Abstract
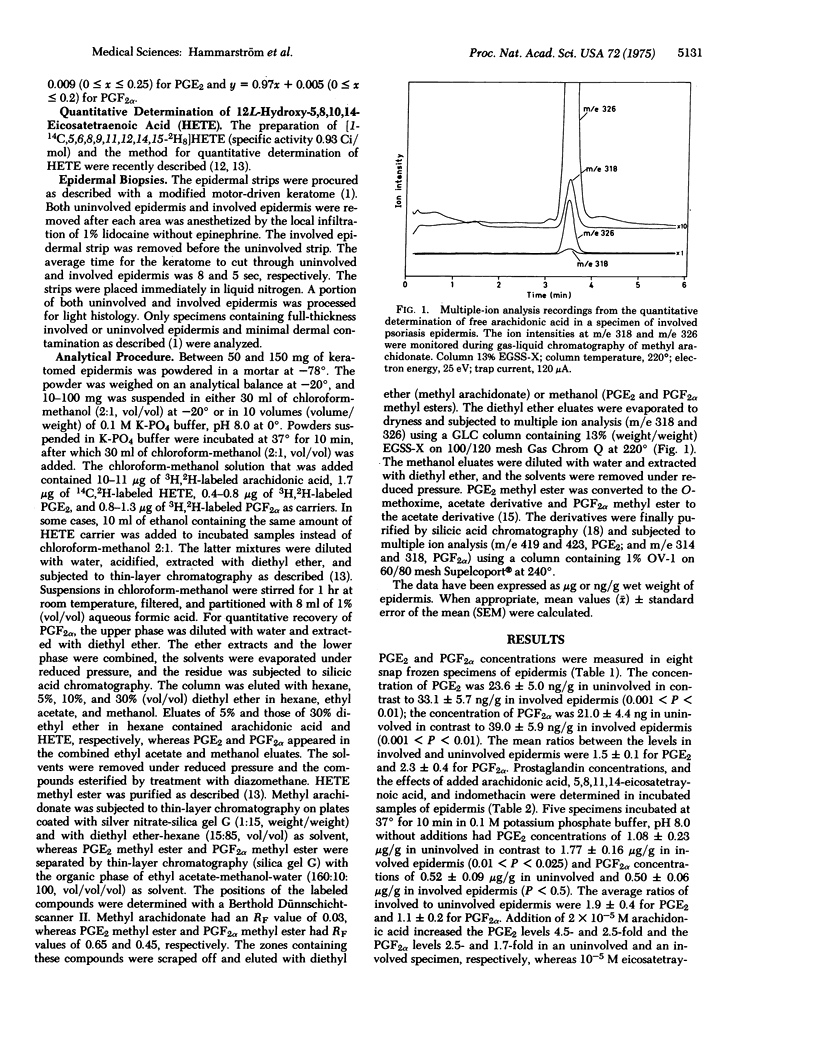
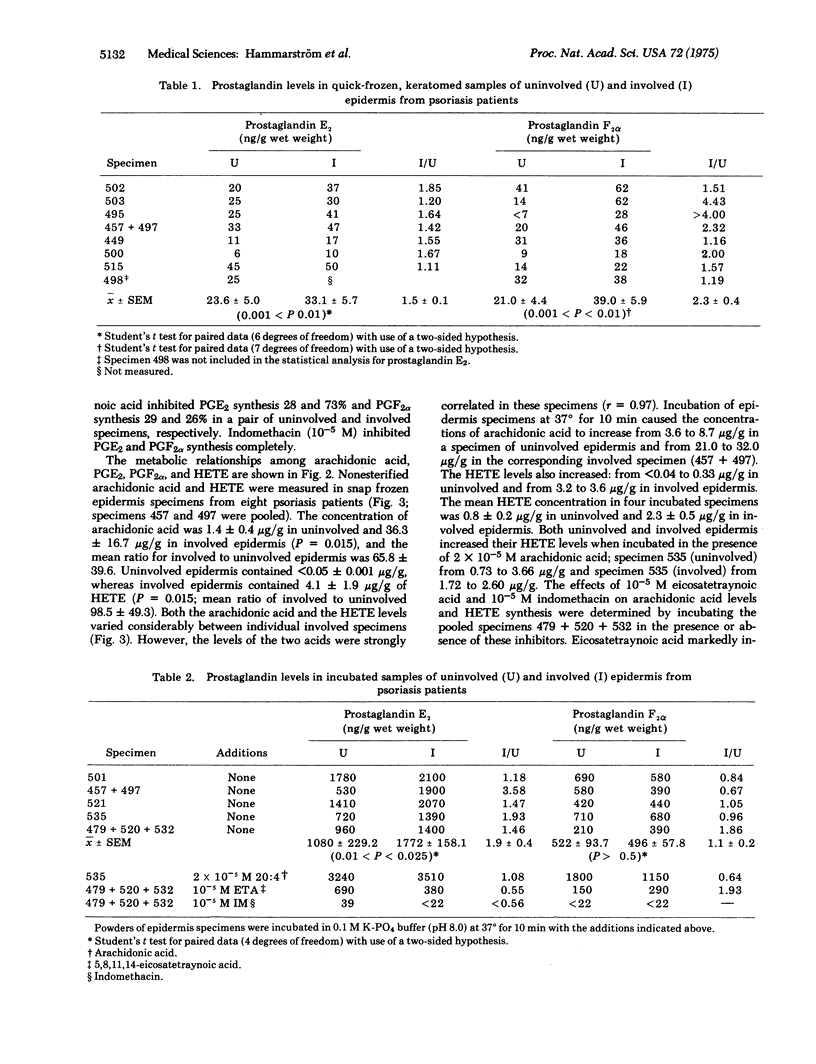
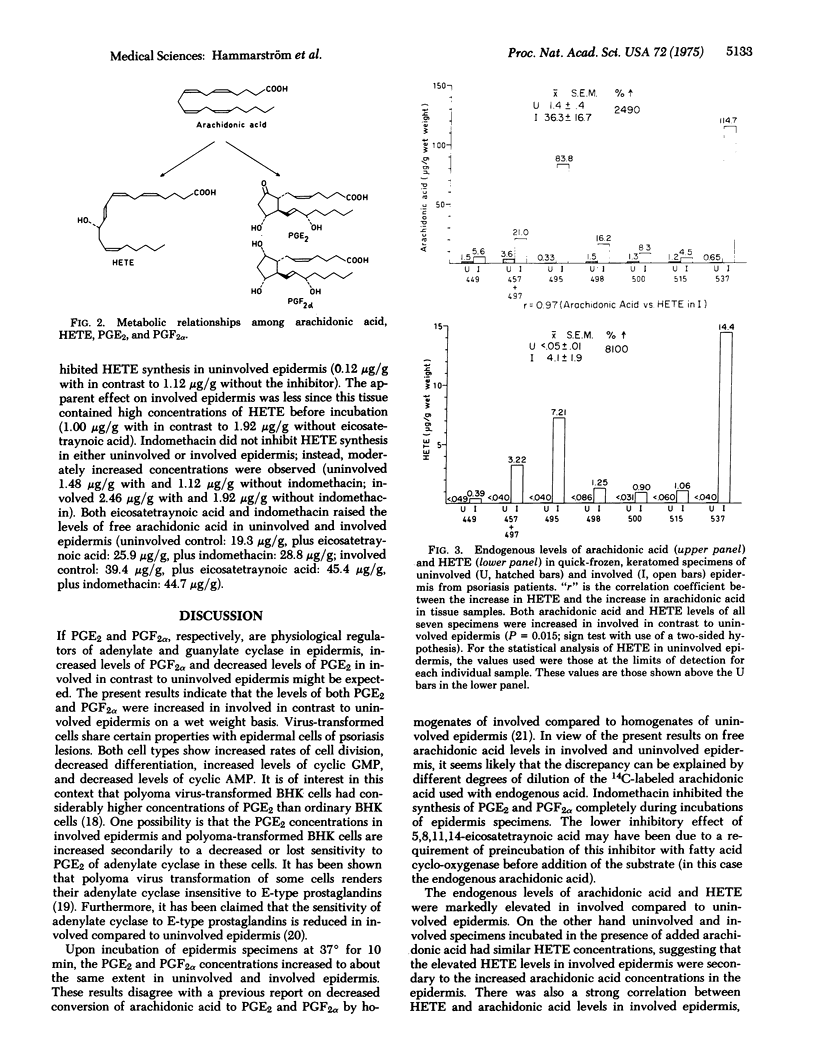
Lesional epidermis of psoriasis has a probable reduction in the cyclic AMP/cyclic GMP ratio. This altered ratio may in part be responsible for the characteristic glycogen storage, rapid cell proliferation, and reduced differentiation in lesional epidermis. The concentrations of prostaglandins E2 and F2alpha, free arachidonic acid, and 12L-hydroxy-5,8,10,14-eicosatetrawnoic acid in specimens of uninvolved and involved epidermis of psoriasis were measured with deuterium-labeled carriers and multiple ion analysis. Snap frozen specimens contained: 1.4 +/- 0.4 mug/g (wet weight) of arachidonic acid in uninvolved in contrast to 36.3 +/- 16.7 mug/g in involved epidermis (P = 0.015); less than 0.05 +/- 0.01 mug/g of hydroxyeicosatetraenoic acid in uninvolved in contrast to 4.1 +/- 1.9 mug/g in involved epidermis (P = 0.015); 23.6 +/- 5.0 ng/g of prostaglandin E2 in uninvolved in contrast to 33.1 +/- 5.7 ng/g in involved epidermis (P less than 0.01); and 21.0 +/- 4.4 ng/g of prostaglandin F2alpha in uninvolved in contrast to 39.0 +/- 5.9 ng/g in involved epidermis (P less than 0.01). The arachidonic acid and hydroxyeicosatetraenoic acid levels in involved epidermis were strongly correlated (r = 0.97). The increased levels of arachidonic acid and 12L-hydroxy-5,8,10,14-eicosatetraenoic acid in involved epidermis may have diagnostic and pathophysiological importance.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aso K., Deneau D. G., Krulig L., Wilkinson D. I., Farber E. M. Epidermal synthesis of prostaglandins and their effect on levels of cyclic adenosine 3', 5'monophosphate. J Invest Dermatol. 1975 May;64(5):326–331. doi: 10.1111/1523-1747.ep12512267. [DOI] [PubMed] [Google Scholar]
- Aso K., Orenberg E. K., Farber E. M. Reduced epidermal cyclic AMP accumulation following prostaglandin stimulation: Its possible role in the pathophysiology of psoriasis. J Invest Dermatol. 1975 Oct;65(4):375–378. doi: 10.1111/1523-1747.ep12607628. [DOI] [PubMed] [Google Scholar]
- Dunham E. W., Haddox M. K., Goldberg N. D. Alteration of vein cyclic 3':5' nucleotide concentrations during changes in contractility. Proc Natl Acad Sci U S A. 1974 Mar;71(3):815–819. doi: 10.1073/pnas.71.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN K., SAMUELSSON B. SYNTHESIS OF TRITIUM-LABELED PROSTAGLANDIN E-2 AND STUDIES ON ITS METABOLISM IN GUINEA PIG LUNG. 37. PROSTAGLANDINS AND RELATED FACTORS. J Biol Chem. 1965 May;240:1932–1940. [PubMed] [Google Scholar]
- Granström E., Samuelsson B. The structure of the main urinary metabolite of prostaglandin F2-alpha in the guinea pig. Eur J Biochem. 1969 Oct;10(3):411–418. doi: 10.1111/j.1432-1033.1969.tb00705.x. [DOI] [PubMed] [Google Scholar]
- Gréen K., Granström E., Samuelsson B., Axen U. Methods for quantitative analysis of PGF2 , PGE2, 9 , 11 -dihydroxy-15-keto-prost-5-enoic acid and 9 , 11 , 15-trihydroxy-prost-5-enoic acid from body fluids using deuterated carriers and gas chromatography--mass spectrometry. Anal Biochem. 1973 Aug;54(2):434–453. doi: 10.1016/0003-2697(73)90373-4. [DOI] [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Niehaus W. G., Jr, Samuelsson B. Preparation, isolation, and characterization of a derivative of malonaldehyde. Anal Biochem. 1968 Jan;22(1):145–153. doi: 10.1016/0003-2697(68)90268-6. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Prostaglandin endoperoxides. A new concept concerning the mode of action and release of prostaglandins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3824–3828. doi: 10.1073/pnas.71.10.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarström S., Samuelsson B., Bjursell G. Prostaglandin levels in normal and transformed baby-hamster-kidney fibroblasts. Nat New Biol. 1973 May 9;243(123):50–51. [PubMed] [Google Scholar]
- Johnson G. S., Pastan I. Change in growth and morphology of fibroblasts by prostaglandins. J Natl Cancer Inst. 1971 Dec;47(6):1357–1364. [PubMed] [Google Scholar]
- Jonsson C. E., Anggård E. Biosynthesis and metabolism of prostaglandin E 2 in human skin. Scand J Clin Lab Invest. 1972 May;29(3):289–296. doi: 10.3109/00365517209080244. [DOI] [PubMed] [Google Scholar]
- Lands W. E., Samuelsson B. Phospholipid precursors of prostaglandins. Biochim Biophys Acta. 1968 Oct 22;164(2):426–429. doi: 10.1016/0005-2760(68)90168-9. [DOI] [PubMed] [Google Scholar]
- Otten J., Johnson G. S., Pastan I. Cyclic AMP levels in fibroblasts: relationship to growth rate and contact inhibition of growth. Biochem Biophys Res Commun. 1971 Sep;44(5):1192–1198. doi: 10.1016/s0006-291x(71)80212-7. [DOI] [PubMed] [Google Scholar]
- Peery C. V., Johnson G. S., Pastan I. Adenyl cyclase in normal and transformed fibroblasts in tissue culture. Activation by prostaglandins. J Biol Chem. 1971 Sep 25;246(18):5785–5790. [PubMed] [Google Scholar]
- Seifert W. E., Rudland P. S. Possible involvement of cyclic GMP in growth control of cultured mouse cells. Nature. 1974 Mar 8;248(5444):138–140. doi: 10.1038/248138a0. [DOI] [PubMed] [Google Scholar]
- Sheppard J. R. Difference in the cyclic adenosine 3',5'-monophosphate levels in normal and transformed cells. Nat New Biol. 1972 Mar 1;236(61):14–16. doi: 10.1038/newbio236014a0. [DOI] [PubMed] [Google Scholar]
- Voorhees J. J., Duell E. A., Stawiski M., Harrell E. R. Cyclic nucleotide metabolism in normal and proliferating epidermis. Adv Cyclic Nucleotide Res. 1974;4(0):117–162. [PubMed] [Google Scholar]
- Voorhees J. J., Marcelo C. L., Duell E. A. Cyclic AMP, cyclic GMP, and glucocorticoids as potential metabolic regulators of epidermal proliferation and differentiation. J Invest Dermatol. 1975 Jul;65(1):179–190. doi: 10.1111/1523-1747.ep12598125. [DOI] [PubMed] [Google Scholar]
- Ziboh V. A., Hsia S. L. Prostaglandin E 2 : biosynthesis and effects on glucose and lipid metabolism in rat skin. Arch Biochem Biophys. 1971 Sep;146(1):100–109. doi: 10.1016/s0003-9861(71)80046-2. [DOI] [PubMed] [Google Scholar]



