Abstract
An important poorly understood phenomenon in controlled-release depots involves the strong interaction between common cationic peptides and low Mw free acid end-group poly(lactic-co-glycolic acids) (PLGAs) used to achieve continuous peptide release kinetics. The kinetics of peptide sorption to PLGA was examined by incubating peptide solutions of 0.2-4 mM octreotide or leuprolide acetate salts in 0.1 M HEPES buffer, pH 7.4, with polymer particles or films at 4-37 °C for 24 h. The extent of absorption/loading of peptides in PLGA particles/films was assayed by two-phase extraction and amino acid analysis. Confocal Raman microspectroscopy and stimulated Raman scattering (SRS) and laser scanning confocal imaging techniques were used to examine peptide penetration in the polymer phase. The release of sorbed peptide from leuprolide-PLGA particles was evaluated both in vitro (PBST + 0.02% sodium azide, 37 °C) and in vivo (male Sprague-Dawley rats). We found that when the PLGA-COOH chains are sufficiently mobilized, therapeutic peptides not only bind at the surface, a common belief to date, but can also internalized and distributed throughout the polymer phase at physiological temperature forming a salt with low-molecular weight PLGA-COOH. Importantly, absorption of leuprolide into low MW PLGA-COOH particles yielded ~17 wt% leuprolide loading in the polymer (i.e., ~70% of PLGA-COOH acids occupied), and the absorbed peptide was released from the polymer for > 2 weeks in a controlled fashion in vitro and as indicated by sustained testosterone suppression in male Sprague-Dawley rats. This new approach, which bypasses the traditional encapsulation method and associated production cost, opens up the potential for facile production of low-cost controlled-release injectable depots for leuprolide and related peptides.
Keywords: peptide, sorption, PLGA, kinetics, microencapsulation, controlled release
1. Introduction
Improved delivery of synthetic peptides and biotechnology-derived medicines is critical to realize the potential of this diverse and powerful drug class. Low bioavailability from noninvasive routes (e.g., oral and transdermal), and short serum half-lives motivate such improvements (e.g., changes to the drug molecule such as PEGylation) [1] or to the formulation (e.g., injectable controlled release depots) [2]. Such approaches improve patient compliance, comfort and efficacy relative to daily injections. Injectable depot formulations, which deliver peptides from biodegradable poly(lactic-co-glycolic acid) (PLGA), reduce injection frequency anywhere from once weekly to a meager twice-a-year dosing for treatment of cancer, endometriosis, acromegaly, and diabetes [3].
Although highly successful, issues persist, impeding PLGA depot development on a broader scale. Suboptimal peptide release and stability behavior of the dosage form, elevated manufacturing costs, difficulties associated with organic solvent use [2], and patient-unfriendly needle sizes are amongst the most challenging [2, 4]. Underscoring the need for further mechanistic understanding of PLGA depots is the recent discovery of the important role of polymer pores [4], as they spontaneously seal off the release route for peptides and proteins and initiate the well-known lag phase [5] with little polymer erosion. When the lag phase begins, little peptide/protein is released from the polymer and this can persist for days to months for PLGAs above a critical molecular weight depending on several factors such as the lactic-glycolic acid ratio in the polymer [6].
Another important poorly understood phenomenon in controlled-release depots examined herein involves the strong interaction between common therapeutic cationic peptides and low-molecular-weight free acid end-group PLGAs (PLGA-COOHs), the latter of which are often used in depot formulations to facilitate slow and continuous drug release without a lag phase [6]. Peptide interactions with PLGA-COOHs have been implicated in improving drug microencapsulation efficiency and initial burst release (e.g. with leuprolide) [3, 7] as well as chemically modifying the encapsulated peptide structure [8, 9]. Spontaneous uptake of peptides in PLGA-COOH has been reported to occur via adsorption to the polymer surface driven by ionic interactions between positively charge peptide moieties and negatively charge carboxylate end groups of the polymer [8, 10]. The adsorption assumption is reasonable considering the commonly held belief that molecules > ~600 Da do not partition in and penetrate nonporous films of controlled release polymers such as poly(ethylene-co-vinyl acetate) [11].
We employed two highly water soluble, strongly PLGA-COOH-interacting cationic therapeutic peptides (Mw ~1000 Da), octreotide and leuprolide (Fig. S1), in a series of sorption studies to better understand the nature of the peptide-polymer interaction. Octreotide is a somatostatin analogue, used to treat acromegaly. It is a cyclic octapeptide with a molecular weight of 1019.3 Da, containing an intramolecular disulfide and two amino-groups—one at the n-terminus (pKa 7.8) and one on the lysine side chain (pKa 10.1) [8, 12]—that are potential acylation sites [8, 10]. Leuprolide is a linear nonapeptide with a molecular weight of 1209.4 Da. As a gonadotropin-releasing hormone agonist, leuprolide is used clinically to treat prostate cancer, endometriosis and other hormone-related diseases. It does not contain any acylating amino-groups, but has one positively charged arginine side side chain and an ionizable histidine imidazole (pKa ~6.0), providing its positive charge at neutral pH. We chose octreotide as the primary model peptide for initial studies characterizing the effect of solution and polymer properties on peptide sorption, because the PLGA-COOH-peptide binding is implicated in the peptide acylation reaction [8, 9]. Understanding the peptide-polymer binding may further help determine rational means to inhibit this reaction [9, 10, 13]. Leuprolide was also studied for the purpose of a) confirming the generality of the important cationic peptide-polymer binding data, and b) to test the concept of aqueous-based absorption to encapsulation of a non-acylating peptide at the end of the study. Unexpectedly, we found evidence that in hydrated low-molecular-weight PLGA-COOH at neutral pH and physiological temperature, octreotide and leuprolide could rapidly penetrate the entire PLGA-COOH phase at levels closely predicted by the number of end-groups in PLGA-COOH. We then used this concept to test whether positively charged peptides of Mw ~1000 Da could be encapsulated without organic solvent for later therapeutic controlled release from easily prepared depot formulations by evaluating the long-term testosterone supression in rats following administration of non-acylating leuprolide-absorbed in PLGA-COOH.
2. Materials and methods
2.1. Materials
Octreotide acetate was obtained from Novartis (Basel, Switzerland). Leuprolide acetate (Lot No. 071002) was purchased from Shanghai Shinjn Modern Pharmaceutical Technology Co. (Shanghai, China). PLGAs 50:50 (Resomer® RG 502H, 503H, and 504H) were purchased from Boehringer-Ingelheim GmbH (Ingelheim, Germany). (Hydroxyethyl)-piperazine-(ethanesulfonic acid) (HEPES) was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). Diethylpiperazine (DEPP) was purchased from Acros Organics (Geel, Belgium). All other reagents used were of analytical grade or purer and purchased from commercial suppliers. Male Sprague Dawley rats were purchased from Charles River Laboratories International, Inc. (Wilmington, MA).
2.2. PLGA films and particles for peptide sorption studies
For preparation of PLGA films, solutions of PLGA in acetone were placed on glass slides and spread using a spin coater (SCS G3-8, Indianapolis, IN). The film thickness of two representative samples was determined by scanning electron microscopy (SEM). The density of the film was estimated to be 1.0 g/cm3. This value, and the mass of polymer added, was used to estimate the thickness of the other films. The conditions used to spin-coat PLGA solutions onto glass microscope cover-slides are listed in Table S1. Following spin-coating, nascent PLGA films were dried for 48 h at room temperature and atmospheric pressure followed by 24 h in a vacuum oven at 40°C. PLGA particles were used as received by the manufacturer. Acid number (the content of free carboxylic acid) in PLGA used for sorption was determined by non-aqueous titration. About 100 mg of polymer was dissolved in 20 mL of acetone/tetrahydrofuran (THF) (1:1) mixture. The solution was immediately titrated with 0.01 N methanolic potassium hydroxide to a stable pink color. 0.1 wt% phenopthalein methanol was used as an indicator, 20 mL of acetone/THF (1:1) was used as a control. To determine raw polymer porosity, PLGA Resomer® RG 502H, 503H, and 504H were first subjected to Helium Pycnometry (AccuPyc 1330, Micromeritic, Atlanta, GA) to determine polymer density. Porosity and surface area measurements were made by Porous Materials, Inc. (Ithaca, NY) using an AMP-60K-A-1 mercury porosimeter. Porosity was taken as the ratio of pore volume to total particle volume (sum of pore volume and polymer volume), with pore volume as the product of intrusion volume (cc/g) and sample mass, and polymer volume determined from the sample mass and density. Particle size was estimated by taking the average of particle size recorded in scanning electron micrographs.
2.3. Sorption of peptides to PLGA particles and films
For sorption to polymer particles, 0.2-4.0 mM peptide solutions were combined in 1.5 mL microcentrifuge tubes with 10 mg of PLGA particles, and incubated in 1 mL of 0.1 M HEPES, MES, or DEPP buffer solutions to examine sorption kinetics or isotherms at pH 7.4, 5.5, and 4.0, respectively. Particles were incubated with peptide on a shaker as above for various times and temperatures to study sorption kinetics and to generate quasi-equilibrium isoterms. To recover the supernatant, the particles were centrifuged (2 min at 9.0 rcf) and the supernatant was analyzed by HPLC (described in section 2.5) to determine both peptide solution concentration and amount of peptide sorbed to the polymer (i.e., from loss of peptide from solution). No significant degradation of either peptide was recorded by HPLC during all of the sorption studies. For sorption to polymer films, solutions of octreotide or leuprolide (1 mM, 4.5 mL) in HEPES buffer (0.1M, pH 7.4) were added to PLGA films of varying thicknesses (2, 7, 13, 24, and 40 µm) with constant surface area placed in a Petri-dish with tight-fit lid (Beckton-Dickinson, Franklin Lakes, NJ) and incubated on a rotary shaker (320 rpm) (IKA® KS basic, IKA® Works Inc.,Wilmington, USA) at 25 and 37°C for 24 h. As a very small percentage of the peptide was lost from the solution during sorption to PLGA films, the amount sorbed was determined by recovering sorbed peptide via two-phase extraction.
2.4. Recovery of peptides by two-phase extraction
Two-phase extraction was used to determine the amount of leuprolide or octreotide sorbed to PLGA films (described in section 2.3) or leuprolide sorbed in PLGA particles (described in section 2.11). Briefly, PLGA films or particles were dissolved in 1 mL of methylene chloride and 2 mL of 50 mM sodium acetate (pH 4.0) was added, followed by vortexing for 1 minute. To recover leuprolide from PLGA particles, 1.5 mL of buffer was removed, replaced with 1.5 mL of same buffer (5 extractions) or 50 mM sodium acetate + 1 M NaCl (6 extractions) and similarly extracted for 11 times. Eleven extractions were found to be sufficient to remove leuprolide completely from PLGA particles. To recover octreotide or leuprolide from PLGA films, 2 extractions with 50 mM sodium acetate and 1 extraction with 50 mM sodium acetate + 1 M NaCl was sufficient for complete recovery. The content of octreotide or leuprolide in each extracted fraction was then analyzed and quantified by RP-HPLC.
2.5. Analysis of peptides by HPLC
Assay of octreotide and leuprolide was done by RP-HPLC on a Waters 2695 alliance system (Milford, MA, USA) consisting of a 2996 Photodiode array detector and a personal computer with Empower 2 Software. Injection volumes of 20-100 μL were loaded onto a Nova-Pak® C-18 (3.9 × 150 mm) column (Waters Corp., Milford, MA, USA) and separation of peptide was accomplished using 0.1% trifluroacetic acid (TFA) in acetonitrile (solvent A) and 0.1% TFA in water (solvent B). A linear gradient of 25 to 35% A in 10 m, with a flow rate of 1.0 mL/min was used. Detection of both the peptides was done at 280 nm or 215 nm. Standard curve of peptide was established and concentration of unknown samples was calculated from the standard curve.
2.6. Octreotide quasi-equilibrium sorption studies
For 24-hr sorption isotherms, peptide solutions with initial concentration of 0.2-4.0 mM in 0.1M HEPES buffer, pH 7.4, 0.1M 2-(N-morpholino)ethanesulfonic acid (MES) buffer, pH 5.5, or 0.05 M DEPP buffer, pH 4.0 were used. Samples were removed from the incubator, centrifuged (2 m at 9.0 rcf) (Eppendorf 5415 D), and the supernatant was analyzed by HPLC. The amount of peptide sorbed was determined by the loss of peptide from solution for particles, or by two-phase extraction of sorbed peptide for films. Sorption data were fit to a modified Langmuir equation (by non-linear regression):
| (1) |
where Cf is the final concentration of peptide in solution; Γ is the quasi-equilibrium amount of peptide sorbed per mass of polymer; Γ0 and Γ1 are model parameters related to the amount of peptide sorbed; Γmax = Γ0 + Γ1, and K is the ‘binding affinity’ in the classical sense described by Langmuir.
2.7. Desorption of octreotide from PLGA
Solutions of 1 mM octreotide (1 mL for particles, 4 mL for film) in 0.1 M HEPES buffer, pH 7.4 were added to PLGA particles (10 mg, as received) or a 24 μm PLGA film and incubated (37 °C) on a rotary shaker (320 rpm) (IKA KS 130 basic). The amount initially sorbed as determined by two-phase extraction was ~ 650 nmol for RG 502H particles, ~ 360 nmol for RG 503H particles, and ~ 300 nmol for RG 502H films. After removal of the supernatant and rinsing with ddH2O twice, desorption solutions containing 50 vol% methanol (MeOH) in water, 0.1 M DEPP (pH 4.0), 2 M CaCl2 in 0.1 M HEPES (pH 7.4), 1 mg/mL poly(ethyleneimine) (PEI) in 0.1 M acetate buffer, pH 4.0, 0.1% TFA in 0.1M HEPES, pH 7.0, 5% SDS, or only 0.1M HEPES, pH 7.4 were separately added to the residual PLGA and incubated for 24 h at 37 °C. Additionally, samples containing 0.1 M HEPES, pH 7.4 desorption buffer were incubated for 24 h at 4 °C. All samples except the 5% sodium dodecyl sulfate (SDS) were then removed from the incubator, centrifuged, and the supernatant was analyzed by HPLC. Because SDS interferes with the RPHPLC analysis, desorption was assessed by recovering sorbed octreotide by two-phase extraction after rinsing with ddH2O three times to remove excess SDS. Again, octreotide was found to be stable after 24 h incubation at 37 °C in all solutions.
2.8. Confocal Raman microspectroscopy
Raman spectra of pure PLGA films and pure peptides were measured by confocal Raman microspectroscopy on the same setup of stimulated Raman scattering (SRS) microscopy (described in section 2.10). A spectrometer (Shamrock SR-303i-A, Andor Technology, Belfast, UK) was mounted to the side port of the microscope as reported previously [14]. A long-pass dichroic mirror (720DCSP, Chroma, VT) was used to reflect the Raman signal to the spectrometer. The pump laser was tuned to 707 nm for Raman excitation. A bandpass filter (825/150, Chroma) was placed in front of spectrometer to block the laser line. The signal was detected by a deep-depleted CCD (DU920N-BR-DD, Andor Technology).
2.9. Sectioning and analysis of PLGA films sorbed with octreotide
PLGA films were cast onto specially prepared plastic cylinders (8 × 13 mm) for microtoming. To achieve even sectioning of the PLGA films, a 2 × 2 mm circular groove was cut out from the 8 mm diameter face to leave a 4 mm diameter face. The PLGA film was cast by placing 4 mL of a 22% PLGA in acetone solution on the 4 mm diameter face of the plastic cylinder. The films were then dried at room temperature for 24 h, then for another 24-48 h in a vacuum oven at 40 °C. Cylinders with PLGA films were then immersed into 2 mL of 0.5 mM octreotide acetate in 0.1 M HEPES, pH 7.4 and incubated for 24 h at 37 °C. After incubation, the films were removed from the octreotide solution, rinsed, and dried for 24-48 h in a vacuum oven at 40 °C. The dried films were then sectioned using a Reichert Ultracut-E ultramicrotome (Vienna, Austria). The films were viewed through the microscope on the Ultracut-E and appear to have a very rough morphology as well as some curvature. The thickness of the film was estimated to be ~80 μm by slicing through the entire film in 0.5 μm increments. Two groups of 5-7 films were cut to a depth of ~20 μm and ~40 μm. Each group of sectioned films, as well as a non-sectioned control, were then carefully dissolved in 1 mL methylene chloride and extracted as described above prior to HPLC analysis.
2.10. Stimulated Raman scattering (SRS) imaging of peptide penetration in polymer film
Films with thickness of about 25 μm were created by spin-coating 0.25 mL of a 22% PLGA (RG 502H) solution (in acetone) on glass cover slips. Spin conditions were 1800 rpm for 15 s and dried as described above. The films (with glass backings) were then cut into 1 cm2 sections and placed in 0.5 mL leuprolide acetate solution (4 mM) in 0.1M HEPES buffer (pH 7.4) before incubating at 37 °C on a shaker (320 rpm) for 7 days to allow leuprolide to penetrate the film. After incubation, the films remaining on the glass slide were removed from the peptide solution and placed into vials of ddH2O and shaken at 37 °C for 1 h. This wash was repeated 3 times. The films were then placed into dry vials and allowed to dry in a fume hood for 24 h at room temperature before being moved to a vacuum for an additional 48 h at room temperature.
The setup of SRS microscope was reported previously [14]. Two 5-ps lasers (80 MHz; Tsunami Spectra-Physics, CA) assigned as pump and Stokes beams were synchronized and collinearly combined into an inverted confocal microscope (FV300/IX71, Olympus America Inc, PA). The pump beam intensity was modulated by a Pockels cell (360-80, Con-optics, CT) at a frequency of 1.13 MHz. The two laser beams were focused onto the sample using a 60× water objective (N.A. = 1.2). The forward signal was collected by another 60× water, detected by a large area photodiode (818-BB-40, Newport, CA) after passing a bandpass filter of 850/90 nm filter to block the pump beam. A lock-in amplifier (SR844, Stanford Research Systems, CA) was used to pick up the stimulated Raman gain signal with a function generator modulated at the modulation frequency of 1.13 MHz. The output channel of the lock-in amplifier was positively biased to avoid the feeding of a negative signal into an analog-to-digital converter which had a range from 0 to 5 V. To directly visualize the penetration of peptide in the film by SRS microscope, the film sample was covered by a clean coverglass. Based on the Raman spectra, the images of the PLGA film and peptide were taken at 1764 cm−1 and 1545 cm−1, respectively, with a pump laser tuned at 722 nm. The time constant, filter slope and sensitivity of lock-in amplifier were set as 300 μs, 6 dB and 30 μV, respectively. The images were acquired with 600 μs/pixel. The 3D distribution was recorded by Z-scanning function with a stepsize of 1 m.
2.11. Analysis of sorption of bodipy-labelled octreotide in PLGA particles
To assess penetration of peptides in the PLGA particles, octreotide acetate, which has two primary amino groups for fluorophore labelling was selected. Bodipy FL (Molecular Probes®, Grand Island, NY) was first conjugated to octreotide by co-dissolving in dimethyl sulfoxide (DMSO) at 1:1 molar ratio. The solution was spiked with 1% triethylamine to deprotonate peptide amines, and then protected from light and stirred for 4 h. Unconjugated dye was separated from the product using PD MidiTrap G-10 desalting columns, with a 700 Da exclusion limit (GE Lifesciences, Piscataway, NJ). PBS was used as the elution buffer, and elutions were lyophilized after collection. Purification was verified using Ultra Performance Liquid Chromatography (Acquity®, Waters, Milford, MA), with a gradient elution of 25% to 35% acetonitrile (+0.1% trifluoroacetic acid (TFA)) in water (+0.1% TFA) over 4 min. Purifications were considered successful when a clear peptide absorbance peak was observed at 280 nm, but no absorbance was visible at 504 nm, attributed to free bodipy. Fluorescence was confirmed in the purified elution with a Synergy Neo HTS Multi-mode microplate reader (Biotek, Winsoki, VT) using a 500 nm excitation wavelength with florescence emission detection at 530 nm. 10 mg of PLGA 502H were then added to 1 mL of a 1 mg/mL (in PBS) solution of conjugate, and rotated for 36 h at 37 °C. Samples were then centrifuged and the supernatant removed and replaced with fresh PBS. After 3 additional washes, fluorescence was detected in the samples by imaging with a Nikon A1Rsi Confocal Laser Scanning Microscope (CLSM) using an excitation wavelength of 488 nm and emission detection at 525 nm. Z-stacked images were taken over 130 μm.
2.12. Sorption loading of leuprolide in PLGA particles for in vitro and in vivo evaluation
A solution of leuprolide in HEPES (0.1 M, pH 7.4) from 3 mM peptide solution was added to previously ground and sieved (20-63 μm) PLGA (Resomer® RG 502H) particles (1 mL/10 mg particles). The mixture was then incubated on a rotary shaker at 37 °C. After 6 h of incubation, an additional 1 mM leuprolide was added to boost the concentration gradient and hence leuprolide absorption to PLGA. After 24 h incubation, leuprolide/PLGA particles mixture was centrifuged at 8000 rpm for 10 m and supernatant was removed. The residual particles were washed three times with ddH2O (1 mL water/10 mg particles) and then freeze-dried. Leuprolide-sorbed PLGA particles were passed through sieves to obtain 20-63 μm and stored at −20 °C until further use.
2.13. Analysis of leuprolide loading in PLGA particles
Determination of actual loading of leuprolide in PLGA (Resomer® RG 502H) particles was performed by two analytical methods: two-phase extraction (described in section 2.4) and amino acid analysis (AAA). Amino acid analysis was performed at the Protein Chemistry Laboratory of Texas A&M University. Briefly, all samples (leuprolide-sorbed PLGA particles, standards, and human serum albumin (control) with two internal standards (norvaline and sarcosine respectively for primary and secondary amino acids) were weighed into ampoules and 100 μL of 6M HCl solution was added, followed by incubation at 110 °C for 18 h. Hydrolysates were then taken to dryness and reconstituted in 0.4 N borate buffer to bring the eventual pH to 10 for optimum derivitization and then transferred to the autosampler for automated pre-column derivitization with o-phthalaldehyde (OPA) and 9-fluoromethyl-chloroformate (FMOC) and loading. The separation of derivatized amino acids was accomplished on a Hewlett-Packard AminoQuant II chromatography system (Hewlett-Packard Co., Palo Alto, CA, USA) using a Hypersil AA-ODS reverse phase column (2.1 × 200 mm, 5 μm; Agilent Technologies, Inc., Santa Clara, CA, USA). Elution of derivatized amino acids was achieved using solvent (A) 20 mM sodium acetate containing 0.018% v/v triethylamine, 0.05 mM EDTA and 0.3% v/v tetrahydrofuran (pH 7.2) and (B) 100 mM sodium acetate: acetonitrile: methanol (20:40:40). The solvent gradient from 0 to 60% B over 17 minutes at 0.45 mL/min. Primary (tagged with OPA) and secondary (tagged with FMOC) amino acids were respectively detected at excitation/emission wavelength of 340/450 and 266/324 nm by a fluorescence detector. The observed peak area for each amino acid was compared to an internal and external standard and the resulting concentration values in nano moles were recorded. Analysis was performed in triplicate for each sample.
2.14. Evaluation of in vitro leuprolide release
In vitro release of leuprolide was performed under perfect sink condition. Briefly, about 10 mg leuprolide absorbed PLGA particles were weighed (n = 3) into Eppendorf tubes and 1 mL of PBST + 0.02% sodium azide was added. Eppendorf tubes were then incubated at 37 °C on a rotary shaker at 240 rpm. At specified time points (1-10 days and every two days thereafter), tubes were centrifuged at 8,000 rpm for 5 minutes and 1 mL supernatant was removed and then replaced with pre-warmed (37 °C) release medium. Leuprolide acetate is stable in pH 7.4 solutions for >30 days [15]. Analysis of leuprolide in release samples was performed by RP-HPLC.
2.15. Evaluation of long-term testosterone suppression ability of leuprolide-sorbed PLGA particles in male Sprague-Dawley rats
The efficacy of leuprolide-sorbed PLGA particles to provide long-term in vivo leuprolide release was evaluated by assessing long-term testosterone suppression ability of leuprolidesorbed PLGA particles in male Sprague-Dawley rats. The treatment of experimental animals was in accordance with University committee on use and care of animals (University of Michigan UCUCA), and all NIH guidelines for the care and use of laboratory animals. Male Sprague-Dawley rats of 6 weeks old were housed in cages and given free access to standard laboratory food and water, and allowed one week to acclimate prior to study initiation. Animals were anesthetized with 2-4% isoflurane administered by a calibrated vaporizer (Midmark, Orchard Park, NY, USA). The leuprolide and leuprolide-sorbed PLGA particles in a liquid vehicle (1% w/v carboxymethylcellulose and 2 %w/v mannitol), and commercial Lupron Depot were subcutaneously injected at the back (lower neck portion) of rats (6 animals/study group) at various dosing intervals. The dose of leuprolide acetate was 100 μg/kg/day. Animal body weight considered for dosing leuprolide acetate was 425 g which was the projected body weight of male Sprague Dawley rat at midpoint (day 28) of the study (as per the weight (g)/age (weeks) curve provided by Charles River Laboratories). Blood samples were collected via jugular vein stick before (day −7 and 0 for baseline testosterone level) and after (1, 7, 14, 21, 28, 35, 42, 49, and 56 days) injection of preparations. The collected blood samples were immediately transferred to BD Microtainer® blood collection and serum separation tubes previously incubated in ice, centrifuged at 8,000 rpm for 10 min, and then the serum was removed and stored in microcentrifuge tubes at −20° until further use. Serum testosterone levels were assayed by Radioimmunoassay using a TESTOSTERONE Double Antibody-125I RIA Kit (MP Biomedicals LLC., Solon, OH, USA) at the University of Pennsylvania Diabetes Center (Philadelphia, PA, USA). Lowest detection limit of testosterone was 0.1 ng/mL.
3. Results
3.1. Maximal sorption of peptides is similar to the number of charged polymer end-groups
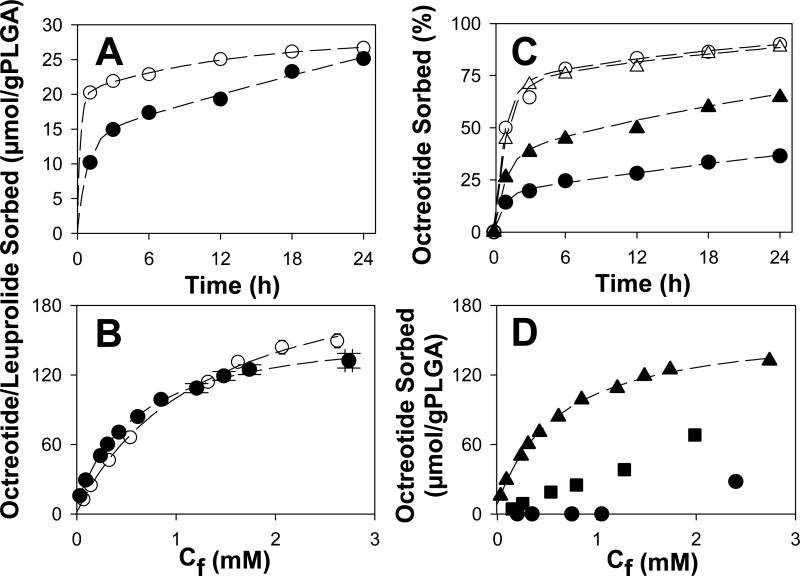
To initially assess peptide sorption, the kinetics and equilibria of the peptide sorption to particles of a commonly used PLGA-COOH (Boehringer Ingelheim RG 502H, Mw = 6.7 kDa) were determined. The kinetics of sorption to ground PLGA-COOH from moderate concentrations of the acetate salts of leuprolide and octreotide in 0.1 M HEPES buffer solution was rapid and extensive at 37°C (Fig. 1A). Leuprolide, which does not chemically react with PLGA-COOH [16], with its roughly single positive charge at this pH, sorbed more rapidly than octreotide, reaching 74% of its 24-h sorption in the first hour. By contrast, octreotide (~ +1.7 charge at this pH), which acylated after 24 h under similar conditions [10], attained a very similar level of sorption as leuprolide by 24 h despite slower sorption (52% after 1 h). As the stable peptide sorption was essentially complete by 24 h, sorption isotherms were obtained at this time (Fig. 1B). Sorption of both peptides followed very similar Langmuir sorption behavior, with binding constants in the vicinity of 1 mM−1 (Table 1). The maximal sorption of the peptides predicted by the Langmuir model was surprisingly similar to the total number of acid end-groups in the polymer (1.24:1 and 0.88:1 mol peptide/mol COOH for leuprolide and octreotide, respectively), as seen in Table 1. The binding constants were on the order of μM, and leuprolide bound more tightly than octreotide as reflected in its lower binding constant, which was also consistent with the need for additional extractions to separate leuprolide from the polymer to quantify peptide sorption directly (see section 2.4). As expected from the similarity in peptide-to-polymer carboxylate ratio predicted for maximal sorption, solution properties affecting charge interactions, namely ionic strength and pH, strongly influenced octreotide sorption. As ionic strength increased, peptide sorption decreased (Fig. 1C). Decreasing the charge on the polymer by protonating the carboxylic acid end-groups steadily decreased peptide sorption, and completely stopped the peptide-polymer interaction by pH 4 (Fig. 1D).
Fig. 1.
Characterization of peptide sorption to PLGA-COOH 50:50 at 37 °C. A) Effect of peptide type [leuprolide (○) vs. octreotide (●)] on sorption kinetics. B) 24-h sorption isotherm of leuprolide (○) and octreotide (●); Cf = final peptide concentration. C) Effect of ionic strength at pH 7 (0.1 M phosphate buffer (236 mM, ●), 0.1 M HEPES buffer (49 mM, ▲), 10 mM phosphate buffer (23 mM, ○) and 10 mM HEPES buffer (4 mM, Δ)) on the kinetics of octreotide sorption from 0.42 mM initial peptide concentration. D) Effect of pH (0.1 M HEPES buffer, pH 7.4 (▲), 0.1 M MES buffer, pH 5.5 (■), and 0.05 M DEPP buffer, pH 4.0 (●) on 24-h octreotide sorption isotherms. Sorption studies of A and B were conducted in 0.1 M HEPES buffer, pH 7. Initial peptide concentration was 0.4 mM in A and C and in the range of 0.2-4.0 mM in B and D. Symbols represent mean of 3 determinations. Dashed lines represent fits to a biexponential (A and C), and Langmuir models (B and D).
Table 1.
Langmuir model fitted parameters (see Eqn 1) and estimated fraction of acids occupied at maximal sorption.
| Peptide | Polymer | K [μM−1] | Γ0 [μmol/g PLGA] | Γmax[a] [μmol/g PLGA] | Total acids [μmol/g PLGA] | FA[b] |
|---|---|---|---|---|---|---|
| Leuprolide | RG 502H | 0.77 | 1.5 | 229 | 185 | 1.24 |
| Octreotide | RG 502H | 1.6 | 8.5 | 163 | 185 | 0.88 |
| Octreotide | RG 503H | 1.2 | 2.9 | 81 | 94 | 0.86 |
| Octreotide | RG 504H | n.d | n.d | ~6[c] | 53 | n.d |
Γmax = Γ0 + Γ1
fraction of acids occupied (Γmax/Total acids).
Γmax estimated visualy from isotherm.
3.2. Sorption of peptides is dependent on polymer-specific molecular properties
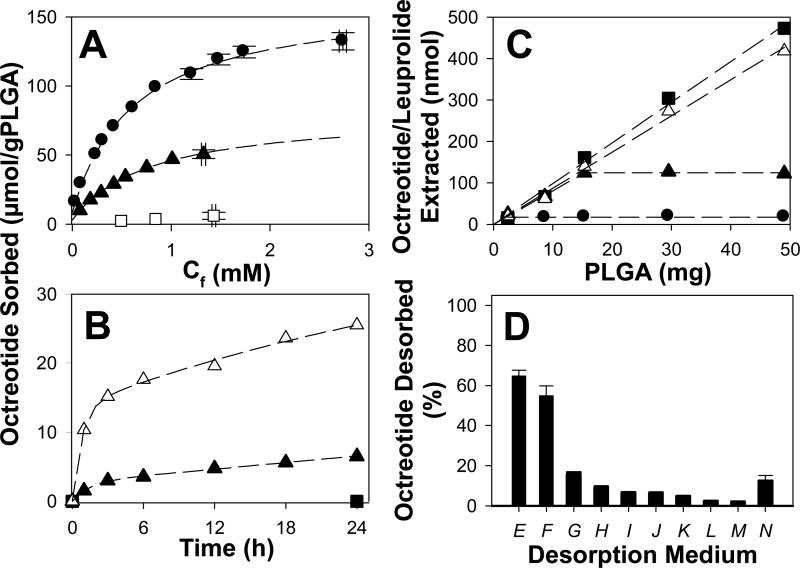
As the sorption isotherms suggested extensive penetration of the peptides into the polymer, we examined two variables that could strongly influence transport of solutes in the polymer phase: polymer molecular weight (Mn) and temperature, using octreotide as a model peptide. As the Mn of the PLGA-COOH was increased, the extent of octreotide sorption strongly decreased (Fig. 2A). Increasing the polymer Mn from 6.7 to 20.9 kDa resulted in a decrease in octreotide sorption proportional to the decreased in the number of COOH endgroups (e.g. the fraction of acids predicted to be occupied at maximal sorption was 0.88 and 0.86, respectively) (Table S2). However, for the highest Mn polymer (31.3 kDa), almost no octreotide sorption was recorded, and less than 12% of the polymer's COOH sites (~30% less acids compared with the Mn = 6.7 kDa polymer) were occupied with peptide. Decreasing water content with increasing Mn is well established [17], strongly suggesting that insufficient moisture was present in the 31 kDa polymer to facilitate peptide penetration. It is noteworthy to mention that particles of each polymer were highly porous (as are common microspheres) with porosity ranging from 74-79% for 502H and 503H and 86% for 504H (Table S5). Because the particle size of the 504H polymer was significantly larger (Table S5), mass transfer issues may also have played a role in the reduced absorption.
Fig. 2.
Effect of polymer Mw [A: Boehringer-Ingelheim Resomer® RG 502H (●), RG 503H (▲), and RG 504H (□)], incubation temperature (B: 37 (Δ), 25 (▲), and 4 (■) °C) and polymer mass/film thickness (C: octreotide sorption at 22 (●), 30 (▲) and 37 (Δ) °C, and leuprolide sorption at 37 °C (■) after 24 h) on peptide sorption to PLGA, and medium on desorption of peptide from PLGA particles or films (D). Sorption of peptides to PLGA was performed at 37 °C in 0.1 M HEPES buffer (pH 7.4) with an initial peptide concentration of 0.4 (A and B) or 1 (C and D) mM. RG 502H polymer was used in sorption and desorption studies unless otherwise specified. Dashed lines represent fits to a biexponential model. Desorption of octreotide from PLGA 50:50 particles (E-M) or films (N) was performed in 5 wt% SDS in water (E); 50 vol% methanol in water (F); 1 mg/mL PEI in 0.1 M acetate buffer, pH 4.0 (G); 0.1 M HEPES, pH 7.4 (H); 0.1% TFA in 0.1 M HEPES, pH 7.0 (I); 0.1 M DEPP, pH 4.0 (J); 0.1 M HEPES, pH 7.4 (K: particles and N: films); 2 M CaCl2 in HEPES (L); 0.1 M HEPES, pH 7.4 (M). Desorption studies of E to L, and N were performed at 37 °C, except N, which was at 4 °C. PLGA RG502H was used in all cases, except H, which used RG503H. Desorption of octreotide in case of SDS (E) was assessed by recovering sorbed octreotide via two-phase extraction. Symbols represent mean of 3 determinations.
Temperature had an equally pronounced influence on octreotide sorption. For example, in particulate PLGA-COOH, moving from 37 to 25 °C reduced 24-h sorption by ~75%, and no significant sorption was detected at 4°C (Fig. 2B). We also investigated the effect of temperature in PLGA films, rather than particles, to be able to adjust the polymer thickness at a constant polymer surface area. The sorption of octreotide from thin films of varying thickness (mass) also showed strong temperature dependence (Fig. 2C). At 37°C, the amount of octreotide or leuprolide sorbed after 24 h was equivalent and linear with film thickness, strongly suggesting peptide absorption into the polymer. However, as temperature was decreased to 30°C, the linear behavior was only observed up until a critical thickness (~13 μm). The discontinuity is consistent with the well-known drop in Tg near the surface of polymers [18]. Furthermore, by 4°C, very small sorption was recorded for all film thickness, strongly suggesting simple adsorption in the immobilized films at a value of ~ 1.4 nmol/cm2.
3.3. Sorbed octreotide has altered solubility properties
The presence of sorbed octreotide in acetonitrile was assessed by 1H-NMR in order test the hypothesis that the octreotide-PLGA ion-pair would have enhanced solubility in low-dielectric solvents, a condition that would appear to be required for partitioning into the bulk of the polymer phase. The characteristic proton shifts at 6.7-7.5 ppm attributable to protons on the aromatic side chains of octreotide [19] present in the octreotide control sample also appeared in the test sample containing PLGA particles incubated with octreotide in acetonitrile (Fig. S2). As free octreotide acetate is not soluble in acetonitrile, the presence of the characteristic octreotide proton shifts in the test sample indicate that octreotide is solubilized in the presence of PLGA, consistent with the hypothesis that upon ion-pairing with PLGA, the solubility of octreotide increases in low dielectric solvents.
Octreotide did not easily desorb from PLGA-COOH. Various desorption solutions were chosen to selectively disrupt ionic interactions (Fig. 2D). The incomplete desorption of octreotide from PLGA, despite a large number of strategies to potentially disrupt ionic interactions, suggest these interactions do not fully explain the irreversibility of sorption. However, the addition of 5% SDS or organic solvent (50 vol% methanol in water) led to the desorption of 65 ± 3 and 55 ± 5 % of the originally sorbed octreotide, respectively, strongly suggesting that additional critical interactions leading to irreversibility of sorption are hydrophobic contacts between octreotide and PLGA.
3.4. Direct evidence for peptide absorption throughout the polymer phase
In order to confirm more directly that the peptides had absorbed into the polymer phase, we performed three experiments to measure peptide directly as a function of position within the polymer. As we sought to employ physical removal of the polymer surface and microscopic methods, we chose polymer films to first evaluate this question. In addition, since the peptides showed essentially equivalent sorption after 1 day in both films and particles after 1-day incubation (see Figs. 1B and 2C), we used single peptides for each of these experiments, and there was no need to repeat these studies for both peptides.
In order to remove the possibility for adsorption, we sorbed octreotide to thin films (1 day, 37 °C) and microtomed two significant fractions of the films away and recovered the remaining peptide. Consistent with the absorption hypothesis, we found steadily less peptide remaining in the polymer as the polymer was sectioned (Table S3). However, as the sorbed-films possessed some slight curvature, we could not discount the possibility that only a portion of the surface of films had been removed. Therefore, a second experiment was also added with stimulated Raman scattering (SRS) as described below.
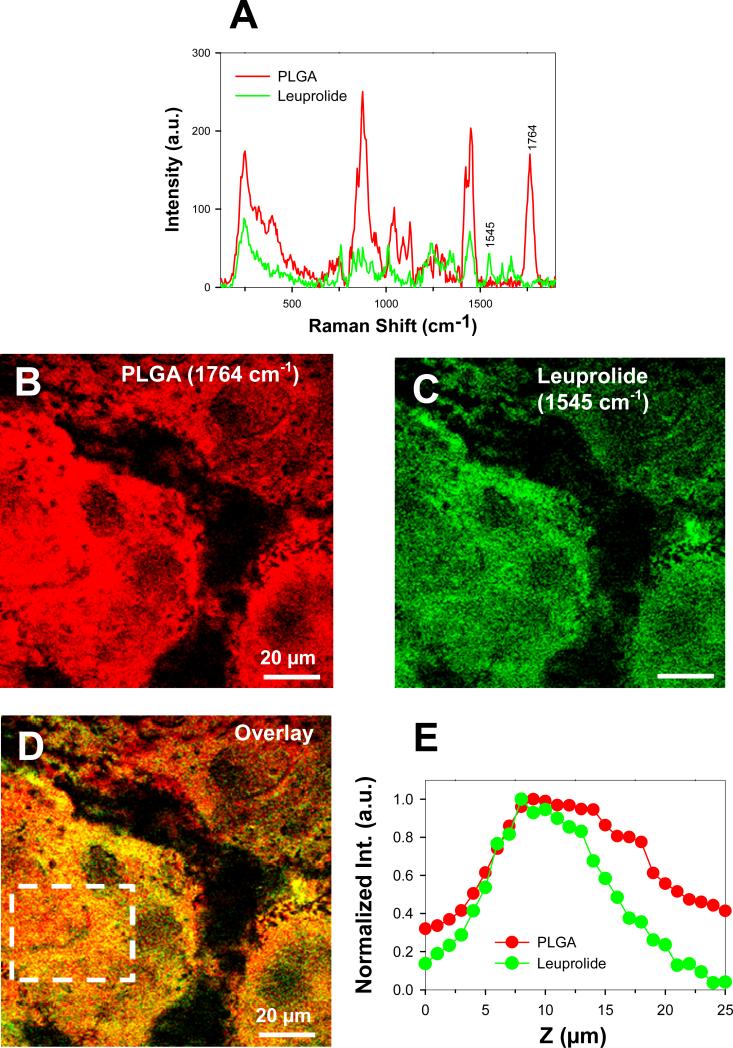
Before evaluating by SRS, we incubated even thinner PLGA films at 37°C for 1 week with peptide to make certain enough time had been allowed for full equilibration of the film. We used leuprolide in this case to avoid significant peptide acylation. The Raman spectra of pure PLGA-COOH and leuprolide (Fig. 3A) had characteristic peaks at 1764 and 1545 cm−1 that were assigned the color red and green, respectively (Fig 3B and 3C). By adjusting the focal point of the microscope into the depth of the film, we were able to image and record the intensity of the leuprolide and PLGA signal throughout the film. Representative images of the PLGA and leuprolide signal, which were taken at 10 μm depth of the film, are respectively shown in Fig. 3B and C; while the overlay image of Fig. 3B and C is shown in Fig 3D. From overlay image (Fig 3D), it appears that leuprolide had distributed throughout the polymer phase. Furthermore, a signal from the characteristic peak of the leuprolide could be detected at all depths of the film, and its intensity followed a trend similar to what was detected for PLGA-COOH (Fig. 3E).
Fig. 3.
Direct visualization of peptide (leuprolide) penetration in PLGA film by SRS imaging. A) Raman spectra from pure PLGA film (red) and leuprolide (green). The peaks at 1764 cm−1 and 1545 cm−1 were identified for selective SRS imaging of PLGA and leuprolide, respectively. B) SRS image of PLGA distribution (red) in the film at 1764 cm−1. C) SRS image of leuprolide distribution (green) at the same location and depth in the film at 1545 cm−1. D) Overlay image of B) and C). E) Average intensity of PLGA and leuprolide in the area indicated by the white box in D as a function of depth along the film. Data was normalized against maximum intensity values. Note that the bell-shaped intensity curves are present for both PLGA and leuprolide, and are an artifact of the SRS imaging method.
Similarly, to demonstrate polymer penetration of peptide in PLGA particles, we labeled octreotide at its reactive primary amino groups with the non-pH sensitive and stable fluorescent dye, bodipy. As expected from the above data and analysis, after incubating the particles for 1.5 days at 37 °C with 1 mg/mL bodipy-octreotide, the dye-labelled peptide was observed throughout the PLGA particles by scanning fluorescent confocal micrsocopy (see Movie S1 and Fig. S5). Collectively, both direct and indirect evidence indicates extensive peptide absorption in low molecular weight PLGA-COOH.
3.5. Aqueous absorption microencapsulation of leuprolide yields high loading and extended testosterone suppression in rats
Considering that maximal sorption of the peptide is ~1:1 mol peptide/mol COOH group, the lowest Mn polymer used has a number averaged molecular weight just below 10 kDa, and the Mw of the peptide is ~1000 Da, then it is reasonable to predict that the peptide could be absorbed at a level greater than 10 wt%. Such a level is commonly found in injectable depots, which are generally formed by complex unit processes with organic solvents under aseptic conditions. Therefore, we sought to determine if an injectable depot could be created simply according to the absorption experiments described above. Again, we chose to use leuprolide to illustrate this principle rather than octreotide in order to avoid potential peptide acylation during release.
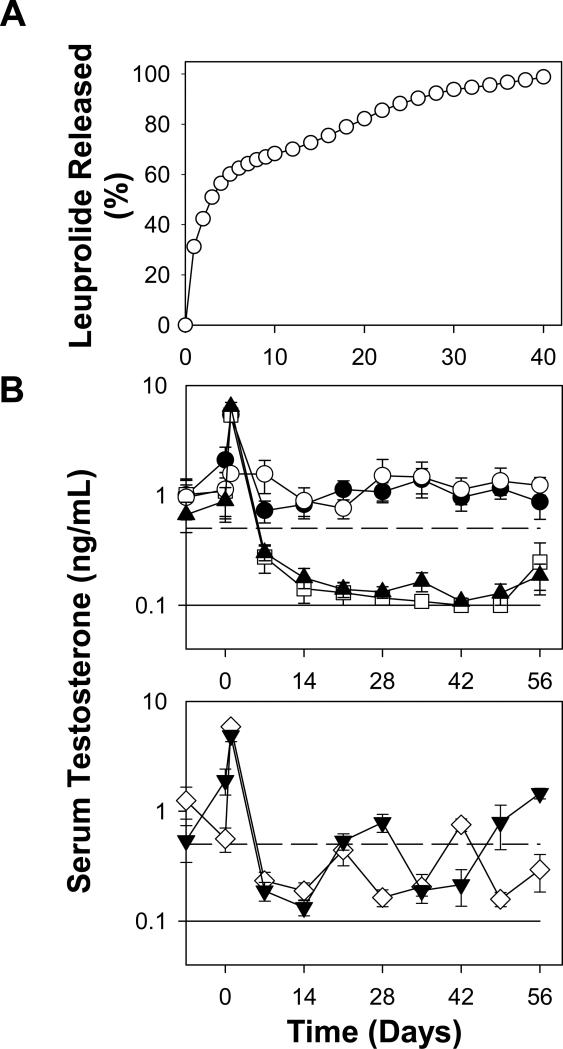
To accomplish absorption microencapsulation of peptide solution, 3 mM leuprolide was added to PLGA 502H particles, and incubated at 37°C. After 6 h, leuprolide equivalent to 1 mM was added to boost the concentration gradient and incubated at the same temperature for additional 18 h. After extensive particle washing, we obtained ~17% (w/w) peptide loading in the polymer as determined either by peptide extraction or amino acid analysis (Table S4). Leuprolide-absorbed PLGA particles exhibited 64, 73, 85, 92 and 99 % leuprolide release respectively after 7, 14, 22, 28, and 40 days of incubation in release buffer, suggesting potential ability of absorption microencapsulation approach to provide long-term slow and continuous peptide release.
The same depot leuprolide-absorbed PLGA-COOH formulation was then injected subcutaneously in rats at three different dosing schedules at 0.1 mg/kg/day to determine the longevity of the pharmacological response to the depot. Extent of plasma testosterone suppression was monitored to determine whether animals would attain therapeutic castration levels (< 0.5 μg/mL), and then if and when animals would escape castration. Control groups included: every four week dosing of the commercial Lupron Depot™ formulation (positive), no-drug blank (negative), and single time 4-week dose of solution leuprolide (negative) (Fig. 4B). Both negative controls did not effectively suppress serum testosterone levels, demonstrating the well-known need for controlled release formulations of leuprolide to attain therapeutic castration. As seen in Fig. 4B, baseline levels of testosterone during the week prior to injecting drug formulations were found to be consistent with the natural variation of that expected (e.g., 1-6 ng/mL [20]). The absorption-loaded leuprolide injected every 3 or 4 weeks resulted in attainment of therapeutic castration, with escape (> 0.5 μg/mL) occurring roughly between 2 and 3 weeks. By contrast, every two-week dosing of the same formulation resulted in very similar testosterone suppression as the positive control, Lupron Depot™, without castration escape over the entire 8-week interval (p > 0.1 for all times). Note that as the release rate from the leuprolide-absorbed PLGA particles was very low after 2 weeks, the in vitro release and the in vivo performance are deemed consistent.
Fig. 4.
Long-term in vitro leuprolide release behavior (A) and in vivo testosterone suppression ability (B) of leuprolide-absorbed PLGA-COOH formulation. Studies were conducted in PBST + 0.02% sodium azide at 37 °C (A) or male Sprague-Dawley rats (B). Animals were injected subcutaneously with soluble leuprolide at day 0 (●), leuprolide-PLGA particles with various dosing intervals (2 week: days 0, 14, 28, and 42 (▲), 3 week: days 21 and 42 (◇), and 4 week: days 0 and 28 (▼)), and blank PLGA particles (○) along with commercial 1-month Lupron Depot® at days 0 and 28 (□). Leuprolide dose was 100 μg/kg/day. Solid and dashed line respectively represents lower testosterone detection limit (0.1 ng/mL) and castration level (0.5 ng/mL). Symbols represent mean ± SEM [n = 3 (A) or 6 (B)] with error bars not visible when smaller than symbols.
4. Discussion
The following aforementioned evidence supports the hypothesis for peptide absorption to PLGA-COOH (6 < Mn < 21 kDa) at physiological temperature and neutral pH. Peptide sorption requires ionic interactions between ionized PLGA-COOH and the cationic peptide at physiological temperature (Fig. 2); the charge-charge directed sorption was extensive, with maximal sorption close to 1:1 mol peptide/COOH (Table 1). It is unreasonable to expect such a high fraction of polymer end-groups could reach the surface from such a low molecular weight polymer to exclusively bind peptide at the polymer surface. Moreover, significant multilayer sorption is also highly unlikely considering the extraordinarily high water-solubility and relatively low Mw of the peptides. Multilayer sorption would decouple the peptide sorption to the number of –COOH groups in the polymer. Peptide sorption as a function of molecular weight (and water uptake) of the polymer was equally compelling. As polymer Mn increased above and water content decreased below critical values, peptide sorption essentially stopped, strongly suggesting the peptide could no longer enter the polymer phase although kinetic limitations could not be ruled out. Similar evidence regarding the influence of temperature and thickness of polymer indicated that as the polymer chains became immobilized the peptide could not penetrate the polymer. We therefore sought direct evidence for peptide penetration. Microtoming PLGA films of penetrated octreotide before analysis, direct SRS analysis of leuprolide-loaded PLGA films, and laser confocal fluorescent imaging of PLGA particles with bodipy-labelled octreotide all provided direct evidence for the concept of extensive peptide absorption into the polymer.
Although it is well known that therapeutic cationic peptides, such as octreotide, interact with PLGAs, data indicating absorption of peptides to PLGAs in addition to adsorption, and the effects of temperature polymer molecular weight on this difference is not well understood. Absorption was required for aqueous-based leuprolide encapsulation. Our data further suggests that certain commercial depot formulations may be actually solid solutions of peptide and polymer, and may not contain domains of pure peptide distributed throughout aqueous pores within the polymer as proposed previously [7]. Although this possibility could be speculated, there is no strong evidence to our knowledge in the literature to strongly support this concept until now.
In addition to these mechanistic findings, the absorption encapsulation of therapeutic peptides by simple mixing of the peptide and the unprocessed polymer in water at physiological temperature is a novel means to achieve high loading (>>10% w/w) for later long-term controlled release. Similar to our recent publication describing self-healing encapsulation [21], i.e., spontaneous pore closing to seal of PLGA pores, encapsulation by absorption is a second mechanism to encapsulate drugs in aqueous media without the need for micronization processes and organic solvents at the time of encapsulation in a manner similar to the “active” or “remote” loading of doxorubicin to form liposomal Doxil [22]. Microencapsulation of peptides in PLGAs continues to have significant clinical significance, as evidenced by the recent 2012 US FDA approval of the once weekly long-acting depot of the GLP-1 analog, exenatide, in the Bydureon® product (Amylin/Alkermes). Our data also indicate that therapeutic depot formulations can be prepared at reduced cost. Elevated manufacturing costs are known to significantly impair depot development. As it is well known that PLGAs can be terminally sterilized by gamma irradiation, according to the approach demonstrated for leuprolide in our paper, commercial manufacture of peptides could reasonably be done with preformed polymers without expensive aseptic manufacture in the presence of organic solvents. While likely impractical for point-of-care encapsulation in most settings in the western world, it is conceivable that a variation on the type of depot formulations such as we describe here could be made in pharmacies in developing countries, which do not have the resources to purchase commercial depot formulations. Furthermore, for encapsulation of experimental peptide drugs, the low-cost method provides an avenue for scientists with low quantities of very costly peptides to prepare very small batch sizes with depot formulations simply from low peptide concentrations (<10 mg/ml), which is not currently feasible with conventional encapsulation methods for microsphere formulation.
5. Conclusions
This study demonstrates that when the PLGA-COOH chains are mobilized (e.g., at 37°C in the swollen polymer) the peptides rapidly form salts with the end-groups throughout the polymer matrix. These absorption data suggested a new and facile means to microencapsulating the therapeutic peptide in PLGA-COOH off the shelf, which was demonstrated with leuprolide to achieve high loading. The resulting non-optimized formulations provided effective chemical castration therapy for 2 months when injected biweekly. Microencapsulation occurs based on polymer-specific phenomena (Mn, end-group chemistry, etc.), suggesting that encapsulation via absorption could be performed in PLGA biomaterials of any shape or form, e.g., tissue engineering scaffolds, drug-eluting stents, sutures, bone screws, and many others. The integral role of the PLGA end-group in determining the ability of the polymer to encapsulate therapeutic peptides from aqueous solution suggests a new avenue of research where interactions between the end group of the polymer and the peptide could be engineered for various peptides and biomedical devices.
Supplementary Material
Acknowledgments
This study was supported by R01 HL 68345 and R21 EB 00873 from NIH and Novartis. We thank Dr. Heather W. Collins, Diabetes Research Center, University of Pennsylvania for her assistance with Radioimmunoassay. We also are greatful to Kari Nieto for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
References
- 1.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 2.Schwendeman SP. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 2002;19:73–98. doi: 10.1615/critrevtherdrugcarriersyst.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- 3.Okada H. One- and three-month release injectable microspheres of the LH-RH superagonist leuprorelin acetate. Adv. Drug Deliv. Rev. 1997;28:43–70. doi: 10.1016/s0169-409x(97)00050-1. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Wang BA, Schwendeman SP. Characterization of the initial burst release of a model peptide from poly(D,L-lactide-co-glycolide) microspheres. J. Control. Release. 2002;82:289–307. doi: 10.1016/s0168-3659(02)00137-2. [DOI] [PubMed] [Google Scholar]
- 5.Kang J, Schwendeman SP. Pore closing and opening in biodegradable polymers and their effect on the controlled release of proteins. Mol. Pharm. 2007;4:104–118. doi: 10.1021/mp060041n. [DOI] [PubMed] [Google Scholar]
- 6.Filde F, Hutchinson F. Delivery means for biologically active agents. 1980 US4235988.
- 7.Okada H, Yamamoto M, Heya T, Inoue Y, Kamei S, Ogawa Y, Toguchi H. Drug-delivery using biodegradable microspheres. J. Control. Release. 1994;28:121–129. [Google Scholar]
- 8.Na DH, DeLuca PP. PEGylation of octreotide: I. Separation of positional isomers and stability against acylation by Poly(D,L-lactide-co-glycolide) Pharm. Res. 2005;22:736–742. doi: 10.1007/s11095-005-2589-4. [DOI] [PubMed] [Google Scholar]
- 9.Na DH, Lee KC, DeLuca PP. PEGylation of octreotide: II. Effect of N-terminal mono-PEGylation on biological activity and pharmacokinetics. Pharm. Res. 2005;22:743–749. doi: 10.1007/s11095-005-2590-y. [DOI] [PubMed] [Google Scholar]
- 10.Sophocleous AM, Zhang Y, Schwendeman SP. A new class of inhibitors of peptide sorption and acylation in PLGA. J. Control. Release. 2009;137:179–184. doi: 10.1016/j.jconrel.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langer R, Folkman J. Polymers for sustained-release of proteins and other macromolecules. Nature. 1976;263:797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- 12.Ghassemi A, Steenbergen M, Barendregt A, Talsma H, Kok R, Nostrum C, Crommelin DA, Hennink W. Controlled release of octreotide and assessment of peptide acylation from Poly(D,L-lactide-co-hydroxymethyl glycolide) compared to PLGA microspheres. Pharm Res. 2012;29:110–120. doi: 10.1007/s11095-011-0517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Schwendeman SP. Minimizing acylation of peptides in PLGA microspheres. J Control Release. 2012;162:119–126. doi: 10.1016/j.jconrel.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slipchenko MN, Chen H, Ely DR, Jung Y, Carvajal MT, Cheng J-X. Vibrational imaging of tablets by epi-detected stimulated Raman scattering microscopy. Analyst. 2010;135:2613–2619. doi: 10.1039/c0an00252f. [DOI] [PubMed] [Google Scholar]
- 15.Herrera LC, Defain Tesoriero MV, Hermida LG. In vitro release testing of PLGA microspheres with franz diffusion cells. Dissolution Technol. 2012;19:6–11. [Google Scholar]
- 16.Na DH, Youn YS, Lee SD, Son MW, Kim WB, DeLuca PP, Lee KC. Monitoring of peptide acylation inside degrading PLGA microspheres by capillary electrophoresis and MALDI-TOF mass spectrometry. J. Control. Release. 2003;92:291–299. doi: 10.1016/s0168-3659(03)00366-3. [DOI] [PubMed] [Google Scholar]
- 17.Hutchinson FG, Furr BJA. Biodegradable polymer systems for the sustained-release of polypeptides. J. Control. Release. 1990;13:279–294. [Google Scholar]
- 18.Kawaguchi D, Tanaka K, Takahara A, Kajiyama T. Surface mobile layer of polystyrene film below bulk glass transition temperature. Macromolecules. 2001;34:6164–6166. [Google Scholar]
- 19.Munk VP, Fakih S, Murdoch P.d.S., Sadler PJ. Reactions of Pt-II diamine anticancer complexes with trypanothione and octreotide. J. Inorg. Biochem. 2006;100:1946–1954. doi: 10.1016/j.jinorgbio.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Mock EJ, Kamel F, Wright WW, Frankel AI. Seasonal rhythm in plasma testosterone and luteinizing-hormone of male laboratory rat. Nature. 1975;256:61–63. doi: 10.1038/256061a0. [DOI] [PubMed] [Google Scholar]
- 21.Reinhold SE, Desai KGH, Zhang L, Olsen KF, Schwendeman SP. Self-healing microencapsulation of biomacromolecules without organic solvents. Angew. Chem. Int. Ed. 2012;51:10800–10803. doi: 10.1002/anie.201206387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haran G, Cohen R, Bar LK, Barenholz Y. Transmembrane ammonium sulfate gradients in liposomes produce efficient and stable entrapment of amphipathic weak bases. Biochim. Biophys. Acta. 1993;1151:201–215. doi: 10.1016/0005-2736(93)90105-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.