Abstract
Herein, we describe the detailed development of a simple and effective method to microencapsulate vaccine antigens in poly(lactic-co-glycolic acid) (PLGA) by simple mixing of preformed active self-microencapsulating (SM) PLGA microspheres in a low concentration aqueous antigen solution at modest temperature (10-38 °C). Co-encapsulating protein-sorbing vaccine adjuvants and polymer plasticizers were used to “actively” load the protein in the polymer pores and facilitate polymer self-healing at temperature > hydrated polymer glass transition temperature, respectively. The microsphere formulation parameters and loading conditions to provide optimal active self-healing microencapsulation of vaccine antigen in PLGA was investigated. Active self-healing encapsulation of two vaccine antigens, ovalbumin and tetanus toxoid (TT), in PLGA microspheres was adjusted by preparing blank microspheres containing different vaccine adjuvant (aluminum hydroxide (Al(OH)3) or calcium phosphate). Active loading of vaccine antigen in Al(OH)3-PLGA microspheres was found to: a) increase proportionally with an increasing loading of Al(OH)3 (0.88-3 wt%) and addition of porosigen, b) decrease when the inner Al(OH)3/trehalose phase to 1 mL outer oil phase and size of microspheres was respectively > 0.2 mL and 63 μm, and c) change negligibly by PLGA concentration and initial incubation (loading) temperature. Encapsulation of protein sorbing Al(OH)3 in PLGA microspheres resulted in suppression of self-healing of PLGA pores, which was then overcome by improving polymer chain mobility, which in turn was accomplished by coincorporating hydrophobic plasticizers in PLGA. Active self-healing microencapsulation of manufacturing process-labile TT in PLGA was found to: a) obviate micronization- and organic solvent-induced TT degradation, b) improve antigen loading (1.4-1.8 wt% TT) and encapsulation efficiency (~ 97%), c) provide nearly homogeneous distribution and stabilization of antigen in polymer, and d) provide improved in vitro controlled release of antigenic TT.
Keywords: PLGA, self-healing, active loading, encapsulation, vaccine delivery, controlled release
1. Introduction
Immunization of children with vaccines such as those against tetanus, diphtheria and pertussis protect them from fatal childhood infections [1], which in turn prevents millions of deaths every year [2-4]. A full immunization schedule typically includes 2-3 parenteral doses of traditional vaccines (e.g., aluminum potassium sulfate-precipitated toxoids) with an interval of 4-6 weeks [1-3, 5]. Accomplishment of the full immunization schedule is often found to be difficult in developing countries. For example, up to 70% drop-out rate after the primary immunization has been reported in developing countries [6]. Further, operational costs in vaccine campaigns contribute to almost 90% of the total costs [3, 6]. Therefore, a single-dose vaccine formulation has become increasingly important to overcome these obstacles [5].
Injectable biodegradable polymer microspheres represent an important approach to obtain controlled release of vaccine antigens, which in turn has shown to reduce the number doses in immunization schedule [7-10]. Injectable microspheres based on poly(DL-lactic-co-glycolic acid) (PLGA) have been studied most widely for this purpose because of: a) impressive safety record of the polymer in humans, b) the ability to inject these particles through a syringe needle with minimal discomfort, and c) excellent ability of PLGA to provide log-term release of vaccine antigens and improved immunogenic responses in mammalian subjects relative to soluble antigen [5, 7, 11, 12]. Despite these benefits, a number challenges still remain with the development of controlled-release PLGA/vaccine microspheres. A key issue include significant instability of vaccine antigens during a) microencapsulation in PLGA, b) drying and storage of encapsulated antigen, and c) long-term release from PLGA microspheres [4, 13-18].
Microencapsulation of vaccine antigens in PLGA is commonly achieved by emulsion-solvent evaporation [17, 18], coacervation [19, 20], or spray drying [19, 21] methods. The harsh conditions such as organic solvent/water interfaces, shear or cavitation forces, excessive heat, and freezing involved in both the encapsulation methods often causes significant degradation of vaccine antigens [5, 15-18]. The mechanistic approaches studied thus far including co-incorporation of stabilizing excipients with antigen in the inner water phase to minimize the effect of organic/aqueous interface [15, 17, 18, 22, 23], freeze-drying of an antigen with a stabilizer to obtain fine particles for anhydrous encapsulation [24, 25], and use of supercritical carbon dioxide in place of organic solvent [26], have been shown to minimize antigen instability during encapsulation. However, the overall complexity associated with the traditional encapsulation methods still remains the same. For example, drop off of the production of Nutropin Depot (prepared by an emulsion method), the first and only FDA approved injectable protein depot [27], is suggestive of lack of feasibility of traditional encapsulation processes for sensitive and expensive vaccines. Hence, new PLGA encapsulation methods are needed to overcome the manufacturing process complexity.
“Self-healing” is a unique phenomenon of polymers, in which the damaged structure (e.g., pores, cracks, and dents) is healed (repaired) through spontaneously rearrangement of the polymer chains [28, 29]. Previously, while characterizing the initial burst release of a model peptide from PLGA microspheres, we observed spontaneous PLGA pore closing in the surface of the polymer at physiological temperature, sealing off the pore-diffusion pathway and leading to cessation of the initial burst of peptide [30]. Taking advantage of this phenomenon, we recently developed a new method of microencapsulation based on spontaneous polymer pore closing (or self-healing) capacity of PLGA at temperature (T) > glass transition temperature (Tg) of the polymer to microencapsulate biomacromolecules (proteins, peptides, and polysaccharides) in aqueous media [31, 32]. A PLGA self-healing microencapsulation paradigm based on passive loading strategy could provide 1-10% wt% protein and peptide loading without significant loss of protein stability, since no excess mixing (e.g., sonication or homogenization), organic solvent/water interfaces, or protein micronization steps were used. As the passive loading method required addition of large amount of protein in the aqueous loading solution (>100 mg/mL), the encapsulation efficiency of passive self-healing microencapsulation method was found to be in the range of 1.5 to 13% [31, 32].
In order to increase encapsulation efficiency and reduce manufacturing costs, a protein-trapping agent, Al(OH)3 gel, was placed in the PLGA pores to effectively remove protein from the low concentration of antigen (< 1 mg/mL) to increase encapsulation efficiency to near 100% [32, 33]. The purpose of this paper is to describe the detailed development and optimization of the active PLGA self-healing microencapsulation method for vaccine antigens. Employing ovalbumin (OVA) and tetanus toxoid (TT) model antigens, microsphere formulation and loading parameters appropriate for the optimal active self-healing microencapsulation were determined by studying the influence of: a) PLGA concentration, b) inner water phase volume, c) microspheres size, d) adjuvant type (Al(OH)3 or CaHPO4), e) Al(OH)3 loading, f) trehalose (porosigen) loading, g) co-incorporation of hydrophobic plasticizers (diethyl phthalate (DEP) and tributyl acetylcitrate (TBAC)), and h) incubation temperature on the active loading characteristics of active SM PLGA microspheres. In addition, the stability and release behavior of TT was examined as a function of the type of plasticizer and porosigen incorporated in PLGA relative to encapsulation by the standard solvent evaporation method.
2. Materials and methods
2.1. Materials
Aluminum hydroxide gel (Al(OH)3), ovalbumin (OVA, phosphorylated), poly(vinyl alcohol) (PVA) (80% hydrolyzed), succinic acid, DEP, bovine serum albumin (BSA), 4-morpholinepropanesulfonic acid (MOPS), dl-dithiothreitol, urea, hydrochloric acid (30%), ethylenediamine-tetraacetic acid (EDTA), p-nitrophenyl phosphate liquid substrate, and trehalose were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fluorescein-ovalbumin (Fluorescein-OVA) was purchased from Biosearch Technologies Inc. (Novato, CA, USA). Tributyl acetylcitrate (TBAC) was purchased from SAFC supply solutions (St. Louis, MO, USA). PLGA 50:50 [i. v = 0.57 dl/g, Mw = 51 kDa, end group = lauryl ester] was purchased from (Medisorb, Alkermes, Cambridge, MA, USA). Tetanus toxoid (3120 Lf/mL) was received from Serum Institute of India Ltd. (Pune, India). Equine tetanus antitoxin was received as a gift sample from U.S. Food and Drug Administration (Silver Spring, MD). Human tetanus immune globulin (HyperTET™ S/D, 250 units) was purchased from Talecris Biotherapeutics, Inc. (Research Triangle Park, NC, USA). Goat anti-human IgG-alkaline phosphatase was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). All other reagents and solvents were of analytical grade or purer and purchased from commercial suppliers.
2.2. Preparation of blank active self-microencapsulating PLGA and traditional TT/PLGA microspheres
Blank active SM PLGA (Al(OH)3- or CaHPO4-PLGA-hydrophobic plasticizer) and traditional TT/PLGA microspheres were prepared by a w/o/w emulsion-solvent evaporation method. For active SM PLGA microspheres preparation, an inner water phase contained varying amount of Al(OH)3 or CaHPO4 and trehalose in 25 mM succinate buffer (pH 4.0). Purified TT in normal saline (3120 Lf/mL) was used as an inner water phase to prepare traditional TT/PLGA microspheres. Two hundred or three hundred microliters of inner water phase was added to 1 mL of 250 or 350 mg/mL PLGA (with or without a hydrophobic plasticizer (DEP or TBAC) and MgCO3) in methylene chloride. The mixture was homogenized at 17 000 rpm with a Tempest IQ2 homogenizer (The VirTis Company, Gardiner, NY, USA) equipped with a 10 mm shaft in an ice/water bath for 1 min to prepare the first emulsion. Two mL of 5% (w/v) PVA solution was immediately added to the primary w/o emulsion (PVA at 5% w/v concentration was used as an emulsifier to stabilize w/o/w emulsion), and the mixture was vortexed (Genie 2, Fisher Scientific Industries, Inc., Bohemia, NY, USA) for 50 s to produce the w/o/w double emulsion. The resultant emulsion was poured into 100 mL of 0.5% (w/v) chilled (plasticizer- loaded active SM PLGA microspheres) or unchilled (plasticizer-free active SM PLGA and traditional TT/PLGA microspheres) PVA solution under rapid stirring and hardened at room temperature for 3 h (PVA at 0.5% w/v concentration was used as an emulsifier to prevent agglomeration of particles). Hardened microspheres were collected by sieve (20-63 and 63-90 μm), washed repeatedly with double-distilled (dd) H2O, and freeze-dried.
2.3. Determination of Al(OH)3 loading by ICP-OES
Actual loading of Al(OH)3 in PLGA microspheres was determined by ICP-OES. Briefly, ~ 15 mg microspheres of Al(OH)3-PLGA microspheres were added to 1.5 mL ethyl acetate, vortexed for 2 min, and centrifuged at 8,000 rpm for 10 min. The supernatant polymer solution was then removed and the remaining residue was washed twice with 1-mL ethyl acetate. The residue was dried at 30 °C using a vacufuge™ concentrator 5301 (Eppendorf International, Barkhausenweg 1, Hamburg, Germany). One milliliter of 30% HCl was added to each sample, incubated at 70 °C for 1 h, and vortexed for 1 min before diluting with distilled water to a suitable volume (10-75 mL). The dilution was done in such a way that the theoretical concentration of Al ion in the samples fell between 1-15 ppm. The concentration of Al ion was then analyzed by ICP-OES (Perkin-Elmer Optima 2000 DV with Winlab software, Waltham, Massachusetts, USA) at the detection wavelength of 396.85 nm in the radial plasma mode. Each sample measurement was an average of three scans and ion analysis was done in triplicate.
2.4. Modified Bradford protein assay
A modified Bradford assay was used to determine antigen concentrations. Briefly, appropriate volume of standard or sample was mixed with Coomassie Plus® reagent (Thermo Fisher Scientific, Rockford, IL, USA) in a 96-well plate (Nalge Nunc International, Rochester, NY, USA). Then the absorbance was read at 595 nm within 30 min using a Dynex II MRX microplate reader (Dynex Technology Inc., Chantilly, VA, USA).
2.5. Effect of microencapsulation process on protein adsorption characteristics of Al(OH)3
Extraction of Al(OH)3-trehalose fraction from about 20 mg microspheres (3.2 wt% Al(OH)3/3.5 wt% trehalose/PLGA microsphere formulation) and subsequent drying was performed as per the method described in the determination of Al(OH)3 content. Then, the residual Al(OH)3-trehalose or control (unencapsulated Al(OH)3) was incubated with 0.4 mL of 1 mg/mL OVA solution at 25 and 42 °C for 24 and 48 h, respectively. At different incubation times (3, 6, 24, and 72 h), the Al(OH)3/trehalose/OVA mixture was centrifuged at 6,000 rpm for 10 min and the remaining OVA mass in the supernatant was determined by modified Bradford assay. To study the effect of drying process on the antigen adsorption characteristics of Al(OH)3, the unencapsulated Al(OH)3 with and without trehalose was similarly dried and used for the adsorption study.
2.6. Scanning electron microscopy (SEM)
The surface and inner morphology of microspheres was examined by taking SEM images using a Hitachi S3200N scanning electron microscope (Hitachi, Tokyo, Japan). Briefly, microspheres were fixed previously on a brass stub using double-sided adhesive tape and then were made electrically conductive by coating, in a vacuum, with a thin layer of gold (approximately 3 to 5 nm) for 100 s at 40 W. The images of microspheres surface or cross-section were taken at an excitation voltage of 8-10 kV.
2.7. Active self-healing encapsulation of vaccine antigens in PLGA microspheres
Active self-encapsulation of vaccine antigens (OVA and TT) in PLGA microspheres was achieved in two phases: incubation of porous active SM PLGA microspheres (Al(OH)3- or CaHPO4-PLGA microspheres with and without hydrophobic plasticizer) with antigen solution at T < Tg of the polymer to allow active antigen sorption by Al(OH)3 or CaHPO4 in PLGA pores and raising the T > Tg of the polymer to irreversibly close the pores of the polymer. Briefly, ~20 mg active SM PLGA microspheres were taken into microcentrifuge tube and then OVA/fluorescein-OVA (0.4 mL of 0.5-1 mg/mL antigen in 10 mM MOPS) or TT (0.5 mL of 0.64-1 mg/mL TT in normal saline (pH 6.8)) solution was added and mixed carefully. The microcentrifuge tubes were first incubated at 10 and 25 °C for 24-48 h (loading phase), and then at 37-43 °C for 30-48 h (pore closing/healing phase) under constant rotation on a rigged rotator (Glas-Col, Terre Haute, IN, USA) to obtain antigen self-encapsulated PLGA microspheres (see Fig. 1). To monitor the mass loss of TT from TT/active SM PLGA microspheres mixture as a function of incubation duration, the mixture was passed through a low protein binding Durapore (PVDF) membrane-based syringe-driven filter unit (Millipore Corporation, Billerica, MA, USA) after specified duration, diluted suitably with normal saline (pH 6.8), and remaining TT in the supernatant was quantified by a modified Bradford assay. Active loading of vaccine antigens in Al(OH)3-PLGA or CaHPO4-PLGA microspheres was then calculated by subtracting the OVA or TT mass remaining in the mixture from the initial incubated mass.
Fig. 1.
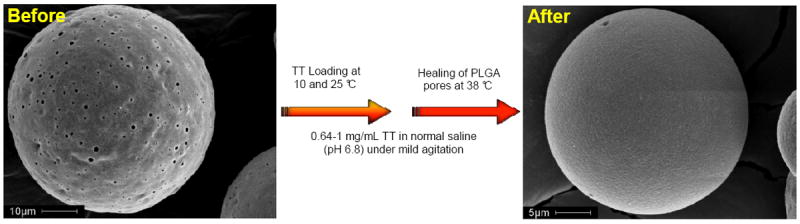
Active self-healing encapsulation of tetanus toxoid (TT) in PLGA microspheres. The surface morphology of 3.2 wt% Al(OH)3-PLGA-3.5 wt% trehalose-5 wt% diethyl phthalate microspheres (ASM-1, Table 1) before and after active self-healing encapsulation of TT.
2.8. Determination of adsorption capacity of Al(OH)3 accessible after PLGA microencapsulation for antigen loading
Required quantity (mass equivalent to about 20 mg active SM PLGA microsphere formulations) of unencapsulated Al(OH)3 was taken and OVA adsorption study was performed as described in active self-healing encapsulation of vaccine antigens. Using the OVA mass sorbed by active SM PLGA microsphere formulations and respective unencapsulated Al(OH)3, the adsorption capacity of Al(OH)3 in self-healing PLGA microspheres was then determined as follows:
2.9. Differential scanning calorimetry
The Tg of Al(OH)3-PLGA microspheres with and without hydrophobic plasticizers (DEP and TBAC) was measured by a differential scanning calorimeter (Perkin-Elmer® DSC7, Waltham, USA). Microspheres samples (~7 mg) were sealed in aluminum hermetic pans and thermograms were determined by first cooling the sample to -40 °C, then heating to 80 °C at a heating rate of 10 °C/min under purged nitrogen atmosphere. The startpoint of the second run was used for Tg calculation.
2.10. Analysis of soluble and insoluble TT in PLGA microspheres prepared by traditional w/o/w emulsion-solvent evaporation
Soluble and insoluble TT in PLGA microspheres prepared by a w/o/w emulsion-solvent evaporation method was determined after extraction of the antigen, as described previously [17], before analysis by a modified Bradford assay.
2.11. Enzyme-linked immunosorbent assay (ELISA) for assay of antigenically active TT
Antigenically active TT was determined by the ELISA as described previously with a slight modification [4]. In the final step, 100 μl of ready-to-use p-nitrophenyl phosphate liquid substrate was added and incubated for 30 min at 37 °C. The absorbance of samples was read at 405 nm on a Dynex II MRX microplate reader (Dynex Technology Inc., Chantilly, VA, USA) equipped with Revelation 3.2 Software. A Log/Logit curve-fitting model was used to plot the standard curve and calculate unknown concentration of TT in test samples.
2.12. Evaluation of in vitro release of antigenic tetanus toxoid from PLGA microspheres
About 20 mg of TT/PLGA microspheres prepared by the traditional w/o/w emulsion-solvent evaporation method were incubated in 1 mL of PBST + 0.2% BSA at 37 °C under constant agitation (240 rpm/min). At different incubation times (1, 3, 7, 14, 21, and 28 days), the mixture was centrifuged at 8,000 rpm for 10 min and supernatant was taken out, followed by the addition of fresh medium (1 mL). Analysis of released antigenic TT was performed by ELISA.
2.13. Statistical analysis
The results are expressed as mean ± SEM (n = 3) with error bars not listed when smaller than symbols. An unpaired Student’s t-test was used to compare the means for each study parameter at the different sample times and assess statistical significance. Results were considered statistically significant if p<0.05.
3. Results and discussion
3.1. Identification of protein antigen-trapping agents for active self-healing microencapsulation in PLGA
One approach to increase encapsulation efficiency of PLGA self-healing microencapsulation is to identify protein-trapping agents, which are placed in the PLGA preformed microspheres. Several criteria for this new excipient type include: a) reversible sorption of the protein antigen from aqueous solution, b) the ability to retain protein antigen stability when bound, c) biocompatibility, d) the ability to dry, e) acceptable excipient to protein antigen binding ratio, and f) the ability to encapsulate the excipient in preformed PLGA suitable for self-healing encapsulation. Al(OH)3 and CaHPO4 are commercially available vaccine adjuvants to augment the immune response of vaccines, which in turn enhance protective immunity against the targeted disease [34, 35]. However, clinically used vaccine preparations (e.g., TT adsorbed: suspension of TT-aluminum potassium sulfate) have not been considered so far as delivery systems for long-term release [36]. PLGA on the other hand is a polymeric and non-immunogenic vaccine adjuvant with a long history of safe use in humans to achieve long-term release of vaccine antigens [5, 7, 37-39]. The two adjuvants appear to fit the expected criteria for successful trapping agent with the exception that Al(OH)3 is not easily dried. However, polyol lyoprotectants such as sorbitol and trehalose can be use used to retain the adjuvants protein-binding function upon drying [4, 40]. In order to test the trapping agents, they first needed to be encapsulated in PLGA to eventually create an interconnected pore-network.
3.2. Effective encapsulation of vaccine adjuvants (Al(OH)3 or CaHPO4) in PLGA microspheres for vaccine antigen sorption
Classical vaccine adjuvants were successfully encapsulated in PLGA microspheres by a w/o/w emulsion method with excellent encapsulation efficiency of 87-98% (see Table 1). Trehalose was co-encapsulated as a lyoprotectant to Al(OH)3 or CaHPO4 during freeze-drying of microspheres [40] and porosigen. Adsorption of OVA onto Al(OH)3 before and after PLGA microencapsulation was determined to find out the effect of encapsulation processing on antigen adsorption property of Al(OH)3 (see Fig. 2). Unencapsulated Al(OH)3 in the hydrated state adsorbed OVA very rapidly (615 and 629 μg OVA/mg Al(OH)3 after 3 and 72 h incubation, respectively). To determine the adsorption characteristics of Al(OH)3 after microencapsulation, the polymer was extracted with ethyl acetate and residual Al(OH)3-trehalose was vacuum-dried at 30 °C before incubating with OVA solution. The rate of OVA adsorption to Al(OH)3 extracted from the polymer was found to be slower (251 and 568 μg OVA/mg Al(OH)3 after 3 and 72 h incubation, respectively) than unencapsulated hydrated Al(OH)3. To understand further the role of initial state (hydrated or dry) of Al(OH)3, unencapsulated Al(OH)3 was similarly vacuum-dried with appropriate amount (equivalent to encapsulated Al(OH)3-trehalose sample) of trehalose and then the OVA adsorption trend was evaluated (see Fig. 2). There was no significant difference in OVA adsorption between vacuum-dried unencapsulated and encapsulated Al(OH)3. It is noteworthy to mention that vacuum-dried Al(OH)3 without the trehalose did not adsorb any OVA. These results indicated effective microencapsulation of Al(OH)3 in PLGA without significant loss of its adsorption capacity. The slow OVA adsorption property of vacuum-dried samples compared to unencapsulated hydrated Al(OH)3 can be attributed to slow and steady hydration of vacuum-dried Al(OH)3, which appears to be governed by the incubation temperature and duration. After the 72 h incubation, both dried Al(OH)3 specimens absorbed 92-94% OVA relative to the non-dried Al(OH)3.
Table 1.
Evaluation of microencapsulation of aluminum hydroxide (Al(OH)3) in PLGA microspheres as a function of inner Al(OH)3/trehalose phase-to-1 mL oil phase ratio (ASM-2 and ASM-3), microspheres size (ASM-4 and ASM-5), PLGA concentration (ASM-6 and ASM-7), adjuvant type (ASM-8 to ASM-9), Al(OH)3 loading (ASM-10 to ASM-12), and trehalose loading (ASM-13 to ASM-16), and co-incorporation of hydrophobic plasticizers (ASM-1 and ASM-20 to ASM-26).
| Formulation | PLGA concentration (mg/mL) | Inner Al(OH)3/trehalose phase-to-1 mL oil phase ratio (mL) | Trehalose loading (wt%) | Hydrophobic plasticizer loading (wt%) | Microspheres size (μm) | Al(OH)3/CaHPO4 loading (wt%)
|
Al(OH)3 encapsulation efficiency (%)* | Tg (°C) | |
|---|---|---|---|---|---|---|---|---|---|
| Theoretical | Actual Al(OH)3 loading*a | ||||||||
| ASM-1 | 250 | 0.2 | 3.5 | 5 (DEP) | 20-63 | 3.20 | 3.10 ± 0.02 | 96.8 ± 0.7 | 37.4 |
| ASM-2 | 350 | 0.2 | 7.8 | - | 20-63 | 1.85 | 1.80 ± 0.03 | 97.3 ± 1.4 | - |
| ASM-3 | 350 | 0.3 | 7.8 | - | 20-63 | 2.80 | 2.69 ± 0.07 | 96.0 ± 2.6 | - |
| ASM-4 | 350 | 0.2 | 7.8 | - | 20-63 | 1.85 | 1.80 ± 0.03 | 97.3 ± 1.4 | - |
| ASM-5 | 350 | 0.2 | 7.8 | - | 63-90 | 1.90 | 1.79 ± 0.01 | 94.1 ± 0.7 | - |
| ASM-6 | 250 | 0.2 | 7.8 | - | 20-63 | 3.20 | 3.10 ± 0.07 | 96.8 ± 2.1 | - |
| ASM-7 | 350 | 0.2 | 7.8 | - | 20-63 | 3.10 | 2.97 ± 0.02 | 95.8 ± 0.6 | - |
| ASM-8 | 250 | 0.2 | 4.0 | - | 20-63 | 3.20 | - | - | - |
| ASM-9 | 250 | 0.2 | 4.0 | - | 20-63 | 3.20b | - | - | - |
| ASM-10 | 350 | 0.2 | 7.8 | - | 20-63 | 0.88 | 0.83 ± 0.02 | 93.9 ± 2.5 | 42.5 |
| ASM-11 | 350 | 0.2 | 7.8 | - | 20-63 | 1.85 | 1.80 ± 0.03 | 97.3 ± 1.4 | 42.6 |
| ASM-12 | 350 | 0.2 | 7.8 | - | 20-63 | 3.04 | 2.82 ± 0.03 | 92.7 ± 1.0 | 42.7 |
| ASM-13 | 250 | 0.2 | 0 | - | 20-63 | 3.20 | 3.10 ± 0.05 | 96.8 ± 1.5 | - |
| ASM-14 | 250 | 0.2 | 2.5 | - | 20-63 | 3.20 | 3.07 ± 0.09 | 95.8 ± 2.9 | - |
| ASM-15 | 250 | 0.2 | 4.0 | - | 20-63 | 3.10 | 3.02 ± 0.06 | 97.6 ± 2.0 | - |
| ASM-16 | 250 | 0.2 | 10.4 | - | 20-63 | 2.90 | 2.51 ± 0.04 | 86.6 ± 1.3 | - |
| ASM-17 | 350 | 0.2 | 4.0 | 20-63 | 3.20 | ||||
| B-1 | 350 | 0.2 | 4.0 | - | 20-63 | - | - | - | 42.7 |
| ASM-18 | 350 | 0.2 | 4.0 | 20-63 | 3.20 b | - | - | - | |
| ASM-19 | 250 | 0.2 | 3.5 | 0 | 20-63 | 3.20 | 3.04 ± 0.04 | 95.0 ± 1.3 | 42.7 |
| ASM-20 | 250 | 0.2 | 3.5 | 2.5 (DEP) | 20-63 | 3.20 | 3.11 ± 0.04 | 97.2 ± 1.2 | 39.0 |
| ASM-21 | 250 | 0.2 | 3.5 | 10 (DEP) | 20-63 | 3.20 | - | - | 29.1 |
| ASM-22 | 250 | 0.2 | 3.5 | 20 (DEP) | 20-63 | 3.20 | - | - | 20.3 |
| ASM-23 | 250 | 0.2 | 3.5 | 2.5 (TBAC) | 20-63 | 3.20 | - | - | 37.6 |
| ASM-24 | 250 | 0.2 | 3.5 | 5 (TBAC) | 20-63 | 3.20 | - | - | 32.7 |
| ASM-25 | 250 | 0.2 | 3.5 | 10 (TBAC) | 20-63 | 3.20 | - | - | 24.4 |
| ASM-26 | 250 | 0.2 | 3.5 | 20 (TBAC) | 20-63 | 3.20 | - | - | 13.2 |
| PLGA | - | - | - | - | - | - | - | - | 43.0 |
Fig. 2.
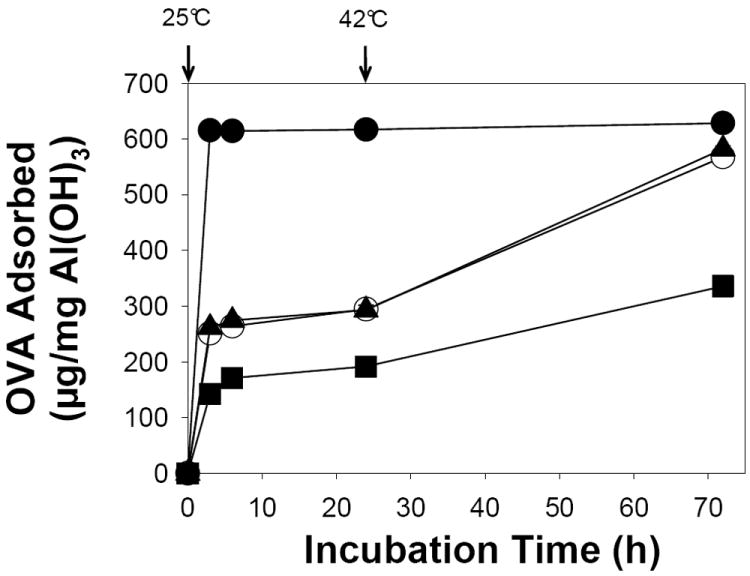
Retention of Al(OH)3-sorption capacity after encapsulation in PLGA microspheres. Effect of initial state (hydrated or dry) of Al(OH)3 on ovalbumin (OVA) adsorption as a function of incubation time. Samples studied were unencapsulated Al(OH)3 in hydrated state (●), vacuum-dried (with trehalose as a stabilizer) Al(OH)3 before encapsulation (▲), vacuum-dried Al(OH)3 after encapsulation and polymer removal (○), and 3.2 wt% Al(OH)3/3.5 wt% trehalose/5 wt% DEP/PLGA microspheres (ASM-1, Table 1) (■). All the samples were incubated with 0.4 mL of 1 mg/mL OVA in 10 mM MOPS buffer (pH 7.4) under mild agitation at 25 and 42 °C respectively for 24 and 48 h. The mass of Al(OH)3 in all the samples was about 0.6 mg and symbols represent mean ± SEM, n = 3.
3.3. Development of PLGA microsphere formulation and incubation parameters for optimal active self-healing microencapsulation
Microsphere formulation variables and loading temperature that are required for optimal active self-encapsulation in PLGA microspheres were determined by investigating the effect of initial water phase volume, polymer concentration, microspheres size, type and loading of vaccine adjuvant, trehalose loading, incubation temperature, and co-incorporation of hydrophobic plasticizers on the encapsulation efficiency of Al(OH)3 (see Table 1), active loading of OVA (see Fig. 3), fraction of Al(OH)3 accessible inside PLGA microspheres for OVA adsorption (see Fig. 4), and self-healing of PLGA pores were thoroughly investigated and the same is described below.
Fig. 3.
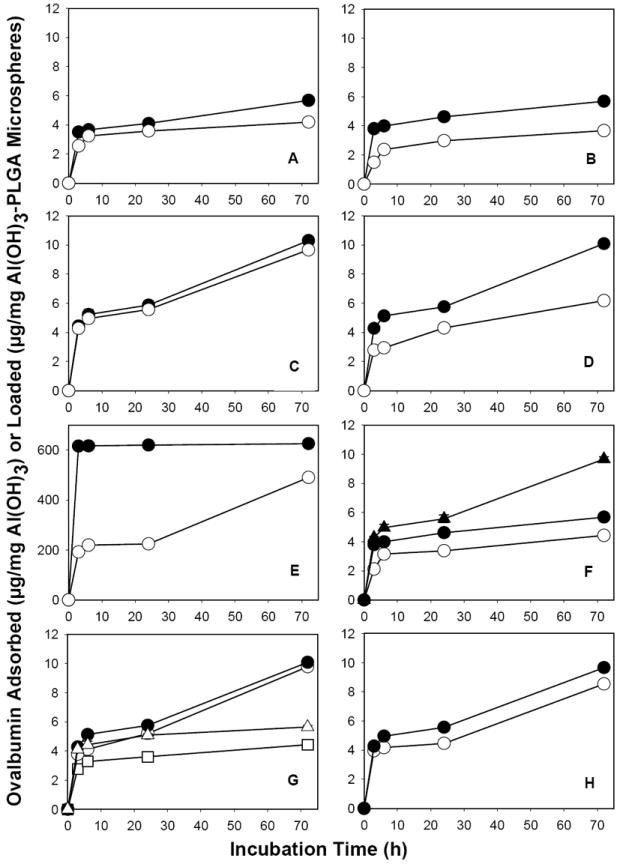
Optimization of active self-microencapsulating (SM) PLGA microsphere formulation variables and antigen loading temperature (ASM-2 to ASM-16, Table 1). Ovalbumin (OVA) sorption by unencapsulated Al(OH)3 control (E: μg OVA/mg Al(OH)3) or active SM PLGA microspheres (A-D and F-H: μg OVA/mg Al(OH)3-PLGA microspheres) as a function of incubation time. Effect of the following variables on OVA sorption: inner water phase volume (A: 0.2 (●) or 0.3 (○) mL inner Al(OH)3/trehalose phase-to-1 mL oil phase ratio), microspheres size (B: 20-63 (●) and 63-90 (○) μm), PLGA concentration (C: 250 (●) and 350 (○) mg/mL), adjuvant type (D and E: Al(OH)3 (●) or CaHPO4 (○) after (D) or before (E) PLGA encapsulation, Al(OH)3 loading (F: 0.88 (○), 1.85 (●), and 3.04 (▲) wt%), trehalose loading (G: 0 (□), 2.5 (○), 4 (●), and 10.4 (Δ) wt%), and initial loading temperature (H: 10 (○) and 25 (●) °C). A: ASM-2 and ASM-3; B: ASM-4 and ASM-5; C: ASM-6 and ASM-7; D: ASM-8 and ASM-9; F: ASM-10 to ASM-12; G: ASM-13 to ASM-16; and H: ASM-8. Mass of unencapsulated Al(OH)3 and microspheres (20-63 μm in size) used in the study was ~0.6 and 20 mg, respectively. All the samples were incubated with 0.4 mL of 1 mg/mL OVA in 10 mM MOPS buffer (pH 7.4) under mild agitation at 25 and 42 °C respectively for 24 and 48 h. Symbols represent mean ± SEM, n = 3.
Fig. 4.
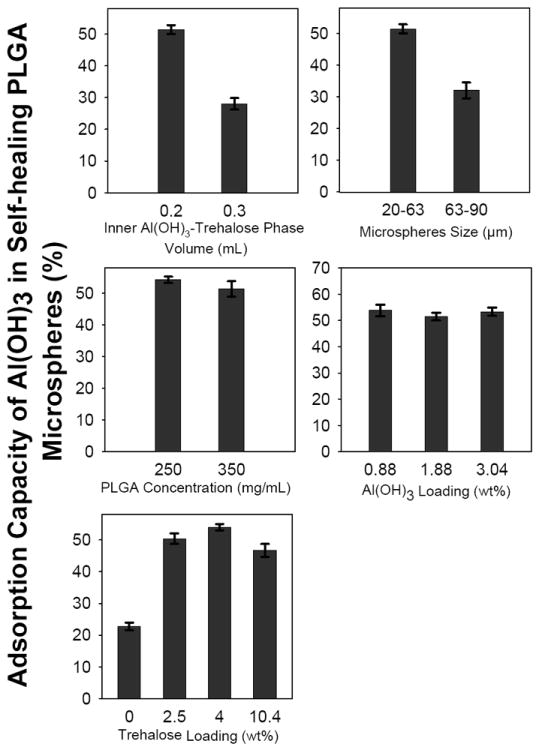
Determination of adsorption capacity of aluminum hydroxide adjuvant (Al(OH)3) accessible in self-healing PLGA microspheres (ASM-2 to ASM-7 and ASM-10 to ASM-16, Table 1) for ovalbumin (OVA) loading as a function of the following microsphere formulation variables: inner Al(OH)3-trehalose phase volume (0.2 (ASM-2) and 0.3 (ASM-3) mL), microspheres size (20-63 (ASM-4) and 63-90 (ASM-5) μm), PLGA concentration (250 (ASM-6) and 350 (ASM-7) mg/mL), Al(OH)3 loading (0.88 (ASM-10), 1.85 (ASM-11), and 3.04 (ASM-12) wt%), and trehalose loading (0 (ASM-13), 2.5 (ASM-14), 4 (ASM-15) and 10.4 (ASM-16) wt%). Fraction of Al(OH)3 accessible in PLGA microspheres for OVA adsorption was calculated by dividing the mass of OVA sorbed by active SM microspheres/mass of Al(OH)3 in microspheres with mass of OVA adsorbed by unencapsulated Al(OH)3/mass of unencapsulated Al(OH)3. Twenty mg microspheres (20-63 μm in size) or unencapsulated Al(OH)3 (mass equivalent to the actual content of Al(OH)3 in 20 mg microspheres) were incubated with 0.4 mL of 1 mg/mL OVA in 10 mM MOPS buffer (pH 7.4) under mild agitation at 25 and 42 °C respectively for 24 and 48 h. Bars represent mean ± SEM, n = 3.
3.3.1. Ratio of inner Al(OH)3/outer polymer (primary w/o-emulsion) phase
Al(OH)3 was encapsulated in PLGA microspheres by a double emulsion (w/o/w)-solvent evaporation method. The primary w/o-emulsion was formed by adding 0.2 and 0.3 mL of inner Al(OH)3/trehalose phase to 1 mL outer oil phase (350 mg PLGA in 1 mL CH2Cl2). With almost same amount of particles, OVA sorption capacity of microspheres prepared with 0.2 mL inner Al(OH)3/trehalose phase-to-1 mL oil phase ratio was significantly higher (p<0.05) than those prepared with 0.3 mL inner Al(OH)3/trehalose phase-to-1 mL oil phase ratio (see Fig. 3A). In the latter active SM formulation, Al(OH)3 was not effectively encapsulated in PLGA microspheres (see Supplementary Fig. 1 for the presence of free Al(OH)3 aggregates along with microspheres). This poor Al(OH)3 microencapsulation can be attributed to formation of a less stable primary w/o-emulsion with higher fraction (0.3 mL) of inner phase, which is known to originate from inner phase droplet coalescence and agglomeration [41-43], resulting in drainage of Al(OH)3-trehalose from PLGA. Then, extensive washing of blank active SM microspheres to remove PVA before freeze-drying also removed lyoprotectant trehalose from Al(OH)3 remaining outside PLGA microspheres (see Supplementary Fig. 1), thereby leading to loss of adsorbing capacity of the non encapsulated Al(OH)3 due to freeze-drying without sufficient trehalose [40]. In addition, the adsorption capacity of Al(OH)3 accessible in preformed microspheres prepared with 0.3 mL inner Al(OH)3/trehalose phase-to-1 mL oil phase ratio was significantly lower (p<0.05) when compared to those prepared with a 0.2 mL inner Al(OH)3/trehalose phase-to-1 mL oil phase ratio (see Fig. 4A). As expected from the percolation theory, these results suggest the necessity of an appropriate inner Al(OH)3/trehalose phase-to-1 mL oil phase ratio to achieve effective microencapsulation of Al(OH)3 in PLGA and protect its adsorbing capacity.
3.3.2. Size of PLGA microparticles
Al(OH)3-PLGA microspheres of different size distributions (20-63 and 63-90 μm) were obtained by passing the microspheres through 20, 63, and 90 μm sieves. The influence of size of preformed Al(OH)3-PLGA microspheres on active OVA loading capacity and accessibility of Al(OH)3 adsorption capacity in preformed microspheres for OVA loading was then determined. OVA loading (see Fig. 3B) and Al(OH)3 accessibility in preformed PLGA microspheres for OVA loading (see Fig. 4B) was significantly higher (p<0.05) for 20-63 μm microspheres than those of 63-90 μm. Since the microspheres of both the size distributions had similar Al(OH)3 loading (see Table 1). However, the lower OVA loading exhibited by the larger size distribution can be attributed to an increased difficulty of the OVA to access the protein-trapping agent deep within the larger microspheres.
3.3.3. PLGA concentration
Polymer concentration can potentially affect the distribution of the inner water phase droplets in the polymer phase during the formation of primary w/o-emulsion and hence drug-loading in the polymer [41, 43]. We investigated the influence PLGA concentration (250 and 350 mg/mL) on encapsulation efficiency of Al(OH)3, active loading of OVA, and Al(OH)3 accessibility in preformed PLGA microspheres for OVA loading. There was no significant difference (p>0.05) in encapsulation efficiency of Al(OH)3 (see Table 1), active loading of OVA (see Fig. 3C), and post microencapsulation accessibility of Al(OH)3 (see Fig. 4C) between 250 and 350 mg/mL polymer concentration-based Al(OH)3-PLGA microsphere formulations.
3.3.4. Type of vaccine adjuvant
Effect of type of vaccine adjuvant (Al(OH)3 or CaHPO4) on active loading/adsorption of OVA after and before PLGA encapsulation of adjuvant is shown in Figs. 3D and E, respectively. Adsorption of OVA to Al(OH)3 before and after PLGA encapsulation was correspondingly higher than that of CaHPO4. The key mechanisms responsible for protein’s adsorption to vaccine adjuvants are electrostatic interactions and ligand exchange [34, 35, 44]. Electrostatic interactions occur between a protein with the lowest isoelectric point (pI) and an adjuvant having the highest points of zero charge, and vice versa [34, 35, 44]. Points of zero charge for Al(OH)3 and CaHPO4 are 11 and 5.5, respectively [34, 35]. The pI of OVA is 4.6 [34]. OVA, Al(OH)3 and CaHPO4 respectively carry negative, positive and negative charges in loading buffer (10 mM MOPS) having pH 7.4 [34, 35]. Hence, stronger (higher) OVA adsorption to Al(OH)3 before and after PLGA microencapsulation of Al(OH)3 can be attributed to both electrostatic interaction (between negatively charged OVA and positively charged Al(OH)3) and ligand exchange (ligand exchange of phosphate groups of phosphorylated OVA with surface hydroxyl groups of Al(OH)3) [44]. Since OVA and CaHPO4 both carry a negative charge at pH 7.4, adsorption of OVA to CaHPO4 before and after PLGA encapsulation was likely governed by ligand exchange via phosphate groups of OVA with hydroxyl groups of CaHPO4 adjuvant, in agreement with the report of Jiang et. al. [35]. Jiang et. al. studied the adsorption of alpha casein (which also has pI of 4.6) to CaHPO4 adjuvant at pH 7.4, and found that adsorption of alpha casein to CaHPO4 was due to ligand exchange and ruled out significant electrostatic interactions [35]. Hence, these results indicated both the ease and flexibility of the novel strategy to actively load different vaccine antigens in PLGA by placing different protein trapping substances in PLGA pores.
3.3.5. Al(OH)3 loading
The quantity of Al(OH)3 required for optimal antigen loading in PLGA microspheres was investigated by preparing 0.88, 1.85, and 3.04 wt% Al(OH)3 loaded PLGA microspheres. As the loading of Al(OH)3 was increased from 0.88 to 3.04 wt%, the fraction of OVA sorbed by the microspheres increased significantly (p<0.05), indicating excellent antigen sorbing ability of Al(OH)3-PLGA microspheres. For example, about 20 mg of 0.88, 1.85, and 3.04 wt% Al(OH)3/PLGA microspheres respectively sorbed 88, 114 and 194 μg OVA (4.4, 5.7 and 9.7 μg OVA/mg Al(OH)3-PLGA microspheres, respectively) after active self-healing microencapsulation (see Fig. 3F). Note that there was no significant difference (p>0.05) in encapsulation efficiency (see Table 1) and post microencapsulation accessibility of Al(OH)3 (see Fig. 4D) when the loading of Al(OH)3 in PLGA was increased over the entire range studied (0.88 to 3.04 wt%).
3.3.6. Porosigen (trehalose) loading
In order to create optimal pores in the polymer so that maximal antigen loading and pore closing is achieved, varying amounts (2.5, 4, and 10.4 wt%) of porosigen (trehalose) was co-encapsulated with Al(OH)3 in PLGA microspheres (Supplementary Fig. 2). As expected, co-incorporation of trehalose (2.5-10.4 wt%) in Al(OH)3-PLGA microspheres significantly improved post microencapsulation accessibility of Al(OH)3 for OVA loading relative to the control formulation (see Fig. 4E), thereby increasing the OVA loading significantly (p<0.05) in PLGA microspheres (see Fig. 3G). For example, about 20 mg of Al(OH)3-PLGA microspheres loaded with 0, 2.5, 4, and 10.4 wt% trehalose respectively sorbed 88, 196, 202, and 112 μg OVA (4.4, 9.8, 10.1 and 5.6 μg OVA/mg Al(OH)3-PLGA microspheres, respectively). However, there was no significant enhancement in post microencapsulation capacity of Al(OH)3 for OVA loading above 4 wt% trehalose loading, suggesting that 2.5 to 4 wt% trehalose appears to be optimal composition for maximal antigen loading in 3 wt% Al(OH)3/PLGA microspheres.
3.3.7. Initial loading temperature
The influence of initial loading temperature on OVA sorption by plasticizer-free Al(OH)3-PLGA microspheres was investigated by incubating the microspheres separately at 10 and 25 °C for 24 h (see Fig. 3H). After 24 h of incubation, Al(OH)3-PLGA microspheres incubated at 25 °C exhibited slightly higher OVA loading (9.7 μg OVA/mg Al(OH)3-PLGA microspheres) than those incubated at 10 °C (8.5 μg OVA/mg Al(OH)3-PLGA microspheres). However, there was no significant difference (p>0.05) in OVA adsorption to unencapsulated Al(OH)3 after 24 h incubation at 10 and 25 °C (617 and 620 μg OVA adsorption/mg Al(OH)3 at 10 and 25 °C, respectively). It is noteworthy to mention here that, when we incubated Al(OH)3-PLGA microspheres containing 5% of the hydrophobic plasticizer, DEP, at 25 °C, pores in the polymer began to heal (due to low Tg of the polymer, see Table 4), thereby reducing OVA loading in the microspheres (data not shown). Hence, a temperature ramp from 10 to 37/38 °C was used to maximize the vaccine antigen loading in hydrophobic plasticizer loaded Al(OH)3-PLGA microspheres.
Table 4.
Evaluation of tetanus toxoid (TT) active self-healing microencapsulation in PLGA microspheres as a function of initial concentration of vaccine antigen in loading solution (ASM-1), and type of the plasticizer (ASM-24) and porosigen (ASM-27) incorporated.
| Formulation | TT massa | TT loading based on
|
Encapsulation efficiency (%)* | |||
|---|---|---|---|---|---|---|
|
|
Mass loss from loading solution (μg)* | Polymer content
|
||||
| Initial (μg) | Remaining (μg)* | (μg)* | (wt%)* | |||
| ASM-1 | 400 | 70 ± 3 | 330 ± 3 | 308 ± 43b | 1.38 ± 0.2b | 77 ± 11b,c |
| ASM-1 | 320 | 6 ± 2 | 314 ± 2 | 309 ± 10b | 1.38 ± 0.1b | 97 ± 3b,c |
| ASM-24 | 320 | 38 ± 4 | 282 ± 4 | ND | 1.35 ± 0.03d | 88 ± 1d |
| ASM-27 | 320 | 81 ± 3 | 239 ± 3 | ND | 1.11 ± 0.02d | 75 ± 1d |
: volume = 0.5 mL;
: Mean ± SEM, n = 3;
: TT content in the polymer was determined by amino acid analysis;
: based on the antigen content in the polymer;
: based on the TT mass loss from loading solution
ASM-1: 3.2 wt% Al(OH)3/3.5 wt% trehalose/5 wt% DEP/PLGA microspheres; ND: Not determined
ASM-24: 3.2 wt% Al(OH)3/3 wt% trehalose/5 wt% TBAC/PLGA microspheres
ASM-27: 3.2 wt% Al(OH)3/1.5 wt% trehalose/1.5 wt% MgCO3/5 wt% TBAC/PLGA microspheres
Active self-healing encapsulation of TT was performed by incubating ~20 mg microspheres (20-63 μm in size) with 0.5 mL of 0.64-0.8 mg/mL TT in normal saline (pH 6.8) under mild agitation at 10, 25 and 38 °C respectively for 24, 24, and 40 h.
The Al(OH)3-PLGA or CaHPO4-PLGA microsphere studied above demonstrated a strong ability to absorb vaccine antigen selectively from very low concentration (1 mg/mL) aqueous antigen solution. The above studied parameters can be summarized as follows. Active loading of vaccine antigen was a) manipulated by encapsulating different adjuvant (Al(OH)3 or CaHPO4), b) found to increase proportionally with an increasing loading of Al(OH)3 (0.88-3.04 wt%) and significantly by co-incorporation of 2.5-4 wt% trehalose, c) found to decrease when the inner Al(OH)3/trehalose phase to 1 mL outer oil phase and size of microspheres was > 0.2 mL and 63 μm, respectively, and d) slightly influenced by the polymer concentration and initial loading temperature.
3.4. Al(OH)3 suppresses self-healing of PLGA
The effect encapsulation of Al(OH)3 or CaHPO4 in PLGA microspheres on pore-closing/self-healing of PLGA is shown in Fig. 5. The pores in the polymeric matrix of Al(OH)3-PLGA microspheres did not close/self-heal under the selected healing conditions (see Fig. 5A). In contrast, almost all pores on the surface of PLGA microspheres without Al(OH)3 or CaHPO4 healed under similar conditions (see Fig. 5B), indicating a suppression of PLGA healing by Al(OH)3. This was further evident when this effect was not exhibited by the other adjuvant studied, CaHPO4, where almost all pores on the surface of CaHPO4-PLGA microspheres appeared to close under similar loading and healing conditions (see Fig. 5C). To understand further, the Tg of 0, 0.88, 1.85, and 3.04 wt% Al(OH)3 loaded PLGA microspheres was measured (see Table 1) and found to be unaffected by the Al(OH)3 loading level (42.7, 42.5, 42.6, and 42.7 °C, respectively). Therefore, interactions of the gel with PLGA did not affect bulk PLGA chain mobility in the dry state, although changes to the PLGA chain mobility in the wet state cannot be ruled out. It is also possible that gel/polymer surface interactions may be responsible. Further studies are underway to understand the kinetics and mechanism of PLGA self-healing.
Fig. 5.
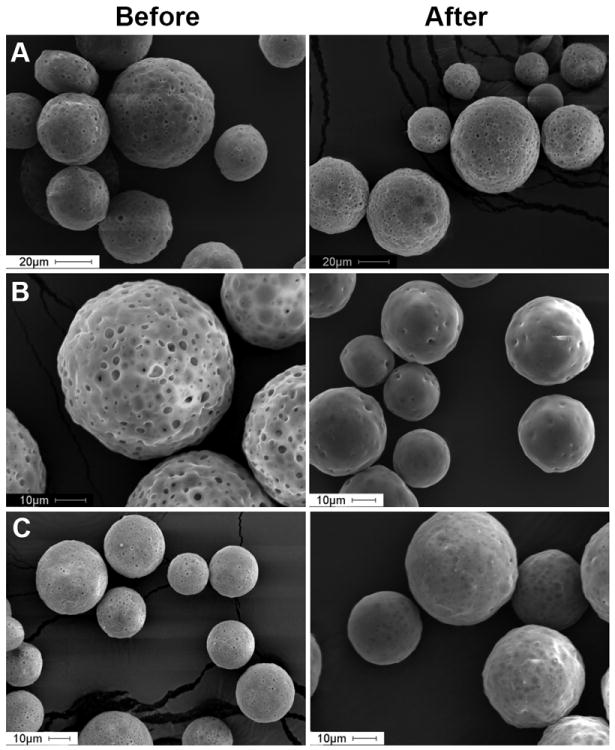
Aluminum hydroxide (Al(OH)3) suppresses self-healing of PLGA. Effect of encapsulation of aluminum hydroxide (Al(OH)3) or calcium phosphate (CaHPO4) in PLGA microspheres on PLGA healing. Scanning electron microscopic images of Al(OH)3-trehalose-PLGA (A), blank (trehalose/PLGA) (B), and CaHPO4/trehalose/PLGA (C) microspheres before and after active self-healing microencapsulation process. Image A, B, and C respectively correspond to ASM-17, B-1, and ASM-18 in Table 1. Microspheres (20-63 μm in size) were incubated with 0.4 mL of 1 mg/mL OVA in 10 mM MOPS buffer (pH 7.4) under mild agitation at 25 and 43 °C respectively for 24 and 48 h.
3.5. Hydrophobic plasticizers overcome Al(OH)3-induced self-healing suppression
In order to overcome the self-healing suppression induced by Al(OH)3 (see Fig. 6A), we sought to increase PLGA mobility by co-incorporating hydrophobic plasticizers in the polymer. For example, DEP or TBAC equivalent to 2.5, 5, 10, and 20 wt% based on polymer mass was incorporated in the polymer. As expected, with increasing content of DEP or TBAC in PLGA, Tg of the dry polymer was reduced considerably (see Table 1), thereby improving polymer chain mobility and providing self-healing of PLGA pores in the polymeric matrix of Al(OH)3-PLGA at 37 °C (see Fig. 6B and C). A similar finding was observed with another hydrophobic plasticizer (TBAC) (Supplementary Fig. 3). This finding indicates that any suppression of self-healing by Al(OH)3 could be overcome by the hydrophobic plasticizer. However, interparticle self-healing observed with 10 and 20 wt% DEP loaded PLGA microspheres (see Fig. 6D and E) indicating the necessity of appropriate quantity of plasticizer for optimal self-healing. Importantly, successful employment of plasticizer to reduce the required healing temperature opens-up the door to self-encapsulate temperature-sensitive molecules in higher Mw PLGA at or below physiological temperature.
Fig. 6.
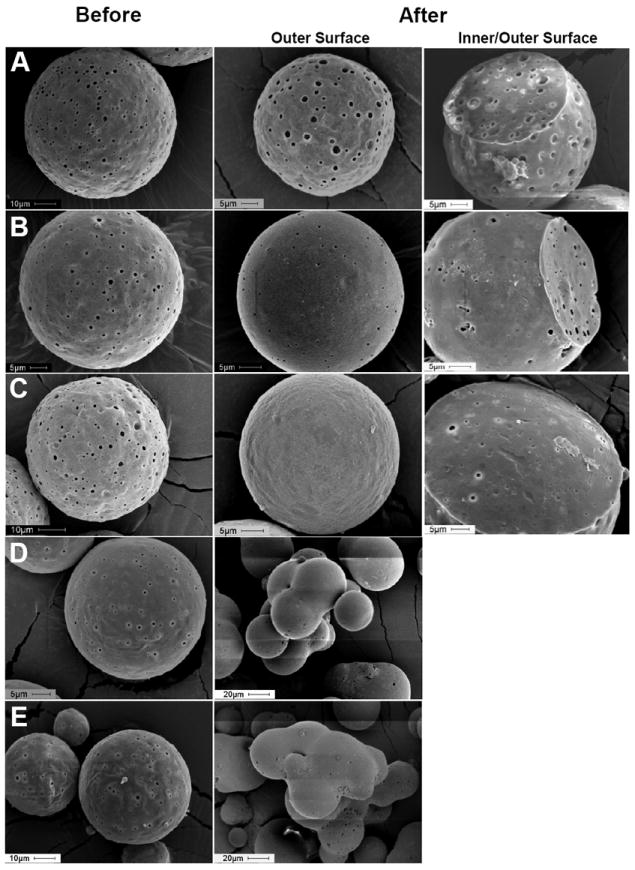
Co-incorporation of hydrophobic plasticizer (diethyl phthalate (DEP)) in PLGA overcomes self-healing suppression induced by Al(OH)3. Scanning electron microscopic image of 0 (A), 2.5 (B), 5 (C), 10 (D), and 20 (E) wt% DEP loaded Al(OH)3-PLGA microspheres before and after active self-healing microencapsulation. Image A, B, C, D, and E respectively correspond to ASM-19, ASM-20, ASM-1, ASM-21 and ASM-22 in Table 1. Microspheres (20-63 μm in size) were incubated with 0.4 mL of 1 mg/mL OVA in 10 mM MOPS buffer (pH 7.4) under mild agitation at 10, 25 and 37 °C respectively for 48, 24, and 30 h.
3.6. Distribution of vaccine antigen in PLGA microspheres after active self-healing microencapsulation
To examine vaccine antigen distribution inside active self-microencapsulated PLGA microspheres, fluorescein-OVA was similarly self-encapsulated using 0.5 mg/mL loading solution and observed under a confocal microscope. Fluorescein-OVA appears to be distributed homogeneously inside microsphere (see Fig. 7, Supplementary Fig. 4 and Supplementary Video), indicating uniformity of encapsulated material by active self-microencapsulation paradigm. Importantly, fluorescein-OVA was characterized by SE-HPLC before encapsulation to check the presence of free fluorescein dye in the sample and found to be free from of un-conjugated/free fluorescein dye (data not shown). Hence, homogeneous fluorescence observed inside active self-microencapsulated PLGA microspheres was mainly due to effective and uniform encapsulation of fluorescein-OVA and not because of free dye, which is known to partition in the PLGA polymer phase [45, 46].
Fig. 7.
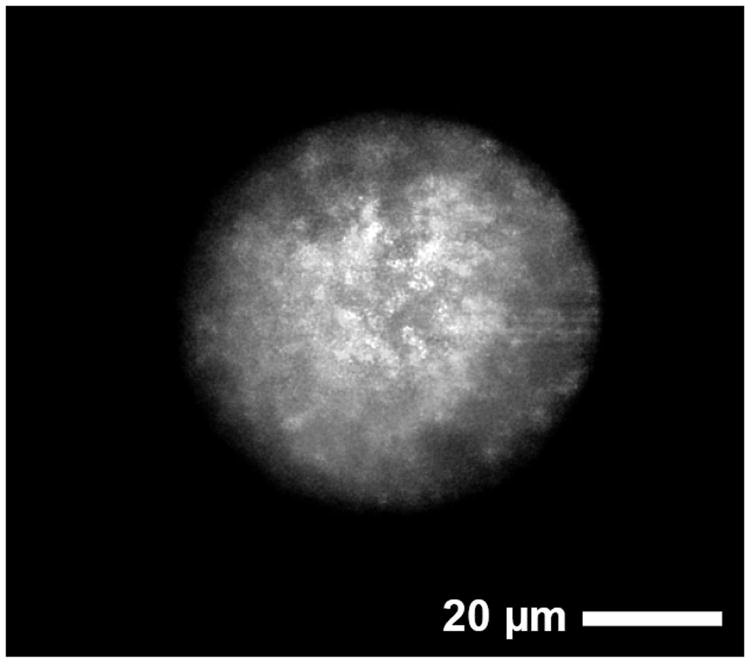
Distribution of vaccine antigen in 3.2 wt% Al(OH)3/3.5 wt% trehalose-PLGA-5 wt% diethyl phthalate microspheres (ASM-1) after active self-healing encapsulation of FITC-OVA. Confocal microscopic image of FITC-OVA, which was encapsulated in a self-healing PLGA microsphere. Active self-healing microencapsulation of fluorescein-OVA was performed by incubating 20 mg microspheres (20-63 μm in size) with 0.4 mL of 0.5 mg/mL fluorescein-OVA in 10 mM MOPS buffer (pH 7.4) under mild agitation at 10, 25 and 37 °C respectively for 48, 24, and 30 h.
3.7. Active PLGA self-healing microencapsulation overcomes tetanus toxoid instability relative to the double emulsion-solvent evaporation method
The stability of manufacturing process-labile TT after encapsulation by the traditional w/o/w emulsion-solvent evaporation was evaluated and compared with that obtained by active self-healing microencapsulation. The fraction of soluble, insoluble (covalent and non-covalent aggregates), and antigenically active TT remaining in PLGA after microencapsulation by w/o/w emulsion-solvent evaporation method is given in Table 2. As expected, the encapsulation of manufacturing process sensitive TT in PLGA microspheres by the traditional method resulted in significant loss of stability of TT (only about 27% of the encapsulated TT was antigenically active). The cause for TT instability during encapsulation has been attributed to harsh manufacturing conditions such as water/methylene chloride interface, homogenization, and freeze-drying [17, 18]. In contrast, active PLGA self-healing microencapsulation obviated this key instability issue, as indicated by 96-99% antigenically active TT release from DEP and TBAC loaded Al(OH)3-PLGA microspheres (formulations with optimal release behavior) after 28 days of incubation in PBST + 0.2% BSA (see in vitro TT release curve). It is important to note that the antigenic release fraction recorded for self-encapsulated microsphere formulations was normalized by TT loading determined by mass loss from loading solution. The results of in vitro release and loading assays (mass loss from loading solution and amino acids analysis of microspheres) indicate that > 96% of the self-encapsulated TT was antigenically active. Therefore, further determination of soluble and insoluble fraction of self-encapsulated TT by polymer extraction was not necessary to compare with the stability results of w/o/w emulsion-solvent evaporation. Further, the integrity of OVA after active self-microencapsulation process was also assessed by size exclusion chromatography. The chromatogram of OVA after active self-microencapsulation was similar to that of native OVA (before encapsulation) (Supplementary Fig. 5). Also, there were no additional peaks in the chromatogram, suggesting the absence of any soluble OVA aggregates or fragments. These results strongly suggest that Al(OH)3-adsorbing vaccine antigens can now be encapsulated in PLGA microspheres with minimal loss of their integrity and antigenicity. Additional in vivo potency will be needed in future studies to confirm these in vitro data.
Table 2.
Evaluation of stability of tetanus toxoid (TT) in PLGA microspheres prepared by traditional w/o/w emulsion-solvent evaporation method.
| Concentration of TT in WP (mg/mL) | TT loading (wt%)
|
Encapsulation efficiency (%)a*
|
|||||
|---|---|---|---|---|---|---|---|
| Theoretical | Actual*
|
Antigenic TT | Total TT | ||||
| Solublea,b | Antigenica,c | Insolublea,b | Totala,b | ||||
| 6.24 | 0.5 | 0.36 ± 0.03 | 0.12 ± 0.01 | 0.11 ± 0.02 | 0.47 ± 0.01 | 24.6 ± 2.2 | 94.0 ± 1.3b |
WP: inner water phase;
Mean ± SEM, n = 3;
: based on TT content in polymer;
: determined by modified Bradford assay
: determined by ELISA
3.8. Kinetics of active PLGA self-microencapsulation of manufacturing process-labile tetanus toxoid
Previously, the kinetics of active PLGA self-microencapsulation was investigated using a model vaccine antigen (ovalbumin) [32]. These characteristics were further investigated in this study with manufacturing process-labile TT. As shown in Table 3, 3.2 wt% Al(OH)3-3.5 wt% trehalose-PLGA-5 wt% DEP microspheres actively sorbed TT as a function of incubation time and temperature from 1 mg/mL aqueous solution with a maximal loading of ~ 18 μg TT/mg Al(OH)3-PLGA-DEP microspheres (~1.8 wt% loading), a significant improvement compared to OVA loading (~ 10 μg OVA/mg Al(OH)3-PLGA-DEP microspheres or 1.0 wt% OVA loading) [32]. The Al(OH)3 adsorption capacity accessible in preformed PLGA microspheres for TT loading was similarly higher (~80%) compared to that of OVA (~55%). Higher TT loading exhibited by active SM PLGA microspheres can be attributed to higher adsorption capacity of Al(OH)3 for TT. The encapsulation efficiency of the same formulation was further evaluated using 0.64 and 0.8 mg/mL TT solution (see Table 4). Active SM PLGA microspheres exhibited maximal encapsulation efficiency of 97% for sensitive TT (see Table 4). In contrast, encapsulation TT by traditional w/o/w emulsion-solvent evaporation required significantly higher concentration of TT (6.24 mg/mL TT) in inner water phase but resulted in significantly less TT loading (0.47 wt%) in the polymer (see Table 2).
Table 3.
Tetanus toxoid (TT) mass loss kinetics in the presence of antigen/unencapsulated Al(OH)3 and antigen/3.2 wt% Al(OH)3-3.5 wt% trehalose-5 wt% DEP-PLGA microspheres (ASM-1) mixture during temperature ramping in contrast (10-38 °C).
| Incubation temperature (°C) and duration (h)
|
Initial TT massa (μg) | Formulation
|
||||
|---|---|---|---|---|---|---|
| Remaining TT mass in solutiona (μg)*
|
TT loaded (μg)*
|
|||||
| °C | h | Unencapsulated Al(OH)3 | ASM | Unencapsulated Al(OH)3 | ASM | |
| 10 | 3 | 500 | 18 ± 2 | 374 ± 14 | 482 ± 2 | 126 ± 14 |
| 6 | 500 | 15 ± 1 | 338 ± 13 | 485 ± 1 | 162 ± 13 | |
| 24 | 500 | 12 ± 1 | 300 ± 18 | 488 ± 1 | 200 ± 18 | |
| 48 | 500 | ND | ND | ND | ND | |
| 25 | 24 | |||||
| + | ||||||
| 38 | 40 | 500 | 10 ± 1 | 99 ± 5 | 490 ± 1 | 401 ± 5 |
: volume = 0.5 mL; ASM: active SM PLGA microspheres;
: Mean ± SEM, n = 3; ND: Not determined
3.9. Effect of encapsulation methods, hydrophobic plasticizers, and mixed porosigens on in vitro release of antigenic tetanus toxoid from PLGA microspheres
Continuing from our previous study reporting initial in vitro release of antigens from one active self-microencapsulating PLGA microsphere formulation [32], we further studied some important aspects relevant to active self-healing microencapsulation and their probable effects on long-term antigenic TT release. Additional studies were performed to understand in vitro antigenic TT release behavior from: a) TT/PLGA microspheres prepared by w/o/w emulsion-solvent evaporation to compare with that of provided by active self-healing encapsulation, b) active self-microencapsulation of TT with a chemically different and significantly less water soluble (0.005 mg TBAC/mL at 20°C compared to 1 mg DEP/mL at 25°C) plasticizer (TBAC) instead of previously studied DEP, and c) mixed porosigens (trehalose and MgCO3) with varying water solubility. Using the actual content of antigenic TT in traditionally encapsulated PLGA microspheres (see Table 2) or TT mass loss from active SM PLGA/TT mixture, the cumulative amount of antigenic TT released from PLGA microspheres as function of time was calculated (see Fig. 8). All the active self-microencapsulated PLGA microspheres exhibited significantly improved in vitro controlled antigenic TT release (significantly less initial burst and slow and continuous antigenic TT release thereafter over a period of 28 days) when compared to those prepared by traditional w/o/w emulsion-solvent evaporation method, indicating the ability of active PLGA self-healing microencapsulation to provide improved long-term stable release of sensitive vaccine antigens. Poor controlled release of antigenic TT from PLGA microspheres (noncontinuous and incomplete release) prepared by the traditional w/o/w solvent evaporation has been attributed to instability of TT in the polymer matrix due to protein damage by organic solvent and homogenization exposure during encapsulation, the PLGA acidic microclimate pH and formaldehyde-induced inactivation [17]. It is important to note that the release fraction recorded for the solvent evaporation microsphere formulation was normalized by the extractable antigenic TT (Table 2). In fact most of the TT originally loaded in this formulation was not antigenically active (Table 2). In vitro release of antigen from active self-encapsulated PLGA microspheres was not significantly affected by the type of plasticizer used to achieve self-healing of PLGA pores (moderately water soluble DEP or nearly insoluble TBAC). However, coincorporation of MgCO3 in place of a portion of trehalose was found to exhibit slower antigen release compared to control, suggesting that the base was less successful in creating a pore network in the pre-formed microspheres. In addition, the TT adsorbed to the Al(OH)3 in the PLGA appeared to be spared the damaging acidic microclimate pH often presence in PLGAs.
Fig. 8.
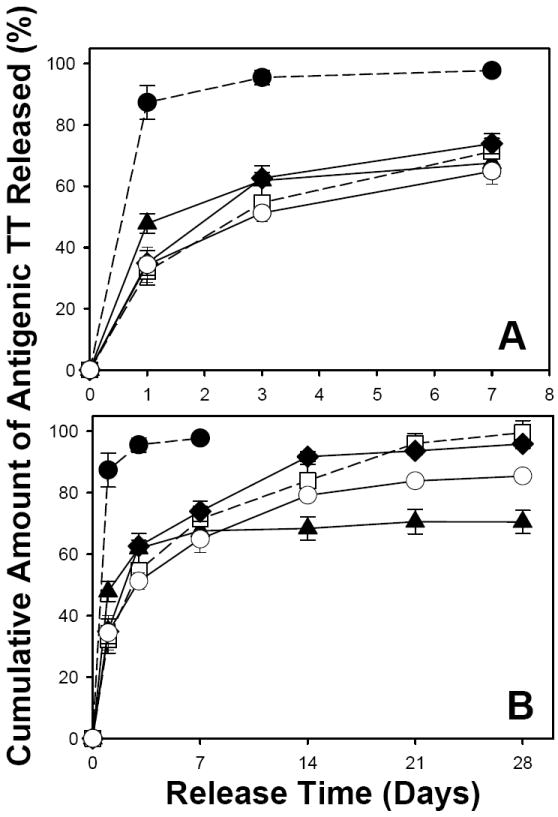
Effect of encapsulation methods (traditional w/o/w emulsion-solvent evaporation vs. active self-healing encapsulation), type of plasticizer (slightly water-soluble diethyl phthalate (DEP) vs. insoluble tributyl acetylcitrate (TBAC)), and mixed porosigens (3 wt% trehalose vs. 1.5 wt% trehalose + 1.5 wt% MgCO3) on initial burst (A) and long-term (B) in vitro antigenic TT release kinetics from PLGA microspheres. The TT release kinetics were from unencapsulated Al(OH)3 (●), TT/PLGA microspheres prepared from w/o/w emulsion solvent evaporation (▲), and TT/adjuvant/plasticizer/PLGA microspheres after self-healing microencapsulation (□: 3.2 wt% Al(OH)3/3.5 wt% trehalose/5 wt% DEP/PLGA microspheres; ◆: 3.2 wt% Al(OH)3/3 wt% trehalose/5 wt% TBAC/PLGA microspheres; ○: 3.2 wt% Al(OH)3/1.5 wt% trehalose/1.5 wt% MgCO3/5 wt% TBAC/PLGA microspheres). The release curves with dashed line are reproduced from [32] for comparison. In vitro release was evaluated in PBST + 0.2% BSA at 37 °C, and symbols represent mean ± SEM, n = 3.
4. Conclusions
The optimization of a novel active self-healing method to encapsulate protein antigens in PLGA [32, 33] is described. Effective and stable encapsulation of protein trapping agent (vaccine adjuvant) in PLGA microspheres was governed by the inner adjuvant/trehalose phase to outer oil phase ratio during formation of primary emulsion. Active loading of vaccine antigens in preformed active SM PLGA microspheres was found to be adjustable by: a) placing different adjuvant in PLGA pores, b) varying the loading of vaccine adjuvant in preformed active SM PLGA microspheres, c) co-incorporating a porosigen, and d) changing the vaccine antigen. The self-healing suppression induced by interaction between vaccine adjuvant and PLGA could be overcome, and temperature required for self-healing was suitably reduced by co-encapsulating hydrophobic plasticizers. Active PLGA self-healing microencapsulation obviated instability of TT caused by traditional w/o/w emulsion-solvent evaporation method, and provided improved controlled release of antigenic TT. Using this self-healing approach, further efforts are underway to develop appropriate active SM PLGA microspheres for delivery of important therapeutic proteins and protein antigens.
Supplementary Material
Acknowledgments
This study was funded by NIH R01 HL 68345 and R21 EB 08873. We greatly appreciate Mrs. Shobha Churi (Dept. of Pharmacy Practice) and Dr. Manjunatha (Registrar, JSS University, Mysore, India) for their help with the procurement of TT sample from Serum Institute of India. We thank Dr. Rajesh Gupta, FDA, for sending Equine tetanus antitoxin sample and assisting with experimental design. We also thank Samuel Straight, Dept. of Microbiology and Immunology at University of Michigan for his assistance with confocal microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diwan M, Khar RK, Talwar GP. Tetanus toxoid loaded ‘preformed microspheres’ of cross-linked dextran. Vaccine. 2001;19:3853–3859. doi: 10.1016/s0264-410x(01)00140-2. [DOI] [PubMed] [Google Scholar]
- 2.Wilson-Welder JH, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B. Vaccine adjuvants: Current challenges and future approaches. J Pharm Sci. 2009;98:1278–1316. doi: 10.1002/jps.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Hagan DT, Rappuoli R. Novel approaches to pediatric vaccine delivery. Adv Drug Deliv Rev. 2006;58:29–51. doi: 10.1016/j.addr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Schwendeman SP, Tobio M, Joworowicz M, Alonso MJ, Langer R. New strategies for the microencapsulation of tetanus vaccine. J Microencapsulation. 1998;15:299–318. doi: 10.3109/02652049809006859. [DOI] [PubMed] [Google Scholar]
- 5.Jiang WL, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57:391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Kersten GFA, Donders D, Akkermans A, Beuvery EC. Single shot with tetanus toxoid in biodegradable microspheres protects mice despite acid-induced denaturation of the antigen. Vaccine. 1996;14:1627–1632. doi: 10.1016/s0264-410x(96)00145-4. [DOI] [PubMed] [Google Scholar]
- 7.Alonso MJ, Gupta RK, Min C, Siber GR, Langer R. Biodegradable microspheres as controlled-release tetanus toxoid delivery systems. Vaccine. 1994;12:299–306. doi: 10.1016/0264-410x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 8.Coombes AGA, Lavelle EC, Jenkins PG, Davis SS. Single dose, polymeric, microparticle based vaccines: The influence of formulation conditions on the magnitude and duration of the immune response to a protein antigen. Vaccine. 1996;14:1429–1438. doi: 10.1016/s0264-410x(96)00077-1. [DOI] [PubMed] [Google Scholar]
- 9.Men Y, Thomasin C, Merkle HP, Gander B, Corradin G. A single administration of tetanus toxoid in biodegradable microspheres elicits T cell and antibody responses similar or superior to those obtained with aluminum hydroxide. Vaccine. 1995;13:683–689. doi: 10.1016/0264-410x(94)00046-p. [DOI] [PubMed] [Google Scholar]
- 10.Singh M, Li XM, Wang HY, McGee JP, Zamb T, Koff W, Wang CY, Ohagan DT. Immunogenicity and protection in small-animal models with controlled-release tetanus toxoid microparticles as a single-dose vaccine. Infect Immun. 1997;65:1716–1721. doi: 10.1128/iai.65.5.1716-1721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguado MT, Lambert PH. Controlled-release vaccines-biodegradable polylactide polyglycolide (PL/LG) microspheres as antigen vehicles. Immunobiology. 1992;184:113–125. doi: 10.1016/S0171-2985(11)80470-5. [DOI] [PubMed] [Google Scholar]
- 12.Waeckerie-Men Y, Gander B, Groettrup M. Delivery of tumor antigens to dendritic cells using biodegradable microspheres. Adoptive Immunotherapy: Methods and Protocols. 2005;109:35–46. doi: 10.1385/1-59259-862-5:035. [DOI] [PubMed] [Google Scholar]
- 13.Alonso MJ, Cohen S, Park TG, Gupta RK, Siber GR, Langer R. Determinants of release rate of tetanus vaccine from polyester microspheres. Pharm Res. 1993;10:945–953. doi: 10.1023/a:1018942118148. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez A, Villamayor B, Guo YY, McIver J, Alonso MJ. Formulation strategies for the stabilization of tetanus toroid in poly(lactide-co-glycolide) microspheres. Int J Pharm. 1999;185:255–266. doi: 10.1016/s0378-5173(99)00178-7. [DOI] [PubMed] [Google Scholar]
- 15.Tobio M, Nolley J, Guo YY, McIver J, Alonso MJ. A novel system based on a poloxamer PLGA blend as a tetanus toxoid delivery vehicle. Pharm Res. 1999;16:682–688. doi: 10.1023/a:1018820507379. [DOI] [PubMed] [Google Scholar]
- 16.Schwendeman SP. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit Rev Ther Drug. 2002;19:73–98. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- 17.Jiang WL, Schwendeman SP. Stabilization of tetanus toxoid encapsulated in PLGA microspheres. Mol Pharm. 2008;5:808–817. doi: 10.1021/mp800027f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang AC, Gupta RK. Stabilization of tetanus toxoid in poly(DL-lactic-co-glycolic acid) microspheres for the controlled release of antigen. J Pharm Sci. 1996;85:129–132. doi: 10.1021/js950365v. [DOI] [PubMed] [Google Scholar]
- 19.Johansen P, Tamber H, Merkle HP, Gander B. Diphtheria and tetanus toroid microencapsulation into conventional and end-group alkylated PLA/PLGAs. Eur J Pharm Biopharm. 1999;47:193–201. doi: 10.1016/s0939-6411(98)00095-2. [DOI] [PubMed] [Google Scholar]
- 20.Johansen P, Estevez F, Zurbriggen R, Merkle HP, Gluck R, Corradin G, Gander B. Towards clinical testing of a single-administration tetanus vaccine based on PLA/PLGA microspheres. Vaccine. 2000;19:1047–1054. doi: 10.1016/s0264-410x(00)00343-1. [DOI] [PubMed] [Google Scholar]
- 21.Murillo M, Gamazo C, Goñi MM, Irache JM, Blanco-Príeto MJ. Development of microparticles prepared by spray-drying as a vaccine delivery system against brucellosis. Int J Pharm. 2002;242:341–344. doi: 10.1016/s0378-5173(02)00212-0. [DOI] [PubMed] [Google Scholar]
- 22.Johansen P, Men Y, Audran R, Corradin G, Merkle HP, Gander B. Improving stability and release kinetics of microencapsulated tetanus toxoid by co-encapsulation of additives. Pharm Res. 1998;15:1103–1110. doi: 10.1023/a:1011998615267. [DOI] [PubMed] [Google Scholar]
- 23.Katare YK, Panda AK. Influences of excipients on in vitro release and in vivo performance of tetanus toxoid loaded polymer particles. Eur J Pharm Sci. 2006;28:179–188. doi: 10.1016/j.ejps.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 24.Morita T, Sakamura Y, Horikiri Y, Suzuki T, Yoshino H. Protein encapsulation into biodegradable microspheres by a novel S/O/W emulsion method using poly(ethylene glycol) as a protein micronization adjuvant. J Control Release. 2000;69:435–444. doi: 10.1016/s0168-3659(00)00326-6. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Rodriguez C, Montano N, Gonzalez K, Griebenow K. Stabilization of alpha-chymotrypsin at the CH2Cl2/water interface and upon water-in-oil-in-water encapsulation in PLGA microspheres. J Control Release. 2003;89:71–85. doi: 10.1016/s0168-3659(03)00074-9. [DOI] [PubMed] [Google Scholar]
- 26.Baxendale AJ, van Hooff P, Durrant LG, Spendlove I, Howdle SM, Woods HM, Whitaker MJ, Davies OR, Naylor A, Lewis AL, Illum L. Single shot tetanus vaccine manufactured by a supercritical fluid encapsulation technology. Int J Pharm. 2011;413:147–154. doi: 10.1016/j.ijpharm.2011.04.053. [DOI] [PubMed] [Google Scholar]
- 27.Wu F, Jin T. Polymer-based sustained-release dosage forms for protein drugs, challenges, and recent advances. AAPS PharmSciTech. 2008;9:1218–1229. doi: 10.1208/s12249-008-9148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Syrett JA, Becer CR, Haddleton DM. Self-healing and self-mendable polymers. Polym Chem. 1:978–987. [Google Scholar]
- 29.Wool RP. Self-healing materials: A review. Soft Matter. 2008;4:400–418. doi: 10.1039/b711716g. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Wang BA, Schwendeman SP. Characterization of the initial burst release of a model peptide from poly(D,L-lactide-co-glycolide) microspheres. J Control Release. 2002;82:289–307. doi: 10.1016/s0168-3659(02)00137-2. [DOI] [PubMed] [Google Scholar]
- 31.Schwendeman SP, Reinhold SE, Kang J. Methods for encapsulation of biomacromolecules in polymers. US8017155. 2005
- 32.Reinhold SE, Desai KGH, Zhang L, Olsen KF, Schwendeman SP. Self-healing microencapsulation of biomacromolecules without organic solvents. Angew Chem Int Ed. 2012 doi: 10.1002/anie.201206387. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwendeman SP, Desai KGH. Active self-healing biomaterial system. 2011 WO/2011/088229. [Google Scholar]
- 34.Jones LS, Peek LJ, Power J, Markham A, Yazzie B, Middaugh CR. Effects of adsorption to aluminum salt adjuvants on the structure and stability of model protein antigens. J Biol Chem. 2005;280:13406–13414. doi: 10.1074/jbc.M500687200. [DOI] [PubMed] [Google Scholar]
- 35.Jiang DP, Premachandra GS, Johnston C, Hem SL. Structure and adsorption properties of commercial calcium phosphate adjuvant. Vaccine. 2004;23:693–698. doi: 10.1016/j.vaccine.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 36.Gupta RK, Siber GR. Adjuvants for human vaccines--current status, problems and future prospects. Vaccine. 1995;13:1263–1276. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 37.Rosas JE, Hernandez RM, Gascon AR, Igartua M, Guzman F, Patarroyo ME, Pedraz JL. Biodegradable PLGA microspheres as a delivery system for malaria synthetic peptide SPf66. Vaccine. 2001;19:4445–4451. doi: 10.1016/s0264-410x(01)00192-x. [DOI] [PubMed] [Google Scholar]
- 38.Waeckerle-Men Y, Allmen EU-v, Gander B, Scandella E, Schlosser E, Schmidtke G, Merkle HP, Groettrup M. Encapsulation of proteins and peptides into biodegradable poly(d,l-lactide-co-glycolide) microspheres prolongs and enhances antigen presentation by human dendritic cells. Vaccine. 2006;24:1847–1857. doi: 10.1016/j.vaccine.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 39.Igartua M, Hernandez RM, Esquisabel A, Gascon AR, Calvo MB, Pedraz JL. Enhanced immune response after subcutaneous and oral immunization with biodegradable PLGA microspheres. J Control Release. 1998;56:63–73. doi: 10.1016/s0168-3659(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 40.Clausi AL, Merkley SA, Carpenter JF, Randolph TW. Inhibition of aggregation of aluminum hydroxide adjuvant during freezing and drying. J Pharm Sci. 2008;97:2049–2061. doi: 10.1002/jps.21143. [DOI] [PubMed] [Google Scholar]
- 41.Cleland JL, Lim A, Barron L, Duenas ET, Powell MF. Development of a single-shot subunit vaccine for HIV-1. 4. Optimizing microencapsulation and pulsatile release of MN rgp120 from biodegradable microspheres. J Control Release. 1997;47:135–150. [Google Scholar]
- 42.Santander-Ortega MJ, Csaba N, Gonzalez L, Bastos-Gonzalez D, Ortega-Vinuesa JL, Alonso MJ. Protein-loaded PLGA-PEO blend nanoparticles: Encapsulation, release and degradation characteristics. Colloid Polym Sci. 2010;288:141–150. [Google Scholar]
- 43.Ye ML, Kim S, Park K. Issues in long-term protein delivery using biodegradable microparticles. J Control Release. 2010;146:241–260. doi: 10.1016/j.jconrel.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Morefield GL, Jiang D, Romero-Mendez IZ, Geahlen RL, HogenEsch H, Hem SL. Effect of phosphorylation of ovalbumin on adsorption by aluminum-containing adjuvants and elution upon exposure to interstitial fluid. Vaccine. 2005;23:1502–1506. doi: 10.1016/j.vaccine.2004.08.048. [DOI] [PubMed] [Google Scholar]
- 45.Kang JC, Schwendeman SP. Pore closing and opening in biodegradable polymers and their effect on the controlled release of proteins. Mol Pharm. 2007;4:104–118. doi: 10.1021/mp060041n. [DOI] [PubMed] [Google Scholar]
- 46.Kang JC, Schwendeman SP. Determination of diffusion coefficient of a small hydrophobic probe in poly(lactide-co-glycolide) microparticles by laser scanning confocal microscopy. Macromolecules. 2003;36:1324–1330. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


