Abstract
Purpose
Drug interactions are of concern when treating patients co-infected with human immunodeficiency virus (HIV) and tuberculosis. Concomitant use of efavirenz (EFV) with the enzyme inducer rifampicin might be expected to increase EFV clearance. We investigated the influence of concomitant tuberculosis treatment on the plasma clearance of EFV.
Methods
Fifty-eight patients were randomized to receive their EFV-containing antiretroviral therapy either during or after tuberculosis treatment. Steady-state EFV plasma concentrations (n=209 samples) were measured, 83 in the presence of rifampicin. Data were analyzed using a non-linear mixed effects model, and the model was evaluated using non-parametric bootstrap and visual predictive checks.
Results
The patients had a median age of 32 (range 19–55) years and 43.1% were women. There was a bimodal distribution of apparent clearance, with slow EFV metabolizers accounting for 23.6% of the population and having a metabolic capacity 36.4% of that of the faster metabolizers. Apparent EFV clearance after oral administration in fast metabolizers was 12.9 L/h/70 kg whilst off tuberculosis treatment and 9.1 L/h/70 kg when on tuberculosis treatment. In slow metabolizers, the clearance estimates were 3.3 and 4.7 L/h/70 kg in the presence and absence of TB treatment, respectively. Overall there was a 29.5% reduction in EFV clearance during tuberculosis treatment.
Conclusion
Unexpectedly, concomitant rifampicin-containing tuberculosis treatment reduced apparent EFV clearance with a corresponding increase in EFV exposure. While the reasons for this interaction require further investigation, cytochrome P450 2B6 polymorphisms in the population studied may provide some explanation.
Keywords: Pharmacokinetics, Tuberculosis, HIV, Efavirenz, Rifampicin
Introduction
Human immunodeficiency virus (HIV) and tuberculosis co-infection is a serious public health issue in sub-Saharan Africa. The World Health Organization has reported that in South Africa, 53% of patients diagnosed with tuberculosis are also HIV-positive [1]. When treating co-infected individuals, determination of the optimal timing of antiretroviral therapy (ART) initiation in relation to tuberculosis treatment [2] is complicated by the potential for clinically significant drug–drug interactions [3].
Rifampicin, an important component of first-line tuberculosis treatment, induces various hepatic cytochrome P450 (CYP450) enzymes and may decrease plasma concentrations of antiretroviral (ARVs) sharing similar metabolic pathways [4]. Conversely, isoniazid, also part of first-line tuberculosis treatment, is a competitive inhibitor that specifically targets enzymes CYP2C19 and CYP3A [5]. Efavirenz (EFV) is the preferred non-nucleoside reverse transcriptase inhibitor (NNRTI) component of the first-line ARV regimen in South Africa. Metabolism of EFV entails 8-hydroxylation by CYP2B6 with subsequent glucuronidation [6, 7]. EFV is also known to induce CYP3A4 as well as its own metabolism [8]. If EFV plasma concentrations are decreased due to interaction with enzyme inducers, this may increase the potential for therapeutic failure and the development of drug resistance [9]. There are currently conflicting reports in the literature regarding the nature of the interaction, with some studies [10, 11] reporting increased metabolism of EFV in the presence of rifampicin and others reporting just the opposite [12, 13].
In order to better understand the interaction between EFV and tuberculosis treatment, we designed the present study in which a population–pharmacokinetics approach was used to describe EFV clearance in South African patients treated with rifampicin-containing first-line tuberculosis regimens and NNRTI-based ART.
Methods
This study was conducted as part of the “Starting Tuberculosis and Antiretroviral Therapy” (START) study, an open label randomized clinical trial conducted in Durban, South Africa between July 2006 and January 2008. All patients recruited to the START study were ART naive, at least 18 years of age, and then received both ART and standard first-line tuberculosis treatment in a pre-existing directly observed therapy (DOT) program. Only patients with no pre-defined laboratory abnormalities, having received at least 10 but not more than 28 days of tuberculosis treatment were enrolled. The standard tuberculosis treatment comprised rifampicin (R), isoniazid (H), pyrazinamide (Z), and ethambutol (E) dosed daily on weekdays only for 2 months, followed by R and H for a minimum of 4 additional months. Patients weighing ≥50 kg received five tablets daily of a fixed-dose combination of RHZE containing 120/60/300/200 mg respectively, followed by two tablets daily of RH 300/150 mg. Patients weighing <50 kg received four tablets of the RHZE 120/60/300/200 mg daily, followed by three tablets of RH 150/100 mg. Women recruited to the study were required to use both injectable progestogen and barrier methods of contraception. All patients received standard of care, which included multivitamins and co-trimoxazole prophylaxis. No additional drugs thought likely to interact with EFV were permitted.
Participants were randomized to receive both ART and tuberculosis treatment simultaneously (integrated arm) or to initiate ART only on completion of tuberculosis treatment (sequential arm). In both arms, ART comprised once-daily enteric-coated didanosine (400 mg for participants ≥60 kg; 250 mg for participants <60 kg), lamivudine 300 mg, and EFV. Based on the expected interaction, when EFV was administered in the presence of tuberculosis treatment, participants weighing <50 kg received 600 mg and those weighing ≥50 kg received 800 mg daily. After the tuberculosis treatment was successfully completed, all patients received EFV 600 mg. For patients in the sequential arm, ART initiation occurred a median of 7 days [interquartile range (IQR) 6–9 days] after TB treatment completion. EFV concentrations were sampled at least 28 days after the TB treatment was completed; no residual wash-out effects of the TB drugs were expected after this time due to the short half-life of the TB drugs.
At enrolment and follow-up visits, demographic, clinical, and treatment adherence data were collected. Adherence to ART was determined by means of a monthly pill count. Blood samples for trough EFV plasma concentrations were obtained at the end of months 1, 2, and 3 during tuberculosis treatment and at the same time points after tuberculosis treatment was successfully completed. The timing of blood sampling in relation to EFV and tuberculosis treatment dosing was recorded. Samples were drawn a median of 20.3 h post-dose (IQR 14.8–25.2 h). Blood was collected in heparinized tubes, which were stored on ice and separated at 3,000 rpm within 1 h. Samples were then stored at −70°C until analysis. Samples were analyzed in the Division of Clinical Pharmacology, University of Cape Town. Plasma EFV concentrations were determined using a modification of a method by Chi et al. [14] based on liquid chromatography/tandem mass spectrometry; the accuracy ranged from 97.2 to 105.6%. Intraday and interday precisions ranged from 1.3 to 4.6%. The lower limit of quantitation (LLOQ) for EFV concentrations was 0.2 mg/L. In five samples where the concentration was below the LLOQ, the actual concentration was recorded for these observations and used in the analysis. These concentrations represent less than 3% of the data and were determined to be unlikely to influence the final results.
Demographic and clinical data were analyzed using SAS ver. 9.1 (SAS Institute, Cary, NC). Baseline characteristics were compared using the Mann–Whitney, Fisher’s Exact or Student’s t test, as appropriate. A type 1 error (α) of 0.05 was used to reject the null hypothesis.
NONMEM (ver. VI 2.0), with the first-order conditional estimation method and interaction option, was used [15]. A one-compartment model with first-order absorption and elimination was used to describe the data. The model was parameterized in terms of absorption half-life, clearance, and volume of distribution. The apparent clearance (CL/F) after oral administration, where F is the bioavailability, was estimated. As there were no EFV plasma concentrations in the absorption phase, the absorption half-life was fixed to 1 h. Because EFV has a long elimination half-life and measurements were made at steady state, there was no reliable information on the volume of distribution (V/F). Therefore, the V/F was fixed at 267 L/70 kg, as derived from Csajka et al. ([16]. The population parameter values were standardized for a body weight of 70 kg using allometric scaling [CL/F = CL/FPOP × (weight/70)0.75, V/F = V/FPOP × (weight/70)] [17].
Sample times were classified as “occasions” (OCCs), where OCCs 1, 2, and 3 represented EFV concentrations sampled at the end of months 1, 2, and 3, respectively, when EFV and tuberculosis treatment were co-administered in the integrated arm. OCCs 4, 5, and 6 represented EFV concentrations sampled once a month in each of the first 3 months after the completion of tuberculosis treatment in both the integrated and sequential arms. The seemingly random variability (between and within subjects) was modeled as the exponent of the random effects, as pharmacokinetic parameters resemble a log normal distribution. Random residual variability was described using a combined proportional and additive error model. Using a step-wise model building process and subsequent backward deletion, we identified covariates which had a significant influence on CL/F. Mixture models with two and three sub-populations for the distribution of CL/F were evaluated. The statistical comparison of models was based on the difference in the value of the minimum objective function (ΔOBJ). ΔOBJ values are approximately χ2 distributed, with degrees of freedom equal to the difference in the number of parameters between models. A ΔOBJ greater than minus twofold the log-likelihood of the data was considered to be significant, e.g. ΔOBJ of 3.84, α=0.05, 1df. The final model was evaluated using non-parametric bootstrapping and visual predictive checks.
The START study and this pharmacokinetic sub-study were approved by the University of KwaZulu-Natal Biomedical Research Ethics Committee (E183/04), South African Medicines Control Council (20040969) and were registered at ClinicalTrials.gov (NCT00091936). Written informed consent was obtained from each study participant.
Results
A total of 58 patients, randomized equally to either the integrated or sequential treatment arm, were included in the START pharmacokinetic sub-study. There were no significant differences between the groups at baseline (Table 1). Twenty-five patients received EFV 800 mg during the integrated treatment. Mean monthly adherence to ART in the integrated arm was 93.4% during and 87.1% after the tuberculosis treatment. In the sequential arm, mean monthly adherence to ART was 95.0%. There were 209 EFV plasma concentrations available for analysis across six time points, 83 having been drawn during concomitant EFV and tuberculosis treatment. Of the 29 patients in the integrated arm, 16 provided data on EFV concentrations at all six time points, four were missing data at one time point during tuberculosis treatment, seven were missing data at one time point after tuberculosis treatment, and four were unable to provide EFV data for any of the time points after tuberculosis treatment. In the sequential arm, 16 patients provided EFV data at three time points, six provided data at two time points, three provided data at one time point, and a further three patients were unable to provide EFV data at any of the time points. For one participant in the sequential arm, all three time points were classified as BLD (below the limit of detection). In total, there were six samples where the EFV concentration was BLD. During and after TB treatment, 55 and 62% of patients, respectively, had EFV concentrations in the recommended range of 1–4 mg/L, There were no serious EFV-related adverse effects, in any patient with elevated EFV levels.
Table 1.
Patient baseline characteristics
| Variable | Integrated arm (n=29) | Sequential arm (n=29) | P value |
|---|---|---|---|
| Median age in years (range) | 32 (19–54) | 32 (21–55) | 0.45a |
| Females (%) | 10 (34%) | 15 (54%) | 0.29b |
| Mean weight in kg (SD) | 56.3 (7.8) | 58.3 (9.9) | 0.39a |
| Median body mass index in kg/m2 (range) | 21.0 (16.9–28.2) | 22.0 (16.3–33.6) | 0.42c |
| Mean baseline CD4 count in cells/µL (SD) | 281 (178) | 276.2 (128) | 0.71c |
| Median baseline viral load in copies/mL (range) | 49200(503–843000) | 419,00 (685–1,750,000) | 0.62c |
SD, Standard deviation
Students t test
Fishers exact test
Mann–Whitney test
Of the available covariates investigated, sex, age, serum transaminase levels, monthly EFV adherence, baseline CD4 cell count, and baseline viral load did not have a statistically significant effect on EFV concentration. There was a large improvement in the fit of the model when between-occasion variability in CL/F was included. A further improvement in model fit was achieved by assuming a bimodal distribution in CL/F, dividing patients into slow and fast metabolizers using a mixture model. There was no evidence for a third sub-population in the CL/F distribution. The only covariate that further improved the fit of the model was the use of tuberculosis treatment. Stepwise backwards deletion (Table 2) confirmed the role of the covariates in the final model.
Table 2.
Model characteristics showing stepwise backwards deletion
| Model | Covariates included in the model | Objective function (OBJ) |
Change in objective function (ΔOBJ) |
|---|---|---|---|
| 1 | Allometric scaling, between-occasion variability (BOV), tuberculosis treatment, bimodal metabolic capacity (fast and slow metabolizers) | 439.68 | - |
| 2 | Allometric scaling, BOV, tuberculosis treatment | 451.94 | 12.26 |
| 3 | Allometric scaling, BOV, bimodal metabolic capacity (fast and slow metabolizers) | 479.72 | 40.04 |
| 4 | Allometric scaling, tuberculosis treatment, bimodal metabolic capacity (fast and slow metabolizers) | 1354.49 | 914.91 |
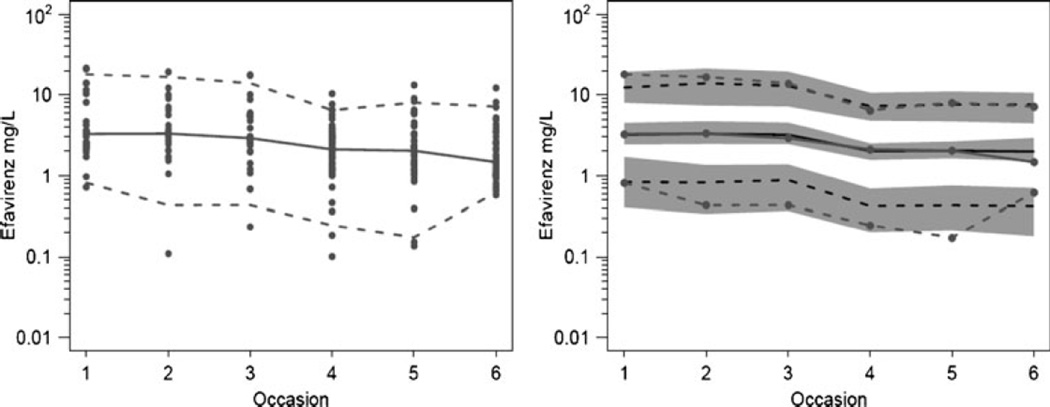
Table 3 shows the final parameter estimates from the original data and the non-parametric bootstrap analysis (n=1,000). The bootstrap estimates are preferred because they will be less influenced by outliers. As can be seen, 23.6% of the population were slow metabolizers with a CL/F that was 36.4% that of the reference group (fast metabolizers). The final model estimate for CL/F was 12.9 L/h/70 kg (95% confidence interval 11.1–15.2) in fast metabolizers not on tuberculosis treatment. Concomitant tuberculosis treatment reduced the overall EFV CL/F to 70.5% of the value without treatment. Based on these estimates, the CL/F for fast metabolizers and slow metabolizers on tuberculosis treatment is 9.1 and 3.3 L/h/70 kg, respectively. For slow metabolizers not on tuberculosis treatment, the CL/F was 4.7 L/h/70 kg. There was more between-occasion variability in relative bioavailability (43.5%) than between-subject variability (19.5%) in EFV CL/F. Residual error in the final model was 11.9% proportional and 0.47 mg/L additive error. The visual predictive check of the final model, as shown in Fig. 1, confirmed the adequacy of the model predictions.
Table 3.
Efavirenz original and bootstrap parameter estimates of the final model (n=1,000)
| Parameter | Original estimate |
Asymptotic RSE |
Bootstrap average |
Bootstrap RSE |
Bootstrap percentile |
|
|---|---|---|---|---|---|---|
| 2.5 | 97.5 | |||||
| Fraction with slow clearance of EFV | 0.227 | 32.9% | 0.236 | 33.6% | 0.105 | 0.413 |
| Ratio of CL/F in slow metabolizers to CL/F in fast metabolizers | 0.365 | 11.3% | 0.364 | 13.4% | 0.291 | 0.464 |
| CL/F for EFV (L/h/70 kg) | 12.8 | 7.50% | 12.9 | 8.05% | 11.1 | 15.2 |
| Ratio of CL/F when taking TB treatment to CL/F without TB treatment | 0.705 | 9.20% | 0.705 | 9.6% | 0.574 | 0.836 |
| Proportional residual error | 0.120 | 31.8% | 0.119 | 43.0% | 0.028 | 0.188 |
| Additive residual error (mg/L) | 0.462 | 26.8% | 0.466 | 28.8% | 0.221 | 0.755 |
| Between-subject variability (CL/F) | 0.215 | 36.5% | 0.197 | 23.8% | 0.091 | 0.278 |
| Between-occasion variability in F | 0.453 | 26.6% | 0.435 | 19.5% | 0.280 | 0.562 |
EFV, Efavirenz; CL/F, apparent clearance, where F is the bioavailability; TB, tuberculosis; RSE, relative standard error
Fig. 1.
Visual predictive check of model for efavirenz concentrations. Right panel 5, 50, and 95 percentiles of observations (gray lines with symbols) and predictions (black lines). Gray shading is 95% confidence interval for 5 and 95 percentile of predictions
Discussion
Although concomitant tuberculosis treatment was expected to decrease EFV exposure by increasing apparent clearance, the results of this study show that concomitant tuberculosis treatment actually reduced EFV CL/F by 29.5%. The initial expectation was based on the known ability of rifampicin to induce CYP2B6 and CYP3A4 and hence to increase the clearance of drugs that are substrates of these isoenzymes. This has been borne out in a number of studies. For example, in a group of 16 Italian patients, the mean CL/F of EFV was found to be significantly higher in the presence of concomitant tuberculosis treatment [11]. In a group of 24 Spanish patients, median peak and trough concentrations of EFV decreased by 24 and 18%, respectively, when tuberculosis treatment was co-administered [10]. In addition, a study of 19 Indian patients found EFV CL/F to be slightly higher in the presence of tuberculosis treatment [18]. As a result, various authors have advocated increased doses of EFV in the presence of tuberculosis treatment [19, 20]. Most recently, in silico prediction of the EFV–R interaction utilizing model input data from the literature (including weight and CYP2B6 phenotype) revealed 50 kg as the preferred weight cutoff for EFV dose increment [21].
In contrast, there are reports that have challenged this view. In a group of 20 black patients from South Africa, despite wide inter-patient variability, the geometric mean plasma concentrations of EFV were similar, whether or not rifampicin was co-administered [13]. Two other South African studies have also found that tuberculosis treatment was not an important determinant of EFV plasma concentrations [22, 23].
Indirectly supporting our finding of a decreased clearance is a case series describing nine patients, seven of whom developed toxicity and required an EFV dose reduction in the presence of tuberculosis treatment [12]. Of the nine patients described, eight were black patients of African origin. Data from the Liverpool Therapeutic Drug Monitoring registry also showed that the co-administration of rifampicin and being black African were important factors influencing EFV concentrations [24]. Without prior adjustment for weight and ethnicity, EFV concentrations were 48% higher when rifampicin was co-administered. It should be noted that the earlier studies, showing an increase in EFV clearance associated with rifampicin co-administration, were all conducted in Caucasian patients [10, 11, 18]
Higher EFV plasma concentrations in black Africans or those of African descent have been associated with the presence of a single nucleotide polymorphism, namely, CYP2B6 516 G > T [25, 26] and CYP2B6c.983 T > C [27, 28]. In 74 Zimbabwean patients receiving no tuberculosis treatment, EFV CL/F could be predicted by genotype, with three groups of varying metabolic capacity [29]. More recently, CYP2A6 has also been shown to independently predict EFV concentrations, a finding that requires further study [30]. Although genotype was not used as a covariate in the present model, the separation of patients into “fast” and “slow” metabolizers contributed to improving the fit of the data. The estimate that 23.6% of the population studied were slow metabolizers was similar to the slow metabolizer genotype prevalence reported in other studies in black African patients [23, 25, 29, 31]. Our model showed that the capacity of the slow metabolizers was 36.4% that of the fast metabolizers.
A Zimbabwean and West African study reported CL/F values of 9.4 and 9.9 and 4.0 and 2.1 L/h in fast and slow metabolizers, respectively [25, 29]. Our population estimate of EFV CL/F was 12.9 L/h standardized for size 70 kg in the fast metabolizers not on tuberculosis treatment. This is similar to previously reported CL/F estimates in a series of cohorts which have ranged from 9.4 to 11.7 L/h but without weight scaling. Because this value is for fast metabolizers, it is expected to be higher than other reported values which did not distinguish these groups [16, 32–34].
In conclusion, this study demonstrated that, by reducing clearance, concomitant tuberculosis treatment increased EFV exposure in black African patients.
Acknowledgments
The authors acknowledge the contribution of START study staff and the staff of the Prince Cyril Zulu Communicable Disease Centre, particularly Ms. Chandraprabha Singh, Dr. Surie Chinnapa, and Sister Jeanne Liebertrau. CAPRISA is supported by the National Institute of Allergy and infectious Disease (NIAID), National Institutes of Health (NIH) (grant no. AI51794). The START study was supported by grant no. U19 AI051794-0453. The antiretroviral drugs provided were funded by the Global Fund to fight AIDS, Tuberculosis and Malaria. Efavirenz assays were funded by a Hasso Platner Foundation scholarship, awarded to TG. TG was also supported by the Columbia University–Southern African Fogarty AIDS International Training and Research Programme (grant no. D43TW00231). Efavirenz assays were performed at the Division of Clinical Pharmacology, University of Cape Town, South Africa. The assistance and advice of Prof. Peter Smith, Dr. Helen McIlleron, and Mr. Emmanuel Chigutsa are acknowledged.
Contributor Information
Tanuja N. Gengiah, Email: gengiaht1@ukzn.ac.za, Centre for the AIDS Programme of Research in South Africa (CAPRISA), University of KwaZulu-Natal, Durban, South Africa; CAPRISA, Doris Duke Medical Research Institute, University of KwaZulu-Natal, Private Bag X7, Congella 4013, South Africa.
Nicholas H. G. Holford, Department of Pharmacology & Clinical Pharmacology, University of Auckland, Auckland, New Zealand
Julia H. Botha, Department of Therapeutics and Medicines Management, University of KwaZulu-Natal, Durban, South Africa
Andrew L. Gray, Centre for the AIDS Programme of Research in South Africa (CAPRISA), University of KwaZulu-Natal, Durban, South Africa Department of Therapeutics and Medicines Management, University of KwaZulu-Natal, Durban, South Africa.
Kogieleum Naidoo, Centre for the AIDS Programme of Research in South Africa (CAPRISA), University of KwaZulu-Natal, Durban, South Africa.
Salim S. Abdool Karim, Centre for the AIDS Programme of Research in South Africa (CAPRISA), University of KwaZulu-Natal, Durban, South Africa Department of Epidemiology, Mailman School of Public Health, Columbia University, Columbia, NY, USA.
References
- 1.World Health Organization. Geneva: WHO; 2008. Global tuberculosis control: surveillance planning financing. [Google Scholar]
- 2.Dean GL, Edwards SG, Ives NJ, Matthews G, Fox EF, Navaratne L, Fisher M, Taylor GP, Miller R, Taylor CB, de Ruiter A, Pozniak AL. Treatment of tuberculosis in HIV-infected persons in the era of highly active antiretroviral therapy. AIDS. 2002;16(1):75–83. doi: 10.1097/00002030-200201040-00010. [DOI] [PubMed] [Google Scholar]
- 3.Gengiah TN, Gray AL, Naidoo K, Karim QA. Initiating antiretrovirals during tuberculosis treatment: a drug safety review. Expert Opin Drug Saf. 2011;10:559–574. doi: 10.1517/14740338.2011.546783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finch CK, Chrisman CR, Baciewicz AM, Self TH. Rifampin and rifabutin drug interactions: an update. Arch Intern Med. 2002;162(9):985–992. doi: 10.1001/archinte.162.9.985. [DOI] [PubMed] [Google Scholar]
- 5.Desta Z, Soukhova NV, Flockhart DA. Inhibition of cytochrome P450 (CYP450) isoforms by isoniazid: potent inhibition of CYP2C19 and CYP3A. Antimicrob Agents Chemother. 2001;45(2):382–392. doi: 10.1128/AAC.45.2.382-392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mouly S, Lown KS, Kornhauser D, Joseph JL, Fiske WD, Benedek IH, Watkins PB. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther. 2002;72(1):1–9. doi: 10.1067/mcp.2002.124519. [DOI] [PubMed] [Google Scholar]
- 7.Ogburn ET, Jones DR, Masters AR, Xu C, Guo Y, Desta Z. Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metab Dispos. 2010;38(7):1218–1229. doi: 10.1124/dmd.109.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith PF, DiCenzo R, Morse GD. Clinical pharmacokinetics of non-nucleoside reverse transcriptase inhibitors. Clin Pharmacokinet. 2001;40(12):893–905. doi: 10.2165/00003088-200140120-00002. [DOI] [PubMed] [Google Scholar]
- 9.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15(1):71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Cortes LF, Ruiz-Valderas R, Viciana P, Alarcon-Gonzalez A, Gomez-Mateos J, Leon-Jimenez E, Sarasanacenta M, Lopez-Pua Y, Pachon J. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin Pharmacokinet. 2002;41(9):681–690. doi: 10.2165/00003088-200241090-00004. [DOI] [PubMed] [Google Scholar]
- 11.Matteelli A, Regazzi M, Villani P, De Iaco G, Cusato M, Carvalho AC, Caligaris S, Tomasoni L, Manfrin M, Capone S, Carosi G. Multiple-dose pharmacokinetics of efavirenz with and without the use of rifampicin in HIV-positive patients. Curr HIV Res. 2007;5(3):349–353. doi: 10.2174/157016207780636588. [DOI] [PubMed] [Google Scholar]
- 12.Brennan-Benson P, Lyus R, Harrison T, Pakianathan M, Macallan D. Pharmacokinetic interactions between efavirenz and rifampicin in the treatment of HIV and tuberculosis: one size does not fit all. AIDS. 2005;19(14):1541–1543. doi: 10.1097/01.aids.0000183519.45137.a6. [DOI] [PubMed] [Google Scholar]
- 13.Friedland G, Khoo S, Jack C, Lalloo U. Administration of efavirenz (600 mg/day) with rifampicin results in highly variable levels but excellent clinical outcomes in patients treated for tuberculosis and HIV. J Antimicrob Chemother. 2006;58(6):1299–1302. doi: 10.1093/jac/dkl399. [DOI] [PubMed] [Google Scholar]
- 14.Chi J, Jayewardene AL, Stone JA, Motoya T, Aweeka FT. Simultaneous determination of five HIV protease inhibitors nelfinavir, indinavir, ritonavir, saquinavir and amprenavir in human plasma by LC/MS/MS. J Pharm Biomed Anal. 2002;30(3):675–684. doi: 10.1016/s0731-7085(02)00357-6. [DOI] [PubMed] [Google Scholar]
- 15.Beal SLSL, Boeckmann A. Division of Pharmacology. San Francisco: University of California; 1999. NONMEM user’s guide. [Google Scholar]
- 16.Csajka C, Marzolini C, Fattinger K, Decosterd LA, Fellay J, Telenti A, Biollaz J, Buclin T. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin Pharmacol Ther. 2003;73(1):20–30. doi: 10.1067/mcp.2003.22. [DOI] [PubMed] [Google Scholar]
- 17.Holford NH. A size standard for pharmacokinetics. Clin Pharmacokinet. 1996;30(5):329–332. doi: 10.2165/00003088-199630050-00001. [DOI] [PubMed] [Google Scholar]
- 18.Ramachandran G, Hemanth Kumar AK, Rajasekaran S, Kumar P, Ramesh K, Anitha S, Narendran G, Menon P, Gomathi C, Swaminathan S. CYP2B6 G516T polymorphism but not rifampin coadministration influences steady-state pharmacokinetics of efavirenz in human immunodeficiency virus-infected patients in South India. Antimicrob Agents Chemother. 2009;53(3):863–868. doi: 10.1128/AAC.00899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabrera SE, Cordero M, Iglesias A, Valverde MP, Dominguez-Gil A, Garcia MJ. Efavirenz–rifampicin interaction: therapeutic drug monitoring to efavirenz dosage optimization in HIV/TBC patients. AIDS. 2008;22(18):2549–2551. doi: 10.1097/QAD.0b013e3283189c07. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Cortes LF, Ruiz-Valderas R, Ruiz-Morales J, Leon E, de Campos AV, Marin-Niebla A, Marquez-Solero M, Lozano F, Valiente R. Efavirenz trough levels are not associated with virological failure throughout therapy with 800 mg daily and a rifampicin-containing antituberculosis regimen. J Antimicrob Chemother. 2006;58(5):1017–1023. doi: 10.1093/jac/dkl357. [DOI] [PubMed] [Google Scholar]
- 21.Rekic D, Roshammar D, Mukonzo J, Ashton M. In silico prediction of efavirenz and rifampicin drug-drug interaction considering weight and CYP2B6 phenotype. Br J Clin Pharmacol. 2011;71(4):536–543. doi: 10.1111/j.1365-2125.2010.03883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren Y, Nuttall JJ, Eley BS, Meyers TM, Smith PJ, Maartens G, McIlleron HM. Effect of rifampicin on efavirenz pharmacokinetics in HIV-infected children with tuberculosis. J Acquir Immune Defic Syndr. 2009;50(5):439–443. doi: 10.1097/QAI.0b013e31819c33a3. [DOI] [PubMed] [Google Scholar]
- 23.Cohen K, Grant A, Dandara C, McIlleron H, Pemba L, Fielding K, Charalombous S, Churchyard G, Smith P, Maartens G. Effect of rifampicin-based antitubercular therapy and the cytochrome P450 2B6 516 G > T polymorphism on efavirenz concentrations in adults in South Africa. Antivir Ther. 2009;14(5):687–695. [PMC free article] [PubMed] [Google Scholar]
- 24.Stohr W, Back D, Dunn D, Sabin C, Winston A, Gilson R, Pillay D, Hill T, Ainsworth J, Pozniak A, Leen C, Bansi L, Fisher M, Orkin C, Anderson J, Johnson M, Easterbrook P, Gibbons S, Khoo S. Factors influencing efavirenz and nevirapine plasma concentration: effect of ethnicity, weight and co-medication. Antivir Ther. 2008;13(5):675–685. [PubMed] [Google Scholar]
- 25.Kwara A, Lartey M, Sagoe KW, Xexemeku F, Kenu E, Oliver-Commey J, Boima V, Sagoe A, Boamah I, Greenblatt DJ, Court MH. Pharmacokinetics of efavirenz when co-administered with rifampin in TB/HIV co-infected patients: pharmacogenetic effect of CYP2B6 variation. J Clin Pharmacol. 2008;48(9):1032–1040. doi: 10.1177/0091270008321790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwara A, Lartey M, Sagoe KW, Court MH. Paradoxically elevated efavirenz concentrations in HIV/tuberculosis-coinfected patients with CYP2B6 516TT genotype on rifampin-containing antituberculous therapy. AIDS. 2011;25(3):388–390. doi: 10.1097/QAD.0b013e3283427e05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wyen C, Hendra H, Vogel M, Hoffmann C, Knechten H, Brockmeyer NH, Bogner JR, Rockstroh J, Esser S, Jaeger H, Harrer T, Mauss S, van Lunzen J, Skoetz N, Jetter A, Groneuer C, Fatkenheuer G, Khoo SH, Egan D, Back DJ, Owen A. Impact of CYP2B6 983 T > C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIVinfected patients. J Antimicrob Chemother. 2008;61(4):914–918. doi: 10.1093/jac/dkn029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehlotra RK, Bockarie MJ, Zimmerman PA. CYP2B6 983 T > C polymorphism is prevalent in West Africa but absent in Papua New Guinea: implications for HIV/AIDS treatment. Br J Clin Pharmacol. 2007;64(3):391–395. doi: 10.1111/j.1365-2125.2007.02884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyakutira C, Roshammar D, Chigutsa E, Chonzi P, Ashton M, Nhachi C, Masimirembwa C. High prevalence of the CYP2B6 516 G– > T(*6) variant and effect on the population pharmacokinetics of efavirenz in HIV/AIDS outpatients in Zimbabwe. Eur J Clin Pharmacol. 2008;64(4):357–365. doi: 10.1007/s00228-007-0412-3. [DOI] [PubMed] [Google Scholar]
- 30.Kwara A, Lartey M, Sagoe KW, Rzek NL, Court MH. CYP2B6 ©.516 G–>T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. Br J Clin Pharmacol. 2009;67(4):427–436. doi: 10.1111/j.1365-2125.2009.03368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haas DW, Ribaudo HJ, Kim RB, Tierney C, Wilkinson GR, Gulick RM, Clifford DB, Hulgan T, Marzolini C, Acosta EP. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18(18):2391–2400. [PubMed] [Google Scholar]
- 32.Kappelhoff BS, Huitema AD, Yalvac Z, Prins JM, Mulder JW, Meenhorst PL, Beijnen JH. Population pharmacokinetics of efavirenz in an unselected cohort of HIV-1-infected individuals. Clin Pharmacokinet. 2005;44(8):849–861. doi: 10.2165/00003088-200544080-00006. [DOI] [PubMed] [Google Scholar]
- 33.Barrett JS, Joshi AS, Chai M, Ludden TM, Fiske WD, Pieniaszek HJ., Jr Population pharmacokinetic meta-analysis with efavirenz. Int J Clin Pharmacol Ther. 2002;40(11):507–519. doi: 10.5414/cpp40507. [DOI] [PubMed] [Google Scholar]
- 34.Pfister M, Labbe L, Hammer SM, Mellors J, Bennett KK, Rosenkranz S, Sheiner LB. Population pharmacokinetics and pharmacodynamics of efavirenz, nelfinavir, and indinavir: Adult AIDS Clinical Trial Group Study 398. Antimicrob Agents Chemother. 2003;47(1):130–137. doi: 10.1128/AAC.47.1.130-137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]