Abstract
In forebrain neurons, knockout of synaptotagmin-1 blocks fast Ca2+-triggered synchronous neurotransmitter release, but enables manifestation of slow Ca2+-triggered asynchronous release. Here, we show using single-cell PCR that individual hippocampal neurons abundantly co-express two Ca2+-binding synaptotagmin isoforms, synaptotagmin-1 and synaptotagmin-7. In synaptotagmin-1 deficient synapses of excitatory and inhibitory neurons, loss-of-function of synaptotagmin-7 suppressed asynchronous release. This phenotype was rescued by wild-type but not mutant synaptotagmin-7 lacking functional Ca2+-binding sites. Even in synaptotagmin-1 containing neurons, synaptotagmin-7 ablation partly impaired asynchronous release induced by extended high-frequency stimulus trains. Synaptotagmins bind Ca2+ via two C2-domains, the C2A- and C2B-domains. Surprisingly, synaptotagmin-7 function selectively required its C2A-domain Ca2+-binding sites, whereas synaptotagmin-1 function required its C2B-domain Ca2+-binding sites. Our data show that nearly all Ca2+-triggered release at a synapse is due to synaptotagmins, with synaptotagmin-7 mediating a slower form of Ca2+-triggered release that is normally occluded by faster synaptotagmin-1-induced release, but becomes manifest upon synaptotagmin-1 deletion.
INTRODUCTION
At a synapse, neurotransmitters are released by Ca2+-triggered synaptic vesicle exocytosis (Katz, 1969) that is mediated by synaptotagmins (Südhof, 2012). Synaptotagmins are membrane-tethered Ca2+-binding proteins containing an N-terminal transmembrane region and two C-terminal cytoplasmic C2-domains. Deletion of synaptotagmin-1 (Syt1) in forebrain neurons blocks fast synchronous release induced by isolated action potentials, but retains an asynchronous, slower and facilitating form of release induced by bursts of action potentials (Geppert et al., 1994; Yoshihara and Littleton, 2002; Maximov and Südhof, 2005; Xu et al., 2012). Although in most synapses asynchronous release becomes manifest only when Syt1 is deleted, in some synapses asynchronous release normally predominates. This is observed in GABAergic synapses formed by CCK-containing dentate gyrus interneurons (Hefft and Jonas, 2005; Daw et al., 2009) and inferior olive interneurons (Best and Regehr, 2009).
The mammalian genome encodes 16 synaptotagmins, 8 of which bind Ca2+ (reviewed in Gustavsson and Han, 2009). Of these 8 synaptotagmins, Syt1, Syt2 and Syt9 are localized to synaptic and neuroendocrine vesicles and function as Ca2+-sensors for fast synaptic and neuroendocrine exocytosis (Perin et al., 1990; Brose et al., 1992; Littleton et al., 1993; Geppert et al., 1994; Sørensen et al., 2003; Pang et al., 2006; Sun et al., 2007; Xu et al., 2007). Syt10, in contrast, is localized to IGF-1 containing vesicles in olfactory bulb neurons and acts as Ca2+-sensor for IGF-1 secretion in these neurons (Cao et al., 2011 and 2013). Among the remaining four Ca2+-binding synaptotagmins, Syt7 is particularly interesting because it is highly expressed in neurons and enriched in synapses (Sugita et al., 2001; Virmani et al., 2003). Surprisingly, Syt7 is dispensable for neurotransmitter release in cultured neurons (Maximov et al., 2008), although it contributes to asynchronous release in zebrafish neuromuscular junctions (Wen et al., 2010). At synapses, Syt7 was not detected in synaptic vesicles but in the synaptic plasma membrane, whereas Syt1 was found in synaptic vesicles (Sugita et al., 2001; Han et al., 2004; Takamori et al., 2006; Maximov et al., 2007). In neuroendocrine cells, however, Syt7 was co-localized with Syt1 on secretory granules, and was shown to mediate Ca2+-triggering of exocytosis similar to Syt1 (Sugita et al., 2001; Shin et al., 2002; Fukuda et al., 2004; Tsuboi and Fukuda, 2007; Schonn et al., 2008; Gustavsson et al., 2008 and 2009; Li et al., 2009; Segovia et al., 2010), albeit with a slower time course (Schonn et al., 2008). Thus, Syt7 is an evolutionarily conserved synaptotagmin highly expressed in brain that functions in neuroendocrine exocytosis, but whose neural function is unclear.
Despite its importance, the identity of the Ca2+-sensor mediating asynchronous release that becomes manifest in Syt1-deficient synapses has remained enigmatic. One study implicated Doc2A as a Ca2+-sensor for asynchronous release (Yao et al., 2011), but other studies failed to detect any role for Doc2 proteins in asynchronous release (Groffen et al., 2010; Pang et al., 2011a). Here, we examined which candidate Ca2+-sensor protein might mediate asynchronous release in hippocampal neurons. We identify a selective essential function for Syt7 in asynchronous release. Our data suggest that multiple synaptotagmins cooperate at a given synapse to mediate the vast majority of all Ca2+-triggered neurotransmitter release.
RESULTS
Individual hippocampal neurons co-express multiple candidate Ca2+-sensors
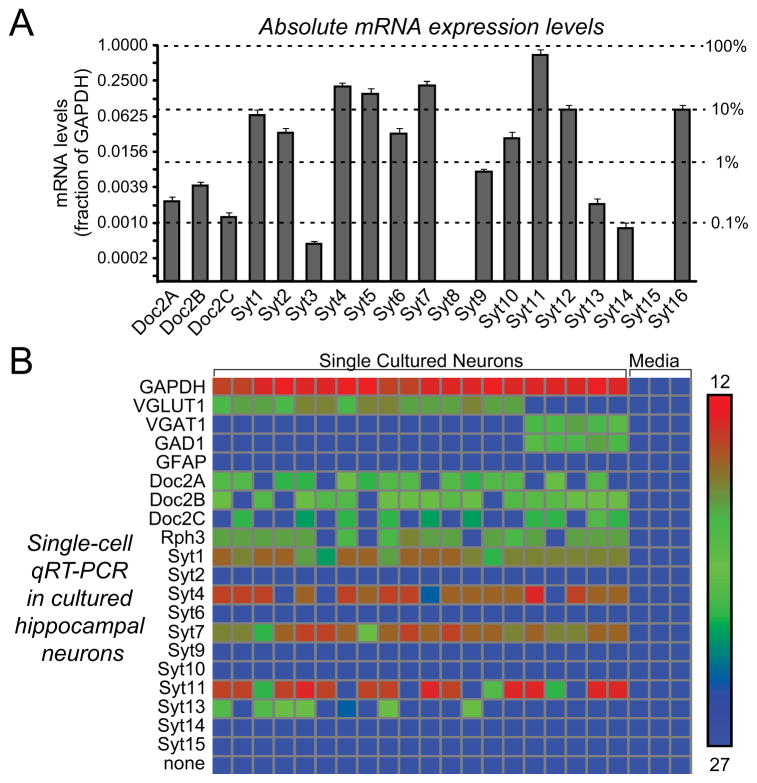
Using quantitative RT-PCR, we measured the expression levels of synaptotagmins and Doc2 proteins in cultured hippocampal neurons. Among the Ca2+-binding protein mRNAs tested, Syt7 (a Ca2+-dependent synaptotagmin [Li et al., 1995; Sugita et al., 2001 and 2002]) was expressed at highest levels, with a ~3-fold greater abundance than Syt1 (Fig. 1A). In addition, Doc2A, 2B, and 2C were co-expressed at ~10-fold lower, but still rather high mRNA levels.
Figure 1. Expression profiling of candidate Ca2+-sensors in hippocampal neurons.
A, mRNA expression levels of candidate Ca2+-sensor genes in cultured hippocampal neurons. mRNA levels were measured by quantitative RT-PCR (normalized to GAPDH). Absolute and relative mRNA levels are depicted on the left and right, respectively, on a logarithmic scale (data are means ± SEM; n=3 independent cultures).
B, mRNA levels of candidate Ca2+-sensor genes measured in individual hippocampal neurons by quantitative RT-PCR using Fluidigm technology (media = negative control). Measurements were performed on cytosol aspirated with a pipette from patched cultured neurons (calibration bar = absolute critical cycle numbers on the Fluidigm chip). Note that most neurons co-express Doc2A, 2B, and 2C together with Syt1, Syt4, Syt7, and Syt11. For the effect of Doc2 KDs on release, see Fig. S1.
Since the analyses in Fig. 1A were performed on mixtures of neurons, we asked whether individual neurons may express subsets of Ca2+-binding proteins. Cytoplasm from individual neurons was collected by aspiration through a patch pipette, and quantitative RT-PCR with Fluidigm technology (Pang et al., 2011b) was used to measure the mRNA levels of 20 genes in 20 single neurons (Fig. 1B). We found that all neurons co-expressed the Ca2+-binding synaptotagmins Syt1 and Syt7 at high levels, and most neurons additionally co-expressed two other synaptotagmins, Syt4 and Syt11, that do not bind Ca2+ (von Poser et al., 1997; Dai et al., 2004). Furthermore, most neurons co-expressed Doc2A, 2B, and 2C at similar, substantial levels (Fig. 1B). No significant expression differences were observed between various Doc2 isoforms in single neurons. Thus, individual hippocampal neurons co-express multiple synaptotagmin and Doc2 isoforms at high levels.
The high and universal expression of Syt7 in all neurons is intriguing given the lack of a known neuronal function for Syt7. However, the finding that Doc2A and Doc2B are co-expressed in hippocampal neurons differs from a previous finding suggesting that hippocampal neurons express only Doc2A (Yao et al., 2011), although it is consistent with other previous studies (Verhage et al., 1997). This finding is important because only the Doc2A KD but not the Doc2B KD was found to decrease asynchronous neurotransmitter release in hippocampal neurons (Yao et al., 2011). The selective effect of the Doc2A KD led to the proposal that Doc2 proteins are general Ca2+-sensors for release, and that the Doc2A KD had a selective effect on release in hippocampal neurons because only Doc2A is expressed in these neurons. Other studies, however, did not observe a change in evoked neurotransmitter release in Doc2A- and Doc2B-deficient neurons (Groffen et al., 2010; Pang et al., 2011a), and our finding of co-expression of Doc2A and Doc2B in hippocampal neurons is also at odds with this hypothesis.
This discrepancy prompted us to directly compare the effects of different previously reported Doc2 shRNAs on neurotransmitter release. We found that one published Doc2A shRNA, but not another similarly effective published Doc2A shRNA and two different Doc2B shRNAs, altered the intrinsic properties of neurons and impaired neurotransmitter release (Fig. S1). Viewed together, the expression and KD results thus argue against a selective role for Doc2A in Ca2+-triggered asynchronous release in hippocampal neurons, consistent with previous studies (Groffen et al., 2010; Pang et al., 2011a). As a consequence, we focused on Syt7 as the most abundant candidate Ca2+-sensor that is universally expressed in all neurons, and developed multiple shRNAs to knock down Syt7 mRNAs.
Syt7 KD impairs asynchronous release at inhibitory synapses
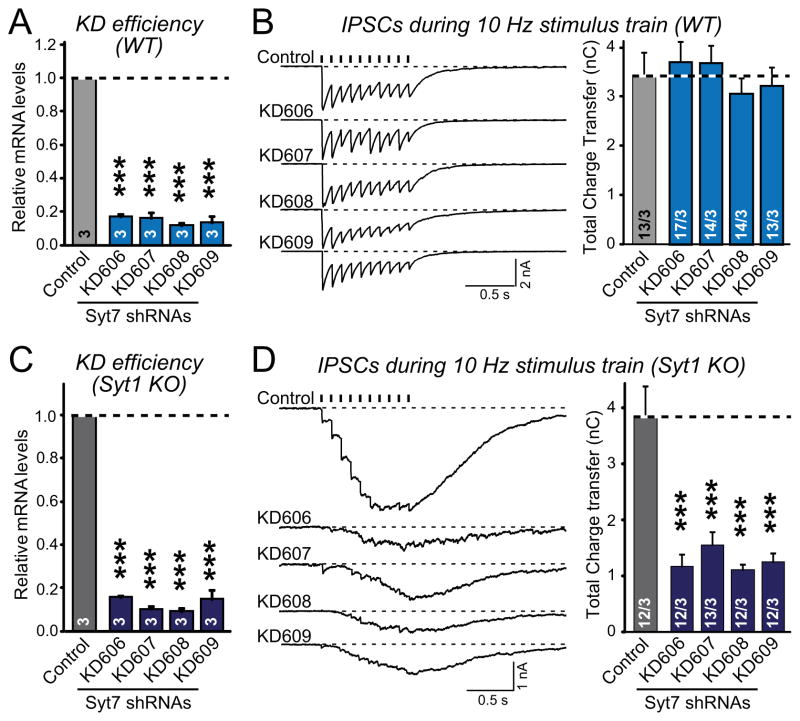
We identified four shRNAs that suppress Syt7 expression by >80% (Fig. 2A). These shRNAs, as well as the Syt7 KO’s discussed below, interfered with expression of all Syt7 splice variants. In wild-type (WT) neurons, the Syt7 shRNAs had no effect on release as measured by inhibitory postsynaptic currents (IPSCs) elicited by a 10 Hz, 1 sec stimulus train (Fig. 2B). In Syt1 KO neurons, however, all four Syt7 shRNAs similarly suppressed the remaining asynchronous release by ~70% (Figs. 2C and 2D). The shRNAs had no effect on intrinsic neuronal properties (Fig. S2). The selective effect of the Syt7 shRNAs on release in Syt1 KO neurons but not in WT neurons could potentially be associated with changes in the expression of Syt7 or other candidate Ca2+-sensors in Syt1 KO neurons. However, quantification of all Doc2 and synaptotagmin mRNAs in WT and Syt1 KO neurons failed to uncover a specific change (Fig. S3).
Figure 2. Multiple Syt7 shRNAs selectively suppress asynchronous release in inhibitory Syt1-deficient neurons.
A, KD efficiency of four different Syt7 shRNAs in cultured WT neurons. Cultured hippocampal neurons were infected with control or shRNA-expressing lentiviruses at DIV4, and Syt7 mRNA levels (normalized to GAPDH) were measured by quantitative RT-PCR at DIV14.
B, Effect of the Syt7 KD on IPSCs evoked by a 10 Hz, 1 s stimulus train in WT neurons that were infected with the control lentivirus and shRNA-expressing lentiviruses as described in panel A. Representative traces are shown on the left, and summary graphs of the total synaptic charge transfer (integrated over 5 s) on the right. Tick marks indicate action potential stimuli; recording artifacts were removed for visual clarity.
C, Syt7 KD efficiency in Syt1 KO neurons, measured as in A.
D, Effect of Syt7 KD on IPSCs elicited by stimulus trains in Syt1 KO neurons, measured as in B.
All summary data are means ± SEM. Numbers in bars indicate numbers of neurons/cultures analyzed. Statistical significance was assessed by one-way ANOVA (*** p<0.001). For the effect of the Syt7 KD on intrinsic neuronal properties, see Fig. S2.
KD experiments always give rise to concerns about specificity. Such specificity can be assessed by three tests: First, the same KD phenotype should be obtained with multiple independent shRNAs. Second, the KD phenotype should be rescued by expression of shRNA-resistant WT (WT) mRNA, although this experiment is only valid if the WT rescue – which necessarily involves overexpression of the rescue mRNA – does not in itself produce a phenotype, and does not impair the shRNA-mediated KD by ‘sponging’ up shRNAs. Third, the KD phenotype should be phenocopied by a genetic KO, although there may be genuine differences between the KD and KO phenotypes that could arise from incompleteness of shRNA-mediated KDs or from compensation effects of constitutive KOs.
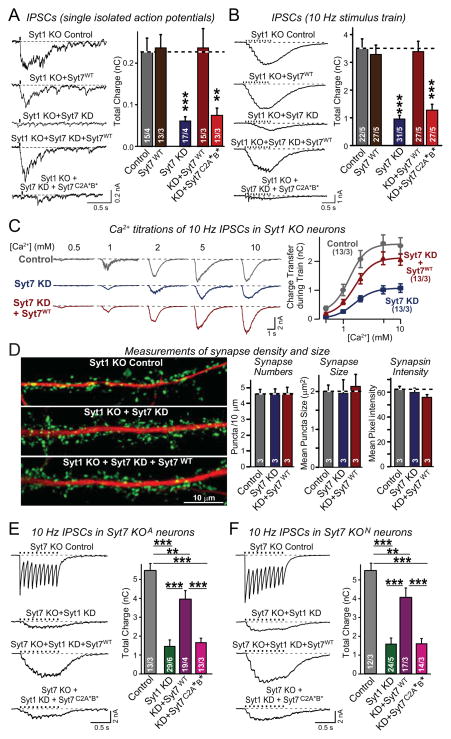
The first criterion for the Syt7 KD phenotype specificity was met in Figs. 2C and 2D, where the lack of an effect of the Syt7 KD in WT neurons (Figs. 2A and 2B) serves as an additional negative control. To test the second criterion, we asked whether the Syt7 KD phenotype can be rescued with WT Syt7 and mutant Syt7 in which Ca2+-coordinating aspartate residues in both the C2A- and the C2B-domains were changed to alanines (Syt7C2A*B*; Fig. S4A), and also examined whether the Syt7 ‘rescue’ by overexpression of WT Syt7 on its own had an effect on IPSCs on Syt1 KO neurons (Figs. 3A and 3B).
Figure 3. Suppression of asynchronous release by Syt7 KD or KO is rescued by WT but not mutant Syt7.
A & B, Syt7 KD impairs asynchronous release (monitored as IPSCs) induced by isolated action potentials (A) and action-potential trains (B) in Syt1 KO neurons. This impairment is rescued by WT Syt7 (Syt7WT) but not by mutant Syt7 containing substitutions in the C2A- and C2B-domain Ca2+-binding sites (Syt7C2A*B*). Cultured hippocampal neurons were infected at DIV4 with control lentivirus, lentivirus expressing WT Syt7 without shRNAs, or lentivirus expressing the Syt7 KD without or with shRNA-resistant WT or mutant Syt7 rescue cDNAs. At DIV14-16, IPSCs were recorded after stimulation by isolated action potentials or by trains of action potentials (10 Hz, 1 s).
C, Ca2+-titrations of asynchronous release in Syt1 KO neurons infected with control lentivirus or lentiviruses expressing the Syt7 KD without or with WT Syt7 rescue. IPSCs elicited with a 10 Hz 1 sec stimulus train were monitored at the indicated concentrations of extracellular Ca2+ (left, representative traces; right, plot of the synaptic charge transfer as a function of the extracellular Ca2+-concentration).
D, Syt7 KD does not alter synapse density or size. Control Syt1 KO neurons or Syt1/Syt7 double-deficient neurons without and with expression of WT Syt7 (obtained as in A) were analyzed by double immunofluorescence labeling for synapsin (green) and MAP2 (red; left = representative images; right = quantifications of synapse number, size, and synapsin staining intensity).
E & F, Impaired asynchronous release in Syt7 KO/Syt1 KD neurons is rescued by WT but not mutant Syt7. Hippocampal neurons cultured from two independent Syt7 KO mouse lines (E, Syt7 KOA from Maximov et al., [2008]; F, Syt7 KON from Chakrabarti et al. [2003]) were infected with control lentivirus or Syt1 KD lentivirus without or with superinfection with a second lentivirus expressing WT or mutant Syt7. IPSCs were elicited by 10 Hz, 1 sec stimulus trains (left, representative traces; right, summary graphs of the total synaptic charge transfer). Syt7 KO neurons exhibit normal synchronous release (control) that is blocked by the Syt1 KD, but in Syt1/Syt7 double-deficient neurons, asynchronous release is also largely absent but can be selectively restored by expression of WT Syt7.
All data are means ± SEM; numbers in bars indicate number of neurons/independent cultures or number of independent cultures analyzed. Statistical significance was assessed by one-way ANOVA (**, p<0.01; ***, p<0.001) comparing test conditions to control (A–D) or to the non-Syt1 KD and the WT Syt7 rescue condition (E and F). For more data, see Figs. S3 and S4.
We found that the Syt7 KD strongly decreased the amplitudes of asynchronous IPSCs elicited in Syt1 KO neurons by single action potentials or by action potential trains (~70% decrease); this phenotype was rescued by WT Syt7, but not by mutant Syt7C2A*B* (Figs. 3A and 3B). Overexpression of Syt7 in Syt1 KO neurons lacking the Syt7 KD had no effect on IPSCs. The various manipulations produced no significant change in the levels of the Doc2’s or synaptotagmin mRNAs except for the Syt1 and Syt7 mRNAs (Fig. S3).
We also tested using extracellular Ca2+-titrations whether the Syt7 KD in Syt1 KO neurons produced a significant shift in the Ca2+-dependence of release, but could not detect a major change (Figs. 3C, S4B, and S4C; note that this approach only reveals large changes in apparent Ca2+-affinity). Moreover, the Syt7 KD in Syt1 KO neurons did not alter the density or apparent size of synapses (Fig. 3D), ruling out effects on synapse formation or maintenance. Together, these experiments suggest that Syt7 is a co-mediator of Ca2+-triggered neurotransmitter release with Syt1, with Syt7 function becoming manifest when Syt1 is deleted because Syt7 operates more slowly than Syt1.
Syt7 KO replicates the Syt7 KD phenotype
Our data meet the first two criteria for specificity of a KD experiment, i.e. the observation of a phenotype with multiple independent shRNAs (Fig. 2) and the rescue of the phenotype with WT protein (Figs. 3A–3D). However, the Syt7 KD effects could still be due to an off-target effect, a concern that is especially relevant because we failed to detect in earlier experiments a role for Syt7 in asynchronous release (Maximov et al., 2008). To completely rule out off-target effects and to address the third specificity criterion mentioned above, we measured synaptic responses in two independent lines of Syt7 KO mice (Chakrabarti et al., 2003; Maximov et al., 2008; Fig. S4D).
Consistent with the Syt7 KD results, Syt7 KO neurons containing Syt1 exhibited apparently normal synchronous release (monitored by evoked IPSCs); this release was suppressed by KD of Syt1 (Figs. 3E and 3F). KD of Syt1 in Syt7 KO neurons, however, suppressed not only synchronous release, but also most asynchronous release. Expression of WT Syt7 in Syt7 KO neurons with the Syt1 KD dramatically increased asynchronous release without restoring synchronous release (Figs. 3E and 3F). Expression of mutant Syt7C2A*B* in the Syt7 KO/Syt1 KD neurons, conversely, did not restore asynchronous release. Expression of WT Syt7 in Syt7 KO neurons without the Syt1 KD had no effect on release (Fig. S4E). All of these results were obtained with both lines of Syt7 KO mice (Figs. 3E and 3F). These data show that WT Syt7 is essential for maintaining most asynchronous release elicited by stimulus trains in Syt1-deficient neurons, and support the notion that the observed Syt7 KD phenotype in asynchronous release is specifically caused by lack of Syt7.
Role of Syt7 in clamping spontaneous mini release
Syt1 not only functions as a Ca2+-sensor for evoked synchronous release, but also as a clamp for spontaneous mini release (Littleton et al., 1993; Maximov and Südhof, 2005). As a result, the Syt1 KO significantly increases (>10-fold) spontaneous mini release. In clamping mini release, Syt1 does not actually clamp fusion, but appears to inhibit a secondary Ca2+-sensor that mediates mini release with a higher Ca2+ sensitivity (Xu et al., 2009; Kochubey and Schneggenburger, 2011). The question thus arises whether Syt7 may represent the secondary Ca2+-sensor that is unclamped in Syt1 KO and KD neurons, or whether Syt7 may conversely also function as a clamp for mini release.
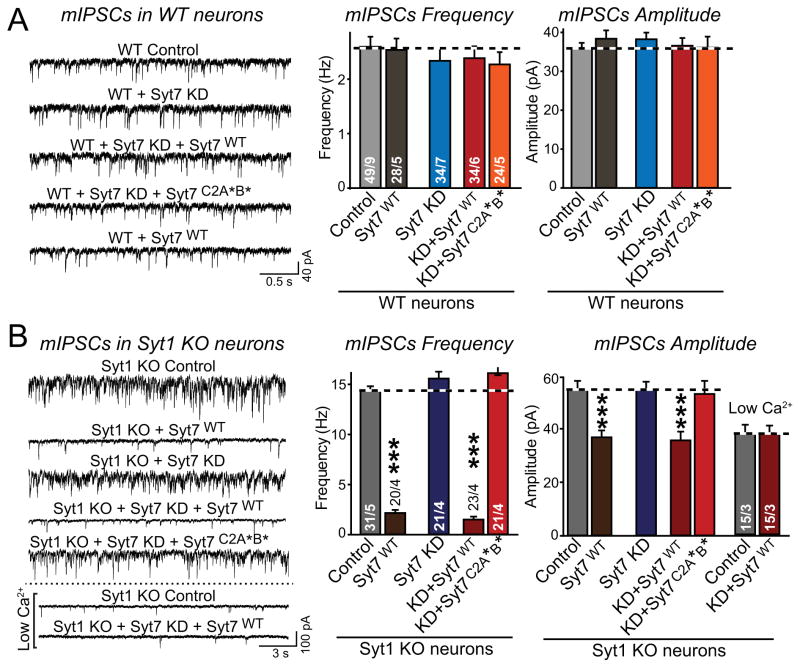
We found that neither Syt7 overexpression nor the Syt7 KD had an effect on the frequency of mIPSCs in WT neurons (Fig. 4A). Moreover, the Syt7 KD did not decrease the increased mIPSC frequency of Syt1 KO neurons (Fig. 4B). Thus, although Syt7 is essential for asynchronous Ca2+-dependent release induced by high-frequency stimulus trains in Syt1 KO neurons, it is not required for the increased Ca2+-dependent spontaneous mini release in these same neurons.
Figure 4. Syt7 KD does not alter spontaneous mIPSCs in WT or Syt1 KO neurons, but Syt7 overexpression clamps mIPSCs in Syt1 KO neurons.
A & B, mIPSCs measured in WT (A) and Syt1 KO neurons (B) as a function of Syt7 KD and/or Syt7 overexpression (left, representative traces; right, summary graphs of the mIPSC frequency and amplitude). Hippocampal neurons were infected with lentiviruses as described for Fig. 3, and mIPSCs were recorded in the presence of TTX (1 μM), CNQX (20 μM), and APV (50 μM). The apparently lower mIPSC amplitude upon Syt7 overexpression in Syt1 KO neurons, but not in WT neurons, resulted from overestimates of the mIPSC amplitudes in the other groups. In these groups, the very large mIPSC frequency makes simultaneous double mIPSCs appear as single large mIPSCs, prompting us to perform mIPSC recordings at low Ca2+-concentrations with decreased mIPSC frequency. These low Ca2+ recordings show that the IPSC amplitude is overestimated during high-frequency conditions (right panel).
All data are means ± SEM; numbers in bars indicate number of neurons/independent cultures analyzed. Statistical significance was assessed by one-way ANOVA (***, p<0.001) comparing test conditions to control.
Strikingly, however, Syt7 overexpression reversed the increased mini frequency in Syt1 KO neurons without or with concurrent Syt7 KD (Fig. 4B; see Fig. S5 for protein quantifications showing that rescue of Syt7 KD neurons with WT Syt7 mediates Syt7 overexpression). Note that in these experiments, the increase in mIPSC frequency in Syt1 KO neurons is likely underestimated because the mIPSC frequency is so high that even custom algorithms do not capture all events (see Methods).
Our data show that Syt7 is not a Ca2+-sensor for the increased mini events in Syt1 KO neurons and does not clamp minis under physiological conditions, but that at increased levels, Syt7 can substitute for Syt1 in clamping mini release. A clamping function by Syt7 may not be apparent under physiological conditions because Syt7 may not be expressed at sufficiently high levels, especially within presynaptic terminals. It is interesting that the ability of overexpressed Syt7 to clamp the increased mini release in Syt1 KO neurons differs remarkably from the inability of overexpressed Syt7 to restore fast synchronous release in Syt1 KO neurons (Figs. 3 and 4B; see also Xue et al., 2010).
None of the Syt1 and/or Syt7 manipulations altered the mIPSC amplitude except for an apparent decrease in mIPSC amplitude upon Syt7 overexpression, which suppressed the increase in mIPSC frequency in Syt1-deficient neurons (Fig. 4B). We hypothesized that this effect on mISPC amplitude may have been due to an overestimation of the mIPSC amplitude under conditions of high mIPSC frequency, when superimposed mIPSCs may not always be detectable. When we measured the mIPSC amplitude in low extracellular Ca2+-concentrations which decrease mIPSC frequency, we found that the Syt1 KO mIPSC amplitude was decreased to the same level as observed for the ‘clamped’ condition (Syt1/Syt7 double-deficient neurons that overexpress WT Syt7; Fig. 4B). Thus, the Syt1 KO does not cause a true change in mIPSC amplitude.
Syt1 and Syt7 exhibit distinct C2-domain requirements
The two C2-domains of Syt1 and Syt7, referred to as the C2A- and C2B-domains, contain multiple Ca2+-binding sites (Fig. 5A). In Syt1, Ca2+-binding to the C2B-domain is essential for Ca2+-triggered synchronous release, whereas Ca2+-binding to the C2A-domain contributes to Ca2+-triggering of release but is not absolutely required (Mackler and Reist, 2001; Mackler et al., 2002; Nishiki and Augustine, 2004; Shin et al., 2009; Lee et al., 2013). To test whether the same principle applies to Syt7, we examined the rescue of synchronous or asynchronous release in Syt1/Syt7 double-deficient neurons by mutant Syt1 or Syt7 containing substitutions in either the C2A- or the C2B-domain Ca2+-binding sites (Fig. S4A).
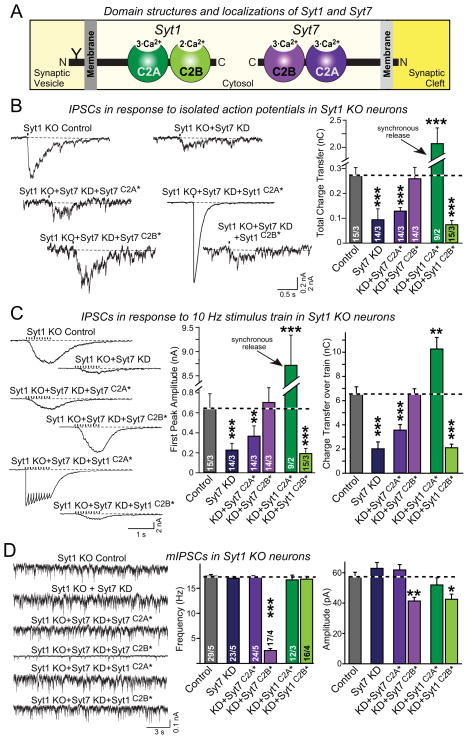
Figure 5. Syt7 function requires intact C2A-domain Ca2+-binding sites whereas Syt1 function requires intact C2B-domain Ca2+-binding sites.
A, Schema of the domain structures and localizations of Syt1 and Syt7. Syt1 resides on synaptic vesicles while Syt7 is found on the plasma membrane. Syt1 contains a glycosylation site (Y).
B, In Syt7, rescue of impaired asynchronous IPSCs evoked by isolated action potentials in Syt1/Syt7 double-deficient neurons requires wild-type C2A-domain Ca2+-binding sites, whereas in Syt1 rescue of synchronous ISPCs requires wild-type C2B-domain Ca2+-binding sites. Syt1 KO neurons were infected with control lentivirus or lentivirus expressing the Syt7 KD shRNA either without (Syt7 KD) or with co-expression of the indicated Syt7 or Syt1 rescue proteins (Syt7C2A* and Syt7C2B*, Syt7 containing mutations in the C2A- or C2B-domain Ca2+-binding sites; Syt1C2A* and Syt1C2B*, same for Syt1; for sequences of the mutations, see Fig. S4A; for expression levels of mutant proteins, see Fig. S5).
C, Rescue experiments of IPSCs evoked by a 10 Hz stimulus train in Syt1/Syt7 double-deficient neurons performed as described for B.
D, Clamping of increased spontaneous mIPSCs in Syt1 KO neurons by Syt7 requires an intact C2A domain, while the C2B domain is dispensable. Mutations in either C2A or C2B of Syt1 prevent clamping of spontaneous mIPSCs.
All data are means ± SEM; numbers in bars indicate number of neurons/independent cultures analyzed. Statistical significance was assessed by one-way ANOVA (*, p<0.05; **, p<0.01; ***, p<0.001) comparing test conditions to control. For sequences of Ca2+-binding site mutants, see Fig. S4.
We examined IPSCs induced by isolated action potentials in Syt1/Syt7 double-deficient neurons, and found that the Syt7 C2A-domain Ca2+-binding sites but not the Syt7 C2B-domain Ca2+-binding sites were essential for rescue of asynchronous release (Fig. 5B). In contrast, in Syt1 the C2B-domain Ca2+-binding sites but not the C2A-domain Ca2+-binding sites were required for synchronous release. The same selective requirement for the Syt7 C2A-domain Ca2+-binding sites for asynchronous release, and for the Syt1 C2B-domain Ca2+-binding sites for synchronous release was observed when release was induced by high-frequency stimulus trains (Fig. 5C). The differential phenotypes of the Syt1 and Syt7 C2A- vs. C2B-domain mutants were not due to differences in protein expression. All of these proteins were overexpressed compared to WT levels during the rescue manipulations; although the degree of overexpression varied between various mutants, it did not correlate with the functional effects of the mutations (Fig. S5). Thus, although Syt1 and Syt7 both appear to function in triggering neurotransmitter release, their mechanism of action differs in terms of the relative importance of their C2-domains, consistent with their differential localization.
To further explore the relative importance of the Syt1 and Syt7 C2A vs. C2B-domains, we also measured the ability of the Syt1 and Syt7 C2-domain mutants to clamp the increased spontaneous mini release in Syt1-deficient neurons (Fig. 5D). Strikingly, the Syt7 C2A-domain mutation but not the Syt7 C2B-domain mutation again blocked clamping, whereas for Syt1 both the intact C2A- and the intact C2B-domain were required as described earlier (Shin et al., 2009; Lee et al., 2013).
Syt7 is also essential for asynchronous release at excitatory synapses
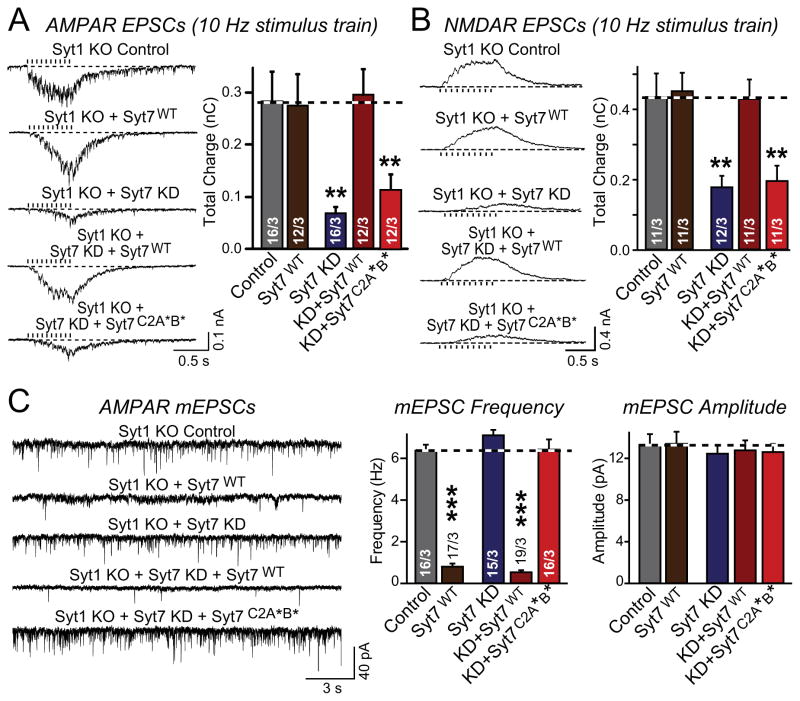
All experiments described up to now were performed in inhibitory synapse (Figs. 1–5). To test whether Syt7 also functions in excitatory synapses, we analyzed the effect of the Syt7 KD on EPSCs induced by stimulus trains in Syt1 KO neurons. In these experiments, we separately measured AMPA-receptor (AMPAR) or NMDA-receptor (NMDAR) mediated EPSCs to ensure that the observed changes are presynaptic since presynaptic changes in glutamate release should equally affect AMPAR- and NMDAR-mediated responses.
We found that in Syt1 KO neurons, the Syt7 KD similarly suppressed AMPAR- and NMDAR-mediated asynchronous EPSCs elicited by stimulus trains (Figs. 6A and 6B). WT Syt7 fully rescued these phenotypes, but had no effect on EPSCs in Syt1 KO neurons that had not been subjected to the Syt7 KD. Mutant Syt7C2A*B* was unable to rescue the phenotype (Figs. 6A and 6B), consistent with a specific effect of the Syt7 KD. As in inhibitory synapses, Syt7 overexpression also reversed the Syt1 KO phenotype of increased mini frequency at excitatory synapses, and the Syt7 KD had no effect on this phenotype (Fig. 6C). Thus, Syt7 performs apparently identical functions in excitatory and inhibitory synapses.
Figure 6. Loss of Syt7 impairs asynchronous release but does not affect spontaneous release in excitatory synapses.
A & B, Syt7 KD blocks most asynchronous EPSCs in hippocampal neurons from Syt1 KO mice; this impairment is rescued by WT Syt7 (Syt7WT) but not by mutant Syt7 containing substitutions in the C2A- and C2B-domain Ca2+-binding sites (Syt7C2A*B*). Both AMPAR-mediated (A) or NMDAR-mediated EPSCs (B) were monitored to ensure a presynaptic effect (left panels = representative traces; right panels = summary graphs of the total synaptic charge transfer).
C, Syt7 KD does not lower the increased frequency of spontaneous miniature EPSCs (mEPSCs) in Syt1 KO neurons, but overexpression of Syt7 clamps these mEPSCs. Representative traces (left) and summary graphs of the mEPSC frequency and amplitude (right) were monitored in TTX (1 μM), picrotoxin (50 μM), and APV (50 μM) in Syt1 KO neurons that were infected with either control lentivirus, lentivirus overexpressing Syt7, or Syt7 KD lentivirus without or with expression of WT or C2AB-domain mutant Syt7 as indicated.
All data are means ± SEM; numbers in bars indicate number of neurons/independent cultures analyzed. Statistical significance was assessed by one-way ANOVA (** p<0.01; *** p<0.001) comparing test conditions to control.
Syt7 KO decreases asynchronous release in WT neurons
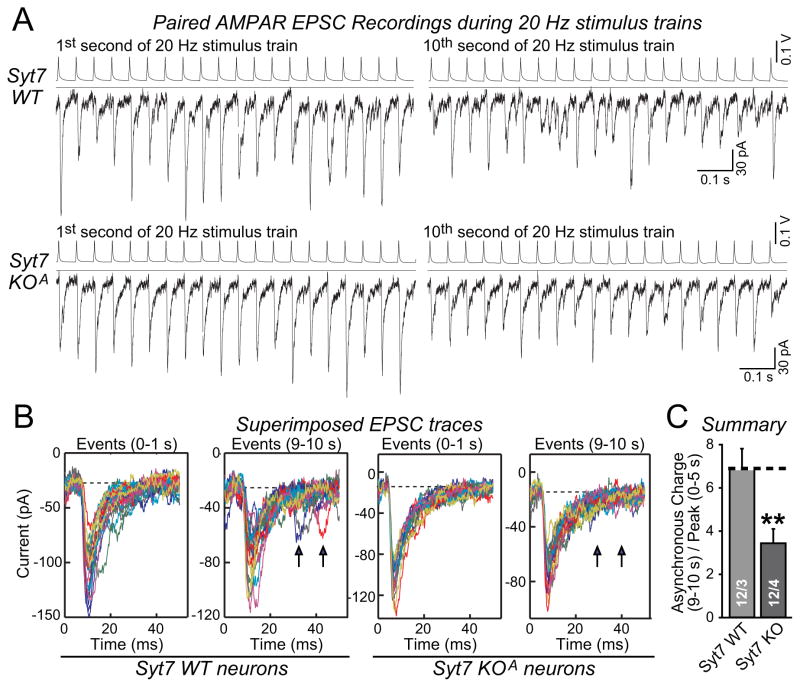
Thus far, we have only detected a phenotype of the Syt7 KD or KO in Syt1-deficient but not in WT neurons. Is it possible that our experimental setups may have obscured a phenotype in neurons lacking only Syt7 but not Syt1? This possibility is suggested by experiments in zebrafish neuromuscular junctions which only exhibited a Syt7-dependent phenotype when asynchronous release was analyzed in the intervals between action potential intervals during extended stimulus trains (Wen et al., 2010). To examine whether the same applies to cultured Syt7 KO neurons, it was necessary to perform paired recordings of EPSCs evoked at high frequency (Fig. 7A). Using this approach, we observed that in sparsely cultured neuronal micro-islands, EPSCs that were not synchronous with action potentials were detectable after a 10 s, 20 Hz stimulus train (Fig. 7B). Strikingly, these EPSCs were decreased by ~50% in Syt7 KO neurons (Fig. 7C). Thus, Syt7 is essential for asynchronous release even in the presence of Syt1 when extended stimulus trains are analyzed.
Figure 7. Syt7 KO suppresses asynchronous release induced by prolonged high-frequency stimulus trains in Syt1-containing neurons.
A, Paired recordings of EPSCs in hippocampal microisland cultures. EPSCs were induced by 20 Hz presynaptic action-potential trains. Sample recordings of presynaptic action potentials (top) and postsynaptic EPSCs (bottom) during the 1st and 10th second are shown.
B, Overlay of all EPSCs during 1st and 10th second. Note asynchronous events in the 9–10 s interval in WT neurons (arrows) that are absent in Syt7 KO neurons.
C, Summary graph of asynchronous release in the 10th second, quantified as the integrated charge transfer during 30–50 ms after a presynaptic action potential, and normalized for the overall synaptic strength by dividing the charge transfer with the average peak amplitudes during the initial 5 s. Data are means ± SEM; numbers in bars indicate number of pairs/independent cultures analyzed. Statistical significance was assessed by one-way ANOVA (** p<0.01) comparing test conditions to control.
Syt7 KD impairs asynchronous neurotransmitter release in vivo
Some properties of cultured neurons differ from those of more physiological preparations, such as acute slices, leading us to ask whether Syt7 is also essential for asynchronous release in situ. In previous studies, we showed that KD of Syt1 in vivo using AAV-mediated shRNA expression blocks synchronous release and amplifies asynchronous release (Xu et al., 2012). Thus, we examined whether the Syt7 KD also impairs asynchronous release in Syt1 KD neurons in vivo.
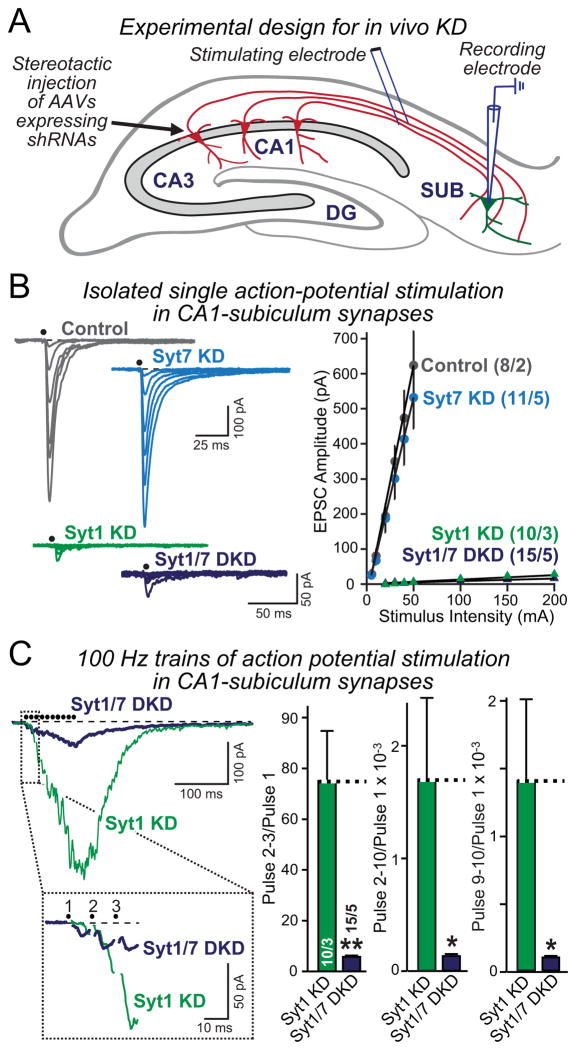
The circuitry of the hippocampus includes abundant projections from the CA1 region to the subiculum (Fig. 8A). We infected CA1 neurons in vivo by stereotactic injection of AAVs expressing either no shRNA (control), Syt1 or Syt7 shRNAs alone, or both shRNAs. Two weeks later, we characterized the effect of Syt1 and Syt7 KDs on presynaptic neurotransmitter release in acute slices using electrophysiological recordings from postsynaptic subicular neurons during stimulation of CA1 inputs (Fig. 8A).
Figure 8. Syt7 KD impairs asynchronous release in Syt1-deficient neurons in vivo.
A, Schematic description of the experimental design for in vivo manipulations of Syt1 and Syt7 expression. The CA1 region of the hippocampus was infected by stereotactic injections at P21 in WT mice with control AAV or AAVs mediating the Syt1 or Syt7 KD alone or the combined Syt1 and Syt7 KD. Acute slices were analyzed at P35 by stimulating presynaptic fibers from the CA1 region and monitoring postsynaptic responses using whole-cell patch-clamp recordings in pyramidal neurons of the subiculum (which was not infected).
B, Measurements of EPSCs elicited by isolated stimuli applied with increasing strength to obtain input/output relationships in control, Syt1 KD, Syt7 KD, and Syt1/7 double KD mice (left, representative traces; right, summary graphs of the EPSC amplitude as a function of stimulus strength).
C, Measurements of EPSCs elicited by a 100 Hz, 0.1 sec stimulus train (left, representative traces with an expansion of the initial response below; right, summary graphs of the facilitation of EPSCs during the train as a measure of the activation of asynchronous release). Summary graphs show the ratios of the cumulative charge transfer for pulses 2–3, pulses 2–10, and pulses 9–10 in the train divided by that of the first pulse.
Summary data are means ± SEM; numbers in bars indicate number of neurons/mice analyzed. Statistical significance was assessed by one-way ANOVA (* p<0.05; ** p<0.01) comparing test conditions to control.
Consistent with previous results (Xu et al., 2012), the Syt1 KD almost completely suppressed synchronous EPSCs induced by single action potentials (Fig. 8B). This phenotype was very large, and no additional effect of the Syt7 KD was detectable. The Syt7 KD by itself had no effect on the size of the EPSC under these conditions (Fig. 8B), mirroring the observations in cultured WT neurons (Fig. 2B).
Although the Syt7 KD did not detectably alter the size of EPSCs induced by single action potentials, a different picture emerged when we measured EPSCs induced by action potential trains (Fig. 8C). After KD of Syt1, typically facilitating asynchronous release was observed. This release was severely impaired (>10-fold) by additional KD of Syt7, as quantified by charge transfer ratios of EPSCs during the stimulus train (Fig. 8C). Thus, Syt7 is required for asynchronous release in Syt1-deficient neurons not only in culture, but also in vivo, confirming its role in asynchronous release.
DISCUSSION
In most neurons, deletion of the Ca2+-sensor Syt1 ablates synchronous neurotransmitter release but it does not block – may even enhance – asynchronous release, which manifests as a facilitating form of release in response to high-frequency stimulus trains (Geppert et al., 1994; Yoshihara and Littleton, 2002; Nishiki and Augustine, 2004; Maximov and Südhof, 2005; Xu et al., 2012). This fundamental observation suggested that additional Ca2+-sensors besides Syt1 (and its functional homologs in fast synchronous release, Syt2 and Syt9; Xu et al., 2007) support neurotransmitter release. However, the identity of the Ca2+-sensors involved has remained elusive.
Here, we propose that Syt7, the most abundant Ca2+-binding synaptotagmin in brain (Fig. 1A), functions as a Ca2+-sensor for asynchronous release. This proposal implies that nearly all Ca2+-triggered release at a synapse is mediated by a synaptotagmin, with different synaptotagmins complementing each other. KD or KO of Syt7 in Syt1-deficient forebrain neurons in culture or in vivo suppressed asynchronous release in Syt1-deficient excitatory or inhibitory neurons, suggesting that asynchronous release requires Syt7 (Figs. 2, 3, 5, 6, and 8). We observed no major phenotype upon ablation of Syt7 in WT neurons when synaptic transmission was elicited by extracellular stimulation, but a major decrease in asynchronous release when synaptic transmission was monitored in paired recordings which allow a more precise measurement of the time course of synaptic responses (Fig. 7). This experiment indicates that most Syt7 function is normally occluded by Syt1, likely because Syt1 acts faster than Syt7 and out-competes Syt7, but that Syt7 is important for asynchronous release during extended stimulus trains even in WT neurons.
Surprisingly, we found that despite their overall similarity, Syt1 and Syt7 exhibit distinct C2-domain requirements for Ca2+-triggered release. Specifically, Ca2+-triggering of synchronous release required the C2B-domain Ca2+-binding sites of Syt1 as shown previously (Mackler and Reist, 2001; Mackler et al., 2002; Nishiki and Augustine, 2004; Shin et al., 2009; Lee et al., 2013), whereas Ca2+-triggering of asynchronous release required the C2A-domain Ca2+-binding sites of Syt7 (Fig. 5). Most of our findings were supported by KD manipulations with multiple independent shRNAs, by rescue experiments with WT and mutant Syt1 and Syt7 cDNAs, and/or by KO experiments for both Syt1 and Syt7. Thus, we propose that Syt1 and Syt7 perform overall similar functions in Ca2+-triggering of release, although with different time courses, C2-domain mechanisms, and efficiencies.
Besides blocking evoked synchronous release, deletion of Syt1 greatly increases spontaneous mini release; this increased mini release is also Ca2+-dependent, but exhibits a different apparent Ca2+-affinity and Ca2+-cooperativity than mini release in WT neurons (Xu et al., 2009). We show that although Syt7 is required for most Ca2+-triggered asynchronous release, the Syt7 KD did not decrease the >10-fold elevated mini frequency in Syt1 KO neurons (Figs. 4 and 6C). In contrast, overexpression of WT Syt7 but not of mutant Syt7 suppressed the elevated mini frequency in Syt1 KO neurons. Even for clamping spontaneous mini release, Syt1 and Syt7 differed in their C2-domain requirements in that the clamping activity of Syt7 required only its WT C2A-domain, whereas the clamping activity of Syt1 require both its WT C2A- and its WT C2B-domain (Fig. 5D).
Our data extend previous studies on Syt1 by confirming its central role as Ca2+-sensor for fast synchronous release (Geppert et al., 1994; Fernandez-Chacon et al., 2001; Mackler et al., 2002). Our results also complement earlier studies on Syt7 which documented a major role for Syt7 in neuroendocrine exocytosis (Sugita et al., 2001; Shin et al., 2002; Fukuda et al., 2004; Tsuboi and Fukuda, 2007; Schonn et al., 2008; Gustavsson et al., 2008 and 2009; Li et al., 2009, Segovia et al., 2010). Moreover, our findings confirm that KO of Syt7 in WT neurons produces no significant phenotype in release elicited by extracellular stimulation (Maximov et al., 2008), and agree with the observation that Syt7 supports asynchronous release during extended stimulus trains in the zebrafish neuromuscular junction (Wen et al., 2010). However, our observations conflict with our own previous finding that constitutive Syt1/Syt7 double KO mice do not exhibit an additional phenotype compared to Syt1 KO mice (Maximov et al., 2008) – indeed, this discrepancy prompted us to institute multiple levels of controls here to confirm the specificity of the observed effects. A possible explanation of this discrepancy is that our earlier experiments involved constitutive KOs that may have elicited developmental compensation.
Our data also argue against a recent suggestion that Doc2A and Doc2B proteins are Ca2+-sensors for asynchronous release, and that a KD of Doc2A alone impairs release in hippocampal neurons because hippocampal neurons express only Doc2A (Yao et al., 2011). Using quantitative RT-PCR, we show that individual hippocampal neurons co-express Doc2A and Doc2B at similarly high levels (Figs. 1A and 1B). Using KD experiments, moreover, we confirm that Doc2A is not required for asynchronous release, and present evidence that the single shRNA to Doc2A that produces a phenotype (Yao et al., 2011) has broad effects on neuronal properties, suggesting a non-specific effect (Fig. S1). Thus, our data are consistent with other studies which did not detect a role for Doc2 proteins in asynchronous release, and support the notion that Doc2 proteins contribute to separate priming and Ca2+-triggering steps in mini release (Verhage et al., 1997; Groffen et al., 2010; Pang et al., 2011a).
Outlook and questions
Syt1 is localized on synaptic vesicles, while Syt7 is largely absent from synaptic vesicles but present, at least in part, on the plasma membrane (Sugita et al., 2001; Takamori et al., 2006; Maximov et al., 2007). We propose that Syt1 and Syt7 perform generally similar, but temporally shifted functions in Ca2+-triggering of evoked release and in clamping mini release with different efficacy. Syt7 appears to be less efficient than Syt1 in both Ca2+-triggering of evoked release and in clamping spontaneous release, and to act more slowly than Syt1. These properties of Syt7 may be due to its predominant localization to the plasma membrane; it is possible that a small percentage of Syt7 is on synaptic vesicles and represents its ‘active’ fraction, with the inefficiency of Syt7 as a Ca2+-sensor for release being due to the inefficiency of its sorting to synaptic vesicles. Alternatively, the properties of Syt7 may be caused by the specific Ca2+-binding properties of its C2-domains, as supported by the differential requirements for the C2A- vs. C2B-domain Ca2+-binding sites for release in Syt1 vs. Syt7, and by the finding that Syt7 C2-domains do not function when transplanted into Syt1 (Xue et al., 2010).
Ca2+-induced activation of Syt1 and Syt7 likely involves Ca2+-dependent phospholipid binding and stimulation of the completion of SNARE-complex assembly from a partially assembled ‘primed’ trans-state to a fully assembled cis-state with fusion-pore opening. The latter activity may be mediated by partial displacement of complexin from the primed SNARE complex (Tang et al., 2006; Südhof and Rothman, 2009). We suggest that in WT synapses stimulated by isolated action potentials, the faster Ca2+-induced activation of Syt1 generally prevails over the slower Ca2+-induced activation of Syt7, thereby occluding Syt7 function and leading to pure synchronous release. In synapses stimulated by action potential trains, Ca2+-transients become longer lasting depending on the Ca2+-dynamics of a particular terminal, activating Syt7 in addition to Syt1, and stimulating at least some asynchronous release. Some synapses normally exhibit extensive asynchronous release even when stimulated at low frequency (Hefft and Jonas, 2005; Best and Regehr, 2009; Daw et al., 2009), possibly due to the effect of additional regulatory mechanisms – for example, it is possible that Syt1 may become inhibited by phosphorylation, or that Syt7 may be constitutively activated by phosphorylation or by alternative splicing (Sugita et al., 2001).
The simple model of Syt1 and Syt7 function we propose accounts for all of the available observations, but raises new questions. What precisely are the mechanisms mediating the functions of Syt1 and Syt7 in Ca2+-triggering of evoked release and in clamping mini release? SNARE- and phospholipid-binding by Syt1 and Syt7 are likely involved (Perin et al., 1990; Bennett et al., 1992; Li et al., 1995; Chapman et al., 1995; Sugita et al., 2002), but the precise nature of the protein complexes that mediate these functions remains to be established. In particular, the clamping function of Syt1 and Syt7 likely operates upstream of Ca2+-influx, and thus implies that Syt1 and Syt7 perform Ca2+-independent actions in release.
A second question regards the identity of the Ca2+-sensor for the increased mini release in Syt1 KO synapses that is not mediated by Syt7. This sensor has a lower apparent Ca2+-cooperativity than the Ca2+-sensor for evoked release (Xu et al., 2009), and is also clamped by complexin (Yang et al., 2010), suggesting that it is not a synaptotagmin. It is tempting to speculate that this Ca2+-sensor may catalyze SNARE-complex assembly, and could be involved in Munc13 function upstream of regular Ca2+-triggering by Syt1 and Syt7 (Augustin et al., 1999; Varoqueaux et al., 2002), but this possibility has not been tested. Finally, not all asynchronous release is blocked in Syt1/Syt7 double-deficient neurons (see Figs. 2, 3, and 6). The residual asynchronous release may be mediated by remaining Syt1 or Syt7 protein, or by other synaptotagmin isoforms. Alternatively, the remaining asynchronous release may reflect an additional, qualitatively different release process that may be driven by the same molecular mechanisms as those that lead to the increased mini release in Syt1 KO synapses.
Finally, our data do not rule out additional functions for Syt1 and Syt7. For example, Syt1 and Syt7 could additionally contribute to vesicle priming and/or regulate the repriming rate of synaptic vesicles. However, the phenotypes we observed cannot be explained solely by a potential function of Syt7 in priming or in regulating repriming. Specifically, impairments in priming or in the Ca2+-dependent regulation of repriming could not account for the sustained increased mini frequency in Syt1/Syt7-double deficient neurons (Fig. 4), for the decrease in isolated single responses in these neurons (Fig. 3), and for the selective loss of asynchronous responses in Syt7 KO neurons still expressing Syt1 (Fig. 7). However, the fact that Syt1 and Syt7 support both Ca2+-triggering of evoked release and clamping of mini release (which is unlikely to involve Ca2+-binding to Syt1 or Syt7), and that the Syt1 and Syt7 Ca2+-binding sites were nevertheless required for both functions, suggests that synaptotagmins do not merely serve as Ca2+-sensors in release. These considerations indicate that synaptotagmins function not only when Ca2+-influx is induced by an action potential, but also prior to Ca2+-influx when synapses are preparing for Ca2+-triggered release. Unraveling these Ca2+-independent functions of synaptotagmins remains a fascinating challenge.
Independent of the answers to these questions, our data suggest that the vast majority of Ca2+-triggered neurotransmitter release under physiological conditions is produced by activation of complementary synaptotagmins, three fast acting isoforms that mediate synchronous release (Syt1, Syt2, or Syt9, which exhibit small differences in kinetics), and a slower acting isoform that mediates most asynchronous release (Syt7). Synaptotagmins, together with complexins, are evolutionarily conserved in all animals from cnidaria to humans, suggesting that the fundamental principle of synaptotagmin function in Ca2+-triggering of exocytosis may be a general principle shared by all animals.
EXPERIMENTAL PROCEDURES
Generation of RNAi vectors
All lentiviral transfection and infection experiments for shRNA expression were performed as described (Pang et al., 2010). The following oligonucleotide sequences were used for KDs: Syt7, KD606 AAAGACAAGCGGGTA GAGAAA, KD607 GATCTACCTGTCCTGGAAGAG, KD608 GTTCAGTGTTGGCTAC AACTT, KD609 AACATCATCAAAGCTCGAAAC; for Syt1 GAGCAAATCCAGAAAGTG CAA (Xu et al., 2012). For standard Syt7 KD experiments, KD607 was used. For rescue experiments, rat Syt7 (NM_021659) and Syt1 cDNAs rendered insensitive to the shRNA were inserted in the KD lentiviral vector and their expression was driven by the synapsin promoter; the vector also contained an internal ribosome entry site followed by GFP to enable monitoring of infection. C2-domain mutants of Syt7 and Syt1 contain the aspartates to alanine substitutions shown in Fig. S4A.
Cultures of hippocampal neurons were produced from WT, Syt1 KO, and Syt7 KO mice as described (Maximov and Südhof, 2005; Pang et al., 2010). Briefly, hippocampi were dissected from P0 pups, dissociated by papain digestion, and plated on Matrigel-coated glass coverslips. Neurons were cultured for 14–16 days in vitro in MEM (Gibco) supplemented with B27 (Gibco), glucose, transferrin, fetal bovine serum, and Ara-C (Sigma).
Lentivirus production and infection of cultured neurons
The production of lentiviruses and infection of neurons with lentiviruses have been described (Pang et al., 2010; Tang et al., 2006). Briefly, supernatant with viruses was collected 48 hr after co-transfection of human embryonic kidney 293T cells with the lentiviral vector and three packaging plasmids, and was used to infect hippocampal neuronal cultures at DIV4. Cultures were analyzed at DIV14–16.
AAV preparation and stereotactic injections
AAV-DJ viruses were prepared as described, and stereotaxic injections with 1.0 × 107 infectious units/μl of virus were performed as described (Xu et al., 2012).
mRNA measurements
For mRNA measurements of bulk cultured neurons, RNA was isolated at DIV14 using the RNAqueous kit (Ambion). RT-PCR reactions were set up in triplicates for each condition (150 ng total RNA) using the LightCycler 480 reagent kit (Roche), gene-specific primers (Roche), and a 7900HT Fast RT-PCR instrument (Applied Biosystems) with GAPDH as internal control. For single-cell gene expression profiling using the Fluidigm system (Pang et al., 2011b), cytoplasm of single cultured neurons was aspirated into patch electrodes, ejected into 2X cells-direct buffer (Invitrogen), and flash-frozen. Thawed cytoplasm was subjected to target-specific reverse-transcription and 18 cycles of PCR pre-amplification with a mix of primers specific to the target genes. These products were processed for real-time PCR analysis on Biomark 48:48 Dynamic Array integrated fluidic circuits (Fluidigm). Alternatively, bulk mRNA from neuronal cultures was reverse-transcribed, amplified, and subjected to Fluidigm analysis as described above. In all cases, the mRNA levels of an empty vector control infection were set as 1.
Electrophysiological recordings
Recordings from cultured neurons were performed essentially as described (Pang et al., 2010; Tang et al., 2006). For paired recordings in microisland cultures, cultures were prepared as described above except that coverslips were coated with Matrigel via an aerosolizing sprayer, and pairs were recorded in two cell microislands to prevent network interference. Current was injected into the presynaptic neuron held under current clamp to induce action potentials, and EPSCs were recorded at −70 mV. For slice electrophysiology, stereotaxic injections using AAVs expressing the Syt1 and/or the Syt7 KD shRNAs and subsequent recordings were performed as described (Xu et al., 2012). All electrophysiological methods are described in detail in the SOMs.
Immunofluorescence staining of cultured neurons
Cultured neurons were fixed in 4% paraformaldehyde, permeabilized in 100% methanol for 1 min, and stained with anti-synapsin (E028 1:1000, Sigma), and anti-MAP2 (1:1000) antibodies. Alexa Fluor 546 anti-mouse and Alexa Fluor 633 anti-rabbit secondary antibodies were used for detection with a confocal microscope. Synaptic puncta were analyzed using a custom Matlab script.
Statistics
All experiments were performed by experimenters unaware of the sample identity. All data are shown as means ± SEM; all statistical analyses were performed by one-way ANOVA.
Supplementary Material
Highlights.
Synaptotagmin-1 deletion uncovers asynchronous release induced by stimulus trains
Synaptotagmin-7 ablation impairs asynchronous release in synaptotagmin-1 KO neurons
Synaptotagmin-7 knockout decreases asynchronous release in wild-type neurons
Synaptotagmin-1 and -7 clamp spontaneous mini release with distinct efficiencies
Acknowledgments
We thank E. Chapman (UW Madison) for providing Doc2A and Doc2B shRNAs, and Ira Huryeva for excellent technical support. This paper was supported by an NINDS NRSA fellowship (F32NS067896 to T.B.) and by grants from the NIH (P50 MH086403 and R01 NS077906 to R.C.M. and T.C.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Augustin I, Rosenmund C, Südhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Best AR, Regehr WG. Inhibitory regulation of electrically coupled neurons in the inferior olive is mediated by asynchronous release of GABA. Neuron. 2009;62:555–565. doi: 10.1016/j.neuron.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N, Petrenko AG, Südhof TC, Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Cao P, Maximov A, Südhof TC. Activity-dependent IGF-1 exocytosis is controlled by the Ca2+-sensor synaptotagmin-10. Cell. 2011;145:300–311. doi: 10.1016/j.cell.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Yang X, Südhof TC. Complexin activates exocytosis of distinct secretory vesicles controlled by different synaptotagmins. J Neurosci. 2013;33:1714–1727. doi: 10.1523/JNEUROSCI.4087-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Kobayashi KS, Flavell RA, Marks CB, Miyake K, Liston DR, Fowler KT, Gorelick FS, Andrews NW. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol. 2003;162:543–549. doi: 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ER, Hanson PI, An S, Jahn R. Ca2+ regulates the interaction between synaptotagmin and syntaxin 1. J Biol Chem. 1995;270:23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- Dai H, Shin OH, Machius M, Tomchick DR, Südhof TC, Rizo J. Structural basis for the evolutionary inactivation of Ca2+ binding to synaptotagmin 4. Nat Struct Mol Biol. 2004;11:844–849. doi: 10.1038/nsmb817. [DOI] [PubMed] [Google Scholar]
- Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. J Neurosci. 2009;29:11112–11122. doi: 10.1523/JNEUROSCI.5760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Südhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kanno E, Satoh M, Saegusa C, Yamamoto A. Synaptotagmin VII is targeted to dense-core vesicles and regulates their Ca2+-dependent exocytosis in PC12 cells. J Biol Chem. 2004;279:52677–52684. doi: 10.1074/jbc.M409241200. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Groffen AJ, Martens S, Diez Arazola R, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Borst JG, et al. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson N, Han W. Calcium-sensing beyond neurotransmitters: functions of synaptotagmins in neuroendocrine and endocrine secretion. Biosci Rep. 2009;29:245–259. doi: 10.1042/BSR20090031. [DOI] [PubMed] [Google Scholar]
- Gustavsson N, Lao Y, Maximov A, Chuang JC, Kostromina E, Repa JJ, Li C, Radda GK, Südhof TC, Han W. Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci U S A. 2008;105:3992–3997. doi: 10.1073/pnas.0711700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson N, Wei SH, Hoang DN, Lao Y, Zhang Q, Radda GK, Rorsman P, Südhof TC, Han W. Synaptotagmin-7 is a principal Ca2+ sensor for Ca2+ -induced glucagon exocytosis in pancreas. J Physiol. 2009;587:1169–1178. doi: 10.1113/jphysiol.2008.168005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Rhee JS, Maximov A, Lao Y, Mashimo T, Rosenmund C, Südhof TC. N-glycosylation is essential for vesicular targeting of synaptotagmin 1. Neuron. 2004;41:85–99. doi: 10.1016/s0896-6273(03)00820-1. [DOI] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Katz B. The release of neuronal transmitter substances. Liverpool University Press; 1969. [Google Scholar]
- Kochubey O, Schneggenburger R. Synaptotagmin increases the dynamic range of synapses by driving Ca2+-evoked release and by clamping a near-linear remaining Ca2+ sensor. Neuron. 2011;69:736–748. doi: 10.1016/j.neuron.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Lee J, Guan Z, Akbergenova Y, Littleton JT. Genetic analysis of synaptotagmin C2 domain specificity in regulating spontaneous and evoked neurotransmitter release. J Neurosci. 2013;33:187–200. doi: 10.1523/JNEUROSCI.3214-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Ullrich B, Zhang JZ, Anderson RG, Brose N, Südhof TC. Ca2+-dependent and -independent activities of neural and non-neural synaptotagmins. Nature. 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- Li J, Xiao Y, Zhou W, Wu Z, Zhang R, Xu T. Silence of Synaptotagmin VII inhibits release of dense core vesicles in PC12 cells. Sci China C Life Sci. 2009;52:1156–1163. doi: 10.1007/s11427-009-0160-y. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Stern M, Schulze K, Perin M, Bellen HJ. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca2+-activated neurotransmitter release. Cell. 1993;74:1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- Mackler JM, Reist NE. Mutations in the second C2 domain of synaptotagmin disrupt synaptic transmission at Drosophila neuromuscular junctions. J Comp Neurol. 2001;436:4–16. [PubMed] [Google Scholar]
- Mackler JM, Drummond JA, Loewen CA, Robinson IM, Reist NE. The C2B Ca2+-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature. 2002;418:340–344. doi: 10.1038/nature00846. [DOI] [PubMed] [Google Scholar]
- Maximov A, Südhof TC. Autonomous function of synaptotagmin 1 in triggering synchronous release independent of asynchronous release. Neuron. 2005;48:547–554. doi: 10.1016/j.neuron.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Maximov A, Shin O-H, Südhof TC. Synaptotagmin-12, a synaptic vesicle phosphoprotein that modulates spontaneous neurotransmitter release. J Cell Biol. 2007;176:113–124. doi: 10.1083/jcb.200607021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A, Lao Y, Li H, Chen X, Rizo J, Sorensen JB, Südhof TC. Genetic analysis of synaptotagmin-7 function in synaptic vesicle exocytosis. Proc Natl Acad Sci U S A. 2008;105:3986–3991. doi: 10.1073/pnas.0712372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiki T, Augustine GJ. Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J Neurosci. 2004;24:8542–8550. doi: 10.1523/JNEUROSCI.2545-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Melicoff E, Padgett D, Liu Y, Teich AF, Dickey BF, Lin W, Adachi R, Südhof TC. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci. 2006;26:13493–13504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Cao P, Xu W, Südhof TC. Calmodulin controls synaptic strength via presynaptic activation of calmodulin kinase II. J Neurosci. 2010;30:4132–4142. doi: 10.1523/JNEUROSCI.3129-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Bacaj T, Yang X, Zhou P, Xu W, Südhof TC. Doc2 supports spontaneous synaptic transmission by a Ca2+-independent mechanism. Neuron. 2011a;70:244–251. doi: 10.1016/j.neuron.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Kokubu Y, Südhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011b;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perin MS, Fried VA, Mignery GA, Jahn R, Südhof TC. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Schonn JS, Maximov A, Lao Y, Südhof TC, Sorensen JB. Synaptotagmin-1 and -7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proc Natl Acad Sci U S A. 2008;105:3998–4003. doi: 10.1073/pnas.0712373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia M, Ales E, Montes MA, Bonifas I, Jemal I, Lindau M, Maximov A, Südhof TC, Alvarez de Toledo G. Push-and-pull regulation of the fusion pore by synaptotagmin-7. Proc Natl Acad Sci U S A. 2010;107:19032–19037. doi: 10.1073/pnas.1014070107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin OH, Rizo J, Südhof TC. Synaptotagmin function in dense core vesicle exocytosis studied in cracked PC12 cells. Nat Neurosci. 2002;5:649–656. doi: 10.1038/nn869. [DOI] [PubMed] [Google Scholar]
- Shin OH, Xu J, Rizo J, Südhof TC. Differential but convergent functions of Ca2+ binding to synaptotagmin-1 C2 domains mediate neurotransmitter release. Proc Natl Acad Sci U S A. 2009;106:16469–74. doi: 10.1073/pnas.0908798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen JB, Fernández-Chacón R, Südhof TC, Neher E. Examining synaptotagmin 1 function in dense core vesicle exocytosis under direct control of Ca2+ J Gen Physiol. 2003;122:265–276. doi: 10.1085/jgp.200308855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF, Sullivan JM. The synaptotagmin C2A domain is part of the calcium sensor controlling fast synaptic transmission. Neuron. 2003;39:299–308. doi: 10.1016/s0896-6273(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Südhof TC. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol. 2012;4:a011353. doi: 10.1101/cshperspect.a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Shin OH, Han W, Lao Y, Südhof TC. Synaptotagmins form a hierarchy of exocytotic Ca2+ sensors with distinct Ca2+ affinities. EMBO J. 2002;21:270–280. doi: 10.1093/emboj/21.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Han W, Butz S, Liu X, Fernandez-Chacon R, Lao Y, Südhof TC. Synaptotagmin VII as a plasma membrane Ca2+ sensor in exocytosis. Neuron. 2001;30:459–473. doi: 10.1016/s0896-6273(01)00290-2. [DOI] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Südhof TC. A Dual Ca2+-Sensor Model for Neuro-transmitter Release in a Central Synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Tang J, Maximov A, Shin OH, Dai H, Rizo J, Südhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, Fukuda M. Synaptotagmin VII modulates the kinetics of dense-core vesicle exocytosis in PC12 cells. Genes Cells. 2007;12:511–519. doi: 10.1111/j.1365-2443.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, de Vries KJ, Roshol H, Burbach JP, Gispen WH, Südhof TC. DOC2 proteins in rat brain: complementary distribution and proposed function as vesicular adapter proteins in early stages of secretion. Neuron. 1997;18:453–461. doi: 10.1016/s0896-6273(00)81245-3. [DOI] [PubMed] [Google Scholar]
- Virmani T, Han W, Liu X, Südhof TC, Kavalali ET. Synaptotagmin 7 splice variants differentially regulate synaptic vesicle recycling. EMBO J. 2003;22:5347–5357. doi: 10.1093/emboj/cdg514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Poser C, Ichtchenko K, Shao X, Rizo J, Südhof TC. The evolutionary pressure to inactivate. A subclass of synaptotagmins with an amino acid substitution that abolishes Ca2+ binding. J Biol Chem. 1997;272:14314–14319. doi: 10.1074/jbc.272.22.14314. [DOI] [PubMed] [Google Scholar]
- Wen H, Linhoff MW, McGinley MJ, Li GL, Corson GM, Mandel G, Brehm P. Distinct roles for two synaptotagmin isoforms in synchronous and asynchronous transmitter release at zebrafish neuromuscular junction. Proc Natl Acad Sci U S A. 2010;107:13906–13911. doi: 10.1073/pnas.1008598107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mashimo T, Südhof TC. Synaptotagmin-1, -2, and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Xu J, Pang ZP, Shin OH, Südhof TC. Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nat Neurosci. 2009;12:759–766. doi: 10.1038/nn.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Morishita W, Buckmaster PS, Pang ZP, Malenka RC, Südhof TC. Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron. 2012;73:990–1001. doi: 10.1016/j.neuron.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Craig TK, Shin OH, Li L, Brautigam CA, Tomchick DR, Südhof TC, Rosenmund C, Rizo J. Structural and mutational analysis of functional differentiation between synaptotagmin-1 and -7. PLoS One. 2010;5:e12544. doi: 10.1371/journal.pone.0012544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Kaeser-Woo YJ, Pang ZP, Xu W, Südhof TC. Complexin clamps asynchronous release by blocking a secondary Ca2+ sensor via its accessory alpha helix. Neuron. 2010;68:907–920. doi: 10.1016/j.neuron.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Gaffaney JD, Kwon SE, Chapman ER. Doc2 is a Ca2+ sensor required for asynchronous neurotransmitter release. Cell. 2011;147:666–677. doi: 10.1016/j.cell.2011.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M, Littleton JT. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 2002;36:897–908. doi: 10.1016/s0896-6273(02)01065-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.