Abstract
Objective
To estimate and compare performance of diffusion weighted imaging (DWI) with other MR imaging techniques including T2-weighted MR imaging (T2WI) for prostate cancer (PC) detection.
Methods
The PubMed and Scopus electronic databases searches for “prostate”, “cancer”, “diffusion weighted imaging”, “magnetic resonance imaging” were completed up to December 2010. All included studies had histopathological correlation. 2 × 2 contingency data were constructed for each study. A Bayesian receiver operating characteristics (ROC) model was used across studies to determine sensitivity, specificity, and area under full or partial receiver operator curve (AUROC).
Results
Nineteen publications consisting of a total of 5,892 lesions were analysed. Based on a 95% CI, DWI alone yielded significantly better AUROC, sensitivity and specificity (0.85, 0.69, 0.89) than T2WI alone (0.75, 0.6, 0.76). DWI/T2WI (0.73, 0.7, 0.83) showed similar AUROC but significantly better sensitivity and specificity than T2WI alone. DWI and DWI/T2WI yielded similar overall sensitivity, but DWI alone showed better overall specificity than DWI/T2WI. At specificities above 80%, DWI/T2WI yielded similar (partial) AUROC (0.138) to DWI alone (0.129), and was significantly better than T2WI alone (0.07). DWI alone and DWI/T2WI appear superior to dynamic contrast enhanced imaging alone (AUROC 0.79, sensitivity 0.58, specificity 0.82).
Conclusion
DWI appears to improve diagnostic performance and can be a useful adjunct to conventional anatomic imaging for identifying tumour foci in PC.
Keywords: magnetic resonance imaging, diffusion weighted imaging, prostate, cancer
Introduction
High resolution T2-weighted imaging (T2WI) has been the mainstay of magnetic resonance imaging (MRI) in view of its tissue contrast resolution and ability to aid staging, including assessing extraprostatic extension of PC [1, 2]. In the meta-analysis by Sonnad et al, T2WI with T1WI showed a maximum joint sensitivity and specificity rate of 74% for staging of PC [3]. Engelbrecht et al [4] showed similar findings, with a joint sensitivity and specificity rate of 71%. Hence, there exists a need for better ways to diagnose prostate cancer (PC) by MRI, particularly in cases where prostate specific antigen is rising and random utrasound guided needle biopsy (TRUS) is negative.
The introduction of non-surgical local ablative techniques (such as high intensity focused ultrasound and cryotherapy [5]) and advances in radiotherapy (brachytherapy, intensity modulated and proton radiotherapy) theoretically allows for escalation of radiation dose to the dominant intraprostatic lesions without increasing dose to surrounding normal tissues [6–8]. This further increases the need for accurate delineation of PC foci by non-invasive techniques.
However, T2WI can be limited in differentiating between tumour and benign disease such as inflammatory tissue. This has led to substantial interest in MRI techniques such as diffusion weighted imaging (DWI), magnetic resonance spectroscopy (MRS), and dynamic contrast enhanced (DCE) MRI that can be performed concurrently with anatomic imaging (primarily T2WI) by MRI to improve detection of tumour foci in the local assessment of PC.
Compared to the other methodologies, DWI has the combined advantage of short acquisition time, no need for intravenous contrast, and low technical demand for image post-processing. DWI measures restriction of water diffusion in biological tissues, corresponding to properties such as cellular density, membrane permeability, and space between cells [9]. For example, the luminal space in benign human prostate tissue has been reported to average several hundreds of microns wide; whereas, in PC, water molecules diffuse over tens of microns [10]. This may be what makes it possible for DWI to distinguish malignant from benign prostate tissues.
Typically, an ultrafast echoplanar T2-weighted sequence is used for DWI; this can be performed with either an endorectal coil only, endorectal plus external phased array coils or an external phased-array coil only. Diffusion-weighted images are postprocessed to obtain apparent diffusion coefficients (ADCs). In clinical practice, this information is represented in the image form (ADC map). Analysis of the ADCs has the advantages of eliminating the effect of T2 shine-through that can be seen with DWI images, and of objectively measuring tissue diffusivity [11].
A growing number of clinical studies in the recent years have evaluated the utility of DWI either in combination with or in comparison to other MRI techniques for detection of PC and these have reported various sensitivities and specificities. However, a meta-analysis has not yet been performed and, given its widespread availability with most modern MRI scanners, is appropriate. The purpose of this meta-analysis is to estimate and compare performance of diffusion weighted imaging (DWI) with other MR imaging techniques, including T2WI, for the detection of tumour foci in the local assessment of PC.
Materials and methods
This meta-analysis was written using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement as reference [12, 13].
Literature Search
A comprehensive systematic literature review was completed up to December 2010. The PubMed and Scopus electronic databases were searched. The literature search was limited to English publications and human subjects. The following Medical Subject Headings (MeSH) terms and keywords were used in the search: “prostate”, “cancer”, “diffusion weighted imaging”, and “magnetic resonance imaging”. The abstracts of all relevant articles available on the database, prior to December 2010, were reviewed.
The inclusion criteria for articles were as follows: (a) patients with histologically proven PC, (b) the diagnostic tests included DWI, with or without other methods of imaging such as T2WI, MRS and DCE MRI, (c) the reference standard was histological diagnosis, and (d) sufficient data were reported to allow for construction of 2 × 2 contingency tables. Only papers that used histopathological results to directly reference independently derived DWI findings were included in our study; those in which the DWI findings were retrospectively obtained from prefabricated region of interest (ROI) maps using data from either histopathology or T2WI were excluded. Study design inclusion criteria were broad and included retrospective and prospective studies. Articles were excluded if they were editorials, commentaries or case reports.
Data extraction
Using the search terms, the abstracts of a total of 150 publications from the PubMed and 244 publications from the Scopus electronic databases were retrieved. The combined records identified 105 English language scientific publications after removal of duplicates. Of these, 26 articles were excluded: studies performed on extra-prostatic organs (9 publications), non-DWI techniques (11 publications), normal subjects (5 publications), ex-vivo (1 publication). Full text evaluation of the remaining 79 publications was performed. Among these, 60 publications had to be excluded for the following reasons: DWI data retrospectively obtained using T2WI, DCE MRI or histological maps as a guide to draw DWI ROI’s (41 publications), insufficient data to construct 2 × 2 tables (15 publications), TRUS biopsies based on MRI data (2 publications), local tumour stage rather than tumour location used as endpoint (1 publication), raters not blinded from results of histology (1 publication).
Nineteen publications [14–32] in total were included in the meta-analysis, and each reported one or more imaging sequences to classify prostate lesions into benign or malignant based on imaging results. Data from the 19 selected articles were extracted for information on study design, year of publication, number of patients, number of lesions studied (total numbers and numbers positive for PC), number of raters, reference standard (whole mount or step section histopathology, TRUS biopsy), imaging techniques (DWI, T2WI, DCE, MRS), field strength, type of coil (torso surface phased array, endorectal, body coil), imaging parameters (b-value), and criteria for diagnosis (e.g. hyperintensity on DWI images, hypointensity on ADC maps, ADC cut-off values).
For all the 19 studies evaluated, the number of true positive, false positive, true negative, and false negative lesions for each imaging technique had to be available for analysis. AUCROC, sensitivity, specificity and accuracy data were recorded separately. All data for which the 2×2 tables could be constructed was utilised for analysis. We did not exclude any part of the studies included in the meta-analysis. Thereby, a total of 45 raters and 5,892 lesions were included in the meta-analysis. The recorded variables for all of the included studies are presented in Table 1.
Statistical Analysis
Because the majority of the studies included in our analysis reported only sensitivity and specificity values for the modalities that they reported, we fitted a Bayesian receiver operating characteristics (ROC) model to the sensitivity and specificity values reported in the studies in order to compare sensitivity, specificity, and AUROC across studies and modalities [33]. We assumed a binormal model for the generation of the classification of lesions. In this model, a continuous latent trait is associated with the manifestation of “disease” in each image. Because this model was used to construct ROC curves based only on published sensitivity and specificity values, it was necessary to assume homogeneity of the binormal model across studies. To better evaluate the use of DWI as an imaging technique, partial AUROC for specificity more than 80% was performed, since at least this degree of specificity is desirable in clinical practice (specifically for patients with PC, to avoid unnecessary treatment).
We assumed that the latent outcome variable associated with normal lesions was drawn from a standard normal distribution, whereas the latent outcome variable associated with malignant lesions was assumed to follow a normal distribution with mean μd, and a standard deviation of SD. We further assumed that the thresholds used in each study for each threshold to determine positive and negative findings were drawn from a normal distribution with a mean of μthreshold and a standard deviation of SDthreshold.
Using a Metropolis-Hasting algorithm to perform Markov chain Monte Carlo (MCMC) sampling, we obtained the posterior distribution of the parameters specified above. This model allowed us to compute the posterior distribution on the total AUROC and partial AUROC (cut-off point based on specificity) for each image modality across the population of studies considered. Comparisons between modalities were based on 95% credible intervals (CI) for the AUROC and partial AUROC values.
Among the various studies, only a total of 4 imaging techniques could be successfully analyzed: 6 with DCE alone, 10 with DWI alone, 14 with T2WI alone, and 11 with joint T2WI / DWI (henceforth referred to as DWI/T2WI) (Table 2). Four studies reported results for each of the multiple raters. Inexperienced raters were identified by reviewing the original papers, and a sensitivity analysis was carried out to assess the effect of these raters (3 in total) on DWI/T2WI.
Table 2.
Summary of papers reviewed by modality. Papers that could be included in the meta-analysis are highlighted in bold.
| Modality | Number of Papers | Number of Raters | Number of Lesions |
|---|---|---|---|
| T2 | 14 | 18 | 3045 |
| DWI/T2 | 11 | 17 | 2230 |
| DWI | 10 | 13 | 4593 |
| DCE | 6 | 7 | 2570 |
| T2/DWI/DCE | 3 | 3 | 786 |
| DWI/MRS | 2 | 2 | 234 |
| T2/DCE | 2 | 2 | 466 |
| MRS | 1 | 1 | 408 |
| T2/DWI/MRS | 1 | 1 | 201 |
Analysis of MRS alone or in combination with DCE, DWI and T2WI could not be made due to the small number of publications and sample populations (Table 2). Due to the relatively small amount of data, further analysis of the selected studies regarding the use of DWI for the detection of disease in the central zone versus the peripheral zone; the optimal b-value, use of DWI images versus ADC maps and 3T scanners versus 1.5T scanners as factors influencing lesion detection could not be made.
Results
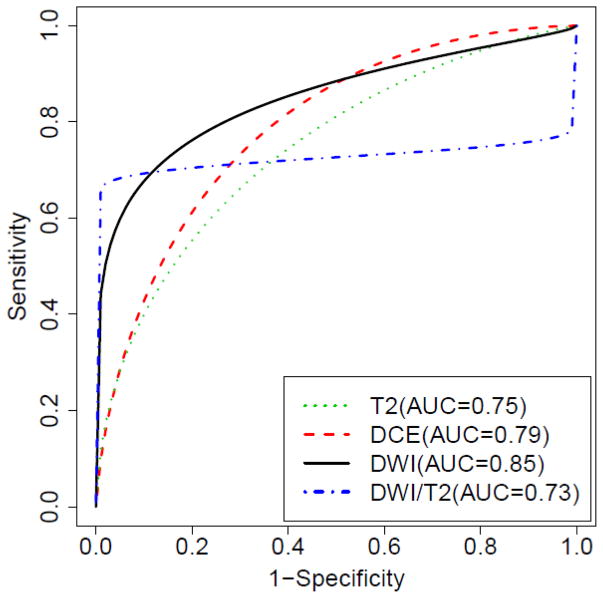
Figure 1 shows the estimated ROC curve for each methodology. Table 3 summarizes the p distributions of all of the parameters.
Figure 1.
Estimated ROC curve for each methodology (T2WI, DCE MRI, DWI and DWI/T2WI).
Table 3.
Overall and random sensitivity and specificity rates, total and partial (specificity >0.8) AUROCs for the various studies grouped by modality. Note: Overall sensitivity and specificity are the expected sensitivity and specificity for all raters, that is, an average of many raters. Therefore, rater variability is eliminated.
| Modality | Number of Studies (Raters) | Parameter | Posterior Mean | Posterior 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| DCE | 6(7) | AUROC | 0.79 | 0.75 | 0.83 |
| Partial AUROC (Spec>0.8) | 0.08 | 0.07 | 0.09 | ||
| Overall sensitivity | 0.58 | 0.53 | 0.62 | ||
| Overall specificity | 0.82 | 0.80 | 0.85 | ||
| T2WI | 14(18) | AUROC | 0.75 | 0.71 | 0.77 |
| Partial AUROC (Spec>0.8) | 0.07 | 0.06 | 0.09 | ||
| Overall sensitivity | 0.60 | 0.57 | 0.62 | ||
| Overall specificity | 0.76 | 0.74 | 0.78 | ||
| DWI | 10(13) | AUROC | 0.85 | 0.82 | 0.87 |
| Partial AUROC (Spec>0.8) | 0.129 | 0.121 | 0.136 | ||
| Overall sensitivity | 0.69 | 0.67 | 0.72 | ||
| Overall specificity | 0.89 | 0.87 | 0.90 | ||
| DWI/T2WI | 11(17) | AUROC | 0.73 | 0.71 | 0.75 |
| Partial AUROC (Spec>0.8) | 0.138 | 0.134 | 0.140 | ||
| Overall sensitivity | 0.70 | 0.69 | 0.72 | ||
| Overall specificity | 0.83 | 0.80 | 0.85 | ||
T2WI vs DWI and DWI/T2WI
Based on a posterior 95% confidence interval (CI), the AUROC for T2WI alone is significantly less than DWI alone, but is not significantly different for joint DWI/T2WI (T2WI: 0.71–0.77; DWI: 0.82–0.87, DWI/T2WI: 0.71–0.75). The overall sensitivity, specificity and partial AUROC (specificities above 80%) of DWI alone and of DWI/T2WI are superior to T2WI alone (sensitivity T2WI: 0.57–0.62, DWI: 0.67–0.72, DWI/T2WI: 0.69–0.72; specificity T2WI: 0.74–0.78, DWI: 0.87–0.90, DWI/T2WI: 0.80–0.85; partial AUROC T2WI: 0.06–0.09, DWI: 0.121–0.136, DWI/T2WI: 0.134–0.140).
DWI vs DWI/T2WI
At extremely low specificity (the right part of the ROC graphs), the unusual shape of the ROC curves for DWI/T2 (all raters) and DWI/T2 (experienced raters) is due to the fact that the 80% sensitivity was relatively constant and little data was available to very low specificity, therefore, the behaviour at extreme values was uncertain.
The overall AUROC for DWI/T2WI is significantly lower than DWI alone. However, when comparing at specificities above 80%, DWI/T2WI yielded similar partial AUROC to DWI alone.
DCE vs T2WI, DWI and DWI/T2WI
Based on the studies, AUROC (0.75–0.83) and overall sensitivity (0.53–0.62) for DCE were not significantly different from T2WI, even though there is increased specificity (DCE: 0.80–0.85). In contrast, DCE was less sensitive than DWI or DWI/T2WI and less specific than DWI; however, the specificity is not significantly different from DWI/T2WI. The partial AUROC of DCE (0.07–0.09) is not significantly different from T2WI, but is significantly less than DWI and DWI/T2WI.
Discussion
DWI for imaging of the prostate is commonplace in most institutions. An understanding of the utility of this sequence is important since it is being used to affect clinical care. A number of clinical studies have evaluated the utility of DWI alone or in combination with other sequences for the assessment of prostate cancers with the majority of the scientific articles published in the last five years. Hence it is both important and appropriate to validate the efficacy of DWI in PC at this point in time.
Our meta-analysis has revealed several important findings that pertain to clinical use of DWI for detection of PC. Firstly, DWI alone yields significantly better AUROC than T2WI alone. This is in line with various published studies at both 1.5T [6, 19, 21, 26, 34, 35] and at 3T [14, 20, 36] that demonstrate both increased sensitivity and specificity of DWI. This appears to represent an inherent advantage of DWI over conventional anatomic imaging alone, consistent with DWI assessing parameters different from T2WI.
Secondly, at a defined specificity greater than 80% (partial AUROC), DWI/T2WI is superior to T2WI alone and DCE alone (Table 3), but is similar to DWI alone. This suggests that to achieve high specificity, DWI should be added as an adjunct to T2WI. Currently T2WI is the principal sequence used for evaluation of extraprostatic involvement in clinical practice.
Thirdly, the total AUROC of DWI/T2WI appears to be worse than DWI alone. A review of the publications indicate the only two comprised direct comparison between DWI and DWI/T2WI [19, 24]. In both studies, the sensitivity of combining DWI with T2WI were higher than that of DWI, even though the specificity rates did not improve. More studies performing such head-to-head experiments are needed.
As the data extraction for this meta-analysis was focused on DWI, results assessing T2WI and DCE are not fully inclusive and need to be considered in relation to the results of DWI for meaningful analysis. Based on our meta-analysis, DCE alone appears to show specificity similar to DWI/T2WI, but is less specific than DWI alone. It is also less sensitive than both DWI alone and DWI/T2WI. This suggests that DWI outperforms DCE. This also raises the question of whether adding more parameters, such as DCE, to multiparametric imaging already including DWI and T2WI, increases diagnostic performance.
Limitations of the Literature
Technical parameters
As the number of publications that met the inclusion criteria was relatively small, there was insufficient data to compare several important DWI parameters, namely: magnet field strength (1.5T vs 3T), type of coil (endorectal vs surface phased array) and b-value. As many as 9 out of 19 studies were performed using a surface phased array coil on a 1.5T scanner, a condition that would be considered suboptimal to the use of endorectal coil imaging [37–39]. Of 19 studies included in the meta-analysis, 5 studies used endorectal coil for imaging on 1.5T scanners [19, 23, 24, 27, 40]; another 5 studies used surface phased array coils for imaging on 3T scanners [14, 17, 20, 25, 32]. While the magnet field strength (1.5T vs 3T) and type of coil are subject to the resources of each institution, the b-value parameter is not. Even so, there has been no consensus on the optimal b-value to date, and the b-value used varies greatly among studies (Table 1). Although higher b-values have been shown to provide better characterisation of PC and treatment response in experimental mouse models [41], they also lead to increased motion and susceptibility artefacts, as well as decreased signal to noise ratio [42]. Studies directly comparing the results of DWI using different technical parameters are needed.
Methods of interpretation
We excluded studies which retrospectively analysed the ADC value of lesions using ROIs drawn from other diagnostic tests (T2WI, DCE MRI or histological maps) because these methods could skew the data and do not allow for proper evaluation of DWI as a diagnostic tool. While this meant that we had to exclude 60 out of 79 studies that were initially considered for full-text evaluation, this allowed us to better analyze the true clinical value of DWI as an imaging test for PC detection. The selection process was thus designed to remove bias and create a more robust data set.
In terms of lesion diagnosis, more than half of the studies utilized ADC maps for interpretation (13 out of 19) either alone or in combination with DWI images, while the remainder relied on DWI images alone. In our experience with prostate DWI, use of ADC maps can be more easily interpreted than DWI images. This is because both tumour and normal tissue can be T2-hyperintense on DWI due to T2 shine-through effects whereas T2 shine-through effects are removed with ADC maps; hence tumour will appear hypointense compared to normal prostatic tissue.
A limitation in the evaluation of the publications pertained to joint DWI/T2WI; it was not explicitly mentioned in most studies what constituted a positive finding, that is, whether DWI detects disease in areas of normal T2 signal or whether it increases diagnostic confidence in areas with low T2 signal. The increased sensitivity of DWI alone compared to T2WI suggests that it does detect disease in areas of T2 signal considered normal when read in isolation. Likely, when read together, diagnostic confidence can also be increased. The results of the meta-analysis seems to suggest that DWI alone is superior to combined T2WI/DWI. However, because only one study [24] directly compared between them, we were not able to come to such a conclusion reasonably.
Reference standard for histological assessment
In 12 of the 19 studies included in this meta-analysis, only TRUS biopsy results were used to correlate with imaging findings (Table 1). Needle biopsies can result in sampling error and false negatives; if taken to be the reference standard, these may result in under-estimation of the sensitivities of the imaging modalities. Furthermore, a per nodule analysis, which requires direct histopathological correlation in all cases with step-section or whole mount techniques, would be the optimal approach. This was performed in 7 of the included studies (including one study that used either TRUS with step section histopathology to correlate with imaging). However, such an approach can be both cost- and labour-intensive, hence routine application of these techniques may not be possible in many studies. Indeed, if we had restricted to only these gold standards, only 6 studies would have qualified, yielding very few studies for a meta-analysis. Such studies that used step-section or whole mount techniques as reference standard revealed findings similar to the overall findings in our meta-analysis: (1) DWI is more specific but less sensitive than DWI/T2WI [19]; (2) DWI/T2 is more sensitive and specific than T2WI [15, 19, 21, 24, 28, 29]; and (3) DWI is more specific than DCE [27] while combined DWI/T2WI is more sensitive and specific than DCE/T2WI [28].
Study end-points
In this meta-analysis, outcomes considered were detection of tumour foci; evaluating the use of DWI for assessing extracapsular extension was not an aim of the majority of the studies. Traditionally, anatomic delineation with T2WI has been considered to be the optimal technique for the local extent of disease. Given the lower spatial resolution of DWI compared to T2WI, it may theoretically fare poorer in assessment for extracapsular extension. In comparison, literature suggests that invasion of other structures such as seminal vesicles [36, 43] and bladder [44] can be detected better on DWI/T2WI than T2WI alone this requires further study. The results of this meta-analysis cannot be used to suggest that DWI is sufficient to replace T2WI and be read in isolation for PC staging assessment.
Comparison of various imaging techniques: DWI, DCE, MRS
As multiparametric MRI assessment of the prostate becomes more widely available, comparison of the use of DWI against or joint with other MRI imaging techniques (such as DCE MRI and MRS) needs to be studied in order to address the issue of MRI protocol optimization.
Based on our meta-analysis, it would appear that DCE MRI is inferior to DWI (either alone or in combination with T2WI), in terms of overall sensitivity and partial AUROC. However, the data did not allow for additional comparisons, such as between MRS and joint T2WI/DCE MRI. To achieve adequate comparison between techniques, it is necessary to include studies that evaluate DCE or MRS without DWI, however, this would be beyond the scope of the current review. The role of multiparametric MRI could not be fully addressed in this study due to the small number studies combining the various imaging techniques. Potentially, more may not be better because there may not be significant test characteristics benefit of three-parameter (DWI, DCE and T2WI) over two-parameter (DWI/T2WI) analysis, as suggested in the recent study by Vilanova et al [45], and because of additional study time and cost.
Potential for future research
Potentially important uses of DWI, such as, improving diagnosis of PC in the central zone and in post-treatment states, could not be evaluated in the meta-analysis due to limited data. A few individual studies suggest that there may be a role. Shimofusa et al found that DWI detected tumour in the central zone in 5 out of 8 (63%) patients, compared with one out of 8 (13%) using T2WI [21]. In patients who underwent high intensity focused ultrasound for PC, DWI/T2WI has been shown to be more specific than DCE (74%–78% vs 63%–68%) in detecting post-treatment tumour progression, although sensitivity may be lower (63–70% vs 80–87%) [14]. Given the relatively longer survival times in PC as compared to other common adult cancers, and the acceptable practice of watchful waiting in patients with early PC and greater than 10 year life expectancy, there is a constant need to avoid over-diagnosis of PC. Currently, how the results of DWI impacts the overall management of PC, for example, in terms of patient morbidity and survival, remains unanswered.
In conclusion, the results of this meta-analysis suggests that DWI is a useful adjunct to conventional anatomic imaging with T2WI, for detecting cancer within the prostate gland.
Table 1. List of papers selected for meta-analysis and the various data recorded. (*=consensus, TRUS = transrectal ultrasound biopsy, ER = endorectal coil, PA = torso phased array coil, ADC = apparent diffusion coefficient, WM = whole mount histopathology, SS = step-section histopathology)
Footnotes
The authors would like to declare that there are no conflicts of interest whilst adhering to ethical standards.
References
- 1.Mullerad M, Hricak H, Kuroiwa K, et al. Comparison of endorectal magnetic resonance imaging, guided prostate biopsy and digital rectal examination in the preoperative anatomical localization of prostate cancer. J Urol. 2005;174:2158–2163. doi: 10.1097/01.ju.0000181224.95276.82. [DOI] [PubMed] [Google Scholar]
- 2.Kundra V, Silverman PM, Matin SF, Choi H. Imaging in oncology from the University of Texas M. D. Anderson Cancer Center: diagnosis, staging, and surveillance of prostate cancer. AJR Am J Roentgenol. 2007;189:830–844. doi: 10.2214/AJR.07.2011. [DOI] [PubMed] [Google Scholar]
- 3.Sonnad SS, Langlotz CP, Schwartz JS. Accuracy of MR imaging for staging prostate cancer: a meta-analysis to examine the effect of technologic change. Acad Radiol. 2001;8:149–157. doi: 10.1016/s1076-6332(01)90095-9. [DOI] [PubMed] [Google Scholar]
- 4.Engelbrecht MR, Jager GJ, Laheij RJ, Verbeek AL, van Lier HJ, Barentsz JO. Local staging of prostate cancer using magnetic resonance imaging: a meta-analysis. Eur Radiol. 2002;12:2294–2302. doi: 10.1007/s00330-002-1389-z. [DOI] [PubMed] [Google Scholar]
- 5.Huang WC, Lee CL, Eastham JA. Locally ablative therapies for primary radiation failures: a review and critical assessment of the efficacy. Curr Urol Rep. 2007;8:217–223. doi: 10.1007/s11934-007-0009-5. [DOI] [PubMed] [Google Scholar]
- 6.Kajihara H, Hayashida Y, Murakami R, et al. Usefulness of diffusion-weighted imaging in the localization of prostate cancer. Int J Radiat Oncol Biol Phys. 2009;74:399–403. doi: 10.1016/j.ijrobp.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Pickett B, Vigneault E, Kurhanewicz J, Verhey L, Roach M. Static field intensity modulation to treat a dominant intra-prostatic lesion to 90 Gy compared to seven field 3-dimensional radiotherapy. Int J Radiat Oncol Biol Phys. 1999;44:921–929. doi: 10.1016/s0360-3016(98)00502-1. [DOI] [PubMed] [Google Scholar]
- 8.Pouliot J, Kim Y, Lessard E, Hsu IC, Vigneron DB, Kurhanewicz J. Inverse planning for HDR prostate brachytherapy used to boost dominant intraprostatic lesions defined by magnetic resonance spectroscopy imaging. Int J Radiat Oncol Biol Phys. 2004;59:1196–1207. doi: 10.1016/j.ijrobp.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 9.Padhani AR, Liu G, Koh DM, et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Humphrey PA, Kibel AS, et al. Magnetic resonance diffusion characteristics of histologically defined prostate cancer in humans. Magn Reson Med. 2009;61:842–850. doi: 10.1002/mrm.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provenzale JM, Engelter ST, Petrella JR, Smith JS, MacFall JR. Use of MR exponential diffusion-weighted images to eradicate T2 “shine-through” effect. AJR Am J Roentgenol. 1999;172:537–539. doi: 10.2214/ajr.172.2.9930819. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CK, Park BK, Park W, Kim SS. Prostate MR imaging at 3T using a phased-arrayed coil in predicting locally recurrent prostate cancer after radiation therapy: preliminary experience. Abdom Imaging. 2010;35:246–252. doi: 10.1007/s00261-008-9495-2. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimitsu K, Kiyoshima K, Irie H, et al. Usefulness of apparent diffusion coefficient map in diagnosing prostate carcinoma: correlation with stepwise histopathology. J Magn Reson Imaging. 2008;27:132–139. doi: 10.1002/jmri.21181. [DOI] [PubMed] [Google Scholar]
- 16.Chen M, Dang HD, Wang JY, et al. Prostate cancer detection: comparison of T2-weighted imaging, diffusion-weighted imaging, proton magnetic resonance spectroscopic imaging, and the three techniques combined. Acta Radiol. 2008;49:602–610. doi: 10.1080/02841850802004983. [DOI] [PubMed] [Google Scholar]
- 17.Kim CK, Park BK, Lee HM, Kim SS, Kim E. MRI techniques for prediction of local tumor progression after high-intensity focused ultrasonic ablation of prostate cancer. AJR Am J Roentgenol. 2008;190:1180–1186. doi: 10.2214/AJR.07.2924. [DOI] [PubMed] [Google Scholar]
- 18.Tamada T, Sone T, Jo Y, et al. Prostate cancer: relationships between postbiopsy hemorrhage and tumor detectability at MR diagnosis. Radiology. 2008;248:531–539. doi: 10.1148/radiol.2482070157. [DOI] [PubMed] [Google Scholar]
- 19.Haider MA, van der Kwast TH, Tanguay J, et al. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol. 2007;189:323–328. doi: 10.2214/AJR.07.2211. [DOI] [PubMed] [Google Scholar]
- 20.Miao H, Fukatsu H, Ishigaki T. Prostate cancer detection with 3-T MRI: comparison of diffusion-weighted and T2-weighted imaging. Eur J Radiol. 2007;61:297–302. doi: 10.1016/j.ejrad.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Shimofusa R, Fujimoto H, Akamata H, et al. Diffusion-weighted imaging of prostate cancer. J Comput Assist Tomogr. 2005;29:149–153. doi: 10.1097/01.rct.0000156396.13522.f2. [DOI] [PubMed] [Google Scholar]
- 22.Portalez D, Rollin G, Leandri P, et al. Prospective comparison of T2w-MRI and dynamic-contrast-enhanced MRI, 3D-MR spectroscopic imaging or diffusion-weighted MRI in repeat TRUS-guided biopsies. Eur Radiol. 2010;20:2781–2790. doi: 10.1007/s00330-010-1868-6. [DOI] [PubMed] [Google Scholar]
- 23.Kumar V, Jagannathan NR, Kumar R, et al. Correlation between metabolite ratios and ADC values of prostate in men with increased PSA level. Magn Reson Imaging. 2006;24:541–548. doi: 10.1016/j.mri.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Lim HK, Kim JK, Kim KA, Kyoung S. Prostate cancer: Apparent diffusion coefficient map with T2-weighted images for detection-a multireader study. Radiology. 2009;250:145–151. doi: 10.1148/radiol.2501080207. [DOI] [PubMed] [Google Scholar]
- 25.Kitajima K, Kaji Y, Fukabori Y, Yoshida Ki, Suganuma N, Sugimura K. Prostate cancer detection with 3 T MRI: Comparison of diffusion-weighted imaging and dynamic contrast-enhanced MRI in combination with T2-weighted imaging. Journal of Magnetic Resonance Imaging. 2010;31:625–631. doi: 10.1002/jmri.22075. [DOI] [PubMed] [Google Scholar]
- 26.Kim CK, Park BK, Lee HM. Prediction of locally recurrent prostate cancer after radiation therapy: incremental value of 3T diffusion-weighted MRI. J Magn Reson Imaging. 2009;29:391–397. doi: 10.1002/jmri.21645. [DOI] [PubMed] [Google Scholar]
- 27.Kozlowski P, Chang SD, Jones EC, Berean KW, Chen H, Goldenberg SL. Combined diffusion-weighted and dynamic contrast-enhanced MRI for prostate cancer diagnosis--correlation with biopsy and histopathology. J Magn Reson Imaging. 2006;24:108–113. doi: 10.1002/jmri.20626. [DOI] [PubMed] [Google Scholar]
- 28.Yoshizako T, Wada A, Hayashi T, et al. Usefulness of diffusion-weighted imaging and dynamic contrast-enhanced magnetic resonance imaging in the diagnosis of prostate transition-zone cancer. Acta Radiologica. 2008;49:1207–1213. doi: 10.1080/02841850802508959. [DOI] [PubMed] [Google Scholar]
- 29.Jeong IG, Kim JK, Cho KS, et al. Diffusion-weighted magnetic resonance imaging in patients with unilateral prostate cancer on extended prostate biopsy: predictive accuracy of laterality and implications for hemi-ablative therapy. J Urol. 2010;184:1963–1969. doi: 10.1016/j.juro.2010.06.136. [DOI] [PubMed] [Google Scholar]
- 30.Iwazawa J, Mitani T, Sassa S, Ohue S. Prostate cancer detection with MRI: is dynamic contrast-enhanced imaging necessary in addition to diffusion-weighted imaging? Diagn Interv Radiol. 2010 doi: 10.4261/1305-3825.DIR.3605-10.1. [DOI] [PubMed] [Google Scholar]
- 31.Arumainayagam N, Kumaar S, Ahmed HU, et al. Accuracy of multiparametric magnetic resonance imaging in detecting recurrent prostate cancer after radiotherapy. BJU Int. 2010;106:991–997. doi: 10.1111/j.1464-410X.2010.09291.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim CK, Park BK, Kim B. High-b-value diffusion-weighted imaging at 3 T to detect prostate cancer: comparisons between b values of 1,000 and 2,000 s/mm2. AJR Am J Roentgenol. 2010;194:W33–37. doi: 10.2214/AJR.09.3004. [DOI] [PubMed] [Google Scholar]
- 33.Johnson VE, Albert JH. Ordinal Data Models. Springer-Verlag; 1999. [Google Scholar]
- 34.Tanimoto A, Nakashima J, Kohno H, Shinmoto H, Kuribayashi S. Prostate cancer screening: the clinical value of diffusion-weighted imaging and dynamic MR imaging in combination with T2-weighted imaging. J Magn Reson Imaging. 2007;25:146–152. doi: 10.1002/jmri.20793. [DOI] [PubMed] [Google Scholar]
- 35.Morgan VA, Kyriazi S, Ashley SE, DeSouza NM. Evaluation of the potential of diffusion-weighted imaging in prostate cancer detection. Acta Radiol. 2007;48:695–703. doi: 10.1080/02841850701349257. [DOI] [PubMed] [Google Scholar]
- 36.Ren J, Huan Y, Wang H, et al. Seminal vesicle invasion in prostate cancer: prediction with combined T2-weighted and diffusion-weighted MR imaging. Eur Radiol. 2009;19:2481–2486. doi: 10.1007/s00330-009-1428-0. [DOI] [PubMed] [Google Scholar]
- 37.Heijmink SW, Futterer JJ, Hambrock T, et al. Prostate cancer: body-array versus endorectal coil MR imaging at 3 T--comparison of image quality, localization, and staging performance. Radiology. 2007;244:184–195. doi: 10.1148/radiol.2441060425. [DOI] [PubMed] [Google Scholar]
- 38.Beyersdorff D, Taymoorian K, Knosel T, et al. MRI of prostate cancer at 1.5 and 3.0 T: comparison of image quality in tumor detection and staging. AJR Am J Roentgenol. 2005;185:1214–1220. doi: 10.2214/AJR.04.1584. [DOI] [PubMed] [Google Scholar]
- 39.Wefer AE, Hricak H, Vigneron DB, et al. Sextant localization of prostate cancer: comparison of sextant biopsy, magnetic resonance imaging and magnetic resonance spectroscopic imaging with step section histology. J Urol. 2000;164:400–404. [PubMed] [Google Scholar]
- 40.Reinsberg SA, Payne GS, Riches SF, et al. Combined use of diffusion-weighted MRI and 1H MR spectroscopy to increase accuracy in prostate cancer detection. AJR Am J Roentgenol. 2007;188:91–98. doi: 10.2214/AJR.05.2198. [DOI] [PubMed] [Google Scholar]
- 41.Roth Y, Tichler T, Kostenich G, et al. High-b-value diffusion-weighted MR imaging for pretreatment prediction and early monitoring of tumor response to therapy in mice. Radiology. 2004;232:685–692. doi: 10.1148/radiol.2322030778. [DOI] [PubMed] [Google Scholar]
- 42.Naganawa S, Kawai H, Fukatsu H, et al. Diffusion-weighted imaging of the liver: technical challenges and prospects for the future. Magn Reson Med Sci. 2005;4:175–186. doi: 10.2463/mrms.4.175. [DOI] [PubMed] [Google Scholar]
- 43.Kim CK, Choi D, Park BK, Kwon GY, Lim HK. Diffusion-weighted MR imaging for the evaluation of seminal vesicle invasion in prostate cancer: initial results. J Magn Reson Imaging. 2008;28:963–969. doi: 10.1002/jmri.21531. [DOI] [PubMed] [Google Scholar]
- 44.Ren J, Huan Y, Li F, et al. Combined T2-weighted and diffusion-weighted MRI for diagnosis of urinary bladder invasion in patients with prostate carcinoma. J Magn Reson Imaging. 2009;30:351–356. doi: 10.1002/jmri.21727. [DOI] [PubMed] [Google Scholar]
- 45.Vilanova JC, Barcelo-Vidal C, Comet J, et al. Usefulness of Prebiopsy Multifunctional and Morphologic MRI Combined With Free-to-Total Prostate-Specific Antigen Ratio in the Detection of Prostate Cancer. AJR Am J Roentgenol. 2011;196:W715–722. doi: 10.2214/AJR.10.5700. [DOI] [PubMed] [Google Scholar]