Abstract
Purpose
To evaluate the anti-apoptotic effect of the antioxidant reaction of anthocyanin on the prostate in an andropause animal model.
Materials and Methods
Sprague-Dawley rats were divided into three groups (n=12 in each): control (Group I), andropause (Group II), andropause treated with anthocyanin (Group III). For induction of andropause, Group II and III underwent bilateral orchiectomy. Group III was treated with daily oral anthocyanin (160 mg/kg) for 8 weeks. After 8 weeks, the rats were sacrificed and their blood and prostates were examined pathohistologically and evaluated for oxidative stress and apoptosis. Oxidative stress was assessed by the activity of superoxide dismutase (SOD) and apoptosis in the prostate was identified by terminal deoxyribonucleotidyl transferase-mediated dUTP-digoxigenin nick end-labelling assay.
Results
Group II showed markedly increased activity of SOD in serum over that observed in Group I, whereas the rats in Group III showed reduced oxidative stress compared to Group II. Despite no significant differences in prostate weight between Group II and III (p=0.078), the apoptotic index was significantly greater in Group II than Group I, and was significantly lesser in Group III than Group II.
Conclusions
We suggest that the oxidative stress caused by low testosterone may be another inducer of apoptosis, and this apoptosis may partly contribute to the overall apoptosis of the prostate in the andropause animal model. Therefore, anthocyanin supplementation may contribute to preventing excessively rapid cell death by apoptosis in the prostate in an animal model of andropause.
Keywords: Andropause, Anthocyanins, Antioxidants, Apoptosis
INTRODUCTION
The average life span of human beings is gradually increasing with the development of science and medicine [1]. As a result, the elderly account for a significant portion of the population, and their quality of life has become a social concern. In the aging process in men, andropause, recently named symptomatic late-onset hypogonadism [2], affects quality of life, causing detrimental physiological and mental effects [3]. The common clinical symptoms of andropause are lethargy, decreased sense of well-being, reduced physical and mental activity, diminished libido, increased sweating, depressive mood, reduced muscle and bone mass or even osteoporosis, erectile dysfunction, and mild anemia [4]. It is believed that these symptoms are caused by reduced production of hormones including testosterone and dihydrotestosterone (DHT) [5]. Therefore, andropause is generally defined by a serum total testosterone level less than 3.5 ng/mL or a free testosterone level less than 72 pg/mL in a man of advancing age with various andropause symptoms [6,7].
It is known that a low level of testosterone, such as that which occurs in andropause, induces rapid cell death in the prostate by the activation of apoptosis and this, in turn, results in the reduction of prostate weight and size [8-10]. This effect is not limited to only the sexual organs but also affects several other organ systems. In addition, this effect is known to be associated with various mental and physical changes in andropause. The activation of apoptosis inducing rapid cell death in the prostate is known to be mediated by a genetically regulated process, one that requires the expression and subsequent action of discrete gene products and coregulatory molecules to proceed [11]. Furthermore, having low testosterone induces oxidative stress [12], which is known to be one of the triggers of apoptosis [13-15]. Considering all this, apoptosis in andropause may be mainly mediated by the activation of the critical signaling pathway of apoptosis, which is genetically regulated. We also suggest that the oxidative stress induced by a low level of testosterone may be another inducer of apoptosis, and this may partly contribute to the overall apoptosis in the prostate under andropausal conditions.
Anthocyanin is a water-soluble natural pigment that appears as red, purple, and blue in plants and belongs to the flavonoid parent class of molecules [16]. It is known that anthocyanin leads to the removal of superoxide, singlet oxygen, peroxide, hydrogen peroxide, and the hydroxyl radical. It also results in stabilizing or inactivating free radicals and preventing cellular oxidative stress [16,17]. Therefore, we presumed that anthocyanin supplementation may be effective in preventing rapid cell death by apoptosis induced by oxidative stress.
To confirm our hypothesis, we evaluated the oxidative stress and apoptosis of the prostate in an animal model of andropause and assessed the beneficial effect of anthocyanin extracted from the seed coat of the black soybean in preventing the induction of apoptosis by decreasing oxidative stress.
MATERIALS AND METHODS
1. Preparation of anthocyanin
The anthocyanin extracted from the seed coat of the black soybean used in our experiments was supplied by the Rural Development Administration (Suwon, Korea). The anthocyanin was extracted and analyzed by the same methods used in our previous study [18].
2. Animal groups and treatment protocol
Thirty-six 12-week-old male Sprague-Dawley rats were treated under a protocol approved by the Institutional Animal Care and Use Committee (CUMC-2010-0152-01) and handled according to NIH guidelines. The rats were divided equally into three groups (n=12 in each): control (Group I), andropause (Group II), and andropause treated with anthocyanin (Group III). To induce a low testosterone state similar to andropause, all of the rats in Group II and III underwent bilateral orchiectomy. After the bilateral orchiectomy, the rats in Group III were treated with oral anthocyanin (160 mg/kg daily) dissolved in 1 mL of distilled water and administered orally through an 8F red Rod-Nel catheter once a day for 8 weeks. After 8 weeks, all of the rats were sacrificed, the blood was sampled by puncturing of the inferior vena cava, and the prostates were excised and weighed. The oxidative stress and apoptosis were analyzed in the serum and prostates from all of the rats.
3. Measurement of oxidative stress in serum
To evaluate the oxidative stress, we measured the total activity of superoxide dismutase (SOD). The total activity of SOD in serum was determined using a SOD assay kit-WST (Dojindo Molecular Technologies, Kumamoto, Japan), which estimates SOD activity by measuring the inhibition of xanthine oxidase activity. Briefly, in a 96-well plate, 20 µL of sample solution was added to each sample and blank #2 well, and 20 µL of double distilled water was added to each blank #1 and blank #3 well. Then 200 µL of WST working solution was added to each well. After mixing, 20 µL of dilution buffer was added to each blank #2 and blank #3 well, and 20 µL of enzyme working solution (15 µL of enzyme mixed with 2.5 µL dilution buffer) was added to each sample and blank #1 well. The plate was incubated at 37℃ for 20 minutes, and the optical density was determined at 450 nm using a microplate reader (Bio-Rad Model 550; Bio-Rad Laboratories, Hercules, CA, USA). The SOD-like activity was calculated by the following equation: SOD activity (%)=[{(A blank1-A blank3)-(A sample-A blank2)}/(A blank1-A blank3)]×100.
4. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay
To assess apoptosis in the prostate tissues, a terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay was performed using the TMR red in situ cell death detection kit (Roche Diagnostics, Mannheim, Germany). Tissue preparation for the detection of apoptotic bodies was performed according to the manufacturer's protocol. After the TUNEL assay, prostate tissue sections were examined using a fluorescent microscope. The apoptotic index was calculated as the ratio of the number of cells per field stained with 4,6-diamidino-2-phenylindole and the number of cells per field with red fluorescence (TUNEL positive).
5. Data analysis
The data were analyzed statistically and expressed as the mean±standard deviation. The statistical analysis was performed using SPSS version 12.0 for Microsoft Windows software (SPSS Inc., Chicago, IL, USA). The Kruskal-Wallis test and Mann-Whitney U test were used for statistical analysis. The level of significance was set at p<0.05.
RESULTS
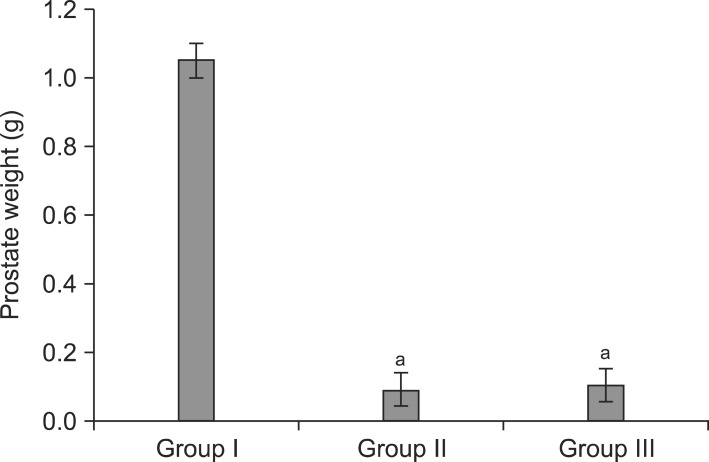
1. Mean prostate weight of andropause and andropause treated with anthocyanin
The mean prostate weights of Group II and III were significantly lower than that of Group I (p<0.001). However, there was no significant difference between Group II and III (p=0.078) (Table 1, Fig. 1).
Table 1.
Changes in prostate weight, activity of SOD and the apoptotic index in the experimental groups
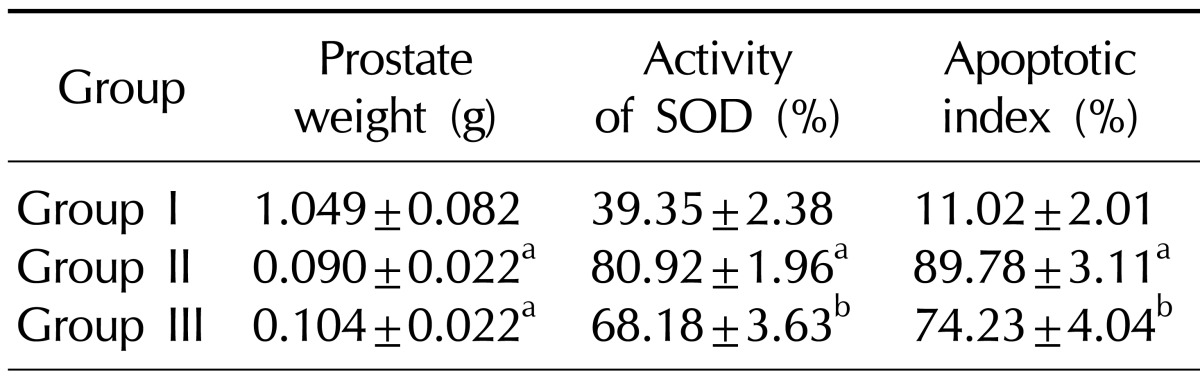
Values are presented as mean±standard deviation.
Group I: control group, Group II: andropause, Group III: andropause treated with anthocyanin.
SOD: superoxide dismutase.
aSignificant difference (p<0.05) compared with the Group I. bSignificant difference (p<0.05) compared with the Group II.
Fig. 1.
Prostate weights in each experimental groups. Group I: control group, Group II: andropause, Group III: andropause treated with anthocyanin. aSignificant difference (p<0.05) compared with the Group I.
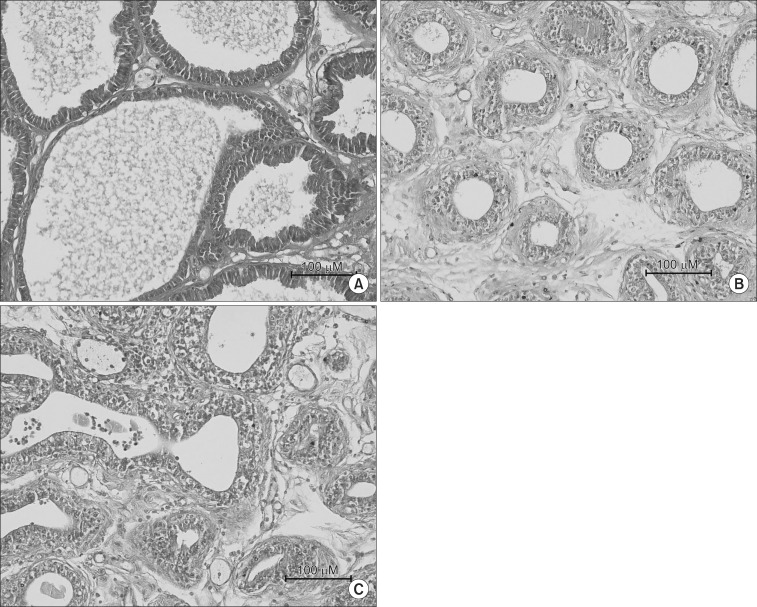
2. Pathohistological findings
In Group I, one layer of low columnar epithelial cells formed a secretory lumen, and the lumen was filled with thin acidophilic materials. Undeveloped epithelial cells forming the prostate gland were arranged as a single layer (Fig. 2A). In Group II and III, all acini of the prostate gland were diffusely atrophic. In Group II, each atrophic acinus formed a relatively defined round shape and were separated from each other by a thick fibrohyaline collar and stromal fibrosis (Fig. 2B). In contrast with Group II, Group III showed variably sized and shaped acini closely packed together and lined by atrophic epithelium. The fibrohyaline collar and stromal fibrosis separating each acinus were reduced (Fig. 2C).
Fig. 2.
H&E stain of the prostate in each experimental groups. (A) It shows normal prostate gland (Group I). (B) All acini of the prostate gland were diffusely atrophic. Each atrophic acini formed a relatively certain round shape and were separated by thick fibrohyaline collar and stromal fibrosis (Group II). (C) All acini of the prostate gland were diffusely atrophic. The variable sized and shaped acini closely packed together and lined by atrophic epithelium. Also fibrohyaline collar and stromal fibrosis, separated each acini, were decreased (Group III).
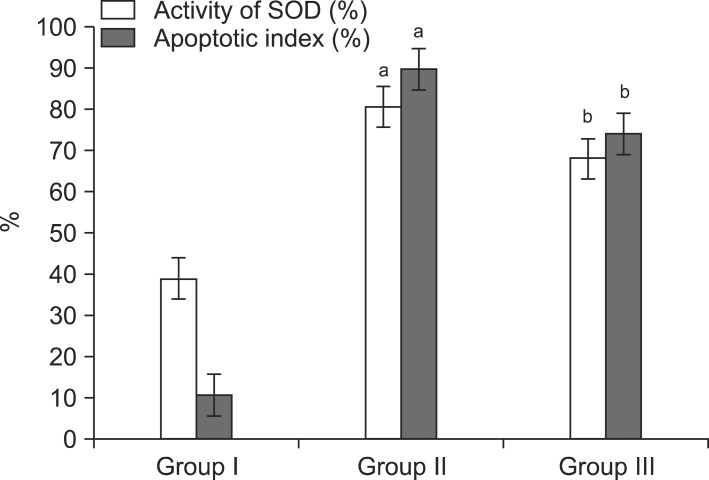
3. Comparison of oxidative stress
The level of oxidative stress in serum was assessed by measuring the total activity of the SOD. A significant increase in oxidative stress was found in Group II compared with Group I (p<0.001). In Group III, however, the oxidative stress was significantly improved compared with Group II (p<0.001) (Table 1, Fig. 3).
Fig. 3.
Comparisons of the activity of superoxide dismutase (SOD) and apoptotic index in each experimental groups. Group I: control group, Group II: andropause, Group III: andropause treated with anthocyanin. aSignificant difference (p<0.05) compared with the Group I. bSignificant difference (p<0.05) compared with the Group II.
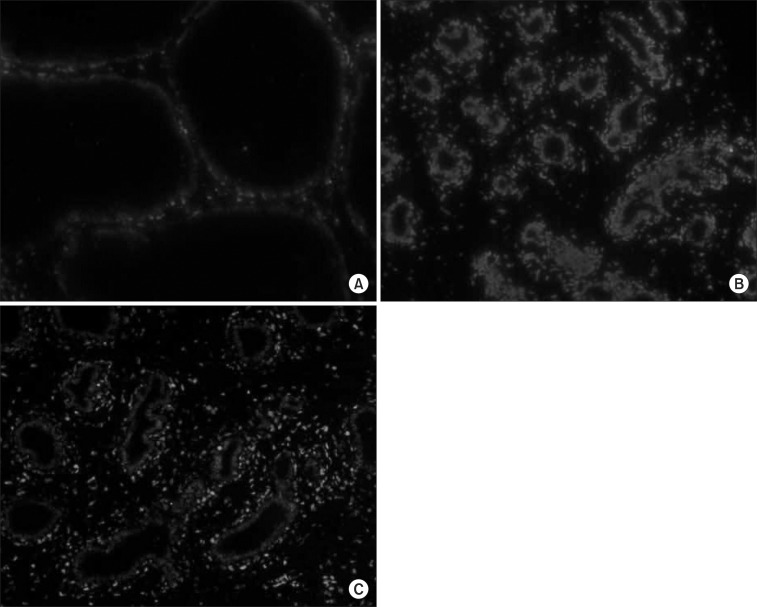
4. TUNEL assay for apoptosis
Group II exhibited a mean apoptotic index that was higher than that of Group I (p<0.001). Compared with Group II, significantly fewer cells were stained positively in the TUNEL assay in Group III (Table 1) (Fig. 3). Representative images are shown in Fig. 4.
Fig. 4.
Terminal deoxyribonucleotidyl transferase-mediated dUTP-digoxigenin nick end-labelling (TUNEL) stain in each experimental groups (×200). (A) A fewer cells were stained positively (Group I). (B) Nearly almost cells were stained positively (Group II). (C) Positively stained cells were decreased in Group III compared with Group II.
DISCUSSION
The prostate is a male exocrine sexual accessory organ composed of secretory and non-secretory epithelial cells, fibroblasts, smooth muscle, nerves, and endothelial cell types interacting to form a branching ductal network [19]. It requires androgenic steroids including testosterone and DHT for its appropriate embryological formation and postpubertal growth, and once at adult size, remains dependent on a continuous supply of androgens for its vitality and function [20]. A reduction of the levels of circulating androgens will rapidly induce apoptosis of the cells of the prostate, leading to extensive glandular regression [8]. Given that prostate growth is entirely dependent on the level of testosterone and that castration by bilateral orchiectomy leads to serum testosterone levels below 1 ng/mL [21] similar to a state of andropause, we concluded that the evaluation of apoptosis and oxidative stress in the prostate were appropriate for our study aims of evaluating the effect of anthocyanin in andropause.
In this study, we hypothesized that with aging, oxidative stress in the human body is increased, and when oxidative injury caused by free radicals cannot be prevented by the antioxidant system, aging occurs [22] and this leads to cellular dysfunction or cell death in various organs including the Leydig cells of the testes. This change, causes decreased testosterone production, which may lead to atrophy of the prostate by the activation of the critical signaling pathway of apoptosis. Furthermore, reduction of the levels of circulating testosterone leads to increasing oxidative stress, and we thought that this condition might be another inducer of apoptosis. This apoptosis may induce andropause and a decrease in prostate weight. In addition, we presumed that when oxidative stress accumulates and protection by endogenous antioxidants is insufficient for maintaining optimal cellular function, it is necessary to supply exogenous antioxidants [23] to help protect the human body from oxidative injury and possibly suppress the progression of aging. Supplementation of antioxidants may also have a protective effect on the oxidative stress induced by a low level of testosterone, and this may prevent rapid cell death in the prostate by activating apoptosis.
The main findings of the present study were as follows: (1) Prostate weight was markedly decreased after bilateral orchiectomy. However, the supplementation of anthocyanin did not prevent the reduction of prostate weight; (2) oxidative stress was markedly increased after bilateral orchiectomy but was reduced after supplementation with anthocyanin; (3) apoptosis was also significantly increased after bilateral orchiectomy, but anthocyanin supplementation reduced the induction of apoptosis in the prostate.
Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell including proteins, lipids, and DNA. This is thought to result in the loss of cellular and tissue function.
Several studies have shown that oxidative stress developed with low testosterone [12]. In our study, oxidative stress was increased by bilateral orchiectomy, and this condition was then improved after supplementation with anthocyanin. We thus concluded that castration by bilateral orchiectomy results in oxidative stress, and the improvement in oxidative stress was due to the antioxidant effect of the anthocyanin.
In this study, we used anthocyanin as an exogenous antioxidant. As described in the introduction, anthocyanin has an antioxidant effect and is known to be a novel antioxidant. It is also known that anthocyanin accelerates the antioxidant response element-regulated phase II enzyme activity, which is important for the protection of normal cells from oxidative stress [24]. We concluded that the reduction of oxidative stress after anthocyanin supplementation observed in our study was likely due to these actions of anthocyanin.
In this study, the apoptotic index was significantly higher in Group II than Group I and was significantly reduced in Group III.
Apoptosis, or programmed cell death, is a naturally occurring cell death process, essential for the normal development and homeostasis of all multicellular organisms [25]. It can be triggered by numerous factors including receptor-mediated signals, withdrawal of growth factors, and anti-tumor drugs; under certain conditions, DNA damage. Also the oxidative stress is known as one of these triggers [13-15].
In apoptosis by castration, several reports have shown that a critical cell signaling pathway, regulated by c-Fos expression [26,27], is present, and this signaling induces an abrupt and transient alteration in the synthesis of Fas antigen, p53, the Bax and Bcl-2 proteins in the androgen receptor-expressing prostate epithelial cells [28-30]. It is also believed that the above changes after castration participate in inducing the activation of apoptosis and result in decreasing the prostate weight.
In our study, oxidative stress and apoptosis were decreased but the prostate weight was not improved after anthocyanin supplementation. Given all these results, we concluded that the apoptosis in the prostate of the castrated rat must be mainly mediated by the activation of the critical signaling pathway of apoptosis induced by a low level of testosterone. We also suggest that the oxidative stress induced by low testosterone may be another inducer of apoptosis, and this may partly contribute to the overall apoptosis in the prostate of the castrated rat. We expected that if oxidative stress participates directly or indirectly in the critical signaling pathway of apoptosis, the prostate weight should be improved after anthocyanin supplementation. However, the prostate weight was not improved. This may indicate that oxidative stress induces apoptosis independently from the activation of the critical signaling pathway of apoptosis. In addition, it may be that the prostate weight was not improved because supplementation with anthocyanin may have a limited effect on the extent of apoptosis induced by oxidative stress among all the factors triggering apoptosis in the prostate of the castrated rat.
We note that our study has some limitations. First, the relationship between apoptosis and the administration of anthocyanin in our previous study and the present study were opposite. In our previous study, we reported that the administration of anthocyanin in rats with induced prostatic hyperplasia increased the induction of apoptosis and resulted in the reduction of the prostate weight [18]. However, in the present study, we found that the supplementation of anthocyanin in the rat model of andropause reduced oxidative stress and decreased the induction of apoptosis. These conflicting results may have arisen from the homeostasis of the living organism. Homeostasis is a process in which the body's internal environment is kept stable, despite changes by external stimulation. Benign prostatic hyperplasia (BPH) and the rapid cell death of the prostate in andropause may be interpreted as an unstable condition. To maintain a stable condition, a series of processes would be needed such as anti-proliferation and an increase in apoptosis with BPH and a decrease in apoptosis with andropause. Furthermore, the antioxidant effect of anthocyanin may be related to those processes. Therefore, we presumed that a living organism may use a given substance to maintain a stable condition and the results of its application, according to the state of the unstable condition (proliferation or apoptosis), could be the opposite under differing conditions despite the same mechanism of the substance. If anthocyanin functions as a stabilizing agent, this could explain the conflicting results of our two studies. To confirm this hypothesis, future studies should involve more in-depth experiments to identify the relationship between homeostasis and the series of processes that occur during the treatment of andropause.
Second, oxidative stress may be another trigger for apoptosis, and the antioxidant effect of anthocyanin might reduce the apoptosis induced by oxidative stress. It is obvious that oxidative stress can be one of the triggering factors of apoptosis [13-15] and under certain conditions, anthocyanin has an antioxidant effect [16,17]. However, the mechanism of apoptosis in the prostate of the castrated rat has not yet been clearly elucidated, and anthocyanin has a varying association with apoptosis. Therefore, to assess the usefulness of anthocyanin as an exogenous antioxidant in andropause, further investigation of the mechanism of apoptosis that occurs with low testosterone and the specific effects of anthocyanin in that context are needed. A better understanding of these relationships may lead to a clinical application of an antioxidant that would be effective in preventing rapid cell death in andropause.
CONCLUSIONS
The findings of this study show that the oxidative stress induced by a low level of testosterone may be one trigger of apoptosis in the prostate in an andropause animal model. Although anthocyanin supplementation did not prevent apoptosis enough to improve decreasing prostate weight, the anti-apoptotic effect induced by the antioxidant reaction of anthocyanin may have still been available at the cellular level. We believe that anthocyanin supplementation could contribute to preventing rapid cell death by apoptosis in andropause.
ACKNOWLEDGEMENTS
This work was supported by a grant from the Next-Generation Biogreen 21 Program (No. PJ009546), Rural Development Administration, Republic of Korea.
References
- 1.Arias E. United States life tables, 2007. Natl Vital Stat Rep. 2011;59:1–60. [PubMed] [Google Scholar]
- 2.Schubert M, Jockenhövel F. Late-onset hypogonadism in the aging male (LOH): definition, diagnostic and clinical aspects. J Endocrinol Invest. 2005;28:23–27. [PubMed] [Google Scholar]
- 3.Morales A. Andropause (or symptomatic late-onset hypogonadism): facts, fiction and controversies. Aging Male. 2004;7:297–303. doi: 10.1080/13685530400016664. [DOI] [PubMed] [Google Scholar]
- 4.Staerman F, Léon P. Andropause (androgen deficiency of the aging male): diagnosis and management. Minerva Med. 2012;103:333–342. [PubMed] [Google Scholar]
- 5.Mahmoud A, Comhaire FH. Mechanisms of disease: late-onset hypogonadism. Nat Clin Pract Urol. 2006;3:430–438. doi: 10.1038/ncpuro0560. [DOI] [PubMed] [Google Scholar]
- 6.Nieschlag E, Swerdloff R, Behre HM, Gooren LJ, Kaufman JM, Legros JJ, et al. Investigation, treatment and monitoring of late-onset hypogonadism in males. Aging Male. 2005;8:56–58. doi: 10.1080/13685530500130969. [DOI] [PubMed] [Google Scholar]
- 7.Lunenfeld B, Saad F, Hoesl CE. ISA, ISSAM and EAU recommendations for the investigation, treatment and monitoring of late-onset hypogonadism in males: scientific background and rationale. Aging Male. 2005;8:59–74. doi: 10.1080/13685530500163416. [DOI] [PubMed] [Google Scholar]
- 8.English HF, Drago JR, Santen RJ. Cellular response to androgen depletion and repletion in the rat ventral prostate: autoradiography and morphometric analysis. Prostate. 1985;7:41–51. doi: 10.1002/pros.2990070106. [DOI] [PubMed] [Google Scholar]
- 9.Sandford NL, Searle JW, Kerr JF. Successive waves of apoptosis in the rat prostate after repeated withdrawal of testosterone stimulation. Pathology. 1984;16:406–410. doi: 10.3109/00313028409084731. [DOI] [PubMed] [Google Scholar]
- 10.Berges RR, Furuya Y, Remington L, English HF, Jacks T, Isaacs JT. Cell proliferation, DNA repair, and p53 function are not required for programmed death of prostatic glandular cells induced by androgen ablation. Proc Natl Acad Sci U S A. 1993;90:8910–8914. doi: 10.1073/pnas.90.19.8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buttyan R, Shabsigh A, Perlman H, Colombel M. Regulation of apoptosis in the prostate gland by androgenic steroids. Trends Endocrinol Metab. 1999;10:47–54. doi: 10.1016/s1043-2760(98)00104-0. [DOI] [PubMed] [Google Scholar]
- 12.Mancini A, Leone E, Festa R, Grande G, Silvestrini A, de Marinis L, et al. Effects of testosterone on antioxidant systems in male secondary hypogonadism. J Androl. 2008;29:622–629. doi: 10.2164/jandrol.107.004838. [DOI] [PubMed] [Google Scholar]
- 13.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::aid-cncr2820730802>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 14.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 15.Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 16.Francis FJ. Food colorants: anthocyanins. Crit Rev Food Sci Nutr. 1989;28:273–314. doi: 10.1080/10408398909527503. [DOI] [PubMed] [Google Scholar]
- 17.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 18.Jang H, Ha US, Kim SJ, Yoon BI, Han DS, Yuk SM, et al. Anthocyanin extracted from black soybean reduces prostate weight and promotes apoptosis in the prostatic hyperplasia-induced rat model. J Agric Food Chem. 2010;58:12686–12691. doi: 10.1021/jf102688g. [DOI] [PubMed] [Google Scholar]
- 19.Kumar VL, Majumder PK. Prostate gland: structure, functions and regulation. Int Urol Nephrol. 1995;27:231–243. doi: 10.1007/BF02564756. [DOI] [PubMed] [Google Scholar]
- 20.Lee C, Sensibar JA, Dudek SM, Hiipakka RA, Liao ST. Prostatic ductal system in rats: regional variation in morphological and functional activities. Biol Reprod. 1990;43:1079–1086. doi: 10.1095/biolreprod43.6.1079. [DOI] [PubMed] [Google Scholar]
- 21.Ward GR, Abdel-Rahman AA. Effect of testosterone replacement or duration of castration on baroreflex bradycardia in conscious rats. BMC Pharmacol. 2005;5:9. doi: 10.1186/1471-2210-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sastre J, Pallardó FV, García de la Asunción J, Viña J. Mitochondria, oxidative stress and aging. Free Radic Res. 2000;32:189–198. doi: 10.1080/10715760000300201. [DOI] [PubMed] [Google Scholar]
- 23.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2:219–236. [PMC free article] [PubMed] [Google Scholar]
- 24.Shih PH, Yeh CT, Yen GC. Effects of anthocyanidin on the inhibition of proliferation and induction of apoptosis in human gastric adenocarcinoma cells. Food Chem Toxicol. 2005;43:1557–1566. doi: 10.1016/j.fct.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Schwartzman RA, Cidlowski JA. Apoptosis: the biochemistry and molecular biology of programmed cell death. Endocr Rev. 1993;14:133–151. doi: 10.1210/edrv-14-2-133. [DOI] [PubMed] [Google Scholar]
- 26.Buttyan R, Zakeri Z, Lockshin R, Wolgemuth D. Cascade induction of c-fos, c-myc, and heat shock 70K transcripts during regression of the rat ventral prostate gland. Mol Endocrinol. 1988;2:650–657. doi: 10.1210/mend-2-7-650. [DOI] [PubMed] [Google Scholar]
- 27.Marti A, Jehn B, Costello E, Keon N, Ke G, Martin F, et al. Protein kinase A and AP-1 (c-Fos/JunD)are induced during apoptosis of mouse mammary epithelial cells. Oncogene. 1994;9:1213–1223. [PubMed] [Google Scholar]
- 28.Suzuki A, Matsuzawa A, Iguchi T. Down regulation of Bcl-2 is the first step on Fas-mediated apoptosis of male reproductive tract. Oncogene. 1996;13:31–37. [PubMed] [Google Scholar]
- 29.Perlman H, Zhang X, Chen MW, Walsh K, Buttyan R. An elevated bax/bcl-2 ratio corresponds with the onset of prostate epithelial cell apoptosis. Cell Death Differ. 1999;6:48–54. doi: 10.1038/sj.cdd.4400453. [DOI] [PubMed] [Google Scholar]
- 30.Colombel M, Radvanyi F, Blanche M, Abbou C, Buttyan R, Donehower LA, et al. Androgen suppressed apoptosis is modified in p53 deficient mice. Oncogene. 1995;10:1269–1274. [PubMed] [Google Scholar]