Abstract
Progressive aging of the population and prolongation of life expectancy have led to the rising prevalence of heart failure (HF). Despite the improvements in medical therapy, the mortality rate of this condition has remained unacceptably high, becoming the primary cause of death in the elderly population. Almost half of patients with signs and symptoms of HF are found to have a nearly normal ejection fraction, which delineates a distinct clinical syndrome, known as HF with preserved ejection fraction (HF-PEF). While early research focused on the importance of diastolic dysfunction, more recent studies reported the pathophysiological complexity of the disease with multiple cardiovascular abnormalities contributing to its development and progression. HF-PEF is a challenging major health problem with yet no solution as there is no evidence-based treatment which improves clinical outcomes. This review summarizes the state of current knowledge on diagnosis, prognosis and treatment of HF-PEF, with particular insights on the pathological characteristics in the elderly population.
Keywords: Diastole, Echocardiography, Mortality, Preserved ejection fraction, Heart failure
1. Introduction
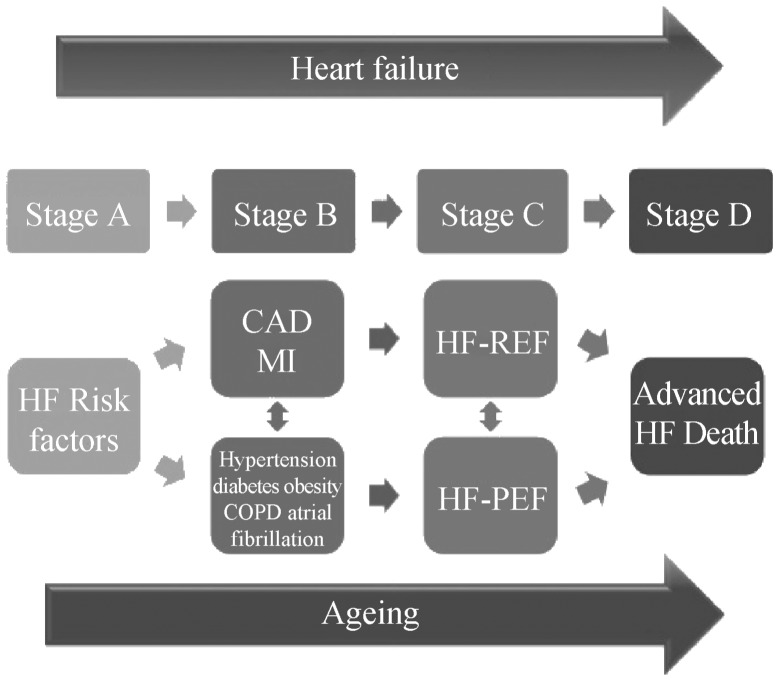
The elderly population (age > 65) has been steeply increasing in the last 3 decades.[1] Given the advances in therapy, the future elderly population can be expected to keep on rising and contribute more actively to society. Heart failure (HF) is a progressive disorder in a continuum superimposed on the aging process, which leads to disability and ultimate death. Progressive changes during aging and the cardiovascular disease evolution contribute to increase the heart failure burden in the elderly, Figure 1.[2] Almost half of the patients with symptomatic heart failure (HF) will be found to have a preserved ejection fraction (PEF); these will more likely be elderly and women, with a history of hypertension, obesity and other co-morbidities.
Figure 1. Heart failure: relationship between disease progression and superimposed ageing.
CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; HF-PEF: Heart failure with preserved ejection fraction; MI: myocardial infarction.
HF can be defined as an abnormality of cardiac structure or function leading to failure of the heart to deliver oxygen at a rate commensurate with the requirements of the metabolizing tissues, despite normal filling pressures (or only at the expense of increased filling pressures).[3] It is a common syndrome, prevalent in aging populations worldwide. Approximately 1%–2% of the adult population in developed countries has HF, with the prevalence rising to ≥ 10% among persons70 years of age or older.[4] HF is the final common pathway of several diseases with an increasing prevalence with population aging and improved treatment.
2. Diagnosis of heart failure
According to the two most recent guidelines on HF published from the European Society of Cardiology (ESC) in 2012[3] and from the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) in 2013,[5] HF can be divided in two clinically distinctive syndromes by widely used measures of pump dysfunction. Typically, this is left ventricular ejection fraction (LVEF) which is easily and often measured using echocardiography. Many major trials have enrolled patients with low LVEF ≤ 35%–40% (so-called heart failure with reduced ejection fraction or HF-REF). However, up to half of all patients with HF present with an ejection function within the normal range (so-called heart failure with preserved ejection fraction (HF-PEF)).[6] HF-PEF has been variably classified due to the differing LVEF cut-off criteria and the challenging diagnostic criteria. Table 1 shows the main differences in definition, classification and diagnosis in HF between the European and the American Guidelines. Of major concern is how the ACCF/AHA staging system for HF focuses more on the progression and worsening of the condition over time and recognizes the need for earlier identification of the contributing underlying conditions. The diagnosis of HF usually occurs in symptomatic patients after a period of dyspnoea or edema.[7] However, HF is usually preceded by other conditions, such as coronary artery disease, diabetes, hypertension or valve disease.[8],[9] HF is often the final, and fatal, stage of disease progression. This classification system moves forward from one stage to the next based on the progression of the disease, with the following proposed stages: (1) Stage A patients at high risk for developing clinical HF (i.e., those with hypertension, diabetes, dyslipidemia, and so on), but without detectable structural heart disease; (2) Stage B patients with detectable structural heart disease (i.e., left ventricular hypertrophy (LVH), left ventricular (LV) dysfunction), but no clinical signs or symptoms of HF; (3) Stage C patients with current or past clinical HF; and (4) Stage D patients with end-stage refractory HF.
Table 1. Major differences in HF definition, classification and HF-PEF diagnosis between the ESC and ACCF/AHA Guidelines.
| 2012 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure | 2013 ACCF/AHA Guideline for the management of heart failure | |
| HF definition | Abnormality of cardiac structure or function leading to failure of the heart to deliver oxygen at a rate commensurate with the requirements of the metabolizing tissues, despite normal filling pressures (or only at the expense of increased filling pressures). | Complex clinical syndrome that results from any structural or functional imparment of ventricular filling or ejection of blood. |
| HF classification | HF-REF Symptoms Signs Reduced LVEF (LVEF < 45%) HF-PEF Symptoms Signs Normal or mildly reduced LVEF and LV not dilated Relevant structural and/or functional heart disease |
Stage A At high risk for HF but without structural heart disease or symptoms Stage B Structural heart disease but without signs or symptoms Stage C Structural heart disease with prior or current symptoms of HF HF-REF (LVEF ≤ 40%) HF-PEF Stage D Refractory HF |
| HF-PEF diagnosis | Requires 4 conditions to be satisfied Typical symptoms of HF Typical signs of HF Preserved LVEF and LV not dilated EF ≥ 45% LVEDVi < 97 mL/m2 LVEDDi < 29 mm2 Relevant structural heart disease and/or diastolic dysfunction LAVi > 34 mL/m2 LVMi > 115 g/m2 (M) LVMi > 95 g/m2 (F) E/E' ≥ 8 E' average < 9 cm/s |
Stage C HF Known structural heart disease Typical Signs and Symptoms Preserved LVEF a. LVEF ≥ 50% HF-PEF b. LVEF 41-50% Borderline HF-PEF |
F: female; HF-REF: heart failure with preserved ejection fraction; LAVi: left atrial volume indexed; LVEDDi: left ventricular end diastolic diameter indexed; LVEDVi: left ventricular end diastolic volume indexed; LVEF: left ventricular ejection fraction; LVMi: left ventricular mass indexed; M: male.
Only the latter two stages qualify for the traditional clinical diagnosis of HF for diagnostic or coding purposes. Moreover, the classification recognizes that there are established risk factors and structural prerequisites for the development of HF and that therapeutic interventions performed even before the appearance of LV dysfunction or symptoms can reduce the morbidity and mortality of HF. The system is complementary to the New York Heart Association (NYHA) functional classification, which primarily gauges the severity of symptoms in patients who are in Stage C or D.
On the other hand, the European Society of Cardiology adopted a complementary classification that underlines the major difference between the two clinical HF syndromes: HF-REF and HF-PEF. These syndromes differ in terms of pathophysiology, epidemiology, diagnosis and prognosis. While HF-REF is the best understood type of HF in terms of pathophysiology and treatment, HF-PEF seems to have a different epidemiological and etiological profile.[10],[11] Patients with HF-PEF are older, more often female and more obese compared with those with HF-REF. They are less likely to have coronary heart disease and more likely to have a history of hypertension and atrial fibrillation.[12] In the past, HF-PEF was described as diastolic HF, and efforts were made to differentiate a cause for diastolic dysfunction, and a subsequent target for therapy.[13] While it is sometimes possible to demonstrate abnormalities in diastolic dysfunction, a specific therapy has thus far remained elusive.[14] It is now recognized, however, that due to effective therapies and remodelling, patients with borderline or normal LVEF may have primarily systolic dysfunction.[15]
3. HF in the elderly
In HF, as in many other cardiovascular diseases, important sexual dimorphisms exist in disease epidemiology and clinical outcomes. While in HF-REF, most studies show that men outnumber women, probably partly because the leading cause of this disease is coronary artery disease. In HF-PEF, females are approximately two times more likely than men to develop heart failure.[16],[17] Many of the cardiovascular alterations seen in HF-PEF are noted to greater extent in women compared with men.[18] For example, women demonstrate more concentric left ventricular remodelling and less ventricular dilatation in response to arterial hypertension;[19] ventricular and arterial stiffness increases with age in both sexes, but the increase is more dramatic in women.[20] And lastly, obesity influences LV geometry substantially more in women than in men- adipose mass is greater in women than men in any weight category, and obese women have greater LV mass than obese men.[21] Recently, age–sex interactions have also been observed in the manner in which LV function changes across the lifespan, wherein systolic and diastolic function and functional reserve become more compromised in women as compared with men in the postmenopausal years, despite similar or enhanced function in women during youth.[22] Finally, regarding outcome, it has been demonstrated that women with HF have a lower risk of death in both HF-REF and HF-PEF when compared to HF men. This better prognosis is more marked in non-ischemic HF.[23]
4. Left ventricular geometry
Left ventricular geometry is often altered in HF patients and there are clear differences in cardiac structure and function comparing patients with HF-REF and HF-PEF. Firstly, in HF-REF there is eccentric remodelling, where the LV is dilated and an adequate stroke volume is maintained at a lower LVEF. This eccentric remodelling is typically mediated through ischemic events.[24] In contrast, in patients presenting with HF-PEF concentric remodelling, the LV end-diastolic volume is not increased relative to the stroke volume, and maintenance of cardiac output is achieved at higher LVEF.[25],[26] Secondly, in HF-REF, LV systolic elastance (the relationship between volume and pressure) is reduced, whereby arterial elastance is elevated, resulting in impaired ventricular–vascular coupling. In contrast, both LV and arterial elastance are increased in HF-PEF, so that the coupling between them is preserved. In fact, the presence of a normal LVEF indicates that the coupling of the LV and arterial system is nearly optimal to convert the energy of contraction into stroke work. Thus, arterial vasodilatation improves LV systolic performance in HF-REF, but not in HF-PEF.[27]–[29] Many therapies are proven to improve outcome in HF-REF, including ACE inhibition,[30],[31] β-blockade,[32]–[34] aldosterone antagonists,[35],[36] and others.[37]–[39] many of which are associated with reverse remodelling. However, none have been demonstrated to be effective in patients with HF-PEF.[40]–[42] In retrospect, probably this is due to the lack of LV dilation in patients with HF-PEF, reflecting different pathological processes.[43]
5. Diagnosis
The diagnosis of HF-PEF is more difficult than the diagnosis of HF-REF because it is largely one of exclusion.[44],[45] Detecting the syndrome is typically done by clinical assessment, echocardiography and supportive evidence from biomarkers (B type natriuretic peptide and its variants). Because of the non-specific symptoms and signs of HF of any type, there has been some doubt about the nature of patients enrolled in the main HF-PEF clinical trials.[46],[47] The higher frequency of obesity and chronic lung disease among patients with HF-PEF has even led to the suggestion that these patients may just be elderly, overweight women who do not even suffer from a real HF.[48]–[51] HF-PEF diagnosis requires objective documentation of a cardiac dysfunction, both systolic and diastolic,[52] as shown in Table 1.
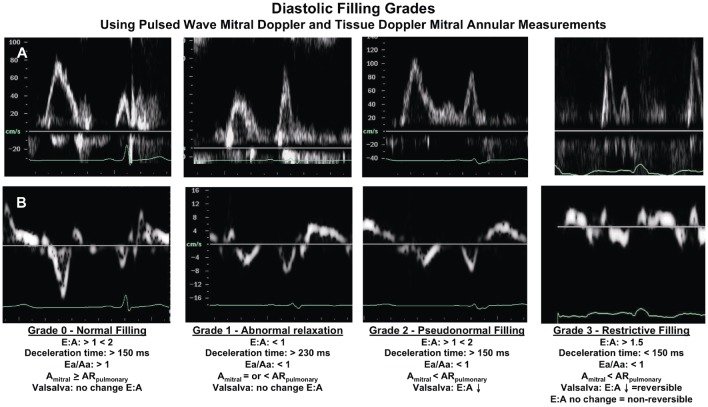
Echocardiography is the main tool used in HF diagnosis; it is used to define etiology and to assess the extent of eccentric remodelling. It has also been widely used to assess the therapeutic impact of various pharmacological interventions upon remodelling.[53] Although echocardiography is exemplary in the setting of HF-REF, there are some limitations in detecting subtle myocardial dysfunction and for quantifying small changes in LVEF. This is especially true in women with small volumes and hypertrophic hearts. In terms of diastolic dysfunction, echocardiography is used to evaluate the dynamics of left ventricular filling, mitral annular motion, and left atrial size. An elevated E: E' ratio and increased left atrial size are, until now, the best diastolic measures used to assist with recognizing HF-PEF in common practice. Moreover, evaluation of diastolic function provides important prognostic information in a wide variety of patients. Using a combination of Doppler measurements, four grades of diastolic function have been defined based upon mitral blood flow (Figure 2). In patients with HF, the stage of diastolic dysfunction is a stronger predictor of mortality than EF.[54] The most severe grades of diastolic dysfunction indicate a 4-fold increase in the risk of death in patients with heart failure and coronary artery disease.[55] Similarly, it was been shown that the presence of a normal filling pattern in community subjects with symptoms of HF, indicates a very good prognosis, while in contrast, an abnormal filling pattern and progressively worse grades of diastolic dysfunction indicate patients with a progressively increased risk of subsequent mortality.[56],[57] Furthermore, the introduction of tissue Doppler indices, in particular the ratio of early mitral flow/early annulus velocity, can be used to indicate poor prognosis in a variety of patients.[58] Tissue Doppler parameters even predict mortality in a general population of patients, most of whom were free of apparent systolic and diastolic dysfunction by conventional echocardiographic methods.[59] Recently, the progressive LV filling abnormalities in outpatients with preserved LV systolic function has been proven to be a strong, independent predictor of all-cause mortality.[60]
Figure 2. The stages of diastolic dysfunction recognized by changes in left ventricle filling dynamics.
(A) Pulsed-wave Doppler of mitral inflow and (B) Pulsed-wave tissue Doppler of mitral annulus in progressive stages. Diastolic filling grades: classification of diastolic filling and representative mitral inflow pulsed wave Doppler and mitral annulus pulsed wave tissue Doppler signals; E/A: ratio of passive early to active late mitral filling velocities; Deceleration time: time from peak to baseline of the mitral E velocity; Ea/Aa: ratio of the early mitral annular velocity to the late annular velocity; Amitral: duration of filling of the late filling velocity of the mitral inflow; ARpulmonary: duration of atrial reversal component of the pulmonary venous inflow. Statement: this figure was adapted from Whalley GA, Wasywich CA, Walsh HJ, Doughty RN. The role of echocardiography in the contemporary management of heart failure. Expert Rev Cardiovasc Ther 2005; 3(1): 51–70, which was authorized by the publisher.
6. Prognosis
The differentiation of HF-PEF and HF-REF is important, since we know that patients with HF-PEF have a better prognosis than those with HF-REF, although it is still very high in both groups. [61] Many studies have enrolled patients with HF-PEF (for example, DIG-PEF,[62] CHARM-Preserved,[63] and I-PRESERVE[64]) providing an interesting comparative group. Campbell, et al.[65] have compared outcomes in HF-PEF with patients of similar age, sex distribution and co-morbidity that were enrolled in trials of hypertension, diabetes mellitus, angina pectoris and atrial fibrillation, and demonstrated that patients in the HF-PEF trials were at higher risk of death and at strikingly higher risk of HF hospitalizations. No data about underlying pathology, including diastolic dysfunction can be made, but it is conceivable that patients with HF-PEF may have more diastolic dysfunction than similar patients without HF-PEF.[66] This diastolic dysfunction occurs in the presence of LV hypertrophy often, but interestingly, the median N-terminal pro– B-type natriuretic peptide concentration was much higher in the I-PRESERVE trial than the LIFE study, which enrolled hypertensive patients, despite the greater LV mass in LIFE suggesting that LV hypertrophy and presumable underlying cardiac function may be important.[67] Similarly, while LV mass in CHARM-Preserved was analogous to that in LIFE, median N-terminal pro-B-type natriuretic peptide was twice that of LIFE.[68] A better understanding of why N-terminal pro–B-type natriuretic peptide is elevated to a greater extent in some patients with HF-PEF than in patients with similar clinical presentation is clearly important, given the prognostic importance of this peptide in HF-PEF.[69]
7. Characterization of LV dysfunction in HF-PEF
As has been previously demonstrated in the MAGGIC study,[70] the implications of missing data can be substantial. In this case, previous studies with up to 70% missing data had erroneously reported that HF-PEF had similar mortality as HF-REF. In fact, the main bias was the missing LVEF among some groups of patients, such as the elderly population. As patients with HF-PEF are older, if some are excluded due to the lack of LVEF measurements, there is a higher probability of systemic selection bias when comparing HF-REF and HF-PEF patients. The impact of this bias has been recently evaluated.[71] Compared to patients with known LVEF, patients missing a EF measurement (HF-mEF) were older, had a greater prevalence of chronic obstructive pulmonary disease (COPD), previous stroke, and were smokers. This group is associated with poor short and long term survival, similar to the HF-REF population.[71] In addition, even if we examine the assessment of diastolic dysfunction in HF-PEF, the data are variable. Assignment of a specific pattern may not be possible, and patients have been excluded from prior studies on this basis. A systematic analysis performed by Narayanan, et al.[72] indicated that even with expert acquisition and interpretation, assignment of LV filling patterns is not possible in up to one-third of patients. A metric that is feasible in all patients, is load independent, yields a single continuous variable number, is quick, reliable, and automated would be desirable, but has thus far been elusive.[73] Therefore, the missing data in both systolic and diastolic disfunction is of major concern, especially in the elderly population which suffers the most from this lack of evaluation and resulting poor outcome, probably due to erroneous diagnosis and treatment.
8. Treatment and implications in the elderly population
No treatment has yet been demonstrated to reduce morbidity and mortality in patients with HF-PEF, as described in HF management guidelines (Table 2). Diuretics are used to control sodium and water retention and relieve breathlessness and edema, as in HF-REF. Adequate treatment of hypertension and myocardial ischemia is also considered to be important, as is control of the ventricular rate in patients with atrial fibrillation.[74] Moreover, randomized trials on elderly HF patients are lacking, although HF-PEF is noted to greater extent in the aging population. However, the use of pharmacological and non-pharmacological therapies is currently recommended by the HF guidelines in both non-elderly and elderly patients, with specific cautions in the latter. The main reasons leading to poor outcome in this population are the suboptimal application of therapy[75] and the lack of mortality benefits in the HF-PEF group, with its higher prevalence especially in the older patients. Sub-optimal therapy is due to co-morbidities and their difficult management, reduced compliance, increased susceptibility to renal dysfunction, impairment of sodium and water excretion, and therapy aggravated postural hypotension and bradyarrhythmias. However, these conditions should not discourage therapy, but be kept in consideration for tailoring treatment.
Table 2. Major differences in HF-PEF treatment recommendations between the ESC and ACCF/AHA Guidelines.
| 2012 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure | 2013 ACCF/AHA Guideline for the management of heart failure | |
| Class 1 | No treatment has yet been shown to reduce morbidity and mortality | Blood pressure control (LOE B) |
| Diuretics for symptoms relief (LOE C) | ||
| Class 2a | Coronary revascularization in CAD (LOE C) | |
| a. Symptomatic or | ||
| b. Myocardial Ischaemia | ||
| AF management (LOE C) | ||
| If hypertension use of ACE inibitors and beta blockers (LOE C) | ||
| Use of PUFA in NYHA 2-4, unless controindicated (LOE B) |
||
| Class 2b | Use of ARBs (LOE C) |
AF: atrial fibrillation; ARBs: angiotensinreceptor blockers; CAD: coronary artery disease; HF-PEF: Heart failure with preserved ejection fraction; LOE: level of evidence; PUFA: omega-3 polyunsatured fatty acid.
9. Conclusions
HF-PEF is a complex and common disease, with a high and constantly increasing prevalence. Detecting the syndrome is still difficult because of its non-specific nature of symptoms and signs, especially in the elderly population. While HF-PEF patients are at higher risk of death and hospitalizations than similar age and co-morbidity profile patients, treatment is still empirical and no therapy has yet shown significant impact on mortality. This is why we believe there is urgent need of increased awareness and clinical research in the field.
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 2.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function: epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 5.Yancy CW, Lopatin M, Stevenson LW, et al. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 6.Lee DS, Gona P, Vasan RS, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 8.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 9.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 10.Lam CS, Donal E, Kraigher-Krainer E, et al. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenzen MJ, Scholte OP, Reimer WJ, et al. Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. Eur Heart J. 2004;25:1214–1220. doi: 10.1016/j.ehj.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 13.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 14.ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd-Jones D, Adams RJ, Brown Tm, et al. Heart disease and stroke statistics- 2010 update: a report for the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2010;121:e1–e170. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 16.Jugdutt BJ. Aging and heart failure: changing demographics and implications for therapy in the elderly. Heart Fail Rev. 2010;15:401–405. doi: 10.1007/s10741-010-9164-8. [DOI] [PubMed] [Google Scholar]
- 17.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–2014. doi: 10.1161/CIRCULATIONAHA.110.954388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol. 2011;26:562–568. doi: 10.1097/HCO.0b013e32834b7faf. [DOI] [PubMed] [Google Scholar]
- 19.Krumholz HM, Larson M, Levy D. Sex differences in cardiac adaptation to isolated systolic hypertension. Am J Cardiol. 1993;72:310–313. doi: 10.1016/0002-9149(93)90678-6. [DOI] [PubMed] [Google Scholar]
- 20.Redfield MM, Jacobsen SJ, Borlaug BA, et al. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 21.De Simone G, Devereux RB, Chinali M, et al. Sex differences in obesity-related changes in left ventricular morphology: the Strong Heart Study. J Hypertens. 2011;29:1431–1438. doi: 10.1097/HJH.0b013e328347a093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foll D, Jung B, Schilli E, et al. Magnetic resonance tissue phase mapping of myocardial motion: new insight in age and gender. Circ Cardiovasc Imaging. 2010;3:54–64. doi: 10.1161/CIRCIMAGING.108.813857. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Selles M, Doughty RN, Poppe K, et al. Gender and survival in patients with heart failure: interactions with diabetes and aetiology. Results from MAGGIC individual patient meta-analysis. Eur J Heart Fail. 2012;14:473–479. doi: 10.1093/eurjhf/hfs026. [DOI] [PubMed] [Google Scholar]
- 24.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 25.Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: with special emphasis on volume, mass, and geometry. J Am Coll Cardiol. 2011;58:1733–1740. doi: 10.1016/j.jacc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 27.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Cardiol Clin. 2011;29:447–459. doi: 10.1016/j.ccl.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Little WC, Pu M. Left ventricular-arterial coupling. J Am Soc Echocardiogr. 2009;22:1246–1248. doi: 10.1016/j.echo.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Little WC, Cheng CP. Left ventricular-arterial coupling in conscious dogs. Am J Physiol. 1991;261:H70–H76. doi: 10.1152/ajpheart.1991.261.1.H70. [DOI] [PubMed] [Google Scholar]
- 30.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 31.The CONSENSUS Trial Study Group Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 32.Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA. 2000;283:1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 33.Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106:2194–2199. doi: 10.1161/01.cir.0000035653.72855.bf. [DOI] [PubMed] [Google Scholar]
- 34.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 35.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 36.Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 37.Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 38.McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 39.Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 40.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 41.Massie BD, Carson PD, McMurray JD, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Little WC, Zile MR. HFpEF: Cardiovascular abnormalities not just comorbidities. Circ Heart Fail. 2012;5:6696–6671. doi: 10.1161/CIRCHEARTFAILURE.112.972265. [DOI] [PubMed] [Google Scholar]
- 44.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 46.Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction: an inconvenient truth. J Am Coll Cardiol. 2010;55:526–537. doi: 10.1016/j.jacc.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 47.Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53:905–918. doi: 10.1016/j.jacc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Henein M, Owen A. HFNEF breathlessness: is it really heart failure? Int J Cardiol. 2010;143:111–112. doi: 10.1016/j.ijcard.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 49.Caruana L, Petrie MC, Davie AP, et al. Do patients with suspected heart failure and preserved left ventricular systolic function suffer from “diastolic heart failure” or from misdiagnosis? A prospective descriptive study. BMJ. 2000;321:215–218. doi: 10.1136/bmj.321.7255.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ingle L, Cleland JG, Clark AL. Perception of symptoms is out of proportion to cardiac pathology in patients with “diastolic heart failure”. Heart. 2008;94:748–753. doi: 10.1136/hrt.2007.131144. [DOI] [PubMed] [Google Scholar]
- 51.Packer M. Can brain natriuretic peptide be used to guide the management of patients with heart failure and a preserved ejection fraction? The wrong way to identify new treatments for a nonexistent disease. Circ Heart Fail. 2011;4:538–540. doi: 10.1161/CIRCHEARTFAILURE.111.963710. [DOI] [PubMed] [Google Scholar]
- 52.Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 53.Doughty RN, Whalley GA, Walsh HA, et al. CAPRICORN Echo Substudy Investigators. Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: the CAPRICORN Echo Substudy. Circulation. 2004;109:201–206. doi: 10.1161/01.CIR.0000108928.25690.94. [DOI] [PubMed] [Google Scholar]
- 54.Little WC, Oh JK. Echocardiographic evaluation of diastolic function can be used to guide clinical care. Circulation. 2009;120:802–809. doi: 10.1161/CIRCULATIONAHA.109.869602. [DOI] [PubMed] [Google Scholar]
- 55.Somaratne JB, Whalley GA, Poppe KK, et al. Pseudonormal mitral filling is associated with similarly poor prognosis as restrictive filling in patients with heart failure and coronary heart disease: a systematic review and meta-analysis of prospective studies. J Am Soc Echocardiogr. 2009;22:494–498. doi: 10.1016/j.echo.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Bella JN, Palmieri V, Roman MJ, et al. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle-aged and elderly adults. The Strong Heart Study. Circulation. 2002;105:1928–1933. doi: 10.1161/01.cir.0000015076.37047.d9. [DOI] [PubMed] [Google Scholar]
- 57.Schillaci G, Pasqualini L, Verdecchia P, et al. Prognostic significance of left ventricular diastolic dysfunction in essential hypertension. J Am Coll Cardiol. 2002;39:2005–2011. doi: 10.1016/s0735-1097(02)01896-x. [DOI] [PubMed] [Google Scholar]
- 58.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 59.Mogelvang R, Sogaard P, Pedersen SA, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation. 2009;119:2679–2685. doi: 10.1161/CIRCULATIONAHA.108.793471. [DOI] [PubMed] [Google Scholar]
- 60.AlJaroudi W, Alraies MC, Halley C, et al. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation. 2012;125:782–788. doi: 10.1161/CIRCULATIONAHA.111.066423. [DOI] [PubMed] [Google Scholar]
- 61.Meta-analysis global group in chronic heart failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750–1757. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 62.Ahmed A, Rich MW, Fleg JL, et al. Effects of Digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left- ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 64.McMurray JJ, Carson PE, Komajda M, et al. Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail. 2008;10:149–156. doi: 10.1016/j.ejheart.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 65.Campbell TR, Jhund PS, Castagno D, et al. What have we learned about patients with heart failure and preserved ejection fraction from DIG-PEF, CHARM-Preserved, and I-PRESERVE? J Am Coll Cardiol. 2012;23:2349–2356. doi: 10.1016/j.jacc.2012.04.064. [DOI] [PubMed] [Google Scholar]
- 66.Zile MR, Gottdiener JS, Hetzel SJ, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 67.Olsen MH, Wachtell K, Nielsen OW, et al. N-terminal brain natriuretic peptide predicted cardiovascular events stronger than high sensitivity C-reactive protein in hypertension: a LIFE substudy. J Hypertens. 2006;24:1531–1539. doi: 10.1097/01.hjh.0000239288.10013.04. [DOI] [PubMed] [Google Scholar]
- 68.Persson H, Lonn E, Edner M, et al. Investigators of the CHARM Echocardiographic Substudy-CHARMES. Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence: results from the CHARM Echocardiographic Substudy-CHARMES. J Am Coll Cardiol. 2007;49:687–694. doi: 10.1016/j.jacc.2006.08.062. [DOI] [PubMed] [Google Scholar]
- 69.Komajda M, Carson PE, Hetzel S, et al. Factors associated with outcome in heart failure with preserved ejection fraction: findings from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE) Circ Heart Fail. 2011;4:27–35. doi: 10.1161/CIRCHEARTFAILURE.109.932996. [DOI] [PubMed] [Google Scholar]
- 70.Somaratne JB, Berry C, McMurrey JJV, et al. The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33:1750–1757. doi: 10.1093/eurheartj/ehr254. [DOI] [PubMed] [Google Scholar]
- 71.Poppe KK, Squire IB, Whalley GA, et al. Meta-analysis global group in chronic heart failure (MAGGIC). Known and missing left ventricular ejection fraction and survival in patients with heart failure: a MAGGIC meta-analysis report. Eur J Heart Fail. 2013;15:1220–1227. doi: 10.1093/eurjhf/hft101. [DOI] [PubMed] [Google Scholar]
- 72.Narayanan A, Aurigemma GP, Hill JC, et al. High prevalence of ‘unclassifiable’ diastolic dysfunction using current criteria. Circulation. 2008;118:787. [Google Scholar]
- 73.Kitzman DW, Little WC. Left ventricle diastolic dysfunction and prognosis. Circulation. 2012;125:743–745. doi: 10.1161/CIRCULATIONAHA.111.086843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology
- 75.Komajda M, Hanon O, Hochadel M, et al. Contemporary management of octogenarians hospitalized for heart failure in Europe: Euro heart failure survey II. Eur Heart J. 2009;30:478–486. doi: 10.1093/eurheartj/ehn539. [DOI] [PubMed] [Google Scholar]