Abstract
Objective
Admission hyperglycemia in acute myocardial infarction (MI) is related with increased in-hospital and long term mortality and major cardiac adverse events. We aimed to investigate how admission hyperglycemia affects the short and long term outcomes in elderly patients (> 65 years) after primary percutaneous coronary intervention for ST elevation myocardial infarction.
Methods
We retrospectively analyzed 677 consecutive elderly patients (mean age 72.2 ± 5.4). Patients were divided into two groups according to admission blood glucose levels. Group 1: low glucose group (LLG), glucose < 168 mg/dL; and Group 2: high glucose group (HGG), glucose > 168 mg/dL.
Results
In-hospital, long term mortality and in-hospital major adverse cardiac events were higher in the high admission blood glucose group (P < 0.001). Multivariate regression analysis showed: Killip > 1, post-thrombolysis in MI < 3 and admission blood glucose levels were independent predictors of in-hospital adverse cardiac events (P < 0.001).
Conclusions
Admission hyperglycemia in elderly patients presented with ST elevation myocardial infarction is an independent predictor of in-hospital major adverse cardiac events and is associated with in-hospital and long term mortality.
Keywords: Admission hyperglycemia, Elderly patients, In-hospital mortality, Long-term mortality, Major adverse cardiac events
1. Introduction
It has been shown that hyperglycemia, also called stress hyperglycemia or stress diabetes, associated with critical illness, even in patients without diabetes mellitus (DM), is a consequence of many factors, including increased cortisol, catecholamines, glucagon, growth hormone, gluconeogenesis, and glycogenolysis.[1] Acute hyperglycemia on admission is common among patients with ST-elevation myocardial infarction (STEMI) and is one of the important predictors of in-hospital and long-term adverse events.[2]–[7] Although previously diagnosed DM has been only 20% to 25% in patients with STEMI, the rate of hyperglycemia may reach up to 50%.[4] When oral glucose tolerance testing is applied to acute myocardial infarction (MI) patients without a history of DM, the prevalence of type-2 DM and impaired glucose tolerance may exceed 65%.[8] Aging is characterized with reduced response capacity to various unusual conditions. The fact that glucose levels after glucose loading tend to be higher in healthy elderly compared to younger subjects render these patients prone to stress diabetes.
Along with the global aging phenomenon, elderly patients represent an increasing population in MI patients and there is limited data on how admission blood glucose (ABG) levels affect the prognosis in this population. The aim of this study was to assess whether ABG levels in STEMI patients older than 65 years is a prognostic indicator of in-hospital and long-term adverse cardiovascular events.
2. Methods
2.1. Patient population
In a prospective design, 677 consecutive patients, who were ≥ 65 years and underwent primary percutaneous coronary intervention (PCI) for STEMI in our thoracic and cardiovascular surgery center between October 2003 and March 2008 were included. The study inclusion criteria were as follows: electrocardiography (ECG) revealing STEMI, defined as more than 30 min of continuous typical chest pain and ST-segment elevation of at least 2 mm in two contiguous ECG leads and/or left bundle branch block within 12 h of symptom onset or up to 18 h if there was evidence of continuing ischemia or hemodynamic instability. Patients were excluded if thrombolytic agents were given for the index STEMI, if they were in cardiogenic shock, had a history of stroke within a month, had end-stage renal disease, or had a life expectancy of < 1 year from a non-cardiac condition. The study protocol was approved by the Ethics Committee of our hospital.
2.2. Analysis of patient data
The demographic information of patients, cardiovascular history and risk factors [smoking, hypercholesterolemia, hypertension (HT) and DM] were obtained from the medical records. A 12-lead ECG was recorded on each patient just after hospital admission, and also the MI type was recorded from ECGs. Blood values, which were determined at hospital admission and on a daily basis during patient stay in hospital, were recorded from medical reports. Results of the first laboratory examination during the index event were used in all statistical analyses, except for the lipid profiles which were recorded from eight hour fasting period blood samples.
2.3. Coronary angiography, primary angioplasty and stenting
All patients received chewable aspirin (300 mg) and clopidogrel (300 mg loading dose) before coronary angiography. Prasugrel and ticagrelor were not prescribed due to unavailability and reimbursement rules of the Ministry of Health. Angiographic data of the patients were evaluated from catheter laboratory records. Emergency coronary angiography and angioplasty were performed by percutaneous femoral approach. Heparin (10000 IU) was administered when arterial access was secured. After visualizing the left and right coronary arteries, 2.5 mg of nitrate was selectively injected into the infarct related artery (IRA) to rule out possible coronary spasm. Angiographic assessments were made by visual assessment. IRA was graded according to thrombolysis in MI (TIMI) classification.[9] Primary angioplasty, including balloon angioplasty and/or stent implantation, was performed only for IRA according to the lesion type. For each procedure, interventional success at the acute phase was defined as reducing obstruction or stenosis to less than 50% of the IRA with TIMI 2 or 3 flow just after primary angioplasty. After angioplasty, all patients were admitted to the coronary intensive care unit, where 500 U/h of intravenous heparin or 1 mg/kg per day of subcutaneous low-molecular weight heparin were given; 100 mg aspirin and 75 mg clopidogrel were continued in all patients. Tirofiban is the only glycoprotein IIb/IIIa inhibitor used in our institution. Tirofiban was used upon high thrombus burden, thrombotic complications or slow or no-reflow conditions. Concomitant medical treatment with beta-blockers, angiotensin converting enzyme inhibitors, and statins were prescribed according to American College of Cardiology/American Heart Association guidelines. Loading doses of statins were not used.
2.4. Definition
Patients were evaluated according to Killip clinical examination classification.[10] Multi-vessel disease was defined as a presence of more than 50% lesion in at least two major epicardial coronary arteries, or left main coronary artery lesions. Patient with DM was defined as any individual with documented DM using either oral hypoglycemic agents, or insulin treatment at admission. Hypercholesterolemia was defined as total cholesterol ≥ 200 mg/dL or use of cholesterol-lowering agents. Anemia was defined as a baseline hemoglobin concentration less than 13 g/dL in men and less than 12 g/dL in women. Cardiovascular death was defined as unexplained sudden death, death owing to acute MI, heart failure, or arrhythmia. Repeat target vessel revascularization (TVR) was defined as the need of PCI, or coronary surgery, because of re-stenosis or re-occlusion of the IRA. Re-infarction was defined as an increase of creatinine kinase-MB (CK-MB) fraction more than two fold of the last value of CK-MB level and ST segment re-elevations. Long term follow-up is defined as > 1 year.
2.5. Follow-up
Follow-up data were obtained from hospital records, or by interviewing the patients (directly or by telephone), their families, or their personal physicians. Major adverse cardiac events (MACEs) were defined as cardiovascular death, re-infarction, or TVR (percutaneous or surgical). Only cardiovascular mortality was recorded.
2.6. Statistical analysis
Quantitative variables were expressed as mean ± SD, and qualitative variables were expressed as percent (%). Comparison of parametric values between two groups was performed using the Student's t-test. Categorical variables were compared by the Chi-square test or Fisher's exact test.
Backward, stepwise, multivariate Cox regression analysis, which included variables with a P value of less than 0.1, was performed to identify independent predictors of in-hospital mortality. Smoking, Killip class > 1, DM, post TIMI < 3, ABG and admission creatinine were included in the model.
The cumulative survival curves for long-term cardiovascular mortality were constructed with the use of the Kaplan-Meier method with differences assessed with the log-rank test. A two sided P value of less than 0.05 was considered statistically significant. All statistical studies were carried out with SPSS program (version 15.0, SPSS, Chicago, Illinois, USA).
3. Results
The data from 677 patients with a mean age of 72.2 ± 5.4 years old who underwent primary PCI for STEMI were assessed. Mean available follow-up time was 18.8 months. Long term follow-up of eight patients in the low glucose group (LGG) and four patients in the high glucose group (HGG) were not included due to communications problems. The subjects were analyzed in subgroups according to ABG measurements (low glucose group, glucose < 168 mg/dL and high glucose group, glucose > 168 mg/dL). The baseline demographical, clinical, and laboratory data are demonstrated in Tables 1–3. The HGG contained more women, diabetics, smokers, and Killip class > 1 patients (P < 0.001, P < 0.001, P = 0.04, and P = 0.001, respectively). Pain onset to balloon time was longer in HGG (P = 0.03). Peak CK-MB level and white blood cell (WBC) count were significantly higher in HGG (P < 0.001 for both). Estimated glomerular filtration rate (eGFR) according to the Modification of Diet in Renal Disease (MDRD) formula.[11] (186 × (serum creatinine)−1.154 × (age)−0.203 × 0.742 (for females)) was lower in HGG. The rate of post TIMI < 3 was significantly lower in HGG (P = 0.02). The rate of unsuccessful procedures was higher and left ventricular ejection fraction (LVEF) was lower in HGG (P = 0.005 and P < 0.001, respectively).
Table 1. Demographic and clinical properties and laboratory findings of groups.
| Low-glucose group (n = 457) | High-glucose group (n = 220) | P | |
| Male gender | 327 (71.5%) | 127 (57.7%) | < 0.001 |
| Diabetes mellitus | 76 (16.6%) | 46 (20.9%) | < 0.001 |
| Hypertension | 257 (56.2%) | 133 (60.4%) | 0.32 |
| Hyperlipidemia | 118 (25.8%) | 59 (26.8%) | 0.77 |
| Smoking | 184 (40.2%) | 75 (34%) | 0.04 |
| CABG | 19 (4.1%) | 8 (3.6%) | 0.76 |
| PCI | 33 (7.2%) | 24 (10.9%) | 0.09 |
| Prior MI | 55 (12%) | 30 (13.6%) | 0.55 |
| Killip > 1 | 27 (5.9%) | 46 (20.9%) | 0.001 |
| Age, yrs | 72.2 ± 5.5 | 72 ± 5.2 | 0.64 |
| Pain-balloon time, h | 3.5 ± 2.5 | 4.1 ± 2.8 | 0.03 |
| Door-balloon time, m | 32 ± 5 | 28 ± 4.5 | 0.76 |
| Laboratory findings | |||
| Baseline glucose, mg/dL | 128.6 ± 22.3 | 248.8 ± 91.7 | < 0.001 |
| Baseline creatinine, mg/dL | 1.08 ± 0.54 | 1.18 ± 0.6 | 0.03 |
| Peak CK-MB, U/L | 205.9 ± 170.6 | 267 ± 181.7 | < 0.001 |
| WBC, /mm3 | 10.9 ± 3.2 | 12.9 ± 4.7 | < 0.001 |
| Hemoglobin, g/dL | 12.8 ± 1.7 | 12.4 ± 1.9 | 0.004 |
| Total cholesterol, mg/dL | 178 ± 36.5 | 177.5 ± 42.9 | 0.97 |
| LDL cholesterol, mg/dL | 111.1 ± 28.7 | 110.8 ± 34.9 | 0.93 |
| HDL cholesterol, mg/dL | 42.9 ± 10.3 | 41.4 ± 10.4 | 0.15 |
| Triglyceride, mg/dL | 115.9 ± 55.4 | 122.5 ± 61.8 | 0.22 |
| eGFR (ml/min per 1.73 m2) | 73.4 ± 21.2 | 66.5 ± 23.5 | < 0.001 |
| Anemia | 183 (40%) | 95 (43%) | 0.38 |
Variables are reported as mean ± SD or n (%). CABG: coronary artery by-pass grafting; CK-MB: creatinine kinase-MB; eGFR; estimated glomerular filtration rate; HDL: high density lipoprotein; LDL: low density lipoprotein; MI: myocardial infarction; PCI: primary coronary intervention; WBC: white blood cell count.
Table 3. Comparison of coronary angiographic findings and ejection fraction of groups.
| Low glucose group (n = 457) | High glucose group (n = 220) | P | |
| Unsuccessful procedure | 58 (12%) | 46 (20%) | 0.005 |
| Tirofiban use | 209 (45.7%) | 94 (42.7%) | 0.51 |
| Stent | |||
| Diameter | 3.1 ± 0.35 | 3.04 ± 0.33 | 0.03 |
| Length | 19.2 ± 6.5 | 20.1 ± 7.5 | 0.13 |
| Ejection fraction | 46.8 ± 10.7 | 41.4 ± 12.6 | < 0.001 |
Variables are reported as mean ± SD or n (%).
In-hospital clinical events are listed in Table 4. The rate of in-hospital mortality, MACE, congestive heart failure (CHF), cardio-pulmonary resuscitation (CPR), inotropic usage, and ventricular tachycardia/ventricular fibrillation (VT/VF) were significantly higher in HGG group (P < 0.001 for all). Stroke and the need for hemodialysis were significantly more frequent in HGG group (P = 0.02 and P = 0.03, respectively). Hospital in-stay time was longer (P = 0.04) and the rate of IABP and transient pacemaker usage were higher in HGG group (P = 0.002 and P < 0.001, respectively).
Table 4. Comparison of in-hospital events of groups.
| Low glucose group (n = 457) | High glucose group (n = 220) | P | |
| Death | 23 (5%) | 41 (18%) | < 0.001 |
| Re-infarction | 10 (2%) | 6 (2.7%) | 0.66 |
| TVR | 19 (4%) | 14 (5.4%) | 0.21 |
| MACE | 38 (8%) | 49 (22.2%) | < 0.001 |
| Stroke | 5 (1%) | 8 (3.6%) | 0.02 |
| CPR | 24 (5.2%) | 47 (21.3%) | < 0.001 |
| Hemodialysis | 4 (0.8%) | 7 (3.1%) | 0.03 |
| VT/VF | 14 (3%) | 40 (18.1%) | < 0.001 |
| CHF | 78 (17%) | 72 (32.7%) | < 0.001 |
| Inotrope use | 41 (8.9%) | 56 (25.5%) | < 0.001 |
| IABP | 9 (1.9%) | 45 (20.5%) | < 0.001 |
| Atrial fibrillation | 21 (4.5%) | 10 (4.5%) | 0.06 |
| Transient pacemaker | 21 (4.5%) | 24 (10.9%) | 0.002 |
| Transfusion | 31 (6.7%) | 23 (10.5%) | 0.09 |
| Hospital in-stay (day) | 7.7 ± 4.3 | 8.6 ± 6.1 | 0.04 |
Variables are reported as mean ± SD or n (%). CPR: cardio-pulmonary resuscitation; IABP: intra-aortic balloon pump; MACE: major adverse cardiac event; TVR: target vessel revascularization, VT/VF; ventricular tachycardia/ventricular fibrillation.
Long term follow-up results of the patients are provided in Table 5. Long term follow-up results of eight patients in LGG and four patients of HGG could not be obtained. Death, re-infarction and MACE were significantly higher in HGG (P < 0.001 for all).
Table 5. Comparison of long-term events of groups.
| Low glucose group (n = 426) | High glucose group (n = 175) | P | |
| Death | 41 (9.6%) | 35 (20%) | 0.001 |
| Stroke | 5 (1.1%) | 2 (1.1%) | 0.95 |
| CHF | 50 (11.7%) | 26 (14.9%) | 0.18 |
| Re-infarction | 26 (6.1%) | 24 (13.7%) | 0.001 |
| TVR | 59 (13.8%) | 24 (13.7%) | 0.76 |
| MACE | 95 (22.3%) | 60 (34.2%) | 0.001 |
Variables are reported as n (%). CHF: congestive heart failure; MACE: major adverse cardiac events; TVR: target vessel revascularization.
Results of univariate and multivariate logistic regression analysis for the prediction of in-hospital MACE are shown in Table 6. Univariate regression analysis showed a correlation between DM, smoking, Killip > 1, post TIMI < 3, ABG, admission creatinine, and in-hospital adverse cardiac events.
Table 6. Effects of multiple variables on in-hospital MACE in univariate and multivariate logistic regression analyses.
| Univariate analysis |
Multivariate analysis |
|||||
| OR | CI | P | OR | CI | P | |
| DM | 5.1 | 2.88–9.03 | < 0.001 | |||
| Smoking | 1.8 | 1.066–3.146 | 0.03 | |||
| KILLIP > 1 | 15.6 | 8.3–26.7 | < 0.001 | 7.96 | 3.7–16.9 | < 0.001 |
| Post TIMI < 3 | 9.04 | 5.2–15.7 | < 0.001 | 4.74 | 2.2–10 | < 0.001 |
| ABG (mg/dL) | 1.009 | 1.007–1.012 | < 0.001 | 1.007 | 1.003–1.011 | < 0.001 |
| AC (mg/dL) | 3.1 | 1.94–4.9 | < 0.001 | 1.78 | 1.18–2.7 | 0.006 |
ABG: admission blood glucose; AC: admission creatinine; DM: diabetes mellitus; TIMI: thrombolysis in myocardial infarction.
Table 2. Comparison of studied groups' coronary angiography results.
| Low glucose group (n = 457) | High glucose group (n = 220) | P | |
| Culprit lesion | |||
| LMCA | 0 (0%) | 2 (0.9%) | |
| LAD | 219 (47.9%) | 119 (54%) | |
| CX | 56 (12.3%) | 18 (8.3%) | 0.07 |
| RCA | 179 (39.1%) | 78 (35.5%) | |
| Others | 3 (0.7%) | 3 (1.3%) | |
| Vessel | |||
| 1 | 149 (32.6%) | 67 (30.4%) | |
| 2 | 162 (35.4%) | 71 (32.2%) | 0.37 |
| 3 | 146 (32%) | 82 (37.4%) | |
| Pre TIMI | |||
| 0−1 | 405 (88.6%) | 198 (90%) | |
| 2 | 35 (7.6%) | 13 (6%) | 0.71 |
| 3 | 18 (3.8%) | 8 (4%) | |
| Post TIMI | |||
| 0−1 | 56 (12.3%) | 44 (20%) | |
| 2 | 26 (5.7%) | 16 (7.3%) | 0.02 |
| 3 | 375 (82%) | 160 (72.7%) | |
| Stent implantation | 360 (78.8%) | 165 (75%) | 0.27 |
Variables are reported as n (%). CX: circumflex artery; LAD: left anterior descendant artery; LMCA: left main coronary artery; RCA: right coronary artery, TIMI: thrombolysis in myocardial infarction.
On the other hand, multivariate regression analysis demonstrated that Killip > 1, post TIMI < 3, ABG, and admission creatinine were independent predictors of in-hospital adverse cardiac events.
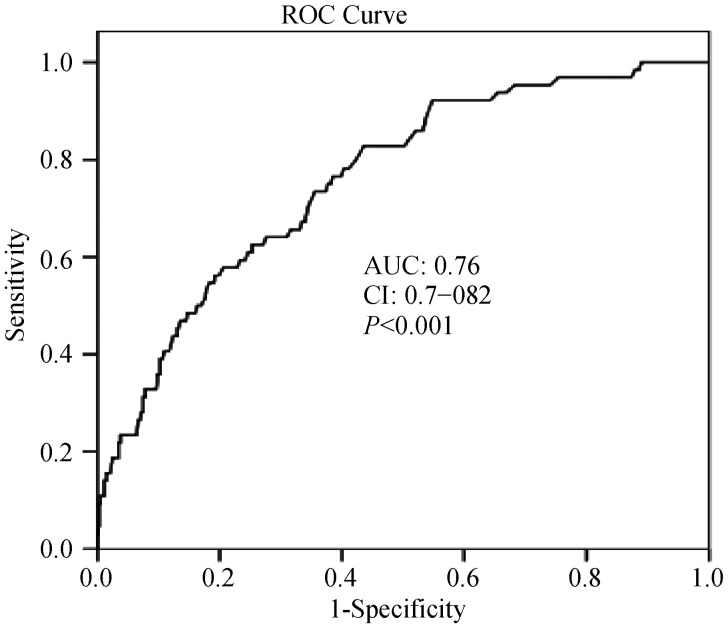
The receive-operating characteristic (ROC) curve with regard to MACE is shown in Figure 1. An ABG level of > 168 mg/dL was identified as an effective cut-point in STEMI of MACE (area under curve = 0.76, 95% confidence interval (CI): 0.7–0.82, P < 0.001). Long term survival curve of the studied groups is found in Figure 2. The rate of low glucose group survival was significantly lower (P < 0.001).
Figure 1. The receive-operating characteristic (ROC) curve of admission blood glucose with regard to major adverse cardiac events.
Figure 2. Long-term survival curve of studied groups.
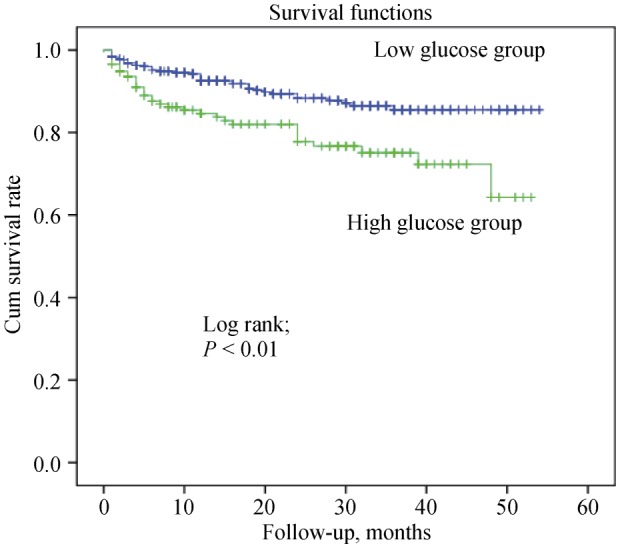
Low glucose group: patients with admission blood glucose level ≤ 168 mg/dL; high glucose group: patients with admission blood glucose > 168 mg/dL. Mean available follow-up time was 18.8 months. Long follow-up of 8 patients in low glucose group and 4 patients in high glucose group were not done due to any communications problems.
4. Discussion
The present study, based on a non-selected cohort of elderly patients hospitalized with STEMI, highlights an important potential relevance of hyperglycemia on admission to accurately identify a group of elderly patients at high risk for both short-term and long-term outcomes. These findings are consistent with various previous studies.[2]–[7] Although ABG is well known as an independent predictor of in-hospital adverse events and mortality in the patients with STEMI, there is limited data in the elderly population which constitute an increasingly important proportion of patients in recent years. Our study indicates that the ABG level may be an independent predictor of worse prognosis in the elderly STEMI patients, as in the general STEMI population
An elevated ABG level is common in patients with ACSs in both diabetic and non-diabetic patients and has been studied in various studies. Dırkali et al.[5] analyzed the relationship of ABG and major complications in patients with ACSs and suggested ABG as an independent and powerful predictor of in-hospital and late outcomes. Kosiborod et al.[6] reported admission hyperglycemia was more accurately associated with in-hospital and long-term morbidity and mortality in non-diabetic patients than in diabetic patients. In three different studies by Hoebers et al.[12], Ishihara et al.[13], and Rasoul et al.[14] it was proposed that a high ABG level was associated with increased in-hospital, but not long-term mortality (> 30 days). Hoebers et al.[12] and Knudsen et al.[15] explained these findings by acute deterioration of glucose metabolism. After the acute phase of MI, glucose intolerance may resolve and long-term mortality does not seem to be affected by glucose metabolism.[12],[16] In our study, we showed that in-hospital and long term mortalities were higher in elderly of the HGG (Figure 2, P < 0.001).
Yang, et al.[17] demonstrated that impaired glucose metabolism is closely related to other cardiovascular risk factors. These subjects with impaired glucose metabolism may easily develop stress diabetes during stressful conditions like acute coronary syndromes. However, subjects in the HGG had less cardiovascular risk factors compared to the individuals in the LGG. This finding may strengthen the importance of ABG as a prognostic indicator in our study.
Post PCI TIMI frame and unsuccessful primary interventions, in our study, were significantly lower in the HGG (P < 0.01 and P = 0.05, respectively). It had been shown that elevated ABG levels in non-diabetic patients is associated with micro-vascular obstruction and causes larger infarcts which result in worse outcomes like left ventricular dysfunction and increased in-hospital MACE.[18]
Even in normal subjects, acute hyperglycemia causes various changes like prolongation of corrected QT interval and reduced nitric oxide availability.[19],[20] These may result in detrimental hemodynamic changes and may lead to cardiac arrhythmias and sudden death. In our study, arrhythmias were more frequently seen in HGG (P < 0.01). One of the reasons for higher mortality in patients with higher glucose levels may be the increased susceptibility to arrhythmias.
In the acute phase of MI, higher glucose levels are usually seen and result in insulin resistance, higher free fatty acid concentrations, and impaired myocardial glucose usage which causes increased oxygen consumption and probably worsens ischemia.[21],[22] In some clinical studies, it has been shown that tight glycemic control with insulin, insulin-glucose and insulin-glucose-potassium infusions may decrease mortality in acute MI and critically ill patients.[23]–[26] However, these treatment strategies may potentially cause volume overload, hyperglycemia and/or hypoglycemia.[27],[28]
A meta-analysis, including 15 trials which examined stress hyperglycemia and in-hospital mortality, showed that stress hyperglycemia in MI is associated with an increased risk of in-hospital mortality in patients with, and without, DM.[29] The risk of congestive heart failure or cardiogenic shock was also increased in patients without DM. Similarly, Ishihara et al.[30] found that higher ABG in acute MI is related with lower LVEF. Heart failure is associated with insulin resistance and glucose intolerance.[31],[32] This may be one of the factors that explain the relation between acute hyperglycemia and increased in-hospital mortality. In this study, patients in HGG had an increased rate of Killip Class >1 (P = 0.001) and lower LVEF (P < 0.001). Furthermore, CHF and inotropic agent usage were significantly more frequent in HGG (P < 0.001 for both). Yang et al.[33] found a linear correlation between higher ABG and Killip classification in a study conducted in elderly patients with acute MI.
The prospective design and the absence of an evaluation of the course of stress hyperglycemia are main limitations of our study. Thus, we could not draw any conclusions about how the course of glycemia affected the long-term outcomes. The unavailability of HgA1c levels hampers discrimination between undiagnosed type 2 diabetes and stress diabetes.
In conclusion, a higher level of ABG may be linked with poor post-procedural myocardial perfusion, lower LVEF, higher in-hospital and long term mortality, and MACE in older patients with STEMI who underwent primary PCI. ABG levels could be used for a better risk stratification of elderly patients.
References
- 1.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 2.Svensson AM, McGuire DK, Abrahamsson P, et al. Association between hyper- and hypoglycaemia and 2-year all-cause mortality risk in diabetic patients with acute coronary events. Eur Heart J. 2005;26:1255–1261. doi: 10.1093/eurheartj/ehi230. [DOI] [PubMed] [Google Scholar]
- 3.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 4.Wahab NN, Cowden EA, Pearce NJ, et al. Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol. 2002;40:1748–1754. doi: 10.1016/s0735-1097(02)02483-x. [DOI] [PubMed] [Google Scholar]
- 5.Dirkali A, van der Ploeg T, Nangrahary M, et al. The impact of admission plasma glucose on long-term mortality after STEMI and NSTEMI myocardial infarction. Int J Cardiol. 2007;1(121):215–217. doi: 10.1016/j.ijcard.2006.08.107. [DOI] [PubMed] [Google Scholar]
- 6.Kosiborod M, Rathore SS, Inzucchi SE, et al. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction. Circulation. 2005;111:3078–3086. doi: 10.1161/CIRCULATIONAHA.104.517839. [DOI] [PubMed] [Google Scholar]
- 7.Stranders I, Diamant M, Van Gelder R, et al. Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med. 2004;164:982–988. doi: 10.1001/archinte.164.9.982. [DOI] [PubMed] [Google Scholar]
- 8.Cao JJ, Hudson M, Jankowski M, et al. Relation of chronic and acute glycemic control on mortality in acute myocardial infarction with diabetes mellitus. Am J Cardiol. 2005;96:183–186. doi: 10.1016/j.amjcard.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 9.Chesebro JH, Knatterud G, Roberts R, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial, phase I: a comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76:142–154. doi: 10.1161/01.cir.76.1.142. [DOI] [PubMed] [Google Scholar]
- 10.Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol. 1967;20:457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Hoebers LP, Damman P, Claessen BE, et al. Predictive Value of Plasma Glucose Level on Admission for Short and Long Term Mortality in Patients With ST-Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. Am J Cardiol. 2012;109:53–59. doi: 10.1016/j.amjcard.2011.07.067. [DOI] [PubMed] [Google Scholar]
- 13.Ishihara M, Kagawa E, Inoue I, et al. Impact of admission hyperglycemia and diabetes mellitus on short- and long-term mortality after acute myocardial infarction in the coronary intervention era. Am J Cardiol. 2007;99:1674–1679. doi: 10.1016/j.amjcard.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 14.Rasoul S, Ottervanger JP, Bilo HJ, et al. Glucose dysregulation in nondiabetic patients with ST-elevation myocardial infarction: acute and chronic glucose dysregulation in STEMI. Neth J Med. 2007;65:95–100. [PubMed] [Google Scholar]
- 15.Knudsen EC, Seljeflot I, Abdelnoor M, et al. Abnormal glucose regulation in patients with acute ST-elevation myocardial infarction-a cohort study on 224 patients. Cardiovasc Diabetol. 2009;8:6. doi: 10.1186/1475-2840-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen CJ, Eberle HC, Nassenstein K, et al. Impact of hyperglycemia at admission in patients with acute ST-segment elevation myocardial infarction as assessed by contrast-enhanced MRI. Clin Res Cardiol. 2011;100:649–659. doi: 10.1007/s00392-011-0290-7. [DOI] [PubMed] [Google Scholar]
- 17.Yang Z, Xing X, Xiao J, et al. Prevalence of Cardiovascular Disease and Risk Factors in the Chinese Population with Impaired Glucose Regulation: the 2007-2008 China National Diabetes and Metabolic Disorders Study. Exp Clin Endocrinol Diabetes. 2013;121:372–374. doi: 10.1055/s-0033-1341520. [DOI] [PubMed] [Google Scholar]
- 18.Eitel I, Hintze S, de Waha S, et al. Prognostic impact of hyperglycemia in non-diabetic and diabetic patients with ST-elevation myocardial infarction: insights from contrast-enhanced magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5:708–718. doi: 10.1161/CIRCIMAGING.112.974998. [DOI] [PubMed] [Google Scholar]
- 19.Giugliano D, Marfella R, Coppola R, et al. Vascular effects of acute hyperglycemia in humans are reversed by L-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia. Circulation. 1997;95:1783–1790. doi: 10.1161/01.cir.95.7.1783. [DOI] [PubMed] [Google Scholar]
- 20.Marfella R, Nappo F, De Angelis L, et al. The effect of acute hyperglycemia on QTc duration in healthy man. Diabetologia. 2000;43:571–575. doi: 10.1007/s001250051345. [DOI] [PubMed] [Google Scholar]
- 21.Tansey MJ, Opie LH. Relation between plasma free fatty acids and arrhythmias within the first twelve hours of acute myocardial infarction. Lancet. 1983;2:419–422. doi: 10.1016/s0140-6736(83)90388-4. [DOI] [PubMed] [Google Scholar]
- 22.Oliver MF. Metabolic causes and prevention of ventricular fibrillation during acute coronary syndromes. Am J Med. 2002;112:305–311. doi: 10.1016/s0002-9343(01)01104-4. [DOI] [PubMed] [Google Scholar]
- 23.Malmberg K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus: DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ. 1997;314:1512–1515. doi: 10.1136/bmj.314.7093.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 25.Malmberg K, Ryden L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26:57–65. doi: 10.1016/0735-1097(95)00126-k. [DOI] [PubMed] [Google Scholar]
- 26.Mehta SR, Yusuf S, Diaz R, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437–446. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- 27.van der Horst IC, Zijlstra F, van't Hof AW, et al. Glucose-insulin-potassium infusion in patients treated with primary angioplasty for acute myocardial infarction: the glucose-insulin potassium study: a randomized trial. J Am Coll Cardiol. 2003;42:784–791. doi: 10.1016/s0735-1097(03)00830-1. [DOI] [PubMed] [Google Scholar]
- 28.Goyal A, Mehta SR, Diaz R, et al. Differential clinical outcomes associated with hypoglycemia and hyperglycemia in acute myocardial infarction. Circulation. 2009;120:2429–2439. doi: 10.1161/CIRCULATIONAHA.108.837765. [DOI] [PubMed] [Google Scholar]
- 29.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara M, Inoue I, Kawagoe T, et al. Impact of acute hyperglycemia on left ventricular function after reperfusion therapy inpatients with a first anterior wall acute myocardial infarction. Am Heart J. 2003;146:674–678. doi: 10.1016/S0002-8703(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 31.Witteles RM, Tang WH, Jamali AH, et al. Insulin resistance in idiopathic dilated cardiomyopathy: a possible etiologic link. J Am Coll Cardiol. 2004;44:78–81. doi: 10.1016/j.jacc.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 32.Suskin N, McKelvie RS, Burns RJ, et al. Glucose and insulin abnormalities relate to functional capacity in patients with congestive heart failure. Eur Heart J. 2000;21:1368–1375. doi: 10.1053/euhj.1999.2043. [DOI] [PubMed] [Google Scholar]
- 33.Yang SW, Zhou YJ, Liu YY, et al. Beijing Elderly Acute Myocardial Infarction Study (BEAMIS) Group. Influence of abnormal fasting plasma glucose on left ventricular function in older patients with acute myocardial infarction. Angiology. 2012;63:266–274. doi: 10.1177/0003319711413893. [DOI] [PubMed] [Google Scholar]