Abstract
For antigen recognition, lampreys use leucine-rich repeats (LRR) instead of immunoglobulin V-(D)-J domains to generate variable lymphocyte receptors (VLR) of three types, VLRA, VLRB, and VLRC. VLRB-bearing lymphocytes respond to immunization with proliferation and differentiation into plasmacytes that secrete multivalent VLRB antibodies. Here we immunized lampreys with B cells from patients with chronic lymphocytic leukemia (CLL) to generate recombinant monoclonal VLRB antibodies, one of which, VLR39, was specific for the donor CLL cells. The target epitope of VLR39 was shown to be the complementarity determining region 3 (CDR3) of the heavy chain variable region (VH) of the B cell receptor. Using this antibody to monitor the CLL donor after chemo-immunotherapy-induced remission, we detected VLR39+ B cells in the patient 51 months later, before significant increase in lymphocyte count or CD5+ B cells. This indication of reemergence of the leukemic clone was verified by VH sequencing. Lamprey antibodies can exhibit exquisite specificity for a protein epitope, a CLL signature VH CDR3 sequence in this case, and offer a rapid strategy for generating anti-idiotype antibodies for early detection of leukemia recurrence.
Keywords: chronic lymphocytic leukemia, lamprey antibodies, tumor cell markers, idiotype, B cell receptor
Introduction
The adaptive immune system of the extant jawless vertebrates, lamprey and hagfish, offers an alternative to monoclonal antibody technology as a method to produce specific binding molecules. Instead of immunoglobulin (Ig)-based antigen receptors, lampreys and hagfish use variable lymphocyte receptors (VLR) comprised of leucine-rich repeat (LRR) modules for antigen binding (1, 2). Similar to the toll-like receptors and many other LRR proteins, the VLR proteins form a curved solenoid structure (3). Antigen binding occurs on the concave surface formed by the parallel β-strands of the “LxxLxLx” motifs in the variable LRR subunits; an important additional contact is contributed by an extended loop of highly variable sequence in the C-terminal LRR (4–8).
Lampreys have three lymphocyte lineages, each of which expresses a different VLR type. The VLRA+ and VLRC+ lymphocytes are T-like cells that exclusively express their VLRs as cell surface molecules (9, 10), whereas VLRB+ lymphocytes resemble mammalian B cells in that they express their antigen receptors on the cell surface, respond to immunization with proliferation, and differentiate into plasma cells that secrete disulfide-linked multimeric VLRB antibodies (4, 11). The VLRB genes are somatically assembled in lamprey B-like cells by a gene conversion-like process in which sequences from flanking LRR gene cassettes are randomly and sequentially incorporated in a piece-wise fashion into an incomplete germline VLRB gene to generate a potential repertoire of >1015 unique VLRB genes (1, 2, 12, 13). Allelic exclusion ensures that each individual lymphocyte assembles and expresses a unique VLRB gene (1, 13, 14). Recombinant monoclonal VLRB antibodies can be produced by making a VLRB cDNA library from immunized lampreys, expressing the derivative VLRB clones in a secretory cell line, and selecting VLRB antibodies based on antigen-specificity and affinity (4, 7, 15, 16). The single chain polypeptide nature of the VLR protein should make them more amenable for molecular engineering compared to Ig-based antibodies, which require the assembly of complementary heavy and light chains (4, 17).
To determine the feasibility of producing lamprey VLRB antibodies with tumor cell specificity, lampreys were immunized with cells from a patient with B cell chronic lymphocytic leukemia (CLL) and derivative VLRB clones were screened for CLL-specificity. Among the lamprey VLRB antibodies produced against human mononuclear blood cells (MNCs), we identified a monoclonal VLRB antibody, VLR39, which preferentially recognized the donor CLL clone. Here we describe the B cell receptor (BCR) idiotope-specificity of VLR39 and the potential use of this anti-idiotype antibody as a monitoring reagent for early detection of CLL recurrence.
Material and Methods
Cells and Cell Lines
Blood samples were obtained with informed consent from CLL patients and healthy adults in studies approved by the Institutional Review Boards of Emory University (Atlanta, GA), the University of Alabama at Birmingham (UAB) (Birmingham, AL), and the North Shore–LIJ Health System (Manhasset, NY), in accordance with the Declaration of Helsinki. MNCs isolated from whole blood by density gradient centrifugation using Lymphocyte Separation Media (Mediatech) were examined immediately or cryopreserved at −150°C in Fetal Bovine Serum (FBS) supplemented with 10% DMSO. HEK-293T cells (generously provided by Dr. Tim Townes, UAB) were maintained in DMEM supplemented with 5% FBS at 37°C in 5% CO2. B cell lines were maintained in RPMI 1640 media supplemented with 10% FBS at 37°C in 5% CO2. The EBV-transformed B cells were kind gifts from Dr. Lou Justement (UAB). The B cell phenotypes of the 697, Daudi, Ramos, and SU-DHL-6 cells were verified by flow cytometry.
Antibodies and flow cytometry
The 4C4 mouse IgG2b/κ monoclonal antibody (mAb) against the invariant VLRB stalk region was described previously (11). R-phycoerythrin (RPE)-conjugated goat anti-mouse IgG polyclonal antibodies (Southern Biotech) were used for detection of 4C4. The 8A5 VLRB-specific mouse IgG1/κ mAb was generated by immunization with full-length VLRB protein, labeled with Alexa Fluor 488 Protein Labeling Kit (Invitrogen), and recognizes 80% of VLRB clones. Mouse anti-human antibodies CD5-FITC, CD5-APC, and CD19-PE, propidium iodide and 7-AAD were from BD Biosciences, and CD19-PECy7 was from Southern Biotech.
Animal maintenance and immunization
Two lamprey larvae, maintained as described (2), received intracoelomic injections of 1×107 blood MNCs from a newly diagnosed CLL patient on days 0, 14 and 28, before sacrifice on day 42 for collection of buffy coat and plasma (2). All experiments were approved by Institutional Animal Care and Use Committee at UAB.
VLRB cDNA library construction, recombinant VLRB expression, and screening for CLL reactivity
Buffy coat leukocytes from an immunized lamprey with the highest titer of donor CLL-reactive VLRB antibodies were used to construct a VLRB cDNA library; individual clones were transfected into HEK-293T cells to obtain recombinant VLRB antibodies (4). To assess the antigen-specificity of the monoclonal VLRB antibodies, MNCs from CLL patients and healthy adults were sequentially incubated with VLRB transfectant supernatants, followed by 4C4 anti-VLRB mAb, and then RPE-conjugated goat anti-mouse IgG polyclonal antibodies, before blocking with 5% mouse serum and staining for CD5 and CD19. Cells were washed in PBS between incubation steps and propidium iodide added before the cells were analyzed by flow cytometry.
Purification of monoclonal VLR39 antibody
Purified VLRB proteins were obtained by PCR cloning of the VLR39 cDNA into a pIRES-puro-2 vector (Clontech) modified to introduce a hexahistidine-tag between the lamprey VLRB signal peptide and LRRNT before transfection into HEK-293T cell lines. Stable transfectants were selected with 2 μg/ml puromycin (Sigma), and recombinant VLRB protein was purified from supernatant via the hexahistidine tag using Ni-NTA agarose (Qiagen). Briefly, 1/10 volume of a 10× binding buffer (500 mM NaH2PO4, pH 8.0, 1.5M NaCl, 100 mM imidazole) was added to the transfectant supernatant before passage over the Ni-NTA agarose column, which was washed with 5 column volumes of wash buffer (50 mM NaH2PO4, pH 8.0, 300 mM NaCl, 20 mM imidazole, 0.05% Tween-20) before elution with 50 mM NaH2PO4, pH 8.0, 300 mM NaCl, 250 mM imidazole, 0.05% Tween-20. The eluate was concentrated by centrifugation using an Amicon Ultra-15 30K Centrifugal Filter Device (Millipore).
Cloning, expression and sequence analysis of CLL donor BCR
RNA from the CLL donor MNCs was converted into cDNA, and expressed Ig heavy chain and light chain variable (VH/VL) regions were sequenced and analyzed as previously described (18, 19). The amplified VH and VL regions from the CLL donor were cloned and expressed as recombinant IgG1 antibodies (20), then papain-treated to yield Fab fragments for use in ELISA analysis of VLR39 binding. RT-PCR reactions, primer sequences, cloning strategy, expression vectors, antibody expression, and purification were as previously described (20, 21). A single-chain variable fragment (scFv) of the CLL donor BCR was constructed by amplifying the VH and VL regions from the recombinant CLL donor IgG1 mAb expression plasmid utilizing overlap-extension PCR with KOD Hot Start DNA polymerase (Novagen) using a published protocol (22). Primer and PCR cycle profile are detailed in Supplementary Materials and Methods. The CLL donor scFv plasmid was transfected into HEK-293T cells using PEI as described (4). Transfected cells were dissociated from culture plates using Cellstripper (Mediatech) and harvested 48 to 72 h post-transfection. Cell surface expression of CLL donor scFv was verified by flow cytometric analysis for myc-tag expression using Anti-Myc Tag, clone 4A6, Alexa Fluor 488 conjugate (Millipore). Primer and PCR cycle profile for constructing the two scFv mutants are described in Supplementary Materials and Methods.
Analysis of VLR39 specificity
For ELISA, 96-Well EIA/RIA Plates (Corning) were coated overnight at 4°C with 5 μg/ml of recombinant Fab fragments either from the donor CLL or from another CLL with unmatched V genes. Non-specific binding was blocked with TBS-T/1% milk before incubation with between 1:20 and 1:20480 PBS dilution of 0.5 mg/ml purified VLR39 protein, which was detected with 4C4 anti-VLRB mAb followed by alkaline phosphatase-conjugated goat anti-mouse IgG polyclonal antibodies (Southern Biotech). For flow cytometric analysis, MNCs from CLL patients and healthy adults were sequentially incubated with 0.04 mg/ml purified VLR39 protein, followed by Alexa Fluor 488-conjugated 8A5 anti-VLRB mAb, and then CD5, CD19, and 7-AAD staining; cells were washed between each incubation step.
Results and Discussion
Identification of a donor CLL-specific monoclonal VLRB antibody
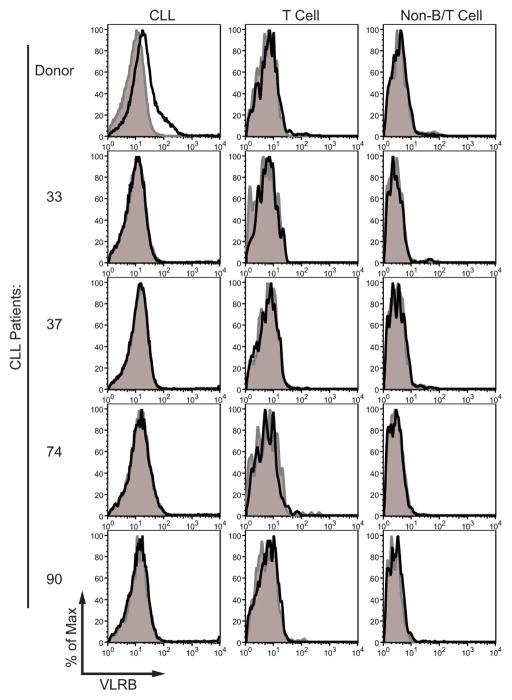
Individual clones selected randomly from the CLL-immunized VLRB cDNA library were transfected into HEK-293T cells, and the transfectant supernatants were assessed by western blot analysis for the presence of secreted VLRB antibodies. Fourteen of 36 transfectants secreted VLRB that was detectable by western blot analysis and these supernatants were analyzed by immunofluorescence for binding to viable donor CLL cells. One monoclonal VLRB antibody, VLR39, reacted with the donor CLL cells and not those from other CLL patients (Figure 1), lymphocytes or other types of blood cells from healthy adults, or with B cell leukemia/lymphoma lines (Figure S1). The low MFI of the VLR39 staining for the donor CLL could be due to the relatively inefficient expression and secretion of VLR into the supernatant by transfected HEK-293T cells. To address this issue, the VLR39 supernatant was pooled, and the purified protein was used for subsequent analyses. Staining with purified VLR39 protein, rather than with supernatant from transiently transfected HEK-293T cells, improved the staining intensity of the donor cells in subsequent experiments.
Figure 1. Flow cytometric analysis of monoclonal VLR39 reactivity.
VLR39 binding (black line) was compared with that of control VLR4 having Bacillus anthracis BclA-specificity (gray shading). Histogram of VLR39 binding to (A) CLL donor and four other CLL patient lymphocytes gated on CLL cells (CD5+/CD19+), T cells (CD5+/CD19−), and non-B/T cells (CD5−/CD19−).
VLR39 is specific for the VH CDR3 sequence of the donor CLL
To examine the possibility that VLR39 recognizes an Ig idiotype (Id) determinant on the donor CLL clone, the VH and VL regions of the leukemic clone from the donor CLL and those from another CLL patient, 141, were sequenced and expressed as recombinant Fab fragments. The donor CLL VH/VL genes were composed of the IGHV1-69*01, IGHD3-3*01, IGHJ4*02, and the IGKV1-33*01 and IGKJ3*01 alleles, whereas the control 141 CLL VH/VL genes were composed of the IGHV4-34*01, IGHJ5*02, IGHD2-2*01, and IGKV1-27*01 and IGKJ2*01 alleles. When tested for reactivity by ELISA, VLR39 was found to react only with the donor CLL Ig Fab and not with the 141 CLL Ig Fab comprising different VH/VL gene sequences (Figure 2A). Id-specificity was tested against other CLL cells selected for partial overlap of their VH sequences with the donor CLL BCR sequence (Table S1). VLR39 failed to react with tumor cells from 26 additional CLL patients (Figure S2) even though the BCRs of seven CLL patients (1153, 1012, 1640, 1324, 1333, 352, 1397) shared identical VH CDR1 and CDR2 amino acid sequences with the donor CLL cells. Moreover, the BCRs of three other CLL patients (1371, 336, 758, 1330) shared identical IGHJ sequences comprising the 3′ end of the VH CDR3 (Figure S3).
Figure 2. ELISA and flow cytometric analysis of VLR39 binding to donor CLL Igs.
(A) Comparison of VLR39 binding to recombinant Fab fragments containing the VH/VL genes of the CLL clone from the patient donor and from another patient with unmatched VH/VL genes. ELISA of VLR39 binding to donor CLL Ig Fab (black squares), 141 CLL Ig Fab (white square), hen egg lysozyme (HEL) (black triangles), and PBS (white circles) coated wells. (B) Sequence alignment of HCDR3 region from donor CLL IgH, 1324 CLL IgH, and the two scFv mutants, 1324 HCDR3 swap and donor HCDR3 swap. The HCDR3 signature sequences of donor CLL (bold, underline text) and the HCDR3 signature sequences of CLL patient 1324 (italic, underline text) were swapped with each other to construct the two scFv mutants. (C) Flow cytometric analysis of VLR39 binding to HEK-293T cells transfected with donor CLL Ig scFv or the two scFv mutants. Myc-tag was co-stained to measure scFv surface expression.
The exquisite Id-specificity of VLR39 was surprising, given that most Ig-based monoclonal anti-Id antibodies cross-react with serum Ig (23) and B cells (24) from healthy individuals. Of note, the CDR3 amino acid sequences for the 26 CLL patients tested were all different from each other, suggesting that the VLR39 antibody recognizes the unique VH CDR3 sequence of the donor CLL. To test this interpretation, the VH/VL components of the CLL BCR from the donor and two mutants (1324 HCDR3 swap and donor HCDR3 swap) were expressed by transfected cells as a single-chain variable fragment (scFv) with a myc-tag (Fig 2B) and evaluated for anti-myc and VLR39 binding by immunofluorescence flow cytometry analysis (Figure 2C). For the scFv mutant 1324 HCDR3 swap, the CDR3 signature sequence of the donor CLL VH was replaced with the CDR3 signature sequence of 1324 CLL VH. For the other scFv mutant, donor HCDR3 swap, the CDR3 signature sequence of the 1324 CLL VH was replaced with the CDR3 signature sequence of the donor CLL VH. The 1324 HCDR3 swap transfectants failed to stain with VLR39, whereas the donor CLL Ig and donor HCDR3 swap transfectants co-stained with VLR39 and anti-myc, confirming that the donor CLL VH CDR3 region is the recognition site for the VLR39 antibody.
VLR39 antibody monitoring for CLL recurrence after chemoimmunotherapy-induced remission
The exquisite specificity of VLR39 for the CLL signature HCDR3 epitope and the absence of native Fc-like binding receptors for the lamprey antibody on human cells suggested that this lamprey antibody would be an ideal reagent for tracking the neoplastic clone in the CLL donor after treatment with multiple infusions of Cyclophosphamide, Fludarabine, Alemtuzumab, and Rituximab (CFAR) over a five day period each month for 6 months. This treatment regimen drastically reduced the T cell population and virtually eliminated circulating B cells in CLL patients. To monitor for leukemia recurrence, blood samples were evaluated for VLR39-reactive CD5+/CD19+ B cells by flow cytometric analysis (Figure 3). B cells were undetectable 4 months after the beginning of therapy, and thereafter progressive reconstitution of both T and B lymphocytes was observed (Figure 4). CD5+/CD19+ cells appeared at very low frequency at 29 months after CFAR therapy, and gradually increased by 56 months. The interpretation of this development was unclear given that some B cells in healthy individuals express CD5. However, VLR39+ B cells were detectable by 51 months after therapy in the CD5+ B cell population (Figure 3), when neither the lymphocyte count nor proportion of CD5+ B cells were significantly increased (Figure 4). To test whether the VLR39+ cells represented recurrence of the leukemic clone, we purified both VLR39+/CD5+ and VLR39−/CD5− B cell populations from the CLL donor for sequence analysis of the VH region (Figure S4). The IGHV gene sequence of the VLR39+/CD5+ sorted B cells proved to be identical to that of the original leukemic clone, whereas the VLR39−/CD5− sorted B cells did not yield a single uniform sequence, indicating their polyclonal nature. The VLR39 idiotope thus provided an early indication of the reemergence of the CLL clone in this patient. When the same blood samples were assayed using quantitative real-time PCR (qPCR) for the CLL donor HCDR3 transcripts, the emergence of the leukemia clone was first detectable at 51 months, in agreement with the VLR39 staining results (Supplementary Table S2).
Figure 3. Monitoring for CLL recurrence.
Blood samples were periodically obtained from CLL donor after CFAR therapy treatment and analyzed by flow cytometry for recurrence of VLR39+/CD5+/CD19+ cells. Each time point is calculated as months after the end of the CFAR treatment regimen. VLR39 binding (black line) was compared with that of control VLR4 having Bacillus anthracis BclA-specificity (gray shading). Anti-CD5 and anti-CD19 staining of CLL donor MNCs after treatment (left column). Histograms of VLR39 binding to CLL donor lymphocytes gated on CD5+ (center column) or CD5−CD19+ B cells (right column).
Figure 4. B cell and absolute lymphocyte count of patient after CFAR therapy treatment.
The blood counts from the CLL donor’s clinical laboratory studies were used to plot absolute lymphocyte counts over time (black circle and line, left Y axis). We calculated the proportions of CD5− B cells (gray bars) and CD5+ B cells (black bars) from our own flow cytometric studies, and plotted them as a percentage of total lymphocytes over time (right Y axis).
There has been longstanding interest in using the tumor-specific nature of anti-Id antibodies for monitoring and treating B cell malignancies (25). One barrier to implementation of this strategy is the arduous nature of generating anti-Id antibodies for each individual patient via hybridoma generation and screening, which can take up to several months after immunizing mice with patient cells. The single chain polypeptide nature of the VLRB protein offers a technological advantage in that the VLRB sequence can be cloned, expressed, and screened within a matter of days after lamprey immunization with patient cells (4). Selection of VLRB antibodies using high-throughput antibody display technologies allows for even more efficient screening of Id-specificity (15). The precise specificities of VLRB antibodies may also facilitate the identification, isolation and characterization of the leukemic clones in patients with B cell malignancies, particularly those with minimal residual disease. Lamprey LRR-based anti-Id antibodies thus offer a complementary biological tool to the classical Ig-based anti-Id antibodies for monitoring the therapy of patients with CLL and other B lineage malignancies.
Supplementary Material
Acknowledgments
Financial Support: This work was supported in part by NIAID/NIH grants R01 AI072435 and R37 AI039816 (M.D.C.) and NCI/NIH grant RO1 CA081554 (N.C.).
We thank Dr. Larry G. Gartland at the University of Alabama at Birmingham and Sommer Durham at the Emory University School of Medicine Flow Cytometry Core Facility for assistance with FACS studies.
Grant Support
This work was supported in part by NIAID/NIH grants R01 AI072435 and R37 AI039816 (M.D.C.) and NCI/NIH grant RO1 CA081554 (N.C.).
Footnotes
Conflicts of interest: The authors declare no competing financial interests.
References
- 1.Pancer Z, Amemiya CT, Ehrhardt GR, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–80. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- 2.Alder MN, Rogozin IB, Iyer LM, Glazko GV, Cooper MD, Pancer Z. Diversity and function of adaptive immune receptors in a jawless vertebrate. Science. 2005;310:1970–3. doi: 10.1126/science.1119420. [DOI] [PubMed] [Google Scholar]
- 3.Kim HM, Oh SC, Lim KJ, Kasamatsu J, Heo JY, Park BS, et al. Structural diversity of the hagfish variable lymphocyte receptors. J Biol Chem. 2007;282:6726–32. doi: 10.1074/jbc.M608471200. [DOI] [PubMed] [Google Scholar]
- 4.Herrin BR, Alder MN, Roux KH, Sina C, Ehrhardt GR, Boydston JA, et al. Structure and specificity of lamprey monoclonal antibodies. Proc Natl Acad Sci U S A. 2008;105:2040–5. doi: 10.1073/pnas.0711619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng L, Velikovsky CA, Xu G, Iyer LM, Tasumi S, Kerzic MC, et al. A structural basis for antigen recognition by the T cell-like lymphocytes of sea lamprey. Proc Natl Acad Sci U S A. 2010;107:13408–13. doi: 10.1073/pnas.1005475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han BW, Herrin BR, Cooper MD, Wilson IA. Antigen recognition by variable lymphocyte receptors. Science. 2008;321:1834–7. doi: 10.1126/science.1162484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velikovsky CA, Deng L, Tasumi S, Iyer LM, Kerzic MC, Aravind L, et al. Structure of a lamprey variable lymphocyte receptor in complex with a protein antigen. Nat Struct Mol Biol. 2009;16:725–30. doi: 10.1038/nsmb.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirchdoerfer RN, Herrin BR, Han BW, Turnbough CL, Jr, Cooper MD, Wilson IA. Variable lymphocyte receptor recognition of the immunodominant glycoprotein of Bacillus anthracis spores. Structure. 2012;20:479–86. doi: 10.1016/j.str.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo P, Hirano M, Herrin BR, Li J, Yu C, Sadlonova A, et al. Dual nature of the adaptive immune system in lampreys. Nature. 2009;459:796–801. doi: 10.1038/nature08068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasamatsu J, Sutoh Y, Fugo K, Otsuka N, Iwabuchi K, Kasahara M. Identification of a third variable lymphocyte receptor in the lamprey. Proc Natl Acad Sci U S A. 2010;107:14304–8. doi: 10.1073/pnas.1001910107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alder MN, Herrin BR, Sadlonova A, Stockard CR, Grizzle WE, Gartland LA, et al. Antibody responses of variable lymphocyte receptors in the lamprey. Nat Immunol. 2008;9:319–27. doi: 10.1038/ni1562. [DOI] [PubMed] [Google Scholar]
- 12.Rogozin IB, Iyer LM, Liang L, Glazko GV, Liston VG, Pavlov YI, et al. Evolution and diversification of lamprey antigen receptors: evidence for involvement of an AID-APOBEC family cytosine deaminase. Nat Immunol. 2007;8:647–56. doi: 10.1038/ni1463. [DOI] [PubMed] [Google Scholar]
- 13.Nagawa F, Kishishita N, Shimizu K, Hirose S, Miyoshi M, Nezu J, et al. Antigen-receptor genes of the agnathan lamprey are assembled by a process involving copy choice. Nat Immunol. 2007;8:206–13. doi: 10.1038/ni1419. [DOI] [PubMed] [Google Scholar]
- 14.Kishishita N, Matsuno T, Takahashi Y, Takaba H, Nishizumi H, Nagawa F. Regulation of antigen-receptor gene assembly in hagfish. EMBO Rep. 2010;11:126–32. doi: 10.1038/embor.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasumi S, Velikovsky CA, Xu G, Gai SA, Wittrup KD, Flajnik MF, et al. High-affinity lamprey VLRA and VLRB monoclonal antibodies. Proc Natl Acad Sci U S A. 2009;106:12891–6. doi: 10.1073/pnas.0904443106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu C, Ali S, St-Germain J, Liu Y, Yu X, Jaye DL, et al. Purification and identification of cell surface antigens using lamprey monoclonal antibodies. J Immunol Methods. 2012;386:43–9. doi: 10.1016/j.jim.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pancer Z, Mariuzza RA. The oldest antibodies newly discovered. Nat Biotechnol. 2008;26:402–3. doi: 10.1038/nbt0408-402. [DOI] [PubMed] [Google Scholar]
- 18.Fais F, Ghiotto F, Hashimoto S, Sellars B, Valetto A, Allen SL, et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J Clin Invest. 1998;102:1515–25. doi: 10.1172/JCI3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghiotto F, Fais F, Valetto A, Albesiano E, Hashimoto S, Dono M, et al. Remarkably similar antigen receptors among a subset of patients with chronic lymphocytic leukemia. J Clin Invest. 2004;113:1008–16. doi: 10.1172/JCI19399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herve M, Xu K, Ng YS, Wardemann H, Albesiano E, Messmer BT, et al. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. J Clin Invest. 2005;115:1636–43. doi: 10.1172/JCI24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 22.Lantto J, Jirholt P, Barrios Y, Ohlin M. Chain shuffling to modify properties of recombinant immunoglobulins. Methods Mol Biol. 2002;178:303–16. doi: 10.1385/1-59259-240-6:303. [DOI] [PubMed] [Google Scholar]
- 23.Miller RA, Hart S, Samoszuk M, Coulter C, Brown S, Czerwinski D, et al. Shared idiotypes expressed by human B-cell lymphomas. N Engl J Med. 1989;321:851–7. doi: 10.1056/NEJM198909283211302. [DOI] [PubMed] [Google Scholar]
- 24.Kiyotaki M, Cooper MD, Bertoli LF, Kearney JF, Kubagawa H. Monoclonal anti-Id antibodies react with varying proportions of human B lineage cells. J Immunol. 1987;138:4150–8. [PubMed] [Google Scholar]
- 25.Miller RA, Maloney DG, Warnke R, Levy R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982;306:517–22. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.