Abstract
Background
Obesity is associated with unfavorable alternations in plasma lipid profile and a broad spectrum of cardio-metabolic disorders. Proprotein convestase subtilisin kexin type 9 (PCSK9) is a novel circulating protein that promotes hypercholesterolemia by decreasing hepatic low lipoprotein density receptor (LDLR) protein. However, the relationship between PCSK9 concentration and lipid profile in an obesity condition has less been investigated.
Objective
To examine the changes of plasma PCSK9 concentration in a rat model fed with high fat diet (HFD) and its correlation to lipid profile, body weight and ageing.
Methods
Twenty male Sprague Dawley (SD) rats were divided into two groups, control group (fed with normal pellet for 4 weeks), and high-fat diet group (fed with 3% cholesterol enrich diet for 4 weeks). Blood samples of rats were obtained before and at days 14, 21, and 28 in both groups. The body weight, plasma metabolic parameters (glucose, lipid profile) and PCSK9 were determined at indicated time points.
Results
The body weights were significantly increased in rats fed with HFD compared to that in rats with normal pellets at day 28. Additionally, total cholesterol (TC), triglyceride (TG), and low density lipoprotein cholesterol (LDL-C) levels in rat fed with HFD were also higher than that in rats fed with control diet while decreased high density lipoprotein cholesterol (HDL-C) levels were found in rats with HFD at day 28. More interesting, there were no differences of plasma PCSK9 concentrations as well as hepatic expression of LDLR between the two groups at day 28.
Conclusions
Although the body weight and LDL-C were significantly increased in rats fed with HFD at 4 weeks, there were no differences of changes in plasma PCSK9 concentration and LDLR expression of liver tissue in both groups at baseline and day 28, suggesting that dyslipidemia in the rat model with HFD appears not to be associated with PCSK9-LDLR pathway but ageing.
Keywords: PCSK9, High fat diet, Dyslipidemia, Ageing
1. Introduction
Being overweight or obesity is associated with alternations in plasma lipids and a broad spectrum of cardio-metabolic disorders, including increases in plasma low-density lipoprotein (LDL)-cholesterol (LDL-C) and triglyceride (TG) concentrations,[1]–[3] which is one of the leading risks for death worldwide. Increasing evidence has suggested that obesity is linked to numerous comorbidity diseases such as type 2 diabetes, hypertension, hypercholesterolaemia, hypertriglyceridemia, and non-alcohol fatty liver disease.[4],[5] Elevated plasma LDL-C levels represent one of the key causal factors for the development of atherosclerosis and subsequent coronary artery disease (CAD).[5] The important role of plasma LDL-C for the development of the atherosclerosis was recently highlighted from studies on subjects with genetically caused reductions of LDL-cholesterol due to variation in the gene for proportion convertase subtilisin/kexin type 9 serine protease (PCSK9), an enzyme that degrades the LDL receptor (LDLR).[6]–[8]
Age is a common risk factor for the development of cholesterol homeostasis.[9] It has been reported that cholesterol metabolism is profoundly modified during normal ageing, and in human plasma LDL-C increases by about 40% from 20 to 60 year of age.[9] A number of metabolic changes have been reported to occur during normal aging in both animals and humans.[9] Reduced physical activity, redistribution of body tissues with a relative increase in adipose over muscle mass, increased insulin resistance, and increased blood pressure are all factors that could contribute to the acceleration of age-related atherosclerosis.[9],[10] Additionally, modern societies are becoming increasingly aged, causing a collision between increased hypercholesterolaemia and increased aging.
PCSK9 is a newly discovered serine protease that plays a key role in LDL-C homeostasis by mediating LDLR breakdown through a post-transcriptional mechanism.[11],[12] Data obtained from studies in cultured cells and rodent models show that PCSK9 is an important regulator of plasma LDL-C concentrations.[13],[14] In recent years, therefore, a number of studies have greatly expounded the physiological role of PCSK9 and reported that PCSK9 inhibition may be a therapeutic strategy for the treatment of dyslipidemia and cardiovascular diseases.[15],[16]
In addition, previous studies have reported that animals fed with four weeks high fat diet (HFD) can cause obesity and abnormal lipid metabolism.[17] Additionally, recent evidence suggests that PCSK9 is associated to LDL-C and a lesser extent plasma TG and may also regulate the very low-density lipoprotein (VLDL) receptor expression and VLDL-TG metabolism.[12],[13] However, whether the dyslipidemia in rats fed with HFD is associated with PCSK9-LDL- receptor pathway has never been evaluated.
In this study, therefore, we examined the changes of plasma PCSK9 concentration in a rat model fed with HFD and its correlation to lipid profile, body weight and ageing.
2. Methods
2.1. Animals
Three-week-young male Sprague-Dawley (SD) rats (initial weight, 50 ± 1 g) were purchased from animal center of Peking University Health Science Center. All animals were fed a standard laboratory diet, and placed in caged (four rats per cage) in an atmosphere of 55% ± 10% relative humidity at 22 ± 2°C with a 12-h light/dark cycle. Rats were given free access to food and water. Body weight and venous blood were recorded and collected once per week. The study protocol was approved by the Ethics Committee on Animal Center of Peking University Health Science.
After adapting to the environment for a week, animals were randomly assigned into the two groups: HFD group and control group (fed with normal diet). The indicated diet purchased from Peking keao Feed Company. Rats in the control group (n = 8) were fed a standard chow diet (3.21 kcal/g, 13% calories from fat, 25% calories from protein, 62% calories from carbohydrate). Those in the HFD group (n = 8) were fed HFD (4.39 kcal/g, 47% calories from fat, 20% calories from protein, 33% calories from carbohydrate) for 4 weeks. Rats underwent an 18 h fasting period prior to sacrificing at four weeks. The blood samples and livers were rapidly collected and stored at –80°C until analysis.
2.2. Serum lipid and PCSK9 measurements
After one week and four weeks of chronic administration of the experimental diet, animals (n = 8 for each group) were fasted 12–15 h and blood sample were collected from eyes veins. Following a centrifugation for 15 min at 1000 r/min, plasma samples were stored at –80°C until analysis. Total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C) were determined on an automatic biochemistry analyzer (Hitachi 7150, Tokyo, Japan). The LDL-C was estimated according to the following formula. LDL-C = TC – HDL-C – TG/5.
PCSK9 concentrations were measured using a high-sensitivity, quantitative sandwich enzyme immunoassay (Quantikine ELISA, R&D Systems Europe Ltd, Sweden). The lower limit of detection was 0.096 ng/mL.
2.3. Real-time polymerase chain reaction (RT-PCR) analysis
Total RNA in liver tissue was isolated from hepatic tissue using the Trizol reagent Kit (Invitrogen, USA) following the manufacture's instructions, and then was measured by spectrophotometry at an absorbance of 260 nm, and designated the purity valid if the ratio of A260/A280 was in the range from 1.8 to 2.0. The integrity of the RNA was checked by denaturing agarose gel electrophoresis and ethidium bromide staining. 3.0 µg of the total RNA was reverse transcribed by revert Aid First Strand cDNA aynthesis kit (Fermentas, CA, USA). The abundances of LDLR and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were analyzed by RT-PCR in the 7500HT RT-PCR system (Applied Biosystems, Foster, CA, USA). Real-time PCR was performed using the SYBR Premix ExTaq (TaKaRa Bio Inc.) according to the manufacturer's instructions. The specific sense and antisence primers were shown as followings: LDLR, sense: 5′-GAT TGG CTA TGA GTG CCT ATG TC-3′, antisense: 5′-GTG AAG AGC AGA AAC CCT ATG G-3′; GAPDH, sense: 5′-ACA GCA ACA GGG TGG TGG AC-3′, antisense: 5′-TTT GAG GGT GCA GCG AAC TT-3′.
Standard curves for each primer pair were generated by serial dilutions of cDNA from a reference sample and used for regression analyses. All PCR assays were performed in triplicate. The variance of the triplicate measurements was < 1%. Results were analyzed using the standard curve method by the SDS (sequence detection systems) software. The data was expressed as the relative levels of mRNA after normalized with GAPDH.
2.4. Statistical analyses
Results are expressed as the means ± SD. Statistical analysis was performed using SPSS 19.0 statistical packages. The significance of differences was evaluated by a one-way analysis of variance (ANOVA) for unpaired data. The significance level was chosen as P < 0.05.
3. Results
3.1. Changes of body weight
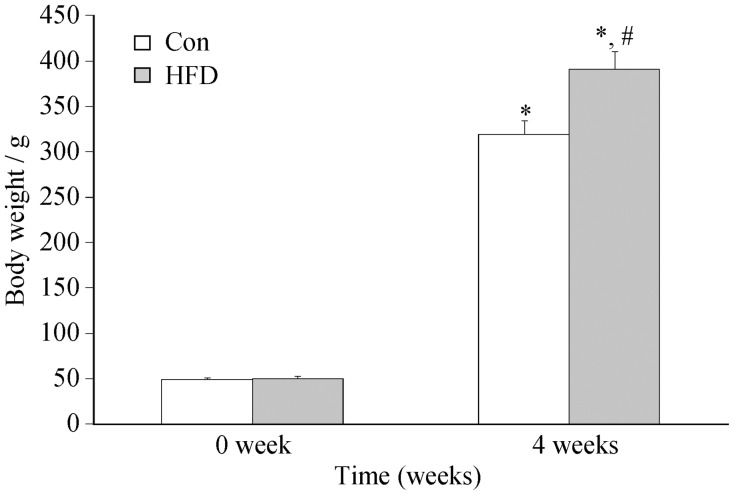
Following a 4-week experimental observation fed with control diet or HFD, body weight of the rats gained significantly. As shown in Figure 1, there was no differences in body weight at baseline between control and HFD group (about 50 g), while the body weight increased at 4 weeks in both groups compared with that at 0 week. However, the body weight in HFD group was higher than that in control group (391 ± 13.09 g vs. 319 ± 8.59 g, P < 0.01), which was increased by 18%.
Figure 1. The changes of body weight of rats fed with high fat diet and control diet.
Results were expressed as mean ± SD. *P < 0.001, compare with 0 week groups; #P < 0.001, compare with control group at 4 weeks. Con: control; HFD: high fat diet.
3.2. Changes of lipid profile
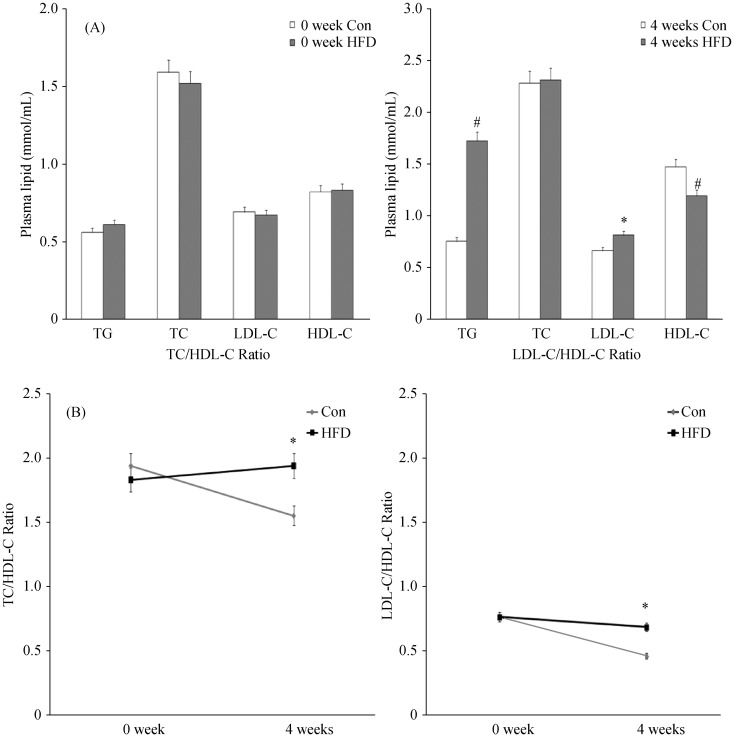
The effects of diets on the levels of serum lipid profile were shown in Table 1 and Figure 2. There were no differences between the control and HFD group at baseline (0 week) regarding serum lipid profile. However, after 4-week treatment with experimental diets, serum TG, TC and LDL-C concentrations of rats were increased while HDL-C concentrations of rats were decreased in both groups. Among those parameters of lipid profile, the serum TG concentration of rats fed with HFD at week 4 were significantly increased compared with that at both baselines (control group: from 0.56 ± 0.09 mmol/L to 0.75 ± 0.15 mmol/L; HFD group: from 0.61 ± 0.03 mmol/L to 1.72 ± 0.19 mmol/L). Apparently, this change was much higher in HFD group compared with that in the control group (1.72 ± 0.19 mmol/L vs. 0.75 ± 0.15 mmol/L, P < 0.01). Although there were no differences at week 4 concerning serum TC concentrations between the two groups (2.28 ± 0.12 mmol/L, and 2.31 ± 0.12 mmol/L, P > 0.05), those values were higher than that at week 0 in both groups (P < 0.05). As shown in Table 1, the levels of LDL-C in HFD group (0.81 ± 0.04) were increased compared with that in control group at 4 weeks, while the levels of HDL-C in HFD group at week 4 was decreased significantly compared with that in control group. The ratio of TC/HDL-C and LDL-C/HDL-C were also presented in Table 1. These ratios at baseline in both groups were no different, but the ratios of TC/HDL-C and LDL-C/HDL-C in HFD group were increased in 4 weeks compared with that in control group, suggesting that more severe dyslipidemia existed in HFD rats. The results were summarized in Table 1 and Figure 2.
Table 1. Changes of serum lipid profile and glucose.
| 0 wk (n = 8) |
4 wks (n = 8) |
0 wk/4wks (HFD) |
|||||
| Con | HFD | P Values | Con | HFD | P Values | P Values | |
| TC (mmol/L) | 1.59 ± 0.07 | 1.52 ± 0.13 | 2.28 ± 0.12 | 2.31 ± 0.12 | < 0.05 | ||
| Changes (%) | −0.07 | > 0.05 | 0.03 | > 0.05 | |||
| TG (mmol/L) | 0.56 ± 0.09 | 0.61 ± 0.03 | 0.75 ± 0.15 | 1.72 ± 0.19 | < 0.01 | ||
| Changes (%) | 0.05 | > 0.05 | 0.97 | < 0.01 | |||
| HDL-C (mmol/L) | 0.82 ± 0.04 | 0.83 ± 0.03 | 1.47 ± 0.10 | 1.19 ± 0.06 | < 0.05 | ||
| Changes (%) | 0.01 | > 0.05 | −0.26 | < 0.05 | |||
| LDL-C (mmol/L) | 0.69 ± 0.02 | 0.67 ± 0.03 | 0.66 ± 0.04 | 0.81 ± 0.04 | < 0.05 | ||
| Changes (%) | −0.02 | > 0.05 | 0.15 | = 0.05 | |||
| LDL-C/HDL-C (mmol/L) | 0.84 ± 0.03 | 0.81 ± 0.06 | 0.45 ± 0.02 | 0.66 ± 0.01 | < 0.05 | ||
| Changes (%) | −0.03 | > 0.05 | 0.21 | < 0.05 | |||
| TC/HDL-C (mmol/L) | 1.96 ± 0.16 | 1.84 ± 0.18 | 1.56 ± 0.02 | 1.95 ± 0.05 | < 0.05 | ||
| Changes (%) | 0.12 | > 0.05 | 0.31 | < 0.05 | |||
| Glucose (mg/dL) | 2.60 ± 0.23 | 2.58 ± 0.25 | 2.77 ± 0.37 | 3.22 ± 0.15 | < 0.01 | ||
| Changes (%) | 0.02 | > 0.05 | 0.45 | < 0.05 | |||
Values are expressed as mean ± SD. Con: control; HDL-C: high density lipoprotein cholesterol; HFD: high fat diet; LDL-C: low density lipoprotein cholesterol; TC: total cholesterol; TG: triglyceride; 0 wk/4wks: 4 weeks group versus with 0 week group.
Figure 2. The changes of serum lipid profile of rats fed with high fat diet and control diet.
(A): Baseline lipid profile at 0 week of both groups (left) and changes of lipid profile at 4 weeks of both groups (right). *P < 0.05, #P < 0.001, compare with controls; (B): Changes of serum TC/HDL-C and LDL-C/HDL-C ratio. *P < 0.05, compare with controls. Results were expressed as mean ± SD. Con: control; HDL-C: high density lipoprotein cholesterol; HFD: high fat diet; LDL-C: low density lipoprotein cholesterol; TC: total cholesterol; TG: triglycerides.
3.3. Change of circulating PCSK9 concentration
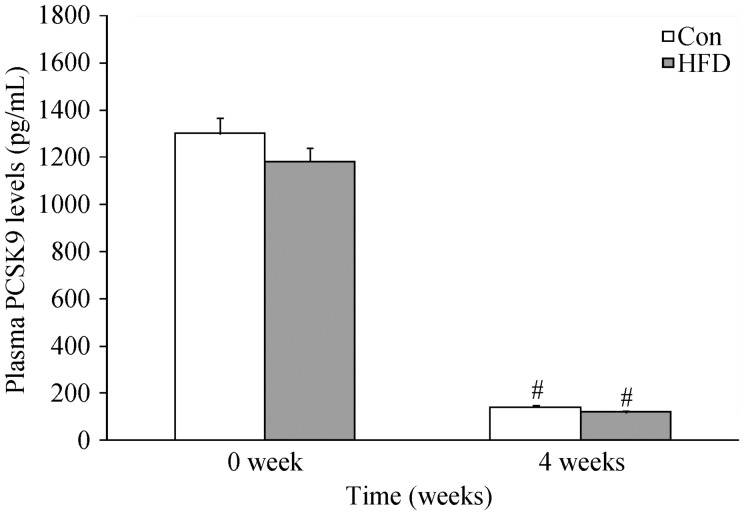
To determine the relationship between PCKS9 and body weight in rats, circulating PCSK9 concentrations of the rats were measured at baseline (0 week) and at 2, 3, 4 weeks in both groups. The data showed that circulating PCSK9 concentrations of rats fed with either control diet or FHD at week 4 were decreased significantly compared with that at 0 week, (P < 0.01 respectively). However, there were no significantly differences between the two groups as shown in Figure 3.
Figure 3. The changes of the plasma PCSK9 concentrations.
Results were expressed as mean ± SD. #P < 0.001, compare with 0 week in both groups. Con: control; HFD: high fat diet.
3.4. LDLR mRNA expression in hepatic tissue
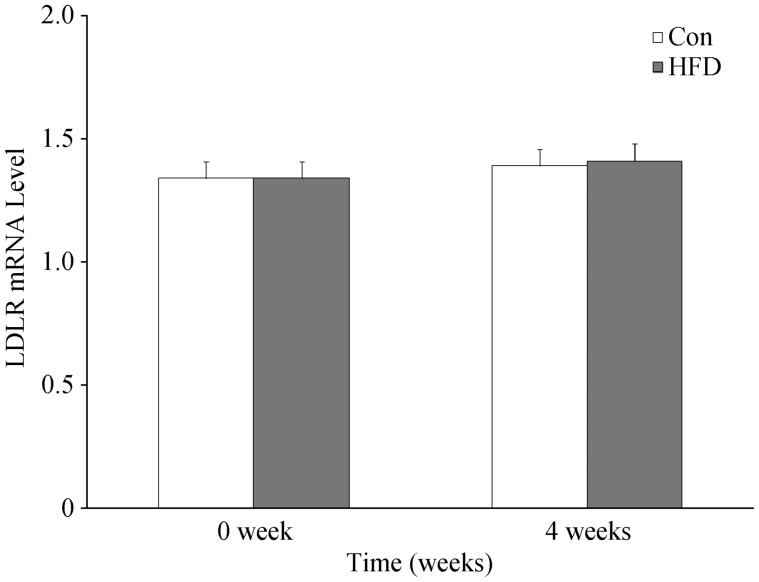
To examine whether the changes of circulating PCSK9 and lipid profile was associated with LDLR mRNA expression, the change of LDLR mRNA expression in hepatic tissue was also assessed. The data indicated that a similar pattern of changes in circulating PCSK9 levels and liver LDLR gene expression were found in both groups. Although the parameters of lipid profile in rats fed with HFD at 4 weeks had a remarked changes, the levels of circulating PCSK9 and liver LDLR gene expression were nearly same, suggesting that dyslipidemia in rat fed with HFD is not associated with PCSK9-LDL-receptor pathway but aging. The detailed results were summarized in Table 2 and Figure 4.
Table 2. Sequences of PCR primers used in real time PCR analysis.
| Genes (Rat) | Forward primer | Reverse primer |
| LDLR | 5′-GATTGGCTATGAGTGCCTATGTC-3′ | 5′-GTGAAGAGCAGAAACCCTATGG-3′ |
| GAPDH | 5′-ACAGCAACAGGGTGGTGGAC-3′ | 5′-TTTGAGGGTGCAGCGAACTT-3′ |
GAPDH: Glyceraldehydes-3-phosphate dehydrogenase; LDLR: Low-density lipoprotein cholesterol receptor.
Figure 4. The changes of the liver tissue LDLR expression by real time-PCR analysis.
Results were expressed as mean ± SD. Con: control; HFD: high fat diet; LDLR: Low-density lipoprotein cholesterol receptor.
3.5. Correlation of serum PCSK9 level with lipid profile
To assess the correlation of circulation PCSK9 concentration with serum metabolic parameters (serum lipids profile and glucose profile), a Person's correlations analysis was performed in this study. As shown in Table 3, the data showed that plasma PCSK9 concentrations were decreased significantly when the body weight of rats increased. However, the results indicated that circulating PCSK9 concentrations were not correlated with the changes of serum lipid profile and glucose (Table 3).
Table 3. Correlations between plasma PCSK9 and glucose.
| 0 wk (n = 8) |
4 wks (n = 8) |
|||
| Correlation with PCSK9 | R-Person | P-value | R-Person | P-value |
| Control | ||||
| TG (mmol/L) | 0.64 | 0.17 | 0.27 | 0.66 |
| TC (mmol/L) | 0.21 | 0.69 | 0.48 | 0.41 |
| HDL-C (mmol/L) | 0.19 | 0.72 | 0.44 | 0.46 |
| LDL-C (mmol/L) | 0.57 | 0.24 | 0.22 | 0.73 |
| Glucose (mg/dL) | 0.32 | 0.53 | 0.09 | 0.88 |
| High fat diet | ||||
| TG (mmol/L) | 0.64 | 0.17 | 0.46 | 0.30 |
| TC (mmol/L) | 0.11 | 0.98 | 0.50 | 0.26 |
| HDL-C (mmol/L) | 0.15 | 0.78 | 0.48 | 0.28 |
| LDL-C (mmol/L) | 0.57 | 0.24 | 0.27 | 0.56 |
| Glucose (mg/dL) | 0.41 | 0.42 | 0.08 | 0.87 |
HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; TC: total cholesterol; TG: triglycerides.
3.6. Effect of ageing on PCSK9
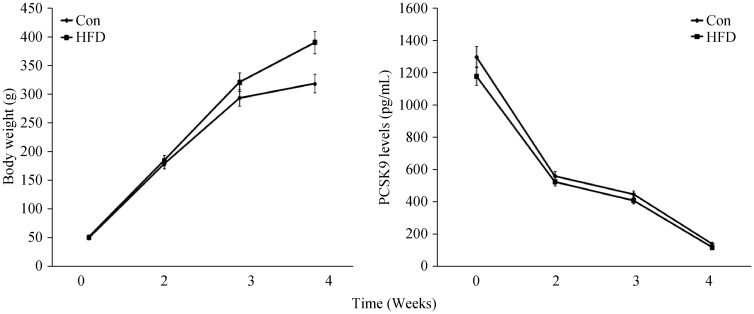
In our study, the data indicated that the increased body weight of rats reached obese levels with ageing and high cholesterol diet. However, circulating PCSK9 concentration was decreased with ageing in either control diet or HFD group compared with that at baseline (P < 0.01 respectively). However, there were no significant difference between HFD group and control group at any indicated time in our time course study (Figure 5).
Figure 5. The time course of the changes in body weight and plasma PCSK9 concentrations.
Results were expressed as mean ± SD. (A): body weight; (B): plasma PCSK9 concentrations. Con: control; HFD: high fat diet.
4. Discussion
Obesity and overweight have been shown to be associated with cardiovascular diseases (CVD), such as arteriosclerosis, stroke and myocardial infarction.[18],[19] These conditions can affect lipoprotein metabolism, and increase body weight and elevates TG and LDL-C levels and reduce HDL-C level.[18]–[20] In the present study, data showed that rats fed with HFD for 4 weeks resulted in a significant increase in body weight. Additionally, serum TC, TG, and LDL-C levels were also enhanced, whereas the serum HDL-C level was significantly decreased. Furthermore, the ratios of TC/HDL-C and LDL-C/HDL-C, the better indexes reflecting the abnormality of lipid metabolism, were also increased compared with that at 0 week. More importantly, the study indicated that there were no differences of plasma PCSK9 concentrations as well as hepatic expression of LDLR between HFD and control group at 4 weeks although the body weight and LDL-C were significantly increased in HFD group compared with that in control group, suggesting that dyslipidemia in the rat model with HFD appears not associated with PCSK9-LDLR pathway but age.
Recent studies have been reported that PCSK9 are associated with hypercholesterolemia and atherosclerosis.[21] It is established that PCSK9 is a natural inhibitor of the LDLR, acting post-transcriptionally.[7],[8],[22] Circulating PCSK9 binds to the epidermal growth factor precursor homology domain A (EGF-A) extracellular domain of the hepatic LDLR and prevents its recycling to the cell surface, thereby influences the circulating level of LDL-C uptake cause to hyperlipidemia.[23],[24] In humans, gain-of-function mutations in PCSK9 result in autosomal hypercholesterolemia and premature atherosclerosis.[25] Conversely, loss-of-function mutations within PCSK9 are associated with a reduction in plasma LDL-C and protection against coronary heart disease.[6],[26] Hence, mechanisms inhibiting PCSK9 expression have potential promise for cholesterol-lowering intervention. Recently, PCSK9 loss-of function mutations were correlated with plasma low levels of HDL-C and elevated plasma TG levels have been also noticed.[27]–[30] Based on those previous observations, we have studied a relationship of circulating PCSK9 concentrations with serum lipids in an obesity condition. The data indicated that circulating PCSK9 concentrations were not correlated with the serum lipids and glucose profile, while it decreased gradually along with ageing (Table 3), suggesting that the change of circulating PCSK9 concentration might be closely correlated with ageing, but not body weight and severity of dyslipidemia.
It is thought that PCSK9 is secreted primarily by the liver; human hepatoma cell lines produce and secret it as well.[6] Once circulating in the blood stream, PCSK9 can modulate the expression of LDLR in several tissues, including the liver, intestines, kidneys, lungs, pancreatic islets and adipose tissue.[8],[11] Physiologically, it has been reported that the serum PCSK9 concentrations were significant modulated by a variety of physiological factors. Firstly, Plasma PCSK9 levels exhibit a diurnal rhythm, with a nadir between 3:00 pm to 9:00 pm, and a peak during the night 4:30 am.[31] Additionally, fasting is another physical factor that can reduce circulating PCSK9 concentrations in healthy volunteers. A 20%–35% decrease was found at 18 h after fasting.[31]–[33] Moreover, PCSK9 concentrations were slightly higher in premenopausal women than in men.[34],[35] Finally, plasma PCSK9 levels are also affected by ageing. It is reported that serum PCSK9 levels were higher in 9-year-old boys than in 13-and 16-year-old male adolescents, paralleling the decrease of LDL-C during puberty in males.[36] In contrast, plasma PCSK9 concentrations increase in girls between 9 and 13–16 years of age, perhaps contributing to the higher LDL-C concentrations observed in girls during puberty compared to boys.[36] Our data provided novel information regarding age-related influence on serum PCSK9 levels.
Ageing is a major cause of hyperlipidemia. Plasma cholesterol increases in normal ageing in both rodents and human.[9] It has been already reported that in aged rats, the hepatic 3-hydroxy-3-methylglutaryl coenzyme A receptor, the rate-limiting enzyme of cholesterol biosynthesis, exhibit slow degradation and cholesterol synthesis is increased.[10],[37] Reduced elimination of cholesterol as bile acids and decreased receptor-mediated clearance of plasma LDL, but the changes that can be reversed by treatment with growth hormone.[38],[39] The decreased LDLR membrane exposure and the following reduced LDL-C uptake could contribute to the hypercholesterolemia characteristic of aged rat as already reported.[30],[40] In addition, the studies found a gradual decline in the fractional clearance of LDL from the circulation with age and the reduced expression of hepatic LDLRs with increasing age in some species.[41]–[44] In a word, ageing causes decreases in LDL-C uptake and LDLR expression. Here, our data have shown that the circulating lipid profile had no effect on the LDLR gene expression, and is not caused by the changes of the LDLR expression with ageing.
In conclusion, in the present study we did not detect a relation of changes in serum PCSK9 levels to increase of body weight and changes of lipid profile in rats fed with HFD, suggesting that dyslipidemia in the rat model with HFD appears not associated with PCSK9-LDLR pathway but ageing.
Acknowledgments
The authors declared no conflict of interest with respect to the research, authorship, and/or publication of this work. There are no ethnical problems in this manuscript. This article is partly supported by National Natural Scientific Foundation (81070171, 81241121), Specialized Research Fund for the Doctoral Program of Higher Education of China (20111106110013), Capital Special Foundation of Clinical Application Research (Z121107001012015) awarded by Dr. Jian-Jun Li.
References
- 1.Levine GN, Keaney JF, Vita JA. Cholesterol reduction in cardiovascular disease-clinical benefits and possible mechanisms. N Engl J Med. 1995;332:512–521. doi: 10.1056/NEJM199502233320807. [DOI] [PubMed] [Google Scholar]
- 2.Lipid Research Clinics Program. The lipid research clinics coronary primary prevention trial results: I. reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 3.Manninen V, Elo MO, Frick MH, et al. Lipid alterations and decline in the incidence of coronary heart disease in the helsinki heart study. JAMA. 1988;260:641–651. [PubMed] [Google Scholar]
- 4.Ghosh A, Bose K, Chakravarti S, et al. Central obesity and coronary risk factors. JR Soc Promot Health. 2004;124:86–90. doi: 10.1177/146642400412400213. [DOI] [PubMed] [Google Scholar]
- 5.Katcher HI, Hill AM, Lanford JL, et al. Lifestyle approaches and dietary strategies to lower LDL-cholesterol and triglycerides and raise HDL-cholesterol. Endocrinol Metab Clin North Am. 2009;38:45–78. doi: 10.1016/j.ecl.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Peterson AS, Fong LG, Young SG. PCSK9 function and physiology. J Lipid Res. 2008;49:1595–1599. doi: 10.1194/jlr.CX00001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen JC, Boerwinkle E, Mosley TH, Jr, et al. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 8.Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci. 2007;32:71–77. doi: 10.1016/j.tibs.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uranga RM, Keller JN. Diet and age interactions with regards to cholesterol regulation and brain pathogenesis. Curr Gerontol Geriatr Res. 2010;219683:1–14. doi: 10.1155/2010/219683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marino M, Pallottini V, D'Eramo C, et al. Age-related changes of cholesterol and dolichol biosynthesis in rat liver. Mech Ageing Dev. 2002;123:1183–1189. doi: 10.1016/s0047-6374(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 11.Chois, Korstanje R Proprotein convertase in high-density liproprotein metabolism. Biomark Res. 2013;18:27–28. doi: 10.1186/2050-7771-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alborn WE, Cao G, Careskey HE, et al. Serum proprotein convertase subtilisin kexin type 9 is correlated directly with serum LDL cholesterol. Clin Chem. 2007;53:1814–1819. doi: 10.1373/clinchem.2007.091280. [DOI] [PubMed] [Google Scholar]
- 13.Abifadel M, Varret M, Rabes JP, et al. Mutation in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 14.Lakoski SG, Lagace TA, Cohen JC, et al. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94:2537–2543. doi: 10.1210/jc.2009-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubuc G, Tremblay M, Pare G, et al. A new method for measurement of total plasma PCSK9: clinical applications. J Lipid Res. 2010;51:140–149. doi: 10.1194/jlr.M900273-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Persson L, Galman C, Angelin B, et al. Importance of proprotein convertase subtilisin/kexin type 9 and in the hormonal and dietary regulation of rat liver low-density lipoprotein receptors. Endocrinology. 2009;150:1140–1146. doi: 10.1210/en.2008-1281. [DOI] [PubMed] [Google Scholar]
- 17.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 18.Zhao HL, Sim JS, Shim SH, et al. Antiobese and hypolipidemic effects of platycodin saponins in diet-induced obese rats: evidences for lipase inhibition and calorie intake restriction. Int J Obes (Lond) 2005;29:983–990. doi: 10.1038/sj.ijo.0802948. [DOI] [PubMed] [Google Scholar]
- 19.Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein JL, Brown MS. The low density lipoprotein pathway and its relation to atherosclerosis. Ann Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- 21.Aviva M, Jennifer S, Eugenie HC, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 22.Maxime D, Jaduiga MW, Ahmed Z, et al. Gene Inactivation of proprotein convertase subtilisin/Kexin Type 9 reduces atheroscleerosis in mice. Circulation. 2012;125:894–901. doi: 10.1161/CIRCULATIONAHA.111.057406. [DOI] [PubMed] [Google Scholar]
- 23.Grefhorst A, McNutt MC, Lagace TA, et al. Plasma PCSK9 preferentially reduces liver LDL receptors in mice. J Lipid Res. 2008;49:1303–13011. doi: 10.1194/jlr.M800027-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang DW, Lagace TA, Garuti R, et al. Binding of PCSK9 to ECF-A repeat of LDL receptor decreases receptor recycling and increases degradation. J Biol Chem. 2007;282:18602–18612. doi: 10.1074/jbc.M702027200. [DOI] [PubMed] [Google Scholar]
- 25.Mcnutt MC, Kwon HJ, Chen C, et al. Antagonism of secreted PCSK9 increases low density lipoprotein receptor expression in HepG2 cells. J Biol Chem. 2009;284:10561–10570. doi: 10.1074/jbc.M808802200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timms KM, Wagner S, Samuels ME, et al. A mutation in PCSK9 causing autosomal-dominant hypercholesterolemia in a Utah pedigree. Nat Genet. 2004;114:349–353. doi: 10.1007/s00439-003-1071-9. [DOI] [PubMed] [Google Scholar]
- 27.Cohen J, Pertsemlidis A, Kotowski IK, et al. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–165. doi: 10.1038/ng1509. [DOI] [PubMed] [Google Scholar]
- 28.Ference BA, Yoo W, Alesh I, et al. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: a Mendelian randomization analysis. J AM Coll Cardiol. 2012;25:2631–2639. doi: 10.1016/j.jacc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 29.Wu NQ, Guo YL, Xu RX, et al. Acute myocardial infarction in an 8-year old male child with homozygous familiar hypercholesterolemia: laboratory findings and response to lipid- lowering drugs. Clin Lab. 2013;59:901–907. [PubMed] [Google Scholar]
- 30.Le May C, Kourimate S, Langhi C, et al. Proprotein convertase subtilisin kexin type 9 null mice are protected from postprandial triglyoeridemia. Arterioscler Thromb Vasc Biol. 2009;29:684–690. doi: 10.1161/ATVBAHA.108.181586. [DOI] [PubMed] [Google Scholar]
- 31.Persson L, Cao G, Stahle L, et al. Circulating proprotein convertase subtilisin kexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humans. Arterioscler Thromb Vasc Biol. 2010;30:2666–2672. doi: 10.1161/ATVBAHA.110.214130. [DOI] [PubMed] [Google Scholar]
- 32.Persson L, Galman C, Angelin B, et al. Importance of proprotein convertase subtilisin/kexin type 9 in the hormonal and dietary regulation of rat liver low-density lipoprotein receptors. Endocrinology. 2009;150:1140–1146. doi: 10.1210/en.2008-1281. [DOI] [PubMed] [Google Scholar]
- 33.Browning JD, Horton JD. Fasting reduces plasma proprotein convertase, subtilisin/kexin type 9 and cholesterol biosynthesis in humans. J Lipid Res. 2010;51:3359–3363. doi: 10.1194/jlr.P009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui Q, Ju X, Yang T, et al. Serum PCSK9 is associated with multiple metabolic factors in a large Han Chinese population. Atherosclerosis. 2010;213:632–636. doi: 10.1016/j.atherosclerosis.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 35.Lakoski SG, Lagace TA, Cohen JC, et al. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94:2537–2543. doi: 10.1210/jc.2009-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baass A, Dubuc G, Tremblay M, et al. Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population-based sample of children and adolescents. Clin Chem. 2009;55:1637–1645. doi: 10.1373/clinchem.2009.126987. [DOI] [PubMed] [Google Scholar]
- 37.Pallottini V, Montanari L, Cavallini G, et al. Mechanisms underlying the impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in aged rat liver. Mech Ageing Develop. 2004;125:633–639. doi: 10.1016/j.mad.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Carpas E, Harman SM, Blackman MR. Human grown hormone and human aging. Endocr Rev. 1993;14:20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- 39.Galman C, Matasconi M, Persson L, et al. Age induced hypercholesterolemia in the rat relates to reduced serum lipids elimination but not increased intestinal absorption of cholesterol. Am J Physiol Endocrinol Metab. 2007;293:734–742. doi: 10.1152/ajpendo.00166.2007. [DOI] [PubMed] [Google Scholar]
- 40.McPherson R, Gauthier A. Molecular regulation of SREBP function: The Insig-SCAP connection and isoform-specific modulation of lipid synthesis. Biochem Cell Biol. 2004;82:201–211. doi: 10.1139/o03-090. [DOI] [PubMed] [Google Scholar]
- 41.Cecilia G, Manuela M, Lena P, et al. Age-induced hypercholesterolemia in the rat relates to reduced elimination but not increased intestinal absorption of cholesterol. Am J Physiol Endocrinol Metab. 2007;293:737–742. doi: 10.1152/ajpendo.00166.2007. [DOI] [PubMed] [Google Scholar]
- 42.Castelli WP, Wilson PW, Levy D, et al. Cardiovascular risk factors in the elderly. Am J Cardiol. 1989;63:12–19. doi: 10.1016/0002-9149(89)90110-0. [DOI] [PubMed] [Google Scholar]
- 43.Choi YS, Ide T, Sugano M. Age-related changes in the regulation of cholesterol metabolism in rats. Exp Gerontol. 1987;22:339–349. doi: 10.1016/0531-5565(87)90032-5. [DOI] [PubMed] [Google Scholar]
- 44.Ericsson S, Eriksson M, Vitols S, et al. Influence of age on the metabolism of plasma low density lipoproteins in healthy males. J Clin Invest. 1991;87:591–596. doi: 10.1172/JCI115034. [DOI] [PMC free article] [PubMed] [Google Scholar]