Abstract
Introduction
Adherence to colorectal cancer screening recommendations is known to vary by state, but less information is available about within-state variability. In the current study, we assess county-level screening rates for Missouri, with the goal of better targeting public health efforts to increase screening.
Methods
Prevalence of colorectal cancer screening among Missouri adults between the ages of 50 and 74 was obtained from 2008 and 2010 Behavioral Risk Factor Surveillance System data. We used multilevel logistic regression to generate county-specific estimates. After excluding 77 counties with fewer than 30 respondents, information was available about 3,739 individuals in 37 counties, representing 78.5 % of the state population.
Results
Across counties, the prevalence of being up-to-date with recommended colorectal cancer screening ranged from 25 to 70 %.
Conclusion
State-level information about colorectal cancer screening masks substantial within-state variability. Assessing and monitoring county-level disparities in screening can guide public health efforts to increase screening and reduce colorectal cancer mortality. More complete population survey data will make such analysis possible.
Keywords: Colorectal cancer, Screening, Primary prevention, Healthcare disparities, Missouri, Community health planning
Introduction
In 2010, an estimated 64.5 % of U.S. adults between the ages of 50 and 75 was up-to-date with recommended colorectal cancer screening [1]. The prevalence of colorectal cancer screening has increased steadily overtime among both men and women [2], bringing us closer to the Healthy People 2020 goal of 70.5 % [3]. The importance of meeting—and eventually exceeding—this goal is clear: screening for colorectal cancer can detect both early-stage cancer and precancerous polyps, and use of recommended screening tests reduces colorectal cancer mortality [4].
Variability in colorectal cancer screening by region provides an important guide for public health efforts to increase screening. States vary notably in both their rates of screening and their rates of colorectal cancer mortality. The highest rates of up-to-date screening—which already exceed the Healthy People 2020 goals—are reported in New England, Maryland, and Washington State [1]. Massachusetts tops the list with 75.2 % of adults up-to-date with colorectal cancer screening. At the other extreme are states with screening prevalence in the 54–56 % range: Oklahoma, West Virginia, and Idaho. Screening rates are strongly correlated with state-specific colorectal cancer mortality: reductions in colorectal cancer mortality overtime tend to be greatest for states with higher levels of screening [5].
As important as this state-level information is, it does not capture the even greater variability that may exist within states. A state with average or even above-average levels of screening may contain areas with screening rates well below the level of even the worst-performing states. To better focus prevention efforts and to bring awareness and access to services for colorectal cancer screening to populations that can benefit most, thereby reducing disparities in mortality, this within-state variability must be described and addressed. To this end, we assess county-level data from Missouri, a state with a rate of colorectal cancer screening that is close to the national average [1].
Methods
The prevalence estimates for colorectal cancer screening were obtained from 2008 and 2010 Behavioral Risk Factor Surveillance System (BRFSS) data. BRFSS data include 78 counties of the total 114 counties and 1 independent city in Missouri. County codes are removed from the BRFSS data if a county has fewer than 50 respondents or an adult population of 10,000 or less.
Only participants between the ages of 50 and 74 were included. Participants were excluded if they did not respond to questions about colorectal cancer screening, or if fewer than 30 people in a county responded to the screening questions in the combined data set. After the exclusions, 3,739 participants in 37 counties remain for analysis. These 37 counties make up 78.5 % of the population of Missouri.
Up-to-date colorectal cancer screening was defined as a fecal occult blood test (FOBT) within the past year, a sigmoidoscopy within the past 5 years and a FOBT within the past 3 years, or a colonoscopy within the past 10 years [4]. Within the BRFSS survey, the participants over the age of 50 were asked the following questions: (1) “A blood stool test is a test that may use a special kit at home to determine whether the stool contains blood. Have you ever had this test using a home kit?” If they answered yes, they were then asked (2) “How long has it been since you had your last blood stool test using a home kit?” If the participant’s answer to the first question was “yes” and the blood stool test was conducted “within the past year,” then the participant is considered to have up-to-date colorectal cancer screening. The participants were also asked, (3) “Sigmoidoscopy and colonoscopy are exams in which a tube is inserted in the rectum to view the colon for signs of cancer or other health problems. Have you ever had either of these exams?” If they answered “yes,” they were then asked, (4) “For a sigmoidoscopy, a flexible tube is inserted into the rectum to look for problems. A colonoscopy is similar, but uses a longer tube, and you are usually given medication through a needle in your arm to make you sleepy and told to have someone else drive you home after the test. Was your most recent exam a sigmoidoscopy or a colonoscopy?” They were then asked (5) “How long has it been since you had your last sigmoidoscopy or colonoscopy?” If the participant answered “yes” to question (1), “within the past 2 years” or “within the past 3 years” to question (2), “yes” to question (3), “sigmoidoscopy” to question (4), and “within the past 5 years,” “within the past 3 years,” “within the past 2 years,” or “within the past year” to question (5), they were considered to have up-to-date colorectal cancer screening. If the participant answered “yes” to question (3), “colonoscopy” to question (4), and “within the past 10 years,” “within the past 5 years,” “within the past 3 years,” “within the past 2 years,” or “within the past year” to question (5), they were considered to have up-to-date colorectal cancer screening.
County screening rates are estimated using the regression approach for small area analysis. Multilevel logistic regression models with random effects were used:
where xij = (χij1,…,χij)′ is the vector of q covariates, β = (β1,…,βq)′ is the corresponding vector of fixed effects, and αi is the random effect for county. The model includes demographic variables: race, sex, and age group, as well as county-level data obtained from the 2010 census: county percent poverty, proportion of adults older than 25 with less than a high school education (county education), the median income of the county compared to the median income in MO (income), and the urban status of the county. The variable “age group” consists of seven categories based on the manner by which census data were divided. The county percent poverty was divided into three groups: (1) <15 % poverty, (2) 15–24 % poverty, and (3) ≥25 % poverty. County education was divided into two groups: (1)<20 % of adults older than 25 have less than a high school education and (2) ≥20 % of adults older than 25 have less than a high school education. The income variable was divided into two groups: (1) counties with a median income ≥$44,306 and (2) counties with a median income <$44,306. County urban status variable was divided into three groups: (1) counties that are ≤25 % urban (>75 % rural), (2) counties that are 25–75 % urban (75–25 % rural), and (3) counties that are ≥75 % urban (25 % rural). The following significant interaction terms were also included: race and sex, race and age group, race and county percent poverty, race and county education, race and income, race and county urban status, sex and age group, sex and county percent poverty, sex and income, sex and county urban status, age group and county education, age group and county percent poverty, age group and income, age group and county urban status, and county percent poverty and county urban status.
After calculating the regression parameter estimates, we estimated the county-level prevalence rates by county. The county-level age by sex by race estimated prevalence is calculated from the predictors given by the regression model as follows:
The county-level prevalence rates by county are then calculated with the following formula:
where p̂i is the estimated prevalence of colorectal cancer screening in county i, n is the number of people in county i that are of race j and belong to age and sex demographic group k, ni = Σi nijk is the total population in county i, and p̂ijk is the estimated prevalence of colorectal cancer screening in county i for race j in demographic group k.
Results
Using all BRFSS data for 2008 and 2010 (n = 5,164), the estimated state-level prevalence of up-to-date colorectal cancer screening is 59.1 %. After our exclusion criteria, our sample (n = 3,739) has a significantly higher screening prevalence of 61.3 % based on a one-sample test for proportion (p = 0.006). This difference is likely due to the exclusion of participants in counties with <30 respondents. These excluded counties are most likely smaller and potentially have lower rates of screening.
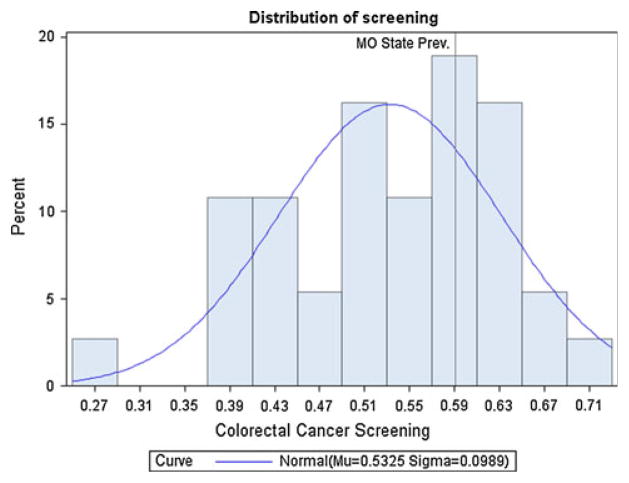
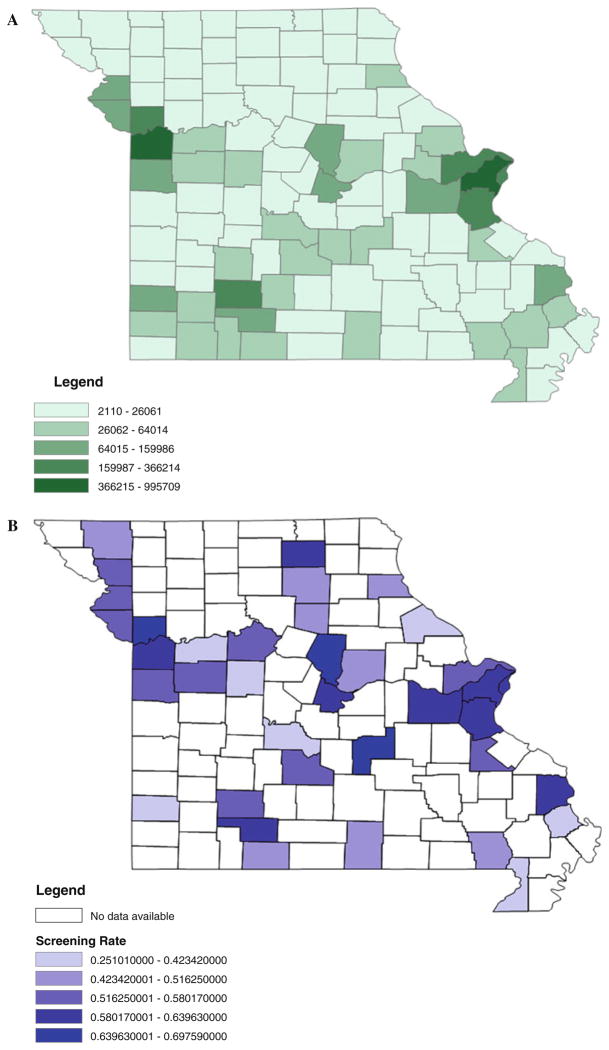
At the county level, the prevalence of up-to-date screening adjusted for age, sex, race, education, and poverty (county level), ranged from a low of 25.1 % to a high of 69.8 % (Table 1). The distribution of county prevalence estimates within the state of Missouri is not normally distributed, with a mean of 53.2 %, standard deviation of 9.9 %, and interquartile range of 14.9 % (Fig. 1). Highly populated urban areas such as St. Louis City, which had a screening rate of 60.1 %, and St. Louis County, which had a screening rate of 64 %, have higher screening rates than rural areas with lower population rates such as Pike County (37.2 %) and Camden County (40.2 %) (Fig. 2). We tested the correlation between each county’s percentage of urban areas as deemed by the 2010 Census and up-to-date colorectal cancer screening and found that they were significantly correlated with a Pearson correlation coefficient of 0.453 and a p value of 0.005.
Table 1.
Adjusted estimated prevalence of up-to-date colorectal cancer screening in Missouri, combined 2008 and 2010 BRFSS data, by county
| n | Mean | Median | SD | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Screening by county | 37 | .532 | .567 | .099 | .251 | .698 |
Fig. 1.
Distribution of county-level prevalence of colorectal cancer screening in Missouri, combined 2008 and 2010 BRFSS data
Fig. 2.
2010 Missouri population (a) and prevalence of up-to-date colorectal cancer screening (b) by county
Discussion
These results demonstrate that colorectal cancer screening rates in Missouri—a state with an overall rate that is close to the national average—vary substantially by county. In the county with the lowest rate, only 1 in 4 eligible adults is up-to-date with colorectal cancer screening. This is well below even the worse state-level data; in Oklahoma, the state with the lowest average screening rates in the nation, more than half of adults are up-to-date with screening [1].
Within-state variability in cancer screening is not unique to Missouri [6] and highlights the importance of looking beyond state-level data when establishing programs and priorities for cancer screening. Focusing only on state-level population averages may exacerbate disparities and diminish efforts to bring awareness and access to populations most in need of screening.
The reasons for the low screening rates in certain counties will vary from county to county and from state to state, but a range of proven approaches to increasing screening is available and can be customized to fit each setting. The CDC’s Guide to Community Preventive Services describes several evidence-based strategies for increasing colorectal cancer screening [7]. At the patient level, these include client reminders, small media (e.g., videos, brochures, or newsletters that can be tailored to specific audiences), one-on-one education, and removal of structural barriers such as the need to travel a long distance to receive care. At the provider level, recommended strategies include provider assessment and feedback and reminder and recall systems. These have been shown to be effective across a range of primary care settings [8, 9].
The challenge, of course, is implementing these strategies. Implementation science continues to evolve, but the need to involve community stakeholders is clear [10]. In the case of cancer screening, these stakeholders include local, state, and federal public health officials; clinicians; advocacy organizations; community groups and businesses; and members of the public. The strategy of collective impact—which involves not only collaboration among these various groups but also a centralized infrastructure to facilitate and structure the collaboration—has made headway against problems in public education [11] and could also play an important role in public health [12]. Coordination among groups is important to ensure that each is working toward a common goal with a shared understanding of the problem at hand. Clear goals and measurement tools have already been established for cancer screening and can be used to motivate involvement and monitor progress. Ongoing efforts to increase colorectal cancer screening include the CDC’s Colorectal Cancer Control Program, which currently funds half of all states and four tribal organizations to bring outreach for colorectal cancer screening to the population [13].
Insurance coverage for colorectal cancer screening is another necessary component of programs to increase screening, but is not sufficient—by itself—to achieve screening goals. Colorectal cancer is second only to lung cancer as a leading cause of cancer death in the USA [14], yet screening rates remain below those of breast and cervical cancer [2]. Medicare has covered colorectal cancer screening since 1998, and screening rates are higher among 65–75 year olds than among 50–65 year olds, but even among older adults roughly one in three are not up-to-date with recommended screening [2].
The utility of county-level health data for guiding prevention efforts extends well beyond cancer screening. Counties also vary in important and modifiable health outcomes such as diabetes and obesity [15]. By assessing within-state variability across a broad range of health indicators, states and other public health stakeholders can focus on those interventions that are likely to have the greatest impact on overall health. Interventions to address obesity, for example, will augment cancer control efforts and will also reduce the risk of many other common chronic diseases [16].
Monitoring county-level data requires statistical methods that generate robust estimates. BRFSS data are commonly used to generate state-level information about screening, but valid county-level estimates cannot be obtained directly. In order to estimate screening prevalence at the county level, we used a multilevel logistic regression model. In a previous study estimating the prevalence of three chronic diseases (asthma, diabetes, and hypertension), this approach showed the least amount of discrepancy when using BRFSS data to generate county-level estimates [17]. The regression approach has also been used to estimate county-level prevalence of disability [18]. This approach can be used by all states with available data and for a broad range of health outcomes. To increase stratum-specific sample sizes and gain stability in our estimates, we averaged data over 2 years (2008 and 2010).
BRFSS data are a probability sample of US households with a telephone. Telephone coverage varies by state and subpopulation, which raises issues of selection bias in BRFSS data collection. Despite its limitations, BRFSS remains the best available health data for estimation at geographic levels smaller than state using small area analysis techniques.
Starting from 2011, BRFSS began sampling cell phones. However, questions on colorectal cancer have not been asked since this sampling began. In the future, it may be possible to obtain estimates for CRC on the local level including those households without a landline through use of this cell phone sampling provided these questions are asked on such future surveys.
A limitation of our study is the small number of counties for which we were able to produce screening estimates (37 out of 114). Even with this small number of counties, however, we identified substantial within-state variability in colorectal cancer screening. While the study include almost 80 % of the state population, the large number of counties with insufficient data to estimate the screening rates makes monitoring the effectiveness of state programs challenging. We note that the counties that were less urban tended to have lower rates of screening; this suggests that the counties with missing screening data will also have lower-than-average rates of screening, since these counties had few respondents or a small population. Efforts to increase screening, therefore, should focus on counties known to have low rates of screening, as well as counties for which screening information is not available due to small numbers.
BRFSS relied on landlines at the time the current data were collected. Within Missouri, we note that the trend away from solely depending on landlines has been led by urban centers including St. Louis City and County. In 2007 for example, 19 % of city residents lived in landline only households compared to 34.5 % of adults (age 18 and over) in the rest of the sate [19]. Switching away from landline use also has increased. We note that only 10.1 % of Missouri households relied on cell phones in 2007. By 2009, that number climbed to 22.4 %. During this time period, the reliance on cell phones was higher in the St Louis City and St. Louis County area, an urban region of high colorectal screening. Thus, BRFSS reliance on landlines at the time of the current data collection should not have biased the estimates of screening prevalence by differentially missing more rural landline users.
BRFSS relies on self-report of colorectal cancer screening. Studies of reliability and validity of questionnaire measures of colorectal screening against administrative data and medical records show high reliability with specificity of colonoscopy at 91 % and FOBT at 82 % [20]. Furthermore, self-report is reasonably accurate across population subgroups [21].
The long-term goal of estimating and monitoring county-level screening rates is to reduce between-county heterogeneity in screening and, ultimately, colorectal cancer mortality. The full potential of screening to reduce colorectal cancer mortality will not be realized until persistent geographic differences in the reach and uptake of screening are addressed. State-level analyses provide important information, but can mask a startling degree of within-state variability. Assessment and monitoring of county-level data can and should play an important role in allocation of public health efforts within a state and within the country as a whole. To achieve the goal of reduced disparities in colorectal cancer screening and speed progress toward the Healthy People 2020 goals, we will need richer county-level data to help focus prevention efforts.
Acknowledgments
The work of Melody Goodman is supported by the Siteman Cancer Center, National Institutes of Health, National Cancer Institute Grant U54CA153460, and Washington University Faculty Diversity Scholars Program. The work of Lucy D’Agostino McGowan, Graham A. Colditz, and Aimee James is supported by the Program for the Elimination of Cancer Disparities, National Institutes of Health, National Cancer Institute grant U54CA153460. The work of Graham Colditz and Kari Bohlke is supported by the Foundation for Barnes-Jewish Hospital, St Louis.
References
- 1.Joseph DA, King JB, Miller JW, Richardson LC. Prevalence of colorectal cancer screening among adults—behavioral risk factor surveillance system, United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(Suppl):51–56. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . Cancer screening—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:41–45. [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services; OoDPaH, editor. Healthy people 2020. Promotion. Washington, D.C: 2010. [Google Scholar]
- 4.U S Preventive Services Task Force. Screening for colorectal cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 5.Naishadham D, Lansdorp-Vogelaar I, Siegel R, Cokkinides V, Jemal A. State disparities in colorectal cancer mortality patterns in the United States. Cancer Epidemiol Biomarkers Prev. 2011;20:1296–1302. doi: 10.1158/1055-9965.EPI-11-0250. [DOI] [PubMed] [Google Scholar]
- 6.Raghunathan TEXD, Schenker N, Parsons VL, Davis WW, Dodd KW, Feuer EJ. Combining Information from two surveys to estimate county level prevalence rates of cancer risk factors and screening. J Am Stat Assoc. 2007;102:474–486. [Google Scholar]
- 7.Prevention CfDCa. The guide to community preventive services (The Community Guide) Atlanta (GA): 2013. [Google Scholar]
- 8.Baron RC, Rimer BK, Coates RJ, et al. Client-directed interventions to increase community access to breast, cervical, and colorectal cancer screening a systematic review. Am J Prev Med. 2008;35:S56–S66. doi: 10.1016/j.amepre.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Wei EK, Ryan CT, Dietrich AJ, Colditz GA. Improving colorectal cancer screening by targeting office systems in primary care practices: disseminating research results into clinical practice. Arch Intern Med. 2005;165:661–666. doi: 10.1001/archinte.165.6.661. [DOI] [PubMed] [Google Scholar]
- 10.Lobb R, Colditz GA. Implementation science and its application to population health. Annu Rev Public Health. 2013;34:235–251. doi: 10.1146/annurev-publhealth-031912-114444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kania J, Kramer M. Collective impact. Stanf Soc Innov Rev. 2011;9:36–41. [Google Scholar]
- 12.Colditz GA, Dart H. Massachusetts leads the nation in colorectal cancer screening: what lessons can we learn from Implementing prevention-translating epidemiology to practice? Epidemiology. 2013;3:e111. [Google Scholar]
- 13.Joseph DA, DeGroff AS, Hayes NS, Wong FL, Plescia M. The colorectal cancer control program: partnering to increase population level screening. Gastrointest Endosc. 2011;73:429–434. doi: 10.1016/j.gie.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 14.American Cancer Society. Cancer facts and figures 2013. American Cancer Society; Atlanta: 2013. [Google Scholar]
- 15.Centers for Disease C and Prevention. Estimated county-level prevalence of diabetes and obesity—United States, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:1259–1263. [PubMed] [Google Scholar]
- 16.Joshu CE, Parmigiani G, Colditz GA, Platz EA. Opportunities for the primary prevention of colorectal cancer in the United States. Cancer Preve Res. 2012;5:138–145. doi: 10.1158/1940-6207.CAPR-11-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman MS. Comparison of small-area analysis techniques for estimating prevalence by race. Prev Chronic Dis. 2010;7:A33. [PMC free article] [PubMed] [Google Scholar]
- 18.Jia H, Muennig P, Borawski E. Comparison of small-area analysis techniques for estimating county-level outcomes. Am J Prev Med. 2004;26:453–460. doi: 10.1016/j.amepre.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Blumberg SJ, Luke JV, Ganesh N, Davern ME, Boudreaux MH, Soderberg K. Wireless substitution: state-level estimates from the National Health Interview Survey, January 2007-June 2010. Natl Health Stat Rep. 2011:1–26. 8. [PubMed] [Google Scholar]
- 20.Vernon SW, Tiro JA, Vojvodic RW, et al. Reliability and validity of a questionnaire to measure colorectal cancer screening behaviors: does mode of survey administration matter? Cancer Epidemiol Biomarkers Prev. 2008;17:758–767. doi: 10.1158/1055-9965.EPI-07-2855. [DOI] [PubMed] [Google Scholar]
- 21.White A, Vernon SW, Eberth JM, et al. Correlates of self-reported colorectal cancer screening accuracy in a multi-specialty medical group practice. Open J Epidemiol. 2013;3:20–24. doi: 10.4236/ojepi.2013.31004. [DOI] [PMC free article] [PubMed] [Google Scholar]