Abstract
We previously reported the chemopreventive potential of kava against 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)- and benzo(a)pyrene (BaP)-induced lung tumorigenesis in A/J mice during the initiation and post-initiation stages. In this study, we investigated the tumorigenesis-stage specificity of kava, the potential active compounds, and the underlying mechanisms in NNK-induced lung tumorigenesis in A/J mice. In the first experiment, NNK-treated mice were given diets containing kava at a dose of 5 mg/g of diet during different periods. Kava treatments covering the initiation stage reduced the multiplicity of lung adenomas by ~ 99%. A minimum effective dose is yet to be defined because kava at two lower dosages (2.5 and 1.25 mg/g of diet) were equally effective as 5 mg/g of diet in complete inhibiting lung adenoma formation. Daily gavage of kava (one before, during, and after NNK treatment) completely blocked lung adenoma formation as well. Kavalactone-enriched Fraction B fully recapitulated kava’s chemopreventive efficacy while kavalactone-free Fractions A and C were much less effective. Mechanistically, kava and Fraction B reduced NNK-induced DNA damage in lung tissues with a unique and preferential reduction in O6-methylguanine (O6-mG), the highly tumorigenic DNA damage by NNK, correlating and predictive of efficacy on blocking lung adenoma formation. Taken together, these results demonstrate the outstanding efficacy of kava in preventing NNK-induced lung tumorigenesis in A/J mice with high selectivity for the initiation stage in association with the reduction of O6-mG adduct in DNA. They also establish the knowledge basis for the identification of the active compound(s) in kava.
Keywords: kava, chemoprevention, lung, tumorigenesis, NNK, DNA damage, A/J mouse
Introduction
Lung cancer is the leading cause of malignancy-related mortality because of its high incidence and the lack of effective treatments (1, 2). Since tobacco usage contributes to 85-90% of its development (3), tobacco cessation is the most straightforward strategy for reducing lung cancer incidence and mortality. However, because of the addictive nature of tobacco (4, 5), limited progress has been achieved in reducing tobacco usage (6, 7). An alternative approach is to block or slow down tobacco carcinogen-induced lung cancer development via chemoprevention (8). Although a number of compounds have been identified as potential chemopreventive agents against lung tumorigenesis in animal models, their in vivo efficacy leaves ample room for improvement. Additional candidates with novel chemical structures, unique mechanisms, and better efficacy therefore need to be identified.
The A/J mice carry the pulmonary adenoma susceptibility 1 (Pas1) gene, tightly linked to the Kras oncogene (9), so that they have high susceptibility to lung tumor development. The A/J mice would develop lung tumor upon aging with high tumor incidence but low tumor multiplicity even without tobacco carcinogen treatment. With appropriate tobacco carcinogen treatment, A/J mice would develop lung tumors with 100% incidence and high multiplicity in a relatively short period of time (8). The tumors induced also have morphological, histological and molecular features similar to human lung adenocarcinomas (10). Therefore, the tobacco carcinogen-treated A/J mouse model is the most commonly used lung tumorigenesis model for evaluating chemopreventive agents with tumor multiplicity being the most practical endpoint.
Kava is an aqueous extract of the roots of Piper methysticum and traditionally serves as a beverage for South Pacific islanders. Kava had also been used to treat anxiety (11, 12), in which case it was prepared as an organic extract. Epidemiological surveillance detected very low cancer incidence rates in several South Pacific countries, including lung cancer (13, 14), and traditional kava usage may be a risk-lowering factor (15). Kava contains a class of unique chemicals, kavalactones (16), which have not been reported to prevent tumorigenesis. Kava, particularly the anxiolytic preparation, also contains chalcones, flavanones, and bornyl esters, which may inhibit cancer development.
We have recently demonstrated that dietary supplement of an ethanol kava extract at a dose of 10 mg/g of diet, during initiation stage or post-initiation stage, effectively reduced lung adenomas multiplicity induced by eight gavage treatment of a mixture of the well-known tobacco carcinogens 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and benzo(a)pyrene (BaP) without adverse side effects in A/J mice (17, 18). Since NNK and BaP induce adenoma formation via different mechanisms, the two-carcinogen model does not provide a feasible system to tackle questions regarding kava’s underlying mechanisms and responsible chemicals. The studies described herein were designed to address these questions by using an NNK-induced lung tumorigenesis A/J mouse model. Similar studies using the BaP-induced lung tumorigenesis models will be carried out in the future.
Materials and methods
Chemicals, reagents, and animal diets
NNK was synthesized (19). The kava product was purchased from Gaia Herbs, Inc. (Brevard, NC). It is an ethanol extract of the wild crafted lateral root from Vanuatu (standardized to 150 mg/mL total kavalactones). The AIN-93 purified diets from Harlan Teklad (Madison, WI) were used herein. The AIN-93G powdered diet started one week before the first dose of NNK and ended one week after the second dose of NNK; thereafter, it was replaced by AIN-93M powdered diet. O6-methylguanine (O6-mG) was purchased from Midwest Research Institute (Kansas City, MO). [CD3]O6-mG was purchased from Toronto Research Chemicals (Toronto, Ontario, Canada). 7-[4–(3-Pyridyl)-4-oxobut-1-yl]guanine (7-pobG), O2-[4–(3-pyridyl)-4-oxobut-1yl]thymidine (O2-pobdT), O6-[4–(3-pyridyl)-4-oxobut-1-yl]-2′-deoxyguanosine (O6-pobdG), and the corresponding [pyridine-D4] analogues were synthetized (20, 21). Micrococcal nuclease and phosphodiesterase II were from Worthington Biochemical Corporation (Lakewood, NJ). Alkaline phosphatase was from Roche Molecular Biochemicals (Indianapolis, IN).
Kava fractionation preparation and characterization
Our previous investigation of traditional kava and kava from Gaia Herbs revealed that the Gaia Herbs preparation contained some minor non-polar constituents with high toxicity (22). We optimized the fractionation protocol of kava from Gaia Herbs using silica gel chromatography, leading to three modalities – Fractions A, B, and C. Briefly, 350 mL of ethanolic kava extract was mixed with 350 g of silica gel. Solvents were evaporated under vacuum. Based on mass balance, 100 gram kava-adsorbed silica gel contained 28 gram kava residue. Kava-adsorbed silica gel (350 g) was loaded on a sample loading chamber and separated by a Biotage Semi-preparative system. The elution method was 28% ethyl acetate (EA) and 72% hexane (Hex) 5 column volumes (CV), followed by 90% EA and 10% Hex 4.1 CV, and then 35% MeOH and 65% EA 5.5 CV. Different eluents were analyzed by thin layer chromatography (TLC) and the desired eluents were combined with solvent removed to generate Fractions A, B, and C. The quantity of each fraction was measured and the integrity of each fraction was characterized by comparing the fingerprints of their 1H-NMR spectra. These fractions were also characterized by HPLC in comparison to traditional kava on a Beckman Coulter System Gold 126 solvent module with a 168 detector. A Clipeus C-18 column (5 μm, 250 × 4.6 mm) was used for the HPLC analyses. The flow rate used was 0.5 mL/min. The mobile phase A was water while B was acetonitrile. The time program used for the analyses was 70% B (0-5 min), 70-95% B (5-30 min), and 95% B (30-35 min). Compounds in Fractions B and C were further separated by normal phase silica gel chromatography and characterized by 1H-NMR and mass spectrometry.
Diet preparation
Different kava modalities in the appropriate quantity were reconstituted in absolute ethanol (50 mL) and then mixed with AIN-93 powdered diet (150 g). Absolute ethanol (50 mL) was also mixed with AIN-93 powdered diet (150 g) for the control diet preparation. The reconstituted diets were dried under vacuum to remove ethanol, ground to a fine powder and mixed with additional AIN-93 powdered diet to the desired dose. The initial dose of kava (5 mg/g of diet) was chosen based on the results of our previous study showing that kava at this dose was well tolerated in A/J mice while its lung cancer chemopreventive efficacy was similar to that at a higher dose (17).
Experiments assessing efficacy of different kava regimens on lung adenoma formation induced by NNK in A/J mice
Female A/J mice, 5–6 weeks of age from the Jackson Laboratory (Bar Harbor, ME), were handled according to protocols approved by IACUC at the University of Minnesota. Upon arrival, mice were housed in the specific pathogen-free animal facilities of Research Animal Resources, University of Minnesota. After one-week acclimation, mice were weighed, randomized into different groups and switched to AIN-93G-powdered diet, defined as Day 1. The number of mice in each group was specified in the Result Section. On Day 7 and Day 14, mice in the negative control groups received 0.1 mL physiological saline solution while mice in the other groups received NNK (100 and 67 mg/kg respectively in 0.1 mL of physiological saline solution) via i.p. injection. At the end of Day 21, mice were switched to AIN-93M-powdered diet until the end of the study. Diet consumption was measured twice weekly and bodyweight was monitored weekly. All mice were euthanized with an overdose of carbon dioxide. The lungs were collected and tumors on the surface of the lung were counted.
For Experiment 1, mice were fed diet supplemented with/without kava at a dose of 5 mg/g of diet during specified periods (Supplementary Figure 1 and Table 1) to define tumorigenesis-stage specificity. This study was terminated at the end of Day 119. For Experiment 2, mice were fed diet supplemented with/without kava at a dose of 5 mg/g of diet during Day 1 – Day 14 (initiation stage only). Half of the mice were terminated at the end of Week 25 (Day 175) and the other half at the end of Week 34 (Day 238). For Experiment 3, mice were given vehicle (PEG400:EtOH 9:1, 200 μL) or kava in the same vehicle (20 mg/mouse/day) via daily gavage with regimens specified in the Result Section. This study was terminated at the end of Day 119. For Experiment 4, mice were fed diet supplemented with kava at lower dosages to define dose-response pattern or different kava fractions at a dose of 2.5 mg/g of diet during Day 1 – Day 14. This study was terminated at the end of Day 119.
Experiments evaluating effect of kava on acute DNA adduct formation by NNK in A/J mice
After one-week acclimation, A/J mice were weighed and randomized into sixteen groups (3 mice per group) and switched to AIN-93G-powdered diet with the date being defined as Day 1. Mice in Groups 1, 2, 4, 6, 8, 10, and 12 were given AIN-93G diet through the study. Mice in Groups 3, 5, 7, 9, 11 and 13 were given AIN-93G diet supplemented with kava at a dose of 5 mg/g of diet through the study except for mice in Groups 9, 11, and 13, which were switched to plain AIN-93G diet one day after NNK injection to mimic stopping kava treatment one day after the last NNK treatment in our long-term lung tumorigenesis studies. Mice in Groups 14 – 16 were given AIN-93G diet supplemented with Fractions A, B, and C respectively at a dose of 2.5 mg/g of diet through the study. On Day 7, mice in Group 1 received 0.1 mL physiological saline solution while mice in the other groups received NNK (100 mg/kg in 0.1 mL physiological saline solution) via i.p. injection. Mice in Groups 1, 2, and 3 were euthanized 4 h after NNK injection. Mice in Groups 4 and 5 were euthanized 8 h after NNK injection. Mice in Groups 6, 7, 14, 15, and 16 were euthanized 24 h after NNK injection. Mice in Groups 8 and 9 were euthanized 48 h after NNK injection. Mice in Groups 10 and 11 were euthanized 96 h after NNK injection. Mice in Groups 12 and 13 were euthanized 2 weeks (336 h) after NNK injection. All mice were euthanized with an overdose of carbon dioxide. The lungs were harvested, snap frozen in liquid N2 and stored at −80°C until DNA isolation.
Isolation and quantification of DNA adducts in the lung tissues by LC-ESI-MS/MS
DNA was isolated from the whole lung tissue of each individual mouse following Puregene DNA isolation protocol (Qiagen Corp, Valencia, CA) (23). 7-pobG, O2-pobdT, O6-pobdG, and O6-mG were quantified by LC-ESI-MS/MS, following established protocols (23, 24).
Lung tumor histopathology
4-μm-thick sections made from formalin-fixed and paraffin embedded lung tissues were stained with hematoxylin and eosin (H&E).
Statistical analyses
Data on lung adenoma multiplicity were reported as mean ± SD (n = 5 – 40). One-way analysis of variance (ANOVA) was used to compare means among NNK and NNK+ kava modality groups for Experiments 1, 3, and 4. Dunnett’s test was used for comparisons of the number of tumors on the surface of the lung between NNK control and kava modality treatment groups. p-value ≤ 0.05 was considered statistically significant. For Experiment 2, unpaired t-test was used for comparison between NNK control and kava treatment groups. Two-sided p-value ≤ 0.05 was considered statistically significant. Data on DNA adducts were reported as mean ± SD (n = 3). For the time-course study, unpaired t-test was used for comparisons between NNK control and kava treatment groups. Two-sided p-value ≤ 0.05 was considered statistically significant: *p < 0.05, **p < 0.01, and *** p < 0.001. For the 24-h time point study, one-way ANOVA was used to compare means. Dunnett’s test was used for comparisons between NNK control and kava modality treatment groups. p-value ≤ 0.05 was considered statistically significant. All analyses were conducted in GraphPad Prism 4 (GraphPad Software, Inc. La Jolla, CA).
Results
Effect of kava treatment schedule with respect to NNK exposure on lung adenoma formation in A/J mice – Experiment 1
To test whether kava inhibited a specific stage of NNK-induced lung tumorigenesis, A/J mice were given two dosages of NNK (100 and 67 mg/kg of bodyweight on Day 7 and Day 14 respectively via i.p. injection). NNK-treated A/J mice were given diet supplemented with kava at a dose of 5 mg/g of diet during different periods of time in reference to NNK exposure. Both the adenoma incidence (presence of one detectable surface adenoma) and the number of adenomas on the lung surface at the end of Day 119 were quantified (Table 1).
Table 1.
Effect of different kava treatment schedules on lung tumor incidence and multiplicity induced by NNK in A/J mice at 119 day end points.
| Group | No. of mice at initiation |
Body Weight at initiation (mean ± SD, g/mouse) |
No. of mice at termination |
Body Weight at termination (mean ± SD, g/mouse) |
Liver weight at termination (mean ± SD g/mouse) |
Lung tumors | |||
|---|---|---|---|---|---|---|---|---|---|
| % of Mice with tumors |
Tumors /mouse (mean ± SD) |
Reduction in tumor multiplicity (%) |
p* | ||||||
| 1 (untreated control) | 10 | 16.3 ± 1.7 | 10 | 26.8 ± 2.7 | 1.24 ± 0.18 | 10 | 0.1 ± 0.3 | - | - |
| 2 (carcinogen control) | 40 | 16.3 ± 1.6 | 40 | 23.4 ± 2.2 | 1.01 ± 0.10 | 100 | 17.5 ± 4.8 | - | - |
| 3 (kava Day 1 – Day 14) | 15 | 16.3 ± 1.0 | 15 | 22.9 ± 1.5 | 1.04 ± 0.09 | 33 | 0.3 ± 0.5 | 98.9 | < 0.01 |
| 4 (kava Day 1 – Day 21) | 15 | 16.1 ± 1.4 | 15 | 23.1 ± 1.7 | 1.04 ± 0.11 | 13 | 0.2 ± 0.6 | 99.4 | < 0.01 |
| 5 (kava Day 1 – Day 119) | 15 | 16.5 ± 1.2 | 15 | 21.9 ± 1.9 | 1.09 ± 0.16 | 33 | 0.3 ± 0.5 | 98.9 | < 0.01 |
| 6 (kava Day 15 – Day 119) | 15 | 16.3 ± 1.2 | 15 | 22.6 ± 2.6 | 1.02 ± 0.12 | 100 | 13.3 ± 4.3 | 24.1 | < 0.05 |
| 7 (kava Day 15 – Day 28) | 15 | 16.3 ± 1.5 | 15 | 23.7 ± 1.8 | 1.06 ± 0.09 | 100 | 15.3 ± 5.4 | 12.7 | > 0.05 |
| 8 (kava Day 22 – Day 119) | 15 | 16.2 ± 1.1 | 15 | 21.3 ± 2.1 | 1.03 ± 0.10 | 100 | 16.1 ± 6.3 | 9.2 | > 0.05 |
| 9 (kava Day 29 – Day 119) | 15 | 16.2 ± 1.4 | 14 | 21.4 ± 1.1 | 1.03 ± 0.11 | 100 | 15.8 ± 6.2 | 10.1 | > 0.05 |
Note: Female A/J mice in Groups 2-9 were treated with NNK (100 and 67 mg/kg bodyweight on Day 7 and Day 14 respectively) in 0.1 mL saline via i.p. injection. Mice were maintained on AIN-93G diet until Day 21 and then shifted to AIN-93M diet for the duration of the experiment. Kava dose was 5 mg/g of diet.
: Compared with Group 2 by Dunnett’s test.
As expected, A/J mice without NNK treatment had low adenoma incidence (10%) and low adenoma multiplicity (0.1 ± 0.3 lung adenoma/mouse) while NNK-treated A/J mice had 100% adenoma incidence and high adenoma multiplicity (17.5 ± 4.8 lung adenoma/mouse). Kava treatment regimens that started after the final NNK treatment (Groups 6 – 9, i.e., post-initiation) had no effect on adenoma incidence. Such treatments also had little effect on adenoma multiplicity, except for the Day 15 – 119 regimen (Group 6), which reduced adenoma multiplicity by 24% (13.3 ± 4.3 lung adenoma/mouse, p < 0.05). On the other hand, kava treatments that preceded and covered the NNK exposure period (Groups 3 – 5, i.e., initiation stage) not only reduced adenoma incidence by 67-87% but also reduced adenoma multiplicity by ~99%, to a level similar to mice without NNK treatment. None of the long-term kava treatment regimens (Groups 5, 6, 8 and 9) caused >10% reduction in bodyweight, and the short-term treatment regimens (Groups 3, 4 and 7) did not reduce bodyweight relative to NNK-treated mice (Group 2). None of the kava treatment regimens caused significant changes in liver weight in comparison to NNK-treated mice (Group 2). These data indicated a complete blocking effect of kava on NNK-induced initiation of lung tumorigenesis, with a modest post-initiation inhibitory efficacy.
Effect of kava on long-term lung tumorigenesis in A/J mice – Experiment 2
To validate the anti-initiation efficacy of the short kava treatment during NNK treatment period (Day 1 – Day 14) and to determine whether such inhibition would persist through later stages of tumorigenesis, we replicated the kava and NNK treatment experiments for the initiation stage and analyzed the tumor status at Week 25 (Day 175) and Week 34 (Day 238). As shown in Table 2, A/J mice without NNK treatment had no adenoma and NNK-treated A/J mice had 100% adenoma incidence and high adenoma multiplicity (18.1 ± 5.1 lung adenoma/mouse) at Week 25. Kava at a dose of 5 mg/g of diet given during Day 1 – Day 14 reduced adenoma incidence by 73% and adenoma multiplicity by 98.5%. As expected of longer duration for tumors to grow, A/J mice at Week 34 had higher adenoma multiplicity (26.5 ± 7.8 lung adenoma/mouse) than those at Week 25. A/J mice without NNK treatment also had higher incidence (25%) and multiplicity (0.5 ± 1.0 lung adenoma/mouse) of spontaneous tumors than those at Week 25. Kava given during Day 1 – Day 14 did not reduce adenoma incidence but dramatically reduced adenoma multiplicity by 97.7% (1.1 ± 0.6 lung adenoma/mouse). Supplementary Figure 2 shows representative photomicrographs of sections of lung from mice without NNK treatment (Supplementary Figure 2A), mice with NNK treatment (Supplementary Figure 2B), and mice with NNK and kava treatment (Supplementary Figure 2C), which confirmed tumor reduction in the lung interior with kava treatment to the same magnitude as enumerated by counting the visible lung surface lesions. This kava treatment regimen caused no changes in mouse bodyweight and liver weight relative to the NNK-control groups. The data from this experiment not only confirmed the initiation-specific inhibitory efficacy of kava on NNK-induced lung tumorigenesis but also demonstrated the long-lasting protective nature of such a brief treatment.
Table 2.
Effect of kava on lung tumor incidence and multiplicity induced by NNK in A/J mice at 25 weeks (175 days) and 34 week (238 days) time point.
| Group | No. of mice at initiation |
Body Weight at initiation (mean ± SD, g/mouse) |
No. of mice at termination |
Body Weight at termination (mean ± SD, g/mouse) |
Liver weight at termination (mean ± SD g/mouse) |
Lung tumors | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| % of Mice with tumors |
Tumors/mouse (mean ± SD) |
Reduction in tumor multiplicity (%) |
p* | |||||||
| 25-week | 1 (untreated control) | 5 | 15.2 ± 1.4 | 5 | 27.5 ± 3.8 | 1.19 ± 0.16 | 0 | 0.0 ± 0.0 | - | - |
| 2 (carcinogen control) | 25 | 15.2 ± 1.4 | 23 | 26.4± 2.9 | 1.11 ± 0.13 | 100 | 18.1 ± 5.1 | - | - | |
| 3 (kava at 5 mg/g diet) | 15 | 15.2 ± 1.3 | 15 | 25.6 ± 2.3 | 1.11 ± 0.18 | 27 | 0.3 ± 0.5 | 98.5 | < 0.01 | |
|
| ||||||||||
| 34-week | 4 (untreated control) | 5 | 15.1 ± 2.1 | 4 | 30.8 ± 2.7 | 1.25 ± 0.05 | 25 | 0.5 ± 1.0 | - | - |
| 5 (carcinogen control) | 25 | 15.2 ± 1.2 | 24 | 27.1 ± 3.0 | 1.16 ± 0.16 | 100 | 26.5 ± 7.8 | - | - | |
| 6 (kava at 5 mg/g diet) | 15 | 15.2 ± 1.4 | 15 | 27.2 ± 3.9 | 1.06 ± 0.13 | 90 | 1.1 ± 0.6 | 97.7 | < 0.01 | |
Note: Female A/J mice in Groups 2, 3, 5, and 6 were treated with NNK (100 and 67 mg/kg bodyweight on Day 7 and Day 14 respectively) in 0.1 mL saline via i.p. injection. The mice were maintained on AIN-93G diet until Day 21 and then shifted to AIN-93M diet for the duration of the experiment. Kava treatment at a dose of 5 mg/g of diet was between Day 1 – Day 14.
: Compared between Groups 2 and 3 and between Groups 5 and 6.
Effect of daily gavage of kava on lung adenoma formation in A/J mice – Experiment 3
Given potential pharmacokinetic differences between kava consumption in humans (most practical as a bolus dose through dietary supplement pill/drink) vs. that of continuous rodent food intake in our experiments so far, we carried out an experiment to explore whether once daily gavage of kava might be as effective in preventing NNK-induced adenoma formation in A/J mice. The dose of kava, 20 mg/mouse/day, was chosen based on the fact that A/J mouse consumes 3 – 4 g of diet/day and the kava dose in diet was 5 mg/g of diet. In one regimen, once daily kava gavage started one day before the first NNK treatment and continued until one day after the second NNK treatment – Group 3 (Table 3). In the second regimen, once daily kava gavage started one day before the first NNK treatment, ended one day after the first NNK treatment, resumed one day before the second NNK treatment and ended one day after the second NNK treatment – Group 4 (Table 3). When the incidence and number of adenoma on the lung surface at the end of Day 119 were quantified (Table 3), A/J mice without NNK treatment had low adenoma incidence (20%) and low adenoma multiplicity (0.2 ± 0.4 lung adenoma/mouse) while NNK-treated A/J mice had 100% adenoma incidence and high adenoma multiplicity (16.6 ± 3.1 lung adenoma/mouse). Both kava gavage regimens reduced adenoma incidence (60-100%) and reduced adenoma multiplicity by ~99%. None of these regimens caused significant bodyweight or liver weight change in comparison to mice in Group 2. These data therefore convincingly established the feasibility of using kava in as few as 3 once-daily bolus treatments (i.e., one dose before, during and after the NNK injection) to block NNK-induced adenoma initiation. It remains to be determined whether a single dose shortly before or concurrent with NNK would be sufficient.
Table 3.
Effect of bolus kava via daily gavage on lung tumor incidence and multiplicity induced by NNK in A/J mice.
| Group | No. of mice at initiation |
Body Weight at initiation (mean ± SD, g/mouse) |
No. of mice at terminatio n |
Body Weight at termination (mean ± SD, g/mouse) |
Liver weight at termination (mean ± SD g/mouse) |
Lung tumors | |||
|---|---|---|---|---|---|---|---|---|---|
| % of Mice with tumors |
Tumors/mouse (mean ± SD) |
Reduction in tumor multiplicity (%) |
p* | ||||||
| 1 (untreated control) | 5 | 15.2 ± 2.1 | 5 | 24.1 ± 4.4 | 1.05 ± 0.17 | 20 | 0.2 ± 0.4 | - | - |
| 2 (carcinogen control) | 5 | 15.2 ± 2.3 | 5 | 22.9 ± 3.3 | 0.92 ± 0.14 | 100 | 16.6 ± 3.1 | - | - |
|
3 (20 mg kava daily Day 6 – Day 15) |
5 | 15.2 ± 1.1 | 5 | 21.7 ± 1.6 | 0.93 ± 0.12 | 40 | 0.4 ± 0.5 | 98.8 | < 0.01 |
|
4 (20 mg kava daily Day 6 – 8 and 13 – 15) |
5 | 15.2 ± 0.6 | 5 | 21.7 ± 1.7 | 0.89 ± 0.08 | 0 | 0.0 ± 0.0 | 100 | < 0.01 |
Note: Female A/J mice in Groups 2-4 were treated with NNK (100 and 67 mg/kg bodyweight on Day 7 and Day 14 respectively) in 0.1 mL saline via i.p. injection. The mice were maintained on AIN-93G diet until Day 21 and then shifted to AIN-93M diet for the duration of the experiment. The mice were given cottonseed oil (0.2 mL) via gavage once a day or kava (20 mg) in cottonseed oil (0.2 mL) on the specified days.
: Compared with Group 2 by Dunnett’s test .
Preparation and characterization of three kava fractions
Kava from Gaia Herbs was separated into three fractions – Fractions A, B, and C with nineteen repeates. The chemical profile of each fraction was characterized by 1H-NMR to ensure the reproducible integrity of each modality (Supplementary Figure 3). The mass of each fraction was determined (Supplementary Figure 3). Fractions A, B, and C accounted for 36.3%, 51.4%, and 9.9% of the mass balance of kava, respectively. The quantitative mass balance (97.7%) suggests that most, if not all, components were recovered. Reconstituted kava from Fractions A, B, and C also revealed no difference in composition from the original kava preparation, based on 1H-NMR and HPLC analyses (Supplementary Figures 3 and 4). HPLC analyses also showed that Fraction C contained chemicals not detectable in traditional kava (Supplementary Figure 4).
The major chemicals in Fraction B and C were isolated, characterized by 1H-NMR and mass spectrometry, and abundance determined (Supplementary Figure 5). All these chemicals have been previously identified from kava products (25). Fraction B contains six kavalactones and one flavanone. Fraction C includes two additional flavanones, two bornyl esters, and two chalcone-based flavokawains A and B. Chemicals in Fraction A were not characterized because our chemopreventive efficacy data showed that Fraction A was the least efficacious and that Gaia Herbs kava’s chemopreventive potential could be recapitulated by Fraction B (Table 4).
Table 4.
Dose-response effect of kava and different kava fractions on lung tumor incidence and multiplicity induced by NNK in A/J mice.
| Group | No. of mice at initiation |
Body Weight at initiation (mean ± SD, g/mouse) |
No. of mice at termination |
Body Weight at termination (mean ± SD, g/mouse) |
Liver weight at termination (mean ± SD g/mouse) |
Lung tumors | |||
|---|---|---|---|---|---|---|---|---|---|
| % of Mice with tumors |
Tumors/mouse (mean ± SD) |
Reduction in tumor multiplicity (%) |
P* | ||||||
| 1 (untreated control) | 5 | 15.2 ± 1.3 | 5 | 24.3 ± 2.7 | 1.08 ± 0.17 | 20 | 0.2 ± 0.4 | - | - |
| 2 (carcinogen control) | 25 | 15.2 ± 1.2 | 25 | 23.5 ± 1.9 | 0.94 ± 0.15 | 100 | 16.0 ± 5.2 | - | - |
| 3 (kava at 5 mg/g diet) | 15 | 15.2 ± 1.5 | 15 | 24.0 ± 3.4 | 1.00 ± 0.13 | 13 | 0.1 ± 0.4 | 100 | < 0.01 |
| 4 (kava at 2.5 mg/g diet) | 15 | 15.2 ± 1.5 | 15 | 23.3 ± 2.0 | 1.00 ± 0.12 | 27 | 0.3 ± 0.5 | 99.4 | < 0.01 |
| 5 (kava at 1.25 mg/g diet) | 15 | 15.2 ± 1.5 | 15 | 23.1 ± 1.5 | 0.89 ± 0.09 | 20 | 0.2 ± 0.4 | 100 | < 0.01 |
| 6 (Fraction A at 2.5 mg/g diet) | 15 | 15.2 ± 2.0 | 15 | 23.3 ± 2.6 | 1.00 ± 0.10 | 100 | 12.0 ± 5.0 | 25.3 | <0.01 |
| 7 (Fraction B at 2.5 mg/g diet) | 15 | 15.2 ± 1.8 | 15 | 23.4 ± 2.9 | 0.97± 0.10 | 7 | 0.1 ± 0.5 | 100 | < 0.01 |
| 8 (Fraction C at 2.5 mg/g diet) | 15 | 15.2 ± 2.0 | 15 | 22.7 ± 2.2 | 0.95 ± 0.11 | 93 | 3.5 ± 2.5 | 70.2 | < 0.01 |
Note: Female A/J mice in Groups 2-8 were treated with NNK (100 and 67 mg/kg bodyweight on Day 7 and Day 14 respectively) in 0.1 mL saline via i.p. injection. The mice were maintained on AIN-93G diet until Day 21 and then shifted to AIN-93M diet for the duration of the experiment. Kava modality treatment was between Day 1 – Day 14.
: Compared with Group 2 by Dunnett’s test.
Estimating minimum effective dosage and searching for active fraction against NNK-induced lung adenoma formation in A/J mice – Experiment 4
To determine the minimum dose of kava that could effectively inhibit NNK-induced lung adenoma formation in A/J mice, we used the same carcinogen protocol as above to initiate tumorigenesis. NNK-treated A/J mice were given diet supplemented with kava at a dose of 5, 2.5, and 1.25 mg/g of diet during Day 1 – Day 14. The incidence and number of adenoma on the lung surface at the end of Day 119 were quantified (Table 4). Similar to previous results, A/J mice without NNK treatment had low adenoma incidence (20%) and multiplicity (0.2 ± 0.4 lung adenoma/mouse) while NNK-treated A/J mice had 100% adenoma incidence and high adenoma multiplicity (16.0 ± 5.2 lung adenoma/mouse). Kava treatments, at all dosages, reduced adenoma incidence by 73 – 87% and reduced adenoma multiplicity by ~99%. These data suggested that future experiments would be needed to explore even lower dosages to define the minimum effective dose of kava to block tumor initiation in this model.
In this study, we also evaluated the efficacy of the three kava fractions at a dose of 2.5 mg/g of diet to rank their anti-initiation efficacy (Table 4, Groups 6-8). Fraction A, equivalent to kava at a dose of 6.9 mg/g of diet based on its abundance in kava, caused no reduction in adenoma incidence and only weakly reduced adenoma multiplicity by 25% (12.0 ± 5.0 lung adenoma/mouse, p < 0.01). Fraction B, equivalent to kava at a dose of 4.9 mg/g of diet, reduced adenoma incidence by 93% and reduced adenoma multiplicity to baseline level (0.1 ± 0.5 lung adenoma/mouse, p < 0.01). Fraction C, equivalent to kava at a dose of 25.2 mg/g of diet, did not reduce adenoma incidence but reduced adenoma multiplicity by 70% (3.5 ± 2.5 lung adenoma/mouse, p < 0.01). None of these regimens caused significant bodyweight or liver weight changes in comparison to mice in Group 2. The data suggest the Fraction B contained the overwhelming majority, if not all, of the active compounds, Fraction C contained minor amount whereas Fraction A contained literally none.
Effect of kava and its fractions on DNA damage induced by NNK in A/J mouse lung tissues
Since our data convincingly established the highly selective anti-initiation efficacy of kava and its Fraction B against NNK-induced lung adenoma formation, we focused next on reduction of NNK-induced DNA damage as a plausible mechanism of chemoprevention. We designed additional experiments to collect lung tissues to characterize the time-course profiles of four NNK-derived DNA adducts (7-pobG, O2-pobdT, O6-pobdG and O6-mG) in the lung tissues of the A/J mice upon kava exposure at a dose of 5 mg/g of diet. As expected, no NNK-derived DNA adducts were detected in the negative control mice (data not shown) while significant amounts of all four DNA adducts were detected in mice with NNK treatment (Figure 1A). Kava treatment reduced the quantity of all four DNA adducts (Figure 1A). When the abundance of each DNA adduct was normalized relative to its time-controlled NNK-treatment group (Figure 1B), the extents of reduction in 7-pobG, O2-pobdT, and O2-pobdG were similar (30-40%), particularly during the first 24 h after NNK treatment when the contribution of DNA repair and intrinsic instability of these adducts are less important. For O6-mG, however, the reduction was 70-80% (Figure 1B). Because there were no differences in the relative abundance of any of these four DNA adducts at different time points after NNK treatment (Figure 1B), kava-induced reduction in these DNA adducts is more likely mediated through the inhibition of their formation instead of the activation of DNA repair mechanisms.
Figure 1.
Characterization of the effect of kava and kava fractions on DNA adducts induced by NNK in the lung of A/J mice (n = 3 each group): * p < 0.05; ** p < 0.01; *** p < 0.001. A. The amount of DNA adducts at different time points after NNK treatment; NNK alone: ; NNK + kava:
; NNK + kava: ). B. Relative amount of DNA adducts in NNK + kava treatment group at different time points after NNK treatment (the amount with kava treatment normalized to that induced by NNK alone at the same time point). C. The amount of DNA adducts with different kava fraction treatment 24 h after NNK treatment. D. Relative amount of DNA adducts by different kava fractions normalized to that induced by NNK alone at the 24 h time point.
). B. Relative amount of DNA adducts in NNK + kava treatment group at different time points after NNK treatment (the amount with kava treatment normalized to that induced by NNK alone at the same time point). C. The amount of DNA adducts with different kava fraction treatment 24 h after NNK treatment. D. Relative amount of DNA adducts by different kava fractions normalized to that induced by NNK alone at the 24 h time point.
We next evaluated the effect of Fractions A, B, and C at a dose of 2.5 mg/g of diet on NNK-induced DNA adducts 24 h after NNK treatment. As shown in Figure 1C, all kava fractions reduced NNK-induced DNA damage. However, the extents of reduction in 7-pobG, O2-pobdT, and O6-pobdG were very similar (Figure 1D) and had no correlation with their distinct capacities in blocking lung adenoma formation. The extents of reduction in O6-mG, on the other hand, were quite different. Fraction B greatly reduced O6-mG (72%) while Fraction A and C had no effect on O6-mG. The extent of reduction in O6-mG correlated with their capabilities in reducing lung adenoma multiplicity (Table 4). These data suggest that blocking the formation of O6-mG (and possibly other methylation adducts) in the lung DNA by active compounds in kava Fraction B was a likely mechanism for its efficacy against NNK-induced lung tumorigenesis in this model.
Discussion
The results from this study clearly demonstrated that kava, when given before and during NNK treatment period (initiation stage), was highly efficacious in preventing lung adenoma formation in A/J mice, with a ~99% reduction in adenoma multiplicity at a dose of as low as 1.25 mg/g of diet. The minimum effective dose remains to be defined. Such treatments also reduced lung tumor incidence. A similar degree of chemopreventive effect was maintained even when the studies were terminated at later stages, suggesting that kava blocks lung tumorigenesis instead of slowing down the process. Furthermore, our data convincingly established the feasibility of using kava in as few as 3 once-daily bolus treatments (i.e., before, during and after the NNK injection) to block the adenoma initiation activity of NNK. While these data further substantiated the chemopreventive potential of kava against tobacco-induced lung cancer, they also demonstrated a new paradigm of highly effective initiation-stage specificity of kava against this carcinogen. Such drastic efficacy has not been reported previously in literature except for several synthetic derivatives of phenethyl isothiocyanate (PEITC) (26).
On the other hand, kava showed much lower efficacy when its treatment started after the second NNK administration, suggesting that kava at this dose and format mainly blocks the initiation of NNK-induced lung tumorigenesis. These results differed from those of our previous studies, which demonstrated that kava at a dose of 10 mg/g of diet decreased NNK- and BaP-induced adenoma formation in A/J mice in the post-initiation stage (i.e., kava treatment started one day after the final dose of carcinogen treatment) as well as in the initiation stage (18). There are several differences between these studies that may account for this apparent discrepancy. First of all, the routes of carcinogen administration and the dose-intensity were different. NNK in this study was given by i.p. injection (2 weekly injections) whereas our previous work involved gavage of NNK and BaP mixture (8 weekly gavages). These may lead to different metabolic processing of the carcinogen(s) and reactive metabolites toward DNA and thereby different pathogenetic alterations in the target tissues in these models. Secondly, the kava treatment regimen in this study (Group 6), most closely mimicking that used in our previous study, displayed a modest reduction in lung adenoma multiplicity (Table 1), albeit to a lesser extent than those in our previous study. This might be explained by the dosage difference in these studies. Finally, kava did not completely block NNK- and BaP-induced lung adenoma formation when it was given during the initiation phase even at a dose of 10 mg/g of diet (18). Compared with the high efficacy against NNK-induced initiation in the current study, it is possible that kava is less effective in blocking BaP-induced lung tumor initiation. Further studies are needed to address these questions.
In our search for the active compound(s), we have developed a highly reproducible fractionation protocol, separating kava into three fractions. Fraction A contains the polar chemicals, Fraction B contains the chemicals with intermediate polarity, and Fraction C contains the non-polar chemicals not detectable in traditional kava. When evaluated at a dose of 2.5 mg/g of diet, Fractions A and C, equivalent doses much higher than kava at 5 mg/g of diet, only weakly reduced lung adenoma multiplicity with no reduction in tumor incidence. Fraction B, on the other hand, completely blocked NNK-induced lung adenoma formation at a dose equivalent to kava at 5 mg/g of diet. These data clearly demonstrate that Fraction B fully recapitulates kava’s lung chemopreventive efficacy and contains the active compounds, whereas Fractions A and C contain none or little. Six kavalactones have been identified in Fraction B, accounting for 94% of its mass balance. Although there had been no report of their efficacy in any in vivo tumorigenesis models, these kavalactones are likely responsible for kava’s efficacy in blocking NNK-induced lung tumorigenesis in A/J mice. It is noteworthy that Fraction B is free of flavokawains A and B that may contribute to kava’s hepatotoxic risk (27). Although flavokawains A and B have revealed anticancer activities in several xenograft models (28-30), results from our current studies indicate that they are not the active compounds against NNK-induced lung tumorigenesis initiation, consistent with the results from our previous study (17).
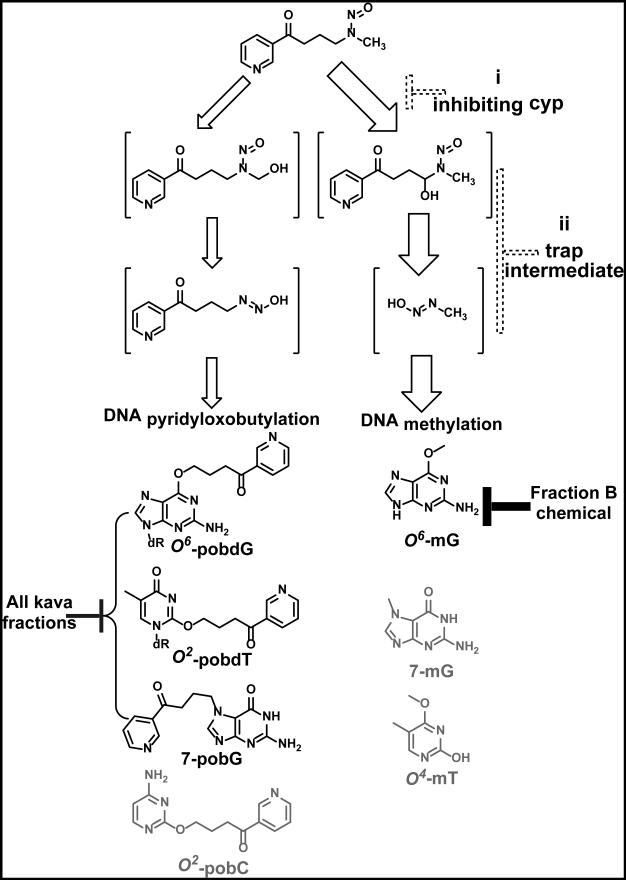
Given the highly selective anti-NNK-initiation action of kava and its fraction B, we characterized their effect on NNK-induced DNA damage in lung tissue as a possible mechanism. NNK, an asymmetrical nitrosamine, can be activated to two types of DNA reactive species via different hydroxylation pathways (Figure 2). Methyl hydroxylation generates 4-oxo-4-(3-pyridyl)-1-butanediazohydroxide, which leads to a panel of DNA adducts, including 7-pobG, O2-[4–(3-pyridyl)-4-oxobut-1-yl]cytidine (O2-pobC), O2-pobdT, and O6-pobdG (23). Methylene hydroxylation generates methanediazohydroxide, leading to another set of DNA adducts, including 7-methylguanine (7-mG), O6-mG, and O4-methylthymidine (O4-mT). The abundance of methylation DNA adducts are typically 10-20 fold more than those of the pob DNA adducts, likely due to a combination of preferential methylene hydroxylation of NNK and higher reactivity of methanediazohydroxide intermediate (31). We analyzed four of these DNA adducts in the lung, 7-pobG, O2-pobdT, O6-pobdG, and O6-mG, because of their better stability, their representation of both pathways of NNK activation, and their potential tumorigenicity (23, 24, 32).
Figure 2.
Two metabolic activation pathways of NNK leading to different methylated- vs. 4–(3-pyridyl)-4-oxobut-1-yl (pob)-DNA adducts. Solid blocks indicate measured reduction in different classes of DNA adducts, as exemplified by O6-mG vs. 7-pobG, O2-pobdT, and O6-pobdG. Dashed blocks indicate hypothetical points of action by kava chemicals or their metabolites: (i) to differentially inhibit cytochrome p450 isoform-mediated NNK metabolic activation or (ii) directly react with NNK methylene hydroxylation metabolites as chemical traps.
Surprisingly, kava treatment causes different extents of reduction in these four DNA adducts with high preference on O6-mG. To our knowledge, kava is the first candidate that demonstrates such a unique mechanism. Given that the POB adducts are generated via methyl hydroxylation of NNK while O6-mG is generated via methylene hydroxylation (Figure 2), kava Fraction B chemicals may preferentially inhibit NNK methylene hydroxylation over methyl hydroxylation. It is also possible that Faction B chemicals better react with and trap methanediazohydroxide over 4-oxo-4-(3-pyridyl)-1-butanediazohydroxide, leading to the observed preferential reduction in O6-mG. Detailed investigation is needed to fully appreciate the underlying molecular and structural bases responsible for such a differential inhibition. Nevertheless, work from Peterson et al. shows that O6-mG has a strong and positive correlation with lung tumor multiplicity in A/J mice (24). A/J mice with increased DNA repair capacity specific to O6-mG are less susceptible to NNK-induced lung tumorigenesis as well (33). In addition, the miscoding properties of O6-mG have been well established (32). These results argue for the high tumorigenicity of O6-mG relative to the POB adducts and the possible cause-effect of its reduction by kava and Fraction B chemicals to their impressive anti-initiation efficacy.
In summary, kava blocks NNK-induced lung tumorigenesis in A/J mice with high selectivity for the initiation stage. Mechanistically kava Fraction B chemicals preferentially reduces NNK-induced O6-mG DNA adduct in the lung tissues. Our results also suggest that kavalactones in Fraction B may be the active compounds.
Supplementary Material
Acknowledgments
Funding
This work was supported by the grant R01 CA142649 by the National Cancer Institute/National Institutes of Health. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Abbreviations
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- BaP
benzo(a)pyrene;
- O6-mG
O6-methylguanine
- 7-pobG
7-[4–(3-pyridyl)-4-oxobut-1-yl]guanine
- O2-pobdT
O2-[4–(3-pyridyl)-4-oxobut-1yl]thymidine
- O6-pobdG
O6-[4–(3-pyridyl)-4-oxobut-1-yl]-2′-deoxyguanosine
- O2-pobC
O2-[4–(3-pyridyl)-4-oxobut-1-yl]cytidine
- 7-mG
7-methylguanine
- O4-mT
O4-methylthymidine
- CV
column volume
- TLC
thin layer chromatography H&E, hematoxylin and eosin
- Pas1
pulmonary adenoma susceptibility 1
- ANOVA
analysis of variance
- PEITC
phenethyl isothiocyanate.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321:323–9. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380:668–79. doi: 10.1016/S0140-6736(12)61085-X. [DOI] [PubMed] [Google Scholar]
- 5.Giovino GA. The tobacco epidemic in the United States. Am J Prev Med. 2007;33:S318–26. doi: 10.1016/j.amepre.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–94. doi: 10.1093/jnci/djn389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enstrom JE, Heath CW., Jr. Smoking cessation and mortality trends among 118,000 Californians, 1960-1997. Epidemiology. 1999;10:500–12. [PubMed] [Google Scholar]
- 8.Hecht SS, Kassie F, Hatsukami DK. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat. Rev. Cancer. 2009;9:476–88. doi: 10.1038/nrc2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'donnell EP, Zerbe LK, Dwyer-Nield LD, Kisley LR, Malkinson AM. Quantitative analysis of early chemically-induced pulmonary lesions in mice of varying susceptibilities to lung tumorigenesis. Cancer Lett. 2006;241:197–202. doi: 10.1016/j.canlet.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Malkinson AM. Primary lung tumors in mice as an aid for understanding, preventing, and treating human adenocarcinoma of the lung. Lung Cancer. 2001;32:265–79. doi: 10.1016/s0169-5002(00)00232-4. [DOI] [PubMed] [Google Scholar]
- 11.Boerner RJ, Sommer H, Berger W, Kuhn U, Schmidt U, Mannel M. Kava-Kava extract LI 150 is as effective as Opipramol and Buspirone in Generalised Anxiety Disorder--an 8-week randomized, double-blind multi-centre clinical trial in 129 out-patients. Phytomedicine. 2003;10(Suppl 4):38–49. doi: 10.1078/1433-187x-00309. [DOI] [PubMed] [Google Scholar]
- 12.Sarris J, Stough C, Bousman CA, Wahid ZT, Murray G, Teschke R, et al. Kava in the Treatment of Generalized Anxiety Disorder: A Double-Blind, Randomized, Placebo-Controlled Study. J Clin Psychopharmacol. 2013 doi: 10.1097/JCP.0b013e318291be67. [DOI] [PubMed] [Google Scholar]
- 13.Henderson BE, Kolonel LN, Dworsky R, Kerford D, Mori E, Singh K, et al. Cancer incidence in the islands of the pacific; Fourth symposium on epidemiology and cancer registries in the pacific basin; 1984. pp. 73–81. [PubMed] [Google Scholar]
- 14.Henderson BE, Kolonel LN, Dworsky R, Kerford D, Mori E, Singh K, et al. Cancer incidence in the islands of the Pacific. Natl. Cancer Inst. Monogr. 1985;69:73–81. [PubMed] [Google Scholar]
- 15.Steiner GG. The correlation between cancer incidence and kava consumption. Hawaii Med. J. 2000;59:420–2. [PubMed] [Google Scholar]
- 16.Rowe A, Narlawar R, Groundwater PW, Ramzan I. Kavalactone pharmacophores for major cellular drug targets. Mini Rev Med Chem. 2011;11:79–83. doi: 10.2174/138955711793564088. [DOI] [PubMed] [Google Scholar]
- 17.Johnson TE, Hermanson D, Wang L, Kassie F, Upadhyaya P, O'sullivan MG, et al. Lung tumorigenesis suppressing effects of a commercial kava extract and its selected compounds in A/J mice. Am J Chin Med. 2011;39:727–42. doi: 10.1142/S0192415X11009202. [DOI] [PubMed] [Google Scholar]
- 18.Johnson TE, Kassie F, O'sullivan MG, Negia M, Hanson TE, Upadhyaya P, et al. Chemopreventive effect of kava on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo[a]pyrene-induced lung tumorigenesis in A/J mice. Cancer Prev Res (Phila) 2008;1:430–8. doi: 10.1158/1940-6207.CAPR-08-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht SS, Lin D, Castonguay A. Effects of alpha-deuterium substitution on the mutagenicity of 4-(methyl-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Carcinogenesis. 1983;4:305–10. doi: 10.1093/carcin/4.3.305. [DOI] [PubMed] [Google Scholar]
- 20.Lao Y, Villalta PW, Sturla SJ, Wang M, Hecht SS. Quantitation of pyridyloxobutyl DNA adducts of tobacco-specific nitrosamines in rat tissue DNA by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Chem Res Toxicol. 2006;19:674–82. doi: 10.1021/tx050351x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturla SJ, Scott J, Lao Y, Hecht SS, Villalta PW. Mass spectrometric analysis of relative levels of pyridyloxobutylation adducts formed in the reaction of DNA with a chemically activated form of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Chem Res Toxicol. 2005;18:1048–55. doi: 10.1021/tx050028u. [DOI] [PubMed] [Google Scholar]
- 22.Shaik AA, Hermanson DL, Xing C. Identification of methysticin as a potent and non-toxic NF-kappaB inhibitor from kava, potentially responsible for kava's chemopreventive activity. Bioorg Med Chem Lett. 2009;19:5732–6. doi: 10.1016/j.bmcl.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urban AM, Upadhyaya P, Cao Q, Peterson LA. Formation and repair of pyridyloxobutyl DNA adducts and their relationship to tumor yield in A/J mice. Chem Res Toxicol. 2012;25:2167–78. doi: 10.1021/tx300245w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson LA, Hecht SS. O6-methylguanine is a critical determinant of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone tumorigenesis in A/J mouse lung. Cancer Res. 1991;51:5557–64. [PubMed] [Google Scholar]
- 25.Duke J., Dr. Duke's phytochemical and ethnobotanical databases. 2013 http://www.arsgrin.gov/duke/
- 26.Alworth WL, Young-Sciame R, Hecht SS. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone mouse lung tumorigenesis by arylalkynes, mechanism-based inactivators of cytochrome P450. Carcinogenesis. 1993;14:1711–3. doi: 10.1093/carcin/14.8.1711. [DOI] [PubMed] [Google Scholar]
- 27.Zhou P, Gross S, Liu JH, Yu BY, Feng LL, Nolta J, et al. Flavokawain B, the hepatotoxic constituent from kava root, induces GSH-sensitive oxidative stress through modulation of IKK/NF-kappa B and MAPK signaling pathways. Faseb Journal. 2010;24:4722–32. doi: 10.1096/fj.10-163311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zi X, Simoneau AR. Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Cancer Res. 2005;65:3479–86. doi: 10.1158/0008-5472.CAN-04-3803. [DOI] [PubMed] [Google Scholar]
- 29.Tang Y, Li X, Liu Z, Simoneau AR, Xie J, Zi X. Flavokawain B, a kava chalcone, induces apoptosis via up-regulation of death-receptor 5 and Bim expression in androgen receptor negative, hormonal refractory prostate cancer cell lines and reduces tumor growth. Int J Cancer. 2010;127:1758–68. doi: 10.1002/ijc.25210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin E, Lin WH, Wang SY, Chen CS, Liao JW, Chang HW, et al. Flavokawain B inhibits growth of human squamous carcinoma cells: Involvement of apoptosis and cell cycle dysregulation in vitro and in vivo. J Nutr Biochem. 2012;23:368–78. doi: 10.1016/j.jnutbio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Peterson LA, Mathew R, Hecht SS. Quantitation of microsomal alpha-hydroxylation of the tobacco-specific nitrosamine, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1991;51:5495–500. [PubMed] [Google Scholar]
- 32.Loechler EL, Green CL, Essigmann JM. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984;81:6271–5. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu L, Qin X, Gerson SL. Reduced lung tumorigenesis in human methylguanine DNA-- methyltransferase transgenic mice achieved by expression of transgene within the target cell. Carcinogenesis. 1999;20:279–84. doi: 10.1093/carcin/20.2.279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.