Abstract
Background
Brain metastases arise in roughly 0.9% of all cases of differentiated thyroid cancer. The median survival of adult patients with thyroid carcinoma that has metastasized to the brain is less than one year. Radioactive iodine treatment is only rarely given because its efficacy is not documented. In children, the situation may be different.
Case description
In 2005, a 15-year-old girl underwent thyroidectomy, and an oxyphilic variant of papillary thyroid carcinoma was found in ectopic thyroid tissue. The patient underwent oral, high-dose radioactive iodine treatment. The post-therapeutic I-131 whole-body scan revealed multiple metastases in the skeleton, lungs, and the soft tissues, along with physiological uptake of the residual thyroid tissue. Magnetic resonance imaging of the head revealed two brain metastases.
Course
When the initial treatment was completed, additional age-adapted high-dose radioactive iodine treatment was given, up to a total activity level of 35 GBq. There followed a complete remission of all metastases in the brain, bones, lungs, and soft tissues. Computed tomography of the chest revealed stable residuals. Over the ensuing 7.5 years of follow-up, the thyroglobulin values steadily declined to less than 2 ng/mL. The patient was asymptomatic at her last follow-up in May 2013. She did not develop any delayed reaction to high-dose radioactive iodine treatment (in particular, she did not develop leukemia or any other secondary malignancy). She remained fertile: after completion of the treatment, she had two healthy children.
Conclusion
In this patient with multifocal thyroid carcinoma, a rare entity, radioactive iodine treatment was successful as the single treatment. This case illustrates the point that a given therapeutic modality might succeed in an individual case despite a total or near-total lack of efficacy for most patients in the same situation.
Differentiated thyroid cancer (DTC), comprising follicular and papillary carcinomas, is the most common endocrine tumor. Despite the high percentage of lymphatic and hematogenous metastases, treatment outcomes are very favorable and, with 10-year survival rates of more than 90%, DTC has an excellent prognosis (1–6). Moreover, high quality clinical trials have brought about significant improvements in survival rates among high-risk patients (7). These carcinomas typically remain limited to the thyroid, with distant metastases occurring in only a few patients. Distant metastases with the most favorable prognosis are found in the lungs, followed by bone metastases (8). The survival rate among patients older than 45 years with distant metastases has been shown to be lower than that in the normal population (9).
Cerebral metastases remain a very rare complication, occurring in 0.15–1.3% of patients with thyroid carcinoma (10– 12). They are located more frequently in the hemispheres and only very rarely in the cerebellum (13, 14) and pituitary gland (15, 16). These metastases are usually asymptomatic, with only few patients presenting with symptoms such as headache, visual disturbances or ocular muscle weakness. Intracranial metastatic disease is associated with a poor prognosis and less than one year survival after diagnosis (11, 17, 18).
The current international guidelines (ATA 2009) recommend complete surgical resection of brain metastases as the first-line treatment (19). If surgical therapy is not feasible, alternative treatments to be discussed include radiotherapy, radioactive iodine (RAI) therapy (provided sufficient concentration of RAI) or a chemotherapy or biological therapy with tyrosin kinase inhibitors within the framework of clinical studies. RAI therapy is not commonly used for the treatment of brain metastases, as there are few data supporting efficacy of this approach.
For the purpose of his study, a literature search in PubMed was conducted using the keywords “differentiated thyroid cancer,“ “radioiodine“ and “brain metastases“ or “brain metastasis.“ Only clinical studies and case reports published between 1977 and 2012 which, among other therapies, involved RAI for the treatment of brain metastases were included in the analysis. Furthermore, patients with skull or scalp metastases extending to the brain and patients with non-differentiated thyroid cancer were excluded from the analysis. The Table provides a summary of the literature review.
Table. Overview of the literature.
| Study | n | Treatment | Brain metastases | Survival (years) | Comments |
|---|---|---|---|---|---|
| Shen; 2012 (25) | 1 | RAI + RS + sorafenib | s | n.e. | |
| Klubo-Gwiezdzinska; 2012 (30) | 2 | RAI | n.e. | n.e. | |
| Miranda; 2010 (26) | 1 | RAI + OP + RTX + RS | m | 10 | |
| Walczyk; 2010 (31) | 1 | RAI + RTX | n.e. | 2 | poorly differentiated hyroid carcinoma |
| Erem; 2004 (32) | 1 | OP + RTX + RAI | s | n.e. | |
| McWilliams; 2003 (33) | 4 | 2 OP + RAI 1 OP + RTX + RAI 1 OP + RS + RAI |
s | 1.5 | |
| Cha; 2000 (34) | 1 | OP + RAI + RTX + RS | s | > 3 | |
| Misaki; 2000 (35) | 9 | 4 RAI + RTX 2 RAI + RS 2 OP + RAI 1 OP + RAI + RTX |
5 s 4 m |
0.8 | no significant intracranial RAI uptake |
| Chiu; 1997 (11) | 24 (+ 8 post mortem) | 7 OP 13 RTX 6 CTX 3 RAI |
11 s 12 m |
1 | only 3/18 patients with RAI uptake |
| Samuel; 1997 (18) | 15 | 3 RAI in combination with OP, RTX and/or CTX |
6 s 9 m |
< 0.5 | |
| Biswal; 1994 (17) | 5 | 2 RTX + RAI 1 RTX 1 OP + RTX + RAI 1 OP + RAI |
s | 2 died 4 mo and 7 yr, resp., after diagnosis; 3 are alive 12–23 mo after treatment |
|
| Miyamoto; 1991 (36) | 2 | RAI | n.e. | n.e. | no RAI uptake and no response to treatment |
| Andrews; 1987 (37) | 1 | OP + RTX + RAI | s | 2.75 | |
| Krishnamurthy; 1977 (24) | 2 | RAI | n.e. | 2 | cerebellum |
n, number of patients; s, solitary; m, multiple; n.e., not examined; RAI, radioactive iodine; OP, operation; RTX, radiotherapy; RS, gamma knife radiosurgery; CTX, chemotherapy
Case report
In October 2005, a 15-year-old Caucasian girl presented with acute dyspnea and left thoracic pain of two weeks’ duration. The chest radiograph revealed a large retrosternal space-occupying lesion, shifting the mediastinum to the right. The CT-guided biopsy could not differentiate between thymoma and thymic carcinoma. Subsequent combined positron emission tomography/computed tomography (PET/CT) with F-18 fluorodeoxyglucose (FDG) as well as magnetic resonance imaging (MRI) of the head and the spine revealed bifrontal brain metastases (4–5 mm), lesions in two thoracic vertebral bodies, a suspicious submental space-occupying lesion with strong FDG uptake, and multiple lung metastases. Because of the unusual distribution of the metastases, a second biopsy of the mediastinal tumors and an isolated pulmonary metastasectomy were performed. While the diagnosis of the thymoma was confirmed, unexpectedly a lung metastasis of a (solid-type) papillary thyroid carcinoma was found (Figure 1). The patient’s past history revealed that she underwent thyroidectomy to treat congenital goiter on the day she was born (histology: follicular adenoma) and a left hemithyroidectomy in 2002 for recurrent goiter (benign histology).
Figure 1.
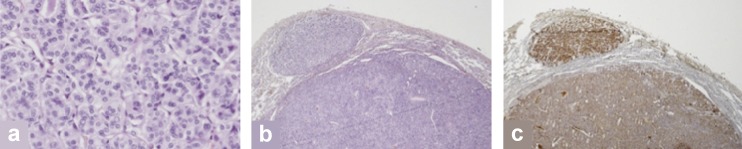
Histology of lung metastasis of papillary thyroid carcinoma (solid and microfollicular types; H&E) (a). Macroscopic view (H&E) (b), and immunohistochemistry of the specimen with significant expression of the specific tumor marker thyroglobulin (c). H&E, hematoxylin and eosin stain
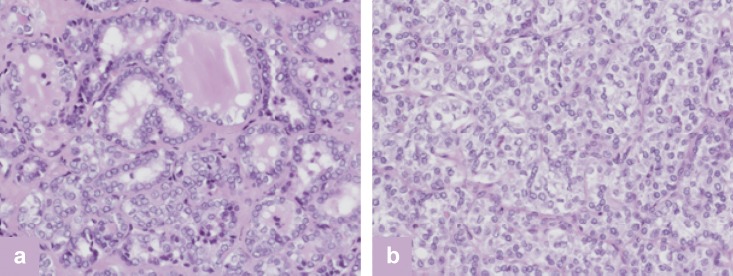
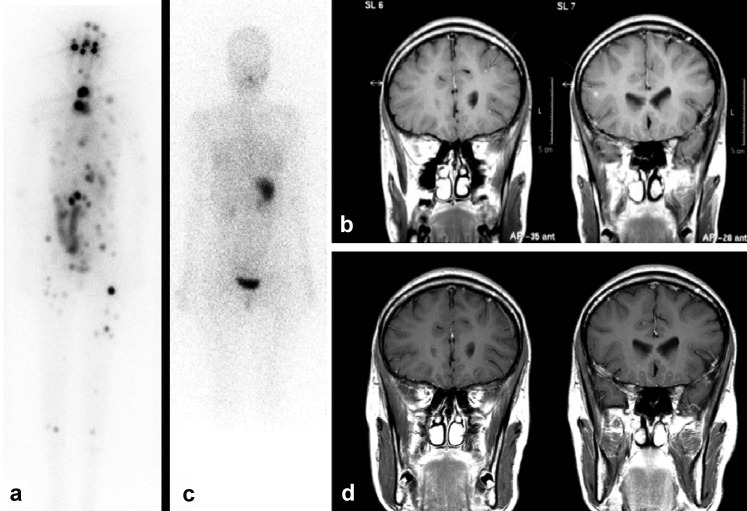
Thyroid scintigraphy revealed ectopic thyroid tissue in the thyroglossal duct, correlating with the FDG-concentrating submental space-occupying lesion on the PET/CT scan. Both the ectopic thyroid tissue and the thymoma were resected in 2005 because of local symptoms (WHO B1). A papillary thyroid carcinoma, oxyphilic type, was found in the ectopic thyroid tissue. Furthermore, reference pathology confirmed the diagnosis of a follicular-type and solid-type papillary thyroid carcinoma in the thyroid tissue resected in 2002 (Figure 2). As the tissue samples obtained in 1990 were no longer available, it was not possible to rule out a congenital papillary thyroid carcinoma. The patient then underwent an oral, age-adapted high-dose RAI therapy (50 MBq/kg body weight [BW]) (20). The post-therapeutic I-131 whole-body scan, including the combination of single-photon emission computed tomography/computed tomography (SPECT-CT) (from head to pelvis), showed multi-focal metastases in the skeletal system, including the skull, in the lungs and soft tissues, as well as normal uptake in the remnant thyroid tissue. Furthermore, the bifocal intracerebral increases in RAI uptake correlated with the brain metastases revealed by MRI (Figures 3 a, b).
Figure 2.
Histology of papillary thyroid carcinoma (follicular [a] and focal solid type [b]; H&E). H&E, hematoxylin and eosin stain
Figure 3.
The first post-therapeutic I-131 whole-body scan shows multifocal metastases in the skeletal system, including the skull, in the lungs and soft tissues and in the brain, in addition to normal uptake in the remnant thyroid tissue (a). Furthermore, bifrontal brain metastases are revealed by magnetic resonance imaging (MRI) (b). (Reprint courtesy of the Institute of Clinical Radiology, University Hospital Münster.) Following repeated RAI therapies (total dose of 35 GBq), complete remission of all metastases in the brain, skeletal system and soft tissues was achieved (c/d)
The international guidelines recommend considering percutaneous radiotherapy as well as glucocorticoid therapy prior to RAI therapy of cerebral metastases to prevent potential TSH-induced and inflammatory swelling of the metastases. In view of the small size of the tumors and the patient’s age, RAI therapy supported by protective glucocorticoid treatment was started without prior radiotherapy. This approach was agreed upon in interdisciplinary discussions and written consent of the patient’s parents was obtained.
A series of age-adapted high-dose RAI treatments (100 MBq/kg BW) with a total activity of 35 GBq achieved complete remission of all metastases in the brain, skeletal system, lungs, and soft tissues (Figures 3 c, d). The patient did not show any early reactions to the treatment, such as painful local swelling of the thyroid, the primary or its metastases, transient gastritis, transient bone marrow changes with thrombocytopenia and leucopenia, or RAI-induced sialadenitis. Likewise, no neurological complications were observed at any time. During the 7.5-year follow-up period, the chest CT scans showed stable residual scars (maximum diameter of 4 mm) with no correlate on 131-iodine whole-body scintigraphy and FDG-PET. The patient’s thyroglobulin levels continued to decline to <2 ng/mL.
At the last follow-up in May 2013, the patient was symptom-free under TSH-suppressive L-thyroxine therapy. The pulmonary changes visualized on the low-dose chest CT scan remained constant (≤4 mm) and the patient continued to show no morphologic signs indicative of cerebral or any other type of distant metastases. Moreover, after completion of her treatment the patient gave birth to two healthy children. Potential late sequelae of repeated high-dose RAI therapy (xerostomia, taste dysfunction, sicca syndrome, permanent bone marrow depression, pulmonary fibrosis, leukemia, and secondary malignancies) were not observed during regular clinical and laboratory follow-up examinations and pulmonary function tests.
A recent meta-analysis (n = 16 502) demonstrated a minor increase in the relative risk of second malignancy among patients after RAI therapy for thyroid cancer, corresponding to the slightly increased incidence of leukemias (21).
Discussion
Brain metastases are a rare complication of DTC, only found in approx. 0.9% of patients while they are alive. Median survival after diagnosis of brain metastasis is typically less than 1 year (11, 18, 22). Since the detection of brain metastases constitutes a negative prognostic factor, it is crucial to tailor the treatment to the special circumstances of the individual patient. Surgery, radiotherapy, RAI therapy, and chemotherapy are the treatment options described in the literature. Traditionally, the mainstays of therapy have been surgical resection and external beam radiotherapy. The current management guidelines for patients with differentiated thyroid cancer issued by the American Thyroid Association recommend to consider, regardless of the patient’s iodine avidity, a complete surgical resection of the brain metastases, as this offers significant survival benefits. Radiosurgery may be employed to limit radiation exposure of the neighboring brain tissue. In cases with multiple metastases, whole-brain and spine irradiation could be considered (19).
So far, only few data are available demonstrating efficacy of RAI therapy. Some metastases, in particular brain metastases, may be undifferentiated and consequently show inadequate RAI uptake, precluding the use of RAI therapy (23). However, in patients with increased risk of perioperative complications or who are considered unsuitable for surgery because of multifocal metastatic disease, RAI therapy, as a monotherapy, appears to be an attractive treatment alternative for small-size cerebral metastases, as described above. It can also be used in combination with other therapies, where indicated.
The five larger studies in patients with brain metastases will be discussed below in more detail. Chiu et al. (11) reported on 24 pre-mortem and 8 post-mortem cases of brain metastases of DTC. Median survival was 12.4 months after diagnosis of brain metastases. Disease-specific mortality was 67%. Patients who underwent surgical excision showed a median disease-specific survival of 22 months, compared with 3.6 months in patients who did not undergo the procedure (p <0.01). In contrast, neither external beam radiotherapy nor chemotherapy appear to have any impact on disease-specific survival. RAI uptake was rarely observed. It was found in only 3 of 18 patients who underwent a whole-body scan to assess RAI uptake.
Two of three patients developed neurological complications after RAI therapy. In the patient without neurological complications, a solitary metastasis had been surgically removed prior to RAI therapy. Overall, approx. 9% of patients with brain metastases of DTC showed a disease-specific survival >36 months. However, the study provides no information about what differentiates these patients from those that died earlier.
Samuel et al. published a series of 15 patients with brain metastases from well-differentiated thyroid carcinoma (18). Except for 3 cases with unifocal brain metastases, in all other cases extensive other metastases were present in various combinations. In only 2 patients, RAI uptake was observed in the brain metastases. However, 3 further patients were treated with RAI because of iodine-concentrating metastases at other anatomic locations. One of these patients received additional chemotherapy, while 2 patients underwent surgery. Of these two patients, one was treated with adjuvant external beam radiotherapy. Two patients died within 2 months after the manifestation of brain metastases. One patient was still alive 18 months after surgical resection of the brain metastasis.
In 1977, Krishnamurthy et al. presented a prospective study evaluating RAI therapy in 54 patients with thyroid cancer (24). Two patients with cerebellar metastases were treated with 6 and 14 GBq RAI, respectively, without achieving complete ablation. Both patients ultimately died of thyroid cancer within 2 years after the initial diagnosis.
The most recent study was the first to evaluate the therapeutic effect of tyrosine-kinase inhibitors in a patient with brain metastases from follicular thyroid carcinoma (25). Shen et al. reported on a 56-year-old patient who underwent thyroidectomy and RAI therapy because of lung metastases. She developed right-sided hemiplegia and other symptoms as the result of a 5-cm lesion in the left parietal lobe. Radiosurgery with a total dose of 28 Gy was unsuccessful. In contrast, the patient’s signs and symptoms improved dramatically and consistently after initiation of treatment with sorafenib. The partial response of the brain metastasis was documented, using imaging studies, over a period of more than a year. The authors concluded that a targeted therapy, such as sorafenib treatment, could be an effective alternative strategy for the treatment of progressive brain metastases from DTC in cases where surgery, external beam radiotherapy and RAI therapy are not suitable or achieve poor results.
The by far longest survival of a patient with brain metastases was reported by Miranda et al. (26). They described the case of a 69-year-old patient with multiple brain metastases from papillary thyroid carcinoma who presented with progressive dizziness, headache, and vomiting. Over a period of 10 years, the patient received a total RAI activity of 44 GBq. In addition, the patient underwent partial surgical resection of the brain metastases and whole-brain radiotherapy with a dose of 44 Gy, followed by two gamma knife radiosurgeries (15 Gy each). At the time of publication, biochemical tests were unremarkable and the patient continued to be asymptomatic. The authors concluded that a combined approach comprising surgical excision, RAI therapy, whole-brain radiotherapy, and gamma knife radiosurgery was successful in treating brain metastases from DTC.
In the rare case of a young female patient with multifocal cerebral and extra-cerebral metastases with sustained avidity for iodine reported here, an excellent result was achieved after a single series of high-dose RAI therapies and maintained over a follow-up period of 7.5 years. Summarizing, a single high-dose RAI therapy could be considered for the treatment of small brain metastases, if surgery is contraindicated and radiotherapy is to be avoided to minimize adverse effects on the developing brains of children and adolescents (27).
Conclusion
Thyroid carcinoma is a rare primary tumor in children and adolescents. Treatment outcomes are favorable despite a significant portion of lymphatic and hematogenous metastases (28, 29). The present case report shows that there is always a chance to encounter tumor patients who can be successfully treated with therapy modalities that show no or only little effect in most patients in the same situation.
Acknowledgments
Translated from the original German by Ralf Thoene, MD
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 2.Lerch H, Schober O, Kuwert T, Saur HB. Survival of differentiated thyroid carcinoma studied in 500 patients. J Clin Oncol. 1997;15:2067–2075. doi: 10.1200/JCO.1997.15.5.2067. [DOI] [PubMed] [Google Scholar]
- 3.Volante M, Collini P, Nikiforov YE, et al. Poorly differentiated thyroid carcinoma: The turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol. 2007;31:1256–1264. doi: 10.1097/PAS.0b013e3180309e6a. [DOI] [PubMed] [Google Scholar]
- 4.Verburg FA, Mader U, Luster M, Reiners C. Primary tumour diameter as a risk factor for advanced disease features of differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2009;71:291–297. doi: 10.1111/j.1365-2265.2008.03482.x. [DOI] [PubMed] [Google Scholar]
- 5.Verburg FA, Mader U, Luster M, Reiners C. Histology does not influence prognosis in differentiated thyroid carcinoma when accounting for age, tumour diameter, invasive growth and metastases. Eur J Endocrinol. 2009;160:619–624. doi: 10.1530/EJE-08-0805. [DOI] [PubMed] [Google Scholar]
- 6.Schlumberger MJ. Papillary and follicular thyroid carcinoma. N Engl J Med. 1998;338:297–306. doi: 10.1056/NEJM199801293380506. [DOI] [PubMed] [Google Scholar]
- 7.Kramer JA, Schmid KW, Dralle H, et al. Primary tumour size is a prognostic parameter in patients suffering from differentiated thyroid carcinoma with extrathyroidal growth: Results of the MSDS trial. Eur J Endocrinol. 2010;163:637–644. doi: 10.1530/EJE-10-0116. [DOI] [PubMed] [Google Scholar]
- 8.Mazzaferri EL, Massoll N. Management of papillary and follicular (differentiated) thyroid cancer: New paradigms using recombinant human thyrotropin. Endocr Relat Cancer. 2002;9:227–247. doi: 10.1677/erc.0.0090227. [DOI] [PubMed] [Google Scholar]
- 9.Vrachimis A, Riemann B, Gerss J, Maier T, Schober O. Peace of mind for patients with differentiated thyroid cancer? Nuklearmedizin. 2013;52:115–120. doi: 10.3413/Nukmed-0563-13-02. [DOI] [PubMed] [Google Scholar]
- 10.Dinneen SF, Valimaki MJ, Bergstralh EJ, Goellner JR, Gorman CA, Hay ID. Distant metastases in papillary thyroid carcinoma: 100 cases observed at one institution during 5 decades. J Clin Endocrinol Metab. 1995;80:2041–2045. doi: 10.1210/jcem.80.7.7608252. [DOI] [PubMed] [Google Scholar]
- 11.Chiu AC, Delpassand ES, Sherman SI. Prognosis and treatment of brain metastases in thyroid carcinoma. J Clin Endocrinol Metab. 1997;82:3637–3642. doi: 10.1210/jcem.82.11.4386. [DOI] [PubMed] [Google Scholar]
- 12.Venkatesh S, Leavens ME, Samaan NA. Brain metastases in patients with well-differentiated thyroid carcinoma: Study of 11 cases. Eur J Surg Oncol. 1990;16:448–450. [PubMed] [Google Scholar]
- 13.Pazaitou-Panayiotou K, Kaprara A, Chrisoulidou A, et al. Cerebellar metastasis as first metastasis from papillary thyroid carcinoma. Endocr J. 2005;52:653–657. doi: 10.1507/endocrj.52.653. [DOI] [PubMed] [Google Scholar]
- 14.Al-Dhahri SF, Al-Amro AS, Al-Shakwer W, Terkawi AS. Cerebellar mass as a primary presentation of papillary thyroid carcinoma: Case report and literature review. Head Neck Oncol. 2009;1 doi: 10.1186/1758-3284-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrisoulidou A, Pazaitou-Panayiotou K, Flaris N, et al. Pituitary metastasis of follicular thyroid carcinoma. Horm Res. 2004;61:190–192. doi: 10.1159/000076387. [DOI] [PubMed] [Google Scholar]
- 16.Yilmazlar S, Kocaeli H, Cordan T. Sella turcica metastasis from follicular carcinoma of thyroid. Neurol Res. 2004;26:74–78. doi: 10.1179/016164104773026561. [DOI] [PubMed] [Google Scholar]
- 17.Biswal BM, Bal CS, Sandhu MS, Padhy AK, Rath GK. Management of intracranial metastases of differentiated carcinoma of thyroid. J Neurooncol. 1994;22:77–81. doi: 10.1007/BF01058357. [DOI] [PubMed] [Google Scholar]
- 18.Samuel AM, Shah DH. Brain metastases in well-differentiated carcinoma of the thyroid. Tumori. 1997;83:608–610. doi: 10.1177/030089169708300226. [DOI] [PubMed] [Google Scholar]
- 19.Cooper DS, Doherty GM, Haugen BR, et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised american thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 20.Franzius C, Dietlein M, Biermann M, et al. Procedure guideline for radioiodine therapy and 131iodine whole-body scintigraphy in paediatric patients with differentiated thyroid cancer. Nuklearmedizin. 2007;46:224–231. doi: 10.1160/nukmed-0288. [DOI] [PubMed] [Google Scholar]
- 21.Sawka AM, Thabane L, Parlea L, et al. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: A systematic review and meta-analysis. Thyroid. 2009;19:451–457. doi: 10.1089/thy.2008.0392. [DOI] [PubMed] [Google Scholar]
- 22.Biswal BM, Bal CS, Sandhu MS, Padhy AK, Rath GK. Management of intracranial metastases of differentiated carcinoma of thyroid. J Neurooncol. 1994;22:77–81. doi: 10.1007/BF01058357. [DOI] [PubMed] [Google Scholar]
- 23.Parker LN, Wu SY, Kim DD, Kollin J, Prasasvinichai S. Recurrence of papillary thyroid carcinoma presenting as a focal neurologic deficit. Arch Intern Med. 1986;146:1985–1987. [PubMed] [Google Scholar]
- 24.Krishnamurthy GT, Blahd WH. Radioiodine I-31 therapy in the management of thyroid cancer. A prospective study. Cancer. 1977;40:195–202. doi: 10.1002/1097-0142(197707)40:1<195::aid-cncr2820400131>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 25.Shen Y, Ruan M, Luo Q, et al. Brain metastasis from follicular thyroid carcinoma: Treatment with sorafenib. Thyroid. 2012;22:856–860. doi: 10.1089/thy.2011.0419. [DOI] [PubMed] [Google Scholar]
- 26.Miranda ER, Padrao EL, Silva BC, De Marco L, Sarquis MS. Papillary thyroid carcinoma with brain metastases: An unusual 10-year-survival case. Thyroid. 2010;20:657–661. doi: 10.1089/thy.2009.0442. [DOI] [PubMed] [Google Scholar]
- 27.Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- 28.Hay ID, Gonzalez-Losada T, Reinalda MS, Honetschlager JA, Richards ML, Thompson GB. Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J Surg. 2010;34:1192–1202. doi: 10.1007/s00268-009-0364-0. [DOI] [PubMed] [Google Scholar]
- 29.Landau D, Vini L, A’Hern R, Harmer C. Thyroid cancer in children: The royal marsden hospital experience. Eur J Cancer. 2000;36:214–220. doi: 10.1016/s0959-8049(99)00281-6. [DOI] [PubMed] [Google Scholar]
- 30.Klubo-Gwiezdzinska J, Burman KD, van Nostrand D, Mete M, Jonklaas J, Wartofsky L. Radioiodine treatment of metastatic thyroid cancer: Relative efficacy and side effect profile of preparation by thyroid hormone withdrawal versus recombinant human thyrotropin. Thyroid. 2012;22:310–317. doi: 10.1089/thy.2011.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walczyk A, Kowalska A, Sygut J. The clinical course of poorly differentiated thyroid carcinoma (insular carcinoma) - own observations. Endokrynol Pol. 2010;61:467–473. [PubMed] [Google Scholar]
- 32.Erem C, Hacihasanoglu A, Sari A, et al. Intrathyroideal papillary thyroid carcinoma presenting with a solitary brain metastasis. Endocrine. 2004;25:187–193. doi: 10.1385/endo:25:2:187. [DOI] [PubMed] [Google Scholar]
- 33.McWilliams RR, Giannini C, Hay ID, Atkinson JL, Stafford SL, Buckner JC. Management of brain metastases from thyroid carcinoma: A study of 16 pathologically confirmed cases over 25 years. Cancer. 2003;98:356–362. doi: 10.1002/cncr.11488. [DOI] [PubMed] [Google Scholar]
- 34.Cha ST, Jarrahy R, Mathiesen RA, Suh R, Shahinian HK. Cerebellopontine angle metastasis from papillary carcinoma of the thyroid: Case report and literature review. Surg Neurol. 2000;54:320–326. doi: 10.1016/s0090-3019(00)00306-2. [DOI] [PubMed] [Google Scholar]
- 35.Misaki T, Iwata M, Kasagi K, Konishi J. Brain metastasis from differentiated thyroid cancer in patients treated with radioiodine for bone and lung lesions. Ann Nucl Med. 2000;14:111–114. doi: 10.1007/BF02988589. [DOI] [PubMed] [Google Scholar]
- 36.Miyamoto S, Kasagi K, Endo K, et al. Results of radioiodine therapy in 47 patients with distant metastases of differentiated thyroid carcinoma. Nihon Igaku Hoshasen Gakkai Zasshi. 1991;51:810–821. [PubMed] [Google Scholar]
- 37.Andrews JT, McGennisken MR. Metastatic differentiated carcinoma of the thyroid and radioiodine. Australas Radiol. 1987;31:41–46. doi: 10.1111/j.1440-1673.1987.tb01780.x. [DOI] [PubMed] [Google Scholar]