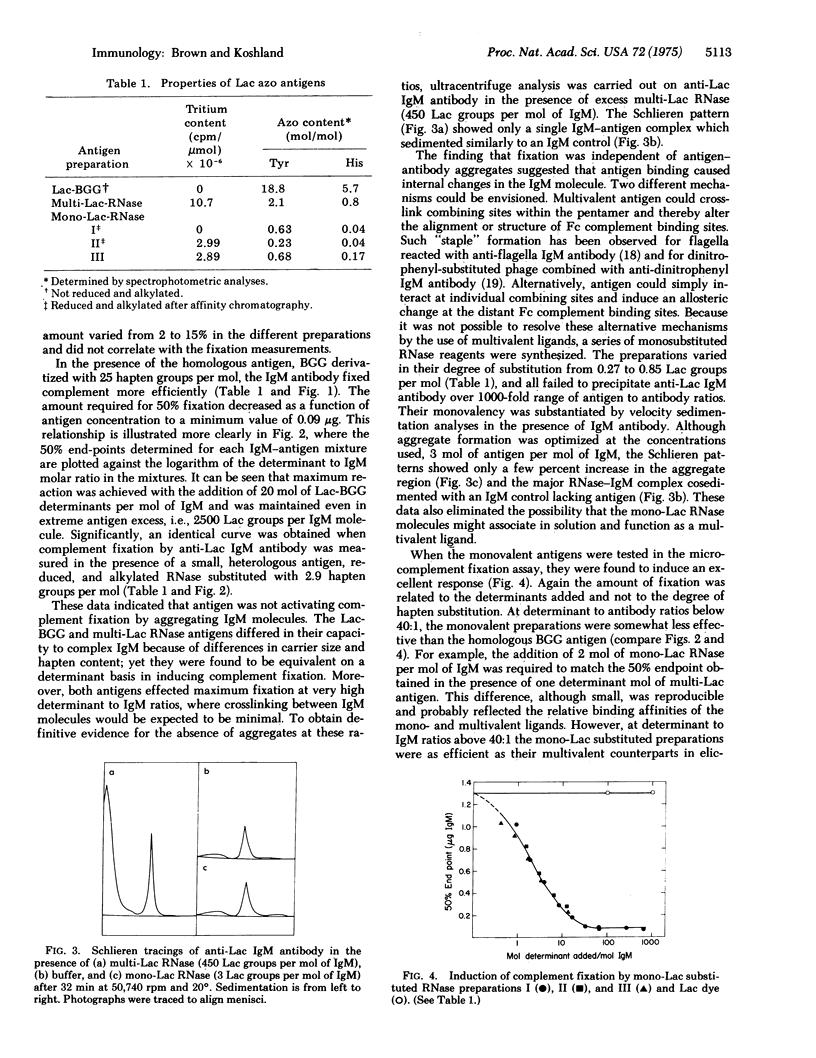
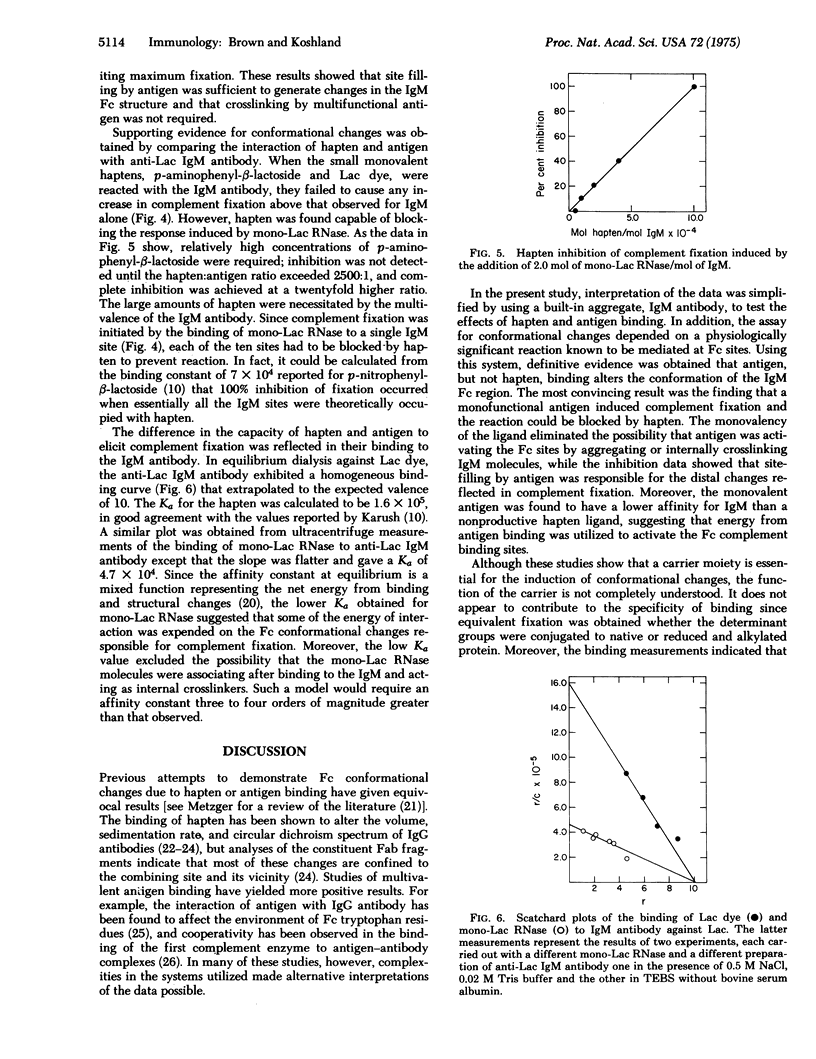
IgM antibody directed against the pheny-beta-lactoside hapten was examined for its capacity to fix complement in the presence of the hapten, monohapten-substituted antigen, and multihapten-substituted antigen. Hapten was found to have no effect; monovalent antigen induced an excellent response which could be inhibited by hapten; and multivalent antigen also induced an excellent response which was related to the number of determinants added and not to the formation of antigen-antibody aggregates. The difference between the activities of hapten and monovalent antigen was reflected in their affinities for the IgM antibody. The monovalent antigen had a lower Ka, indicating that energy from binding was used to activate the Fc complement binding sites. These data show that the expression of IgM Fc function depends on a change in Fc conformation produced by the binding of antigen at the distant Fab combining sites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augener W., Grey H. M., Cooper N. R., Müller-Eberhard H. J. The reaction of monomeric and aggregated immunoglobulins with C1. Immunochemistry. 1971 Nov;8(11):1011–1020. doi: 10.1016/0019-2791(71)90489-7. [DOI] [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Complement fixation on cell surfaces by 19S and 7S antibodies. Science. 1965 Oct 22;150(3695):505–506. doi: 10.1126/science.150.3695.505. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerson J. R., Yasmeen D., Painter R. H., Dorrington K. J. A fragment corresponding to the C(H)2 region of immunoglobulin G (IgG) with complement fixing activity. FEBS Lett. 1972 Aug 15;24(3):318–322. doi: 10.1016/0014-5793(72)80381-8. [DOI] [PubMed] [Google Scholar]
- Feinstein A., Munn E. A., Richardson N. E. The three-dimensional conformation of M and A globulin molecules. Ann N Y Acad Sci. 1971 Dec 31;190:104–121. doi: 10.1111/j.1749-6632.1971.tb13526.x. [DOI] [PubMed] [Google Scholar]
- Feldmann M. Induction of immunity and tolerance in vitro by hapten protein conjugates. I. The relationship between the degree of hapten conjugation and the immunogenicity of dinitrophenylated polymerized flagellin. J Exp Med. 1972 Apr 1;135(4):735–753. doi: 10.1084/jem.135.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. S., Koshland M. E. The stoichiometry of J chain in human secretory IgA. J Immunol. 1973 Dec;111(6):1653–1660. [PubMed] [Google Scholar]
- Holowka D. A., Strosberg A. D., Kimball J. W., Haber E., Cathou R. E. Changes in intrinsic circular dichroism of several homogeneous anti-type 3 pneumococcal antibodies on binding of a small hapten. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3399–3403. doi: 10.1073/pnas.69.11.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornick C. L., Karuch F. Antibody affinity. 3. The role of multivalance. Immunochemistry. 1972 Mar;9(3):325–340. doi: 10.1016/0019-2791(72)90096-1. [DOI] [PubMed] [Google Scholar]
- Hoyer L. W., Borsos T., Rapp H. J., Vannier W. E. Heterogeneity of rabbit IgM antibody as detected by C'1a fixation. J Exp Med. 1968 Mar 1;127(3):589–603. doi: 10.1084/jem.127.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst M. M., Volanakis J. E., Hester R. B., Stroud R. M., Bennett J. C. The structural basis for binding of complement by immunoglobulin M. J Exp Med. 1974 Oct 1;140(4):1117–1121. doi: 10.1084/jem.140.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyslop N. E., Jr, Dourmashkin R. R., Green N. M., Porter R. R. The fixation of complement and the activated first component (C1) of complement by complexes formed between antibody and divalent hapten. J Exp Med. 1970 Apr 1;131(4):783–802. doi: 10.1084/jem.131.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T. IgE and reaginic hypersensitivity. Ann N Y Acad Sci. 1971 Dec 31;190:443–456. doi: 10.1111/j.1749-6632.1971.tb13554.x. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Salmon S., Fudenberg H. Biologic activities of aggregated gamma-globulin. 8. Aggregated immunoglobulins of different classes. J Immunol. 1967 Jul;99(1):82–91. [PubMed] [Google Scholar]
- Ishizaka T., Tada T., Ishizaka K. Fixation of C' and C'la by rabbit gamma-G- and gamma-M-antibodies with particulate and soluble antigens. J Immunol. 1968 Jun;100(6):1145–1153. [PubMed] [Google Scholar]
- Koshland M. E., Davis J. J., Fujita N. J. Evidence for multiple gene control of a single polypeptide chain: the heavy chain of rabbit immunoglobulin. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1274–1281. doi: 10.1073/pnas.63.4.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger H. Effect of antigen binding on the properties of antibody. Adv Immunol. 1974;18:169–207. doi: 10.1016/s0065-2776(08)60310-7. [DOI] [PubMed] [Google Scholar]
- Pilz I., Kratky O., Licht A., Sela M. Shape and volume of anti-poly(D-alanyl) antibodies in the presence and absence of tetra-D-alanine as followed by small-angle x-ray scattering. Biochemistry. 1973 Nov 20;12(24):4998–5005. doi: 10.1021/bi00748a028. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Becka L. N. Structure of Fab' New at 6 A resolution. Nat New Biol. 1972 Feb 2;235(57):137–140. doi: 10.1038/newbio235137a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Steinberg I. Z., Givol D., Hochman J., Pecht I. Antigen-induced conformational changes in antibodies and their Fab fragments studied by circular polarization of fluorescence. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2775–2779. doi: 10.1073/pnas.72.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABACHNICK M., SOBOTKA H. Azoproteins. I. Spectrophotometric studies of amino acid azo derivatives. J Biol Chem. 1959 Jul;234(7):1726–1730. [PubMed] [Google Scholar]
- TABACHNICK M., SOBOTKA H. Azoproteins. II. A spectrophotometric study of the coupling of diazotized arsanilic acid with proteins. J Biol Chem. 1960 Apr;235:1051–1054. [PubMed] [Google Scholar]
- Thompson J. J., Hoffmann L. G. Homotropic cooperative binding of the first component of guinea pig complement to rabbit IgG-erythrocyte complexes: a possible allosteric effect. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2730–2733. doi: 10.1073/pnas.68.11.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- Warner C., Schumaker V., Karush F. The detection of a conformational change in the antibody molecule upon interaction with hapten. Biochem Biophys Res Commun. 1970 Jan 6;38(1):125–128. doi: 10.1016/0006-291x(70)91093-4. [DOI] [PubMed] [Google Scholar]


