Abstract
Background
Depressive disorders are among the more common mental illnesses around the world. About 3% of prepubertal children and 6% of postpubertal children and adolescents are affected. Many physicians are unsure about which treatment approaches are effective and how the treatment should be planned.
Methods
A systematic literature search was carried out in electronic databases and study registries and as a manual search. More than 450 studies (mostly randomized controlled trials [RCTs]) were identified and summarized in five evidence tables. The ensuing recommendations were agreed upon in a consensus conference in which 23 organizations were represented.
Results
The recommended treatment of first choice for children from age 8 onward and for adolescents is either cognitive behavioral therapy (CBT) (Cohen’s d [effect strength]: 0.5–2) or interpersonal psychotherapy (Cohen’s d: 0.5–0.6). Fluoxetine is recommended for drug treatment (Cohen’s d: 0.3–5.6), either alone or in combination with CBT. The analysis revealed a lower level of evidence for psychodynamic or systemic psychotherapy or for drug treatment with escitalopram, citalopram, or sertraline. For mild or moderate depression, psychotherapy is recommended; for severe depression, combination therapy. Particularly for children, there is a lack of adequately informative comparative studies on these treatment approaches as well as on other, complementary interventions (e.g., art therapy, sleep deprivation, youth welfare services).
Conclusion
There is adequate evidence to support some recommendations for the treatment of depressive disorders in adolescents, but evidence for children is lacking. There is a pressing need for intervention research in this area for both children and adolescents.
Depressive disorders are among the more common mental illnesses all over the world, with an estimated 121 million sufferers, according to the World Health Organization (WHO). Depressive disorders are currently the single most important cause of “years lost due to disability” (YLD—a statistical measure of years lived with a disability, multiplied by the severity of the disability). It is projected that, by the year 2020, depressive disorders will also become the second most important cause of loss of “disability-adjusted life years” (DALY) (WHO, 2012) (1).
Depressive disorders can arise early in life: some 3% of prepubertal children and 6% of postpubertal children and adolescents are affected (e1). These disorders manifest themselves in episodes of varying duration and are often chronic. They markedly impair psychosocial development. An adolescent who has had one episode has a 50% to 70% chance of having a second one within five years (e2).
Not all of the affected children and adolescents receive optimal treatment (2). For many of the currently available treatment options, evidence of efficacy is lacking. Recommendations about the treatment of depressive disorders in adults (e3) do not necessarily apply to younger patients, as both the manifestations of disease and the appropriateness and efficacy of various forms of treatment depend on the patient’s age and level of development.
In 2010, the German Society for Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy (Deutsche Gesellschaft für Kinder- und Jugendpsychiatrie, Psychosomatik und Psychotherapie, DGKJP) initiated a project to develop a new evidence- and consensus-based S3 guideline for the treatment of depressive disorders (ICD-10 codes F32, F33, F34.1 and F92.0) in children and adolescents (ages 3–18), so that more of these younger patients can receive optimal treatment. The guideline contains information on the current state of scientific knowledge about treatment and gives recommendations for the selection and planning of effective treatment strategies (3).
Methods
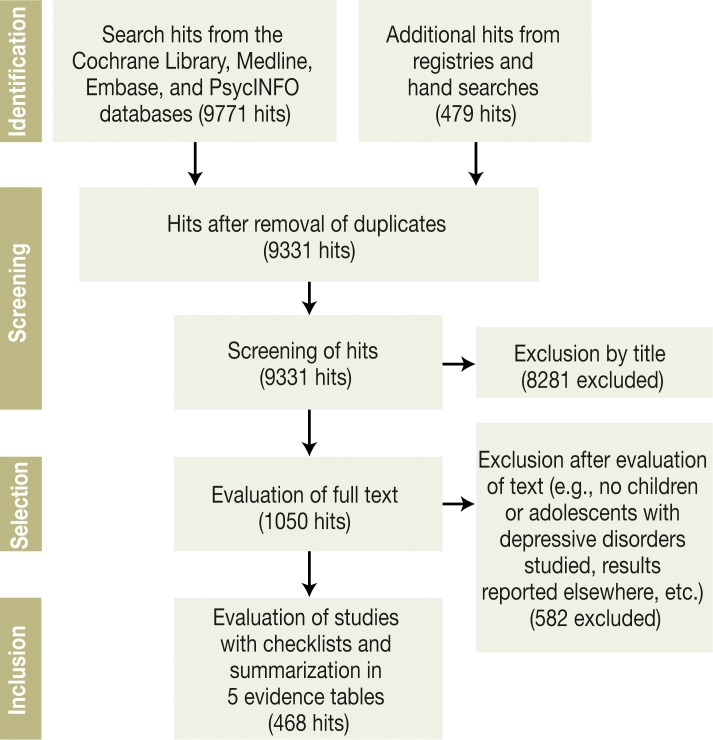
A systematic search for existing guidelines (Figure 1) yielded three of these (4– 7). In addition, searches for relevant publications that appeared from July 2011 to July 2012 were carried out in each of four databases, in clinical trial registries, and (by manual search) in scholarly periodicals and congress proceedings. Modular search filters were used (for an example, cf. eBox 1). Two independent judges selected and evaluated the studies; when their opinions diverged, they reconsidered and discussed the study in question until they reached agreement. Inclusion and exclusion criteria are listed in the Box.
Figure 1.
Results of the systematic literature search and evaluation
eBox 1. An illustrative Medline search filter.
A search for up-to-date randomized controlled trials of psychotherapy from the years 2005–2012
Module 1: population filter
(children and adolescents aged 3–18 with depressive disorders, cf. [4])
1. depression/ or adjustment disorders/ or depressive disorder/ or depressive disorder, major/ or dysthymic disorder/ or mood disorders/
2 (depress$ or dysthym$).mp.
3 1 or 2
4 exp adolescent/ or exp child/ or exp infant/
5 (child$ or adoles$ or teen$ or youth$ or infant$ or toddler$ or kind$ or jugend$ or kleinkind$ or s$ugling$ or enfant$ or jeune$ or nourrisson$).mp.
6 4 or 5
7 3 and 6
8 imit 7 to (“young adult (19 to 24 years)” or “adult (19 to 44 years)” or “young adult and adult (19–24 and 19–44)” or “middle age (45 to 64 years)” or “middle aged (45 plus years)” or “all aged (65 and over)” or “aged (80 and over)”)
9 7 not 8
10. limit 9 to (english or french or german)
11. exp Depression, Postpartum/
12. (postpartum$ or perinatal$ or antenatal$ or postnatal$).mp.
13. 11 or 12
14. 10 not 13
Module 2: method filter (here, for RCTs)
15. Randomized Controlled Trials as Topic/
16. randomized controlled trial/
17. Random Allocation/
18. Double Blind Method/
19. Single Blind Method/
20. clinical trial/
21. clinical trial, phase i.pt
22. clinical trial, phase ii.pt
23. clinical trial, phase iii.pt
24. clinical trial, phase iv.pt
25. controlled clinical trial.pt
26. randomized controlled trial.pt
27. multicenter study.pt
28. clinical trial.pt
29. exp Clinical Trials as topic/
30. or/1–15
31. (clinical adj trial$).tw
32. ((singl$ or doubl$ or treb$ or tripl$) adj (blind$3 or mask$3)).tw
33. PLACEBOS/
34. placebo$.tw
35. randomly allocated.tw
36. (allocated adj2 random$).tw
37. or/17–22
38. 16 or 23
39. case report.tw
40. letter/
41. historical article/
42. or/25–27
43. 24 not 28
Module 3: combination of modules 1 and 2 with a topical filter (here, for psychotherapy)
44. 14 and 43
45. limit 44 to yr=”2005 -Current”
46. exp Psychotherapy/
47. exp Psychoanalysis/
48. (psychotherap* or interpersonH* therap* or $ognitive* therap* or $ognitive behavi* therap* or behavi* therap* or counsel* or problem solv* or psychoanaly* or play therap* or psychodynam* or system* therap* or famil* therap* or relaxation or supportive or psychodram* or role$play* or transaction* or social skill* or client$centered or verhaltenstherap* or spieltherap* or rollenspiel* or beratung* or coaching* or probleml$se* or gespr$chstherap* or gespr$chspsychotherap* or gestalttherap* or sozial* kompeten* or klientenzentrier* or therap* cognitiv$-comportemental* or consultation*).mp.
49. 46 or 47 or 48
50. 45 and 49
51. (depress* or dysthym*).ti.
52. (child$ or adoles$ or teen$ or youth$ or infant$ or toddler$ or kind$ or jugend$ or kleinkind$ or s$ugling$ or enfant$ or jeune$ or nourrisson$ or juvenil$).ti.
53. 50 and 51 and 52
Box. Methods of the systematic literature search.
Inclusion criteria
Publications on the treatment of children and/or adolescents (age 3–18) with a depressive disorder diagnosed according to a classification system
Language: English, German, or French
Systematic reviews:
Time period: 1 January 1991 to 2012
Systematic nature of the review: description of method, specifying (at least) the databases that were searched and the inclusion/exclusion criteria for primary studies
> 50% of the publications included for review concern children and adolescents with depressive disorders, or else separate results are available for this patient group
Primary studies:
Goal of the intervention: reduction of depression and/or suicidality
>50% of the persons included in the study were children and adolescents, or else separate results are available for this patient group
Exclusion criteria
Publications on other types of mental illness (e.g., bipolar disorders)
Publications on depression in association with pregnancy or childbirth
Publications limited to preventive interventions in children and/or adolescents who are not at risk, or at risk, or subclinically affected
All systematic reviews and controlled trials were evaluated by the two judges with the aid of the structured checklists of the Scottish Intercollegiate Guidelines Network (SIGN, available at www.sign.ac.uk/methodology/checklists.html). They were then thematically grouped and summarized in five evidence tables and assigned levels of evidence. The evidence tables and the previously published guidelines were used to develop recommendations for key clinical questions; each recommendation was stated together with a recommendation grade. Evidence levels and recommendation grades were assigned according to the scheme of the Oxford Centre for Evidence-Based Medicine (8) (eTable).
eTable. Evidence levels and recommendation grades for treatment (Oxford Centre for Evidence-Based Medicine, [e8]).
| Question | Step 1 Evidence level 1*1 | Step 2 Evidence level 2*1 | Step 3 Evidence level 3*1 | Step 4 Evidence level 4*1 | Step 5 Evidence level 5 |
|---|---|---|---|---|---|
|
What will happen if we do not offer/add a therapy? (Prognosis) |
Systematic review of inception cohort studies | Inception cohort studies | Cohort study or control arm of RCT | Case-series or case–control studies, or poor quality prognostic cohort study*2 | − |
|
Does this intervention help?
(Treatment Benefits) |
Systematic review of RCTs or n -of-1 trials | RCT or observational study with dramatic effect | Non-randomized controlled cohort / follow-up study | Case-series, case-control studies, or historically controlled studies*2 | Mechanism-based reasoning |
|
What are the COMMON harms? (Treatment Harms) |
Systematic review of RCTs or of nested case–control studies, n-of-1 trial with a patient of the pertinent type, or observational study with dramatic effect | Non-randomized controlled cohort / follow-up study with adequate sample size and follow-up duration | |||
| What are the RARE harms? (Treatment Harms) | Systematic review of RCTs or n-of-1 trial | ||||
| Recommendation grade | A: strong recommendation ↑ ↑ consistent studies*3 with evidence level 1 or 2 | B: Recommendation ↑ consistent studies with evidence level 3 or 4, or extrapolations*3 from studies with evidence level 1 or 2 | 0: open recommendation ↔ studies with evidence level 5, or extrapolations*3 from studies with evidence level 3 or 4 | ||
| Clinical consensus point (CCP): Standard of treatment about which no scientifically conducted trials are feasible or desired | |||||
RCT, radnomized controlled trial: a trial with at least one intervention group and at least one control group, to which subjects are randomly assigned; n -of-1 trial: a single-patient, randomized, controlled study.
*1The evidence level can be lowered because of the study’s poor quality, imprecision, indirect relation to the key question, very low absolute effect strength, or inconsistency with other studies; it can be raised if the measured effect strength is high or very high.
*2Systematic reviews are, in general, preferable to individual studies.
*3This refers to the application of study findings to situations that can differ in clinically significant ways from the situations in which the study was conducted. ↑, recommendation; ↑ ↑, strong recommendation; ↔, open recommendation
In a consensus conference moderated by the Association of Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, AWMF), every recommendation was discussed in a “nominal group process” and then voted on. Each of the 23 participating organizations (ebox 2) had one vote. Each recommendation was issued with a “strong consensus” (>95% agreement), by “consensus” (>75–95% agreement), or by “majority agreement” (>50–75% agreement).
eBox 2. Organizations and persons participating in the creation of this guideline.
Association of Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, AWMF): Dr. rer. hum. biol. Cathleen Muche-Borowski
Steering Committee:
-
German Society for Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy (Deutsche Gesellschaft für Kinder- und Jugendpsychiatrie, Psychosomatik und Psychotherapie, DGKJP):
Prof. Dr. med. Gerd Schulte-Körne (coordinator), PD Dr. med. Michael Kölch, and Prof. Dr. med. Georg Romer
German Association of Hospital Chiefs of Service for Child and Adolescent Psychiatry, Psychosomatics, and Psychotherapy (Bundesarbeitsgemeinschaft der Leitenden Klinikärzte für Kinder- und Jugendpsychiatrie, Psychosomatik und Psychotherapie e. V., BAG KJPP): Dr. med. Martin Jung
German Chamber of Psychotherapists (Bundespsychotherapeutenkammer, BPtK): Dr. phil. Johannes Klein-Hessling and Dipl.-Soz.-Päd. Peter Lehndorfer
Professional Association of Child and Adolescent Psychiatrists, Psychosomatic Therapists, and Psychotherapists in Germany (Berufsverband für Kinder- und Jugendpsychiatrie, Psychosomatik und Psychotherapie in Deutschland e. V., BKJPP): Dr. med. Ute Müller
Expert: Prof. Dr. phil. Sabine Walper
Consent Committee:
Drug Commission of the German Medical Association (Arzneimittelkommision der deutschen Ärzteschaft, AKdÄ): PD Dr. med. Martina Pitzer
Professional Association of Children’s and Young People’s Physicians (Berufsverband der Kinder- und Jugendärzte e. V., BVKJ): Dr. med. Martin Fischer
Professional Association of Children + Adolescent Psychotherapists (Berufsverband der Kinder- und Jugendlichenpsychotherapeutinnen und Kinder- und Jugendlichenpsychotherapeuten e. V., bkj): Silke von der Heyde, M.S.
Association of German Professional Psychologists (Berufsverband Deutscher Psychologinnen und Psychologen e. V., BDP): Dipl.-Psych. Inge Neiser and Dipl.-Psych. Rainer Mannheim-Rouzeaud
Professional Assoication of Arts Therapists (Bundesarbeitsgemeinschaft Künstlerische Therapien, BAG KT): Katja Bonnländer, Titus David Hamdorf, and Prof. Dr. phil. Thomas Staroszynski
Federal Association of Relatives of the Mentally Ill (Bundesverband der Angehörigen psychisch Kranker e. V., BApK): Dipl.-Ing. Karl Heinz Möhrmann
Federal Association of Contract Psychotherapists (Bundesverband der Vertragspsychotherapeuten e. V., bvvp): Dipl.-Soz.-Päd. Ariadne Sartorius
Federal Association for Behavioral Therapy in Childhood and Adolescence (Bundesvereinigung Verhaltenstherapie im Kindes- und Jugendalter e. V., BVKJ): Dr. phil. Maria Elisabeth Ahle and Prof. Dr. rer. nat. Silvia Schneider
German Society of Depth Psychology–Based Psychotherapy (Deutsche Fachgesellschaft für tiefenpsychologisch fundierte Psychotherapie e. V., DFT): Prof. Dr. med. Thomas Löw
German Society of Pediatrics and Adolescent Medicine (Deutsche Gesellschaft für Kinder- und Jugendmedizin e. V. (DGKJ): Dr. med. Torsten Lucas
German Association for Psychiatry and Psychotherapy (Deutsche Gesellschaft für Psychiatrie, Psychotherapie und Nervenheilkunde e. V., DGPPN): Prof. Dr. med. Dr. rer. soc. Frank Schneider and PD Dr. med. Cornelius Schüle
German Psychological Society (Deutsche Gesellschaft für Psychologie e. V., DGPs): Prof. Dr. phil. Wolfgang Ihle and Prof. Dr. phil. Gunter Groen
German Society for Psychosomatic Medicine and Psychotherapy (Deutsche Gesellschaft für Psychosomatische Medizin und Ärztliche Psychotherapie e. V., DGPM): Prof. Dr. med. Thomas Löw
German Society of Social Pediatrics and Youth Medicine (Deutsche Gesellschaft für Sozialpädiatrie und Jugendmedizin e. V., DGSPJ): Dr. med. Ullrich Raupp
German Society for Systematic and Family Therapy (Deutsche Gesellschaft für Systemische Therapie und Familientherapie, DGSF): Dr. med. Filip Caby and Dr. med. Ingo Spitczok von Brisinski
German Society for Behavioral Therapy (Deutsche Gesellschaft für Verhaltenstherapie e. V., DGVT): Dipl.-Psych. Franz Rudolf Merod and Dipl.-Psych. Sonja Stolp
German Association of Psychotherapists (Deutsche PsychotherapeutenVereinigung, DPtV): Michaela Willhauck-Fojkar, Dipl.-Soz.-Päd. (FH)
German College for Psychosomatic Medicine (Deutsches Kollegium für Psychosomatische Medizin, DKPM): Prof. Dr. med. Peter Henningsen and Sigrid Aberl
Association of Analytical Child and Adolescent Psychotherapists in Germany (Vereinigung Analytischer Kinder- und Jugendlichen-Psychotherapeuten in Deutschland e. V., VAKJP): Dipl.-Soz.-Päd. Christine Röpke
Results and recommendations
Treatment setting
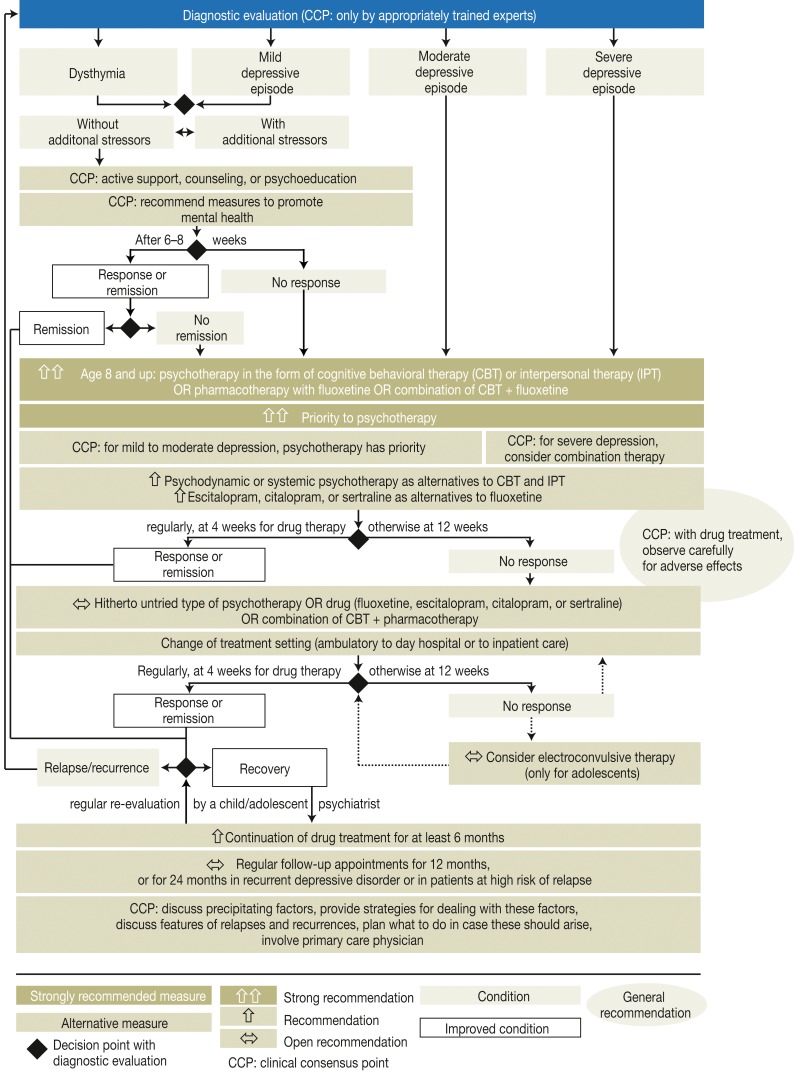
The first necessary step in the treatment of depressive disorders is thorough diagnostic evaluation and classification according to the ICD-10 criteria. The ICD-10 distinguishes three degrees of severity of depressive episodes: mild, moderate, and severe (Figure 2). A mild depressive disorder without comorbidity, significant risk factors, a family history of affective disorders, or warning signs of a likely relapse can be initially managed with watchful waiting for six to eight weeks. This includes support in coping with everyday tasks and counseling or psychoeducation about the manifestations of the disorder, its causes, the expected course, and the options for treatment.
Figure 2.
Treatment algorithm based on the recommendations of the guideline “The Treatment of Depressive Disorders in Children and Adolescents” (3)
Follow-up evaluations should be performed every two weeks. This recommendation also applies to children and adolescents who refuse treatment, as well as to those who are fully able to cope with the demands of everyday life as appropriate for their age group (clinical consensus point [CCP], recommendation issued by consensus).
As a rule, children and adolescents with depressive disorders are treated in the outpatient setting. A prerequisite for outpatient treatment is an appropriate level of psychosocial function (axis VI of the Multi-Axial System [MAS]) (9) (CCP, strong consensus). This means, among other things, that the patient must be able to maintain contact with other persons and cope with everyday tasks, such as regular school attendance. On the other hand, reasons for hospitalization include the following:
acute suicidality, together with the inability to adhere to a non-suicide agreement (i.e., the patient cannot be counted on to seek help if suicidal thoughts arise);
a depressive disorder that is so severe that the patient cannot (for example) attend school any more;
inability to cope with everyday tasks, such as eating and drinking regularly or maintaining a normally structured timetable (getting up in the morning, going to bed at an appropriate time).
These criteria are represented in summary fashion on Axes IV and V of the MAS (9) (CCP, strong consensus). Day hospital care should be considered and, in some cases, recommended in the light of the severity of the episode, the patient’s familial and social resources, and the local conditions of care (CCP, strong consensus).
Treatment recommendations
Data on the efficacy of psychotherapy ara available mainly for adolescents (over age 12). There have been very few studies of German-language psychotherapy programs; there has not been any randomized controlled trial (RCT) to date, and very few studies have directly compared different psychotherapeutic approaches. Cognitive behavioral therapy (CBT) is the type of psychotherapy that has been most commonly studied in RCTs. Psychotherapeutic interventions of all kinds have been found to lessen depression more effectively over the short term (mean, 12 weeks) in children and adolescents (age 6–18) than various alternative management strategies, both active and inactive. This is the case for all degrees of severity of depression, with a trend toward a more marked effect in moderate or severe depression. Further follow-up of the results for up to 24 months yielded inconsistent findings with respect to efficacy. As for suicidality in adolescents, no difference was found between psychotherapeutic intervention on the one hand and control treatment (or no treatment) on the other, although there was a nonsignificant trend toward a greater reduction of suicidality under psychotherapy. The treatments with the highest levels of evidence to date (10, 11) are CBT (with effect strengths [ES] measured by Cohen’s d ranging from 0.5 to 2, depending on the sample, the treatment strategy, the age of the patient, the duration of treatment, and the year of performance of the trial) and interpersonal psychotherapy (10). The latter, however, is not among the guideline techniques in Germany (with ES measured by Cohen’s d ranging from 0.5 to 0.6, depending on the method of assessing treatment outcome).
Fluoxetine is the only drug approved in Germany for the treatment of depressive disorders in children and adolescents aged 8 and above (ES measured by Cohen’s d in the range of 0.3 to 5.6, depending on the method of assessing treatment outcome) (e4). The recommended treatment of first choice for older children and adolescents with depressive disorders of various degrees of severity, and for those with a first episode of a depressive disorder, is either CBT, or interpersonal psychotherapy, or fluoxetine, or a combination of CBT and fluoxetine (12, 13) (strong recommendation, recommendation grade A). Some of the individual associations that belonged to the consensus group (bvvp, bkj, DGSF, BDP, BApK and BAG KT [abbreviations explained in eBox 2]) recommend the use of selective serotonin reuptake inhibitors (SSRIs) as second-line treatment. For mild to moderate depression, psychotherapy should initially be given priority (CCP).
In view of the adverse effects of SSRIs (headache, vomiting, sleep disturbance, fatigue, and loss of appetite at the start of treatment) and their tendency to reinforce suicidal ideation, psychotherapy should be initiated preferentially (e4) (strong recommendation, recommendation grade A). Moreover, it is recommended that patients should be closely observed for adverse drug effects and that the recommended follow-up investigations should be performed (CCP). The initial dose of fluoxetine should be 10 mg/day, and the dose can be raised to 20 mg/day at the end of one week of treatment (4). Other SSRIs should be given initially at half the daily dose for adults, and the dose can then be increased to the full recommended adult dose over the ensuing 2 to 4 weeks. Lower doses can be considered for small children, while higher doses can be considered for older children with near-adult body weight, or if a rapid therapeutic response is desired. These recommendations are largely based on the dosages that have been tested in RCTs, which are also summarized in the Table.
Table. Dosages of the recommended drugs that were used in randomized controlled trials.
| Drug- Trials | Age (years) | Initial dose (mg/day) | Maximal dose (mg/day) | Mean dose (mg/day) | Standard adult dose (mg/day), according to (14) |
|---|---|---|---|---|---|
| Fluoxetine | |||||
|
8–14 | 20 | 20 | 20 | 20–40 |
|
7–17 | 20 | 20 | 20 | |
|
8–17 | 10 in week 1 | 20 | 20 | |
|
13–18 | 20 in week 1, 40 in week 2 |
60 | unknown | |
|
12–18 | 10 initially, 20 in week 1 |
40 | 33.3* | |
| Escitalopram | |||||
|
12–17 | 10 in weeks 1–3 | 20 | 13.2 | 10–20 |
|
6–17 | 10 in weeks 1–4 | 20 | 11.9 | |
| Citalopram | |||||
|
13–18 | 10 | 40 | 26 | 20–40 |
|
7–17 | 20 in weeks 1–4 | 40 | 24 | |
| Sertraline | |||||
|
6–17 | 25 initially, 50 in weeks 1–2 |
200 | 131 | 50–200 |
*mean highest dose
If CBT or interpersonal psychotherapy is not possible for an older child or adolescent, or if an offer of such treatment has been refused, psychodynamic or systemic psychotherapy is recommended as an alternative (recommendation grade B; evidence level 3–4 for both psychodynamic psychotherapy [e5–e7] and systemic psychotherapy [e7, e8]).
If treatment with fluoxetine is not possible or not desired, escitalopram, citalopram, and sertraline are recommended as alternative drugs (recommendation grade B, evidence level 1 [e4, e9]).
Treatment with tricyclic antidepressants is not recommended, as these have been found to be no more effective than placebo (evidence level 1–2 for amitriptyline, clomipramine, desipramine, imipramine, and nortriptyline [e10–e13], evidence level 2–3 for nefazodone [e13, e14]) (strong recommendation, recommendation grade A). Nor should paroxetine, venlafaxine, or mirtazapine be given to children and adolescents with depressive disorders, as these have not been found any more effective than placebo but are associated (particularly venlafaxine) with increased suicidality (evidence level 1 [e4], strong recommendation, recommendation grade A). Moclobemide (a monoamine oxidase inhibitor, MAOI) should not be given to these patients either, as there is no evidence to support its use for this indication (evidence level 3 [e15], recommendation, recommendation grade B).
After a period of remission (i.e., absence of clinically relevant manifestations) of at least two months’ duration, drug treatment should be continued for at least six more months (recommendation, recommendation grade B), as it has been shown, for example, that 24–32 weeks of treatment with fluoxetine prevents and delays relapses in children and adolescents (aged 7–18) more effectively than placebo (evidence level 3 [e16, e17]). Likewise, the prolongation of CBT for a further six months after ten weeks of acute treatment has been found to be associated with a lower cumulative risk of relapse than management in a control group (evidence level 4 [e18]).
Discontinuation of drug treatment can be considered when there has been a period of remission of at least six months’ duration after the first episode of a depressive disorder (CCP). The results of treatment should be regularly rechecked by a child and adolescent psychiatrist (CCP).
Treatment approaches with inadequate evidence
Other treatments that are used in current clinical practice include the following:
client centered psychotherapy
art and music therapy
ergotherapy
youth welfare services
repetitive transcranial magnetic stimulation (rTMS)
vagus nerve stimulation
sleep deprivation
massage.
None of these methods are supported by adequate evidence, however, as clinical trials are either lacking or methodologically flawed (e.g., because of a lack of case definitions according to a classification scheme, lack of standardized inclusion criteria, or lack of standardized assessment of treatment outcome). Thus, no recommendation can be given for or against any of these methods (strong consensus).
The putative efficacy of the botanical substance St. John’s wort (Hypericum perforatum) has not been documented in any controlled trial. Potential adverse effects militate against the use of St. John’s wort (agitation, dry mouth, nightmares) or agomelatine (headache, dizziness, migraine, nausea, diarrhea).
There have not been any comparative clinical trials of electroconvulsive therapy (ECT) versus other types of intervention. There have been uncontrolled trials of ECT for children and adolescents who have not responded to drug treatment, many of whom have also had further complications or comorbidities. Thus, ECT cannot be recommended for children; for adolescents, it can be considered in very severe cases of depression in which the other approaches recommended in this guideline have already been tried without success (evidence level 4–5 [e.g., e19–e21], recommendation grade 0).
Results of treatment
When the treatment is begun, its goals should be clearly set and discussed with the child or adolescent patient and his or her parents, guardian(s), and/or other involved persons. The outcome of treatment should be regularly rechecked. After at least four weeks of treatment, the interim outcome can be assessed by the patients themselves (e.g., with self-assessment questionnaires such as the Depression Test for Children [Depressionstest für Kinder, DTK], e22), by involved persons such as parents (e.g., with the FBB-DES questionnaire, e23), and by the treating specialist (e.g., with the Kinder-DIPS clinical interview, e24). If there has been no clinically significant improvement after 12 weeks of treatment, or if there has been no response to drug treatment (a significant reduction of depressive manifestations for at least one week) in four weeks, then the treatment modality can be changed (CCP, strong consensus).
Children and adolescents with depressive disorders who have no improvement after the first treatment attempt can be treated with a different type of psychotherapy, or with a different medication than the one initially used, if any (fluoxetine, escitalopram, citalopram, or sertraline), or else with a hitherto untried combination of CBT and one of the medications just mentioned. A switch from outpatient treatment to day hospital care or to inpatient treatment can also be considered, according to the recommendations of this guideline (evidence level 5, open recommendation, strong consensus). For older adolescents with a recurrent depressive disorder, the national care guideline for adults with unipolar depression (e3) should be applied (CCP).
A child or adolescent with a depressive disorder who is in remission, i.e., has had no clinically relevant manifestations for at least 2 months, should have regular follow-up by the treating specialist for at least 12 months (open recommendation, strong consensus). A child or adolescent who is in remission after having had two or more episodes of a depressive disorder, or who is at increased risk of relapse because of stress factors that are still present, should have regular follow-up by the treating specialist for at least 24 months (evidence level 5, expert opinion).
Relapse prevention
The continuation of treatment serves to prevent, or at least delay, relapses and recurrent episodes. It was found in a randomized controlled trial of combination therapy to prevent relapses in adolescents that the risk of a relapse was significantly lower if CBT was given for 36 weeks than if fluoxetine was continued without any accompanying CBT (evidence level 3; this RCT did, however, have certain methodological flaws [e25]). As the evidence to date still does not seem to permit any definitive conclusion, the pertinent clinical consensus point (CCT) was stated in terms of “good clinical practice”: persons treating children and adolescents with depressive disorders should
develop strategies, together with the patients and their families and other involved persons, for the prevention of relapses and recurrent episodes (CCP, strong consensus);
inform about the risk of relapses and recurrences and explain what disease features and early-warning signs should be looked for;
develop strategies, together with the patients themselves, for what they ought to do in case these features should arise.
With the patients’ consent, these matters should be brought to the attention of their primary-care physicians (pediatricians and general practitioners), who are more accessible than specialists and thus tend to see the affected children and adolescents when they are in remission, rather than during an acute episode. The goal of involving primary-care physicians is to provide the patients with additional support in carrying out the strategies that were agreed upon (CCP).
The need for further research
Many questions in this area cannot be answered because of the lack of sufficiently informative comparative studies of different treatments for depressive disorders in childhood and adolescence (especially in childhood). In particular, there is a glaring lack of clinical studies in the German health-care system; there have been no studies, for example, of day hospital care or inpatient treatment, nor has there been any study with a follow-up period longer than 12 weeks. All of the recommendations of this guideline are based on the current state of scientific knowledge and may need to be fundamentally revised when an updated version of the guideline is prepared five years from now.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
The creation of this guideline was supported financially by the Erich-Benjamin-Stiftung (a charitable foundation) and by the German Society for Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy (Deutsche Gesellschaft für Kinder- und Jugendpsychiatrie, Psychosomatik und Psychotherapie, DGKJP).
The authors thank all colleagues and organizations that participated in the development of the guideline.
The authors also thank Katharina Galuschka, Lydia Unterberger, Chiara H. Schlenz, Carolina Silberbauer, and Rita Rupprecht for their assistance with the literature search, text versions, and administrative tasks having to do with the development of the guideline.
Footnotes
Conflict of interest statement
The authors state that they have no conflict of interest.
References
- 1.WHO. Depression. www.who.int/mental_health/management/depression/ 2012. (last accessed on 20. October 2013)
- 2.Essau CA. Frequency and patterns of mental health services utilization among adolescents with anxiety and depressive disorders. Depress Anxiety. 2005;22:130–137. doi: 10.1002/da.20115. [DOI] [PubMed] [Google Scholar]
- 3.Dolle K, Schulte-Körne G. Leitlinie Behandlung von depressiven Störungen bei Kindern und Jugendlichen. www.awmf.org/uploads/tx_szleitlinien/028-043l_S3_Depressive_St%C3%B6rungen_bei_Kindern_Jugendlichen_2013-07.pdf. 2005 (last accessed on 20. October 2013) [PubMed] [Google Scholar]
- 4.National Collaborating Centre for Mental Health. www.nice.org.uk/nicemedia/pdf/cg028fullguideline.pdf. London: The British Psychological Society & The Royal College of Psychiatrists; Depression in children and young people. Identification and management in primary, community and secondary care. (last accessed on 20. October 2013) [PubMed] [Google Scholar]
- 5.US Preventive Services Task Force. Screening and treatment for major depressive disorder in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;123:1223–1228. doi: 10.1542/peds.2008-2381. [DOI] [PubMed] [Google Scholar]
- 6.Cheung AH, Zuckerbrot RA, Jensen PS, Ghalib K, Laraque D, Stein RE. Guidelines for adolescent depression in primary care (GLAD-PC): II. Treatment and ongoing management. Pediatrics. 2007;120:e1313–e1326. doi: 10.1542/peds.2006-1395. [DOI] [PubMed] [Google Scholar]
- 7.Zuckerbrot RA, Cheung AH, Jensen PS, Stein RE, Laraque D. Guidelines for adolescent depression in primary care (GLAD-PC): I Identification, assessment, and initial management. Pediatrics. 2007;120:e1299–e1312. doi: 10.1542/peds.2007-1144. [DOI] [PubMed] [Google Scholar]
- 8.Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of evidence working group. The Oxford 2011 Levels of Evidence. www.cebm.net/index.aspx?o=5653] 2011. (last accessed on 20. October 2013)
- 9.Remschmidt H, Schmidt MH, Poustka F. 5 ed. Bern: Verlag Hans Huber; 2006. Multiaxiales Klassifikationsschema für psychische Störungen des Kindes- und Jugendalters nach ICD-10 der WHO. [Google Scholar]
- 10.Watanabe N, Hunot V, Omori IM, Churchill R, Furukawa TA. Psychotherapy for depression among children and adolescents: a systematic review. Acta Psychiatr Scand. 2007;116:84–95. doi: 10.1111/j.1600-0447.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 11.Harrington R, Whittaker J, Shoebridge P, Campbell F. Systematic review of efficacy of cognitive behaviour therapies in childhood and adolescent depressive disorder. BMJ. 1998;316:1559–1563. doi: 10.1136/bmj.316.7144.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubicka B, Elvins R, Roberts C, Chick G, Wilkinson P, Goodyer IM. Combined treatment with cognitive-behavioural therapy in adolescent depression: meta-analysis. Br J Psychiatry. 2010;197:433–440. doi: 10.1192/bjp.bp.109.075853. [DOI] [PubMed] [Google Scholar]
- 13.Cox GR, Callahan P, Churchill R, Hunot V, Merry SN, Parker AG, et al. Psychological therapies versus antidepressant medication, alone and in combination for depression in children and adolescents. Cochrane Database of Systematic Reviews. 2012;11 doi: 10.1002/14651858.CD008324.pub2. CD008324. [DOI] [PubMed] [Google Scholar]
- 14.Arzneimittelkommision der Deutschen Ärzteschaft Depression. Arzneiverordnungen in der Praxis, Band 33, Sonderheft 1 (Therapieempfehlungen) 2006. Therapieempfehlungen der Arzneimittelkommision der Deutschen Ärzteschaft. 2. Auflage. [Google Scholar]
- e1.Costello EJ, Erkanli A, Angold A. Is there an epidemic of child or adolescent depression? J Child Psychol Psychiatry. 2006;47:1263–1271. doi: 10.1111/j.1469-7610.2006.01682.x. [DOI] [PubMed] [Google Scholar]
- e2.Emslie GJ, Rush AJ, Weinberg WA, et al. A double-blind, randomized, placebo-controlled trial of fluoxetine in chldren and adolescents with depression. Arch Gen Psychiatry. 1997;54:1031–1037. doi: 10.1001/archpsyc.1997.01830230069010. [DOI] [PubMed] [Google Scholar]
- e3.Härter M, Klesse C, Bermejo I, Schneider F, Berger M. Unipolar depression: diagnostic and therapeutic recommendations from the current S3/National Clinical Practice Guideline. Dtsch Arztebl Int. 2010;107:700–708. doi: 10.3238/arztebl.2010.0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e4.Hetrick SE, et al. Newer generation antidepressants for depressive disorders in children and adolescents. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD004851.pub3. DOI: 10.1002/14651858.CD004851.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e5.Horn H, et al. Zur Wirksamkeit psychodynamischer Kurzzeitpsychotherapie bei Kindern und Jugendlichen mit Depressionen. Prax Kinderpsychol Kinderpsychiatr. 2005;54:578–597. [PubMed] [Google Scholar]
- e6.Muratori F, et al. A Two-Year Follow-up of Psychodynamic Psychotherapy for Internalizing Disorders in Children. J Am Acad Child Adolesc Psychiatry. 2003;42:331–339. doi: 10.1097/00004583-200303000-00014. [DOI] [PubMed] [Google Scholar]
- e7.Trowell J, et al. Childhood depression: a place for psychotherapy. An outcome study comparing individual psychodynamic psychotherapy and family therapy. Eur Child Adolesc Psychiatry. 2007;16:157–167. doi: 10.1007/s00787-006-0584-x. [DOI] [PubMed] [Google Scholar]
- e8.Diamond GS, et al. Attachment-based family therapy for depressed adolescents: a treatment development study. J Am Acad Child Adolesc Psychiatry. 2002;41:1190–1196. doi: 10.1097/00004583-200210000-00008. [DOI] [PubMed] [Google Scholar]
- e9.von Knorring AL, Olsson GI, Thomsen PH, Lemming OM, Hulten A. A randomized, double-blind, placebo-controlled study of citalopram in adolescents with major depressive disorder. J Clin Psychopharmacol. 2006;26:311–315. doi: 10.1097/01.jcp.0000219051.40632.d5. [DOI] [PubMed] [Google Scholar]
- e10.Hazell P, et al. Tricyclic drugs for depression in children and adolescents. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD002317. CD002317. [DOI] [PubMed] [Google Scholar]
- e11.Maneeton N, Srisurapanont M. Tricyclic antidepressants for depressive disorders in children and adolescents: a meta-analysis of randomized-controlled trials. J Med Assoc Thai. 2000;83:1367–1374. [PubMed] [Google Scholar]
- e12.Papanikolaou K, et al. Efficacy of antidepressants in child and adolescent depression: a meta-analytic study. J Neural Transm. 2006;113:399–415. doi: 10.1007/s00702-005-0340-2. [DOI] [PubMed] [Google Scholar]
- e13.Tsapakis EM, et al. Efficacy of antidepressants in juvenile depression: meta-analysis. Br J Psychiatry. 2008:10–17. doi: 10.1192/bjp.bp.106.031088. [DOI] [PubMed] [Google Scholar]
- e14.Moreno C, et al. Antidepressants in child and adolescent depression: where are the bugs? Acta Psychiatr Scand. 2007;115:184–195. doi: 10.1111/j.1600-0447.2006.00951.x. [DOI] [PubMed] [Google Scholar]
- e15.Avci A, et al. Comparison of moclobemide and placebo in young adolescents with major depressive disorder. Annals of Medical Sciences. 1999;8:31–40. [Google Scholar]
- e16.Emslie GJ, et al. Fluoxetine treatment for prevention of relapse of depression in children and adolescents: a double-blind, placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 2004;43:1397–1405. doi: 10.1097/01.chi.0000140453.89323.57. [DOI] [PubMed] [Google Scholar]
- e17.Emslie GJ, et al. Fluoxetine versus placebo in preventing relapse of major depression in children and adolescents. Am J Psychiatry. 2008;165:459–467. doi: 10.1176/appi.ajp.2007.07091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e18.Kroll LEO, et al. Pilot study of continuation cognitive-behavioral therapy for major depression in adolescent psychiatric patients. J Am Acad Child Adolesc Psychiatry. 1996;35:1156–1161. doi: 10.1097/00004583-199609000-00013. [DOI] [PubMed] [Google Scholar]
- e19.Ghaziuddin N, et al. Electroconvulsive treatment in adolescents with pharmacotherapy-refractory depression. J Child Adolesc Psychopharmacol. 1996;6:259–271. doi: 10.1089/cap.1996.6.259. [DOI] [PubMed] [Google Scholar]
- e20.Ghaziuddin N, Laughrin D, Giordani B. Cognitive side effects of electroconvulsive therapy in adolescents. J Child Adolesc Psychopharmacol. 2000;10:269–276. doi: 10.1089/cap.2000.10.269. [DOI] [PubMed] [Google Scholar]
- e21.Willoughby CL, Hradek EA, Richards NR. Use of electroconvulsive therapy with children: an overview and case report. J Child Adolesc Psychiatr Nurs. 1997;10:11–17. doi: 10.1111/j.1744-6171.1997.tb00409.x. [DOI] [PubMed] [Google Scholar]
- e22.Rossmann P. Göttingen: Hogrefe; 2005. Depressionstest für Kinder (DTK) [Google Scholar]
- e23.Döpfner M, Lehmkuhl G. Göttingen: Hogrefe; 2000. Diagnostik-System für psychische Störungen im Kindes- und Jugendalter nach ICD-10/DSM-IV. [Google Scholar]
- e24.Schneider S, Unnewehr S, Margraf J. (3rd ed.) Heidelberg: Springer Verlag; 2009. Kinder-DIPS - Diagnostisches Interview bei psychischen Störungen im Kindes- und Jugendalter [Kinder-DIPS—diagnostic interview for mental disorders in children and youths] [Google Scholar]
- e25.Kennard BD, et al. Cognitive-behavioral therapy to prevent relapse in pediatric responders to pharmacotherapy for major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:1395–1404. doi: 10.1097/CHI.0b013e31818914a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e26.Almeida-Montes LG, Friederichsen A. Treatment of major depressive disorder with fluoxetine in children and adolenscents. A double-blind, placebo-controlled study. Psiquiatria Biologica. 2005;12:198–205. [Google Scholar]
- e27.Emslie GJ, Heiligenstein JH, Wagner KD, et al. Fluoxetine for acute treatment of depression in children and adolescents: a placebo-controlled, randomized clinical trial. J Am Acad Child Adolesc Psychiatry. 2002;41:1205–1215. doi: 10.1097/00004583-200210000-00010. [DOI] [PubMed] [Google Scholar]
- e28.Simeon JG, Dinicola VF, Ferguson HB, Copping W. Adolescent depression: a placebo-controlled fluoxetine treatment study and follow-up. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:791–795. doi: 10.1016/0278-5846(90)90050-q. [DOI] [PubMed] [Google Scholar]
- e29.March JS. Treatment for Adolescents With Depression Study (TADS) Team: Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression. Treatment for Adolescents With Depression Study (TADS). Randomized controlled trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- e30.Emslie GJ, Ventura D, Korotzer A, Tourkodimitris S. Escitalopram in the treatment of adolescent depression: a randomized placebo-controlled multisite trial. J Am Acad Child Adolesc Psychiatry. 2009;48:721–729. doi: 10.1097/CHI.0b013e3181a2b304. [DOI] [PubMed] [Google Scholar]
- e31.Wagner KD, Jonas J, Findling RL, Ventura D, Saikali K. A double-blind, randomized, placebo-controlled trial of escitalopram in the treatment of pediatric depression. J Am Acad Child Adolesc Psychiatry. 2006;45:280–288. doi: 10.1097/01.chi.0000192250.38400.9e. [DOI] [PubMed] [Google Scholar]
- e32.Wagner KD, Robb AS, Findling RL, Jin J, Gutierrez MM, Heydorn WE. A randomized, placebo-controlled trial of citalopram for the treatment of major depression in children and adolescents. Am J Psychiatry. 2004;161:1079–1083. doi: 10.1176/appi.ajp.161.6.1079. [DOI] [PubMed] [Google Scholar]
- e33.Wagner KD, Ambrosini P, Rynn M, et al. Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: two randomized controlled trials. JAMA. 2003;290:1033–1041. doi: 10.1001/jama.290.8.1033. [DOI] [PubMed] [Google Scholar]