Abstract
Background
Deep inspirations (DIs) can prevent (bronchoprotection-BP) and reverse (bronchodilation-BD) methacholine (Mch)-induced bronchoconstriction, but this effect is reduced or absent in people with asthma or airways hyperresponsiveness (AHR). The mechanisms of this defect are unknown.
Objective
Indirectly examine the role of cGMP by testing the hypothesis that the PDE V inhibitor, sildenafil, would improve DI-induced BP in individuals with AHR.
Methods
Thirty-two individuals were screened and 15 met all inclusion/exclusion criteria (7 subjects with AHR and 8 healthy). A single dose Mch challenge inducing a 20% reduction in FEV1 in the absence of DIs was first identified. Thereafter, every study participant had 4 pairs of visits, each pair testing DI-induced BP and BD against the single dose Mch, with no drug, or pretreatment with 25, 50 and 100 mg of sildenafil, respectively, in consecutive order.
Results
Sildenafil did not influence baseline lung function. However, in the absence of DIs, the drug caused a dose-dependent attenuation of the Mch-induced decrease in FEV1 by 17% (1, 16 (median (25th, 75th percentiles)), 35% (−3, 61), and 37% (13, 79) for the 25mg, 50mg, and 100mg doses respectively (p=0.0004). No differences between the two participant groups were found. There were no effects of sildenafil on DI-induced BP or BD.
Conclusion
We infer from these results that the mechanism responsible for the defective ability of DIs to protect the airways from bronchoconstriction is unlikely to be due to dysregulation of cGMP. Of importance, a potential role for PDE V inhibition as bronchoprotector treatment needs to be explored.
Keywords: asthma, bronchodilation, bronchoprotection, hyperresponsiveness
INTRODUCTION
Deep inspirations (DIs) can prevent (bronchoprotection-BP) and reverse (bronchodilation-BD) methacholine (Mch)-induced bronchoconstriction in healthy individuals, but their effect is reduced or absent in people with asthma [1–3]. The mechanisms behind this defect remain to be determined. In a previous report, our group attempted, with little success, to improve the BP effect of deep inspirations using high doses of inhaled fluticasone [4], with the assumption that the loss of this effect was secondary to airway inflammation. In the current study, we aimed at addressing the role of the biochemical pathway that involves the airway smooth muscle relaxant effects of guanosine 3 ,5 cyclic monophosphate (cGMP).
Airway stretch has been demonstrated as a necessary step to activate the beneficial properties of deep inspiration [5,6]. Airway distension depends on the forces generated during deep inspiration-induced inflation of the lungs. One factor that influences the magnitude of these forces is the change in lung volume with a deep inspiration from functional residual capacity (FRC) to total lung capacity (TLC). Recent work from our laboratory suggests that there is a minimum required stretch of the airway walls that allows the airway to remain dilated even after the stretch is removed [7]. This concept is also supported by ex vivo experimentation and by in vivo animal data [8–12].
Intact ability to stretch the airways in asthma is not adequate for the beneficial effects to DI to fully manifest. People with mild asthma often have complete loss of bronchoprotection by DI [13] despite the fact that their ability to distend their airways with a DI is not different from healthy controls [14]. Downstream neural or biochemical processes may be, therefore, mediating the effectiveness of DI.
Stretch of smooth muscle organs such as the bladder and the esophagus leads to changes in wall tension through nitric oxide (NO)-mediated pathways [15,16]. Previous work from our laboratory showed that airway dilation resulting from lung inflation to relatively high pressure in a canine model was inversed to airway constriction when NG-nitro-L-arginine methyl ester (L-NAME), a nitric oxide synthase (NOS) blocker, was administered [10]. Physiological amounts of NO derived from constitutive NOS (cNOS), modulate underlying pulmonary function and are associated with baseline pulmonary function and decreased airways responsiveness [17]. Recent animal studies suggest an important role for the cNOS isoforms in controlling airways hyperresponsiveness (AHR) [18–21]. NO deriving from constitutive neuronal NOS (Type I) plays a major role in the mediation of nonadrenergic, noncholinergic (NANC) bronchodilation in humans [22–24]. NO increases soluble guananyl cyclase (sGC) which in turn increases cGMP leading to smooth muscle relaxation. Phosphodiesterase (PDE) V degrades cGMP [25]. Thus, inhibitors of PDE V, such as sildenafil, lead to elevated levels of cGMP, and cause smooth muscle relaxation [26,27]. In a lung slice model, NO-induced relaxation of the airways was enhanced by selective inhibitors of cGMP-specific PDE V [28].
The study described herein was designed with the primary objective to test the hypothesis that the PDE V inhibitor, sildenafil, improves the DI-induced BP responses in individuals with AHR. The choice of primary outcome was based on our previous work showing a stronger relationship between BP (over BD) and AHR [4]. Secondary objectives included testing the hypotheses that sildenafil would also increase the BP effect of DI in healthy individuals as well as the BD effects of DI in individuals with AHR and healthy subjects.
MATERIALS AND METHODS
Subjects
A total of 32 individuals were screened for the study and 15 met all inclusion/exclusion criteria. Inclusion criteria for both subject groups (subjects with AHR and healthy control group) were male sex (females were excluded because sildenafil is only approved for males), age 18–50, resting systolic blood pressure ≥100 mmHg and resting heart rate ≥55. Exclusion criteria for both groups included use of antihypertensive treatment or any history of cardiovascular disease. Additionally, current smokers and former smoker with >10 pack-years of smoking as well as endurance athletes (running >20 miles/week or equivalent exercise) were excluded, the latter individuals because we have previously shown that they do not bronchoconstrict to inhaled methacholine even in the absence of DI [29].
Subjects with AHR had to report upper and/or lower respiratory symptoms in the 12 months prior to the study in the absence of upper respiratory infections, have at least 2 positive allergy prick skin tests, an FEV1≥70% predicted, and a positive conventional, multi-dose methacholine inhalation challenge (PC20 ≤ 25 mg/ml). All of these subjects had to demonstrate a bronchoprotective effect of DI (bronchoprotective index) less than 40% (see below for methodology and definition). Exclusion criteria for this group included persistent respiratory symptoms suggesting uncontrolled asthma. Subjects on short acting bronchodilators were asked to withhold these medications for at least 8 hours prior to Mch challenge. One subject was on a long acting bronchodilator inhaler. He was asked to withhold that medication for 48 hours prior to Mch challenge.
Healthy subjects had to have no history of respiratory illness or recurrent upper or lower respiratory symptoms, no more than one positive skin prick test, an FEV1>80% predicted, and an FEV1/FVC ratio no less than 95% of predicted. Also, they had to have < 10% reduction in FEV1 after receiving the top dose (25 mg/ml) of Mch in a conventional, multi- dose challenge. The protocol was approved by the Johns Hopkins University IRB. Informed, written consent was obtained from each subject.
Study Design
Screening
This included a general medical examination with focus on respiratory history, physical examination, skin prick testing with a panel of common aeroallergens, and baseline spirometry followed by a conventional Mch challenge. For spirometry, we used Hankinson (NHANES III) predicted values [30].
Evaluation of the Beneficial Effects of Deep Inspiration
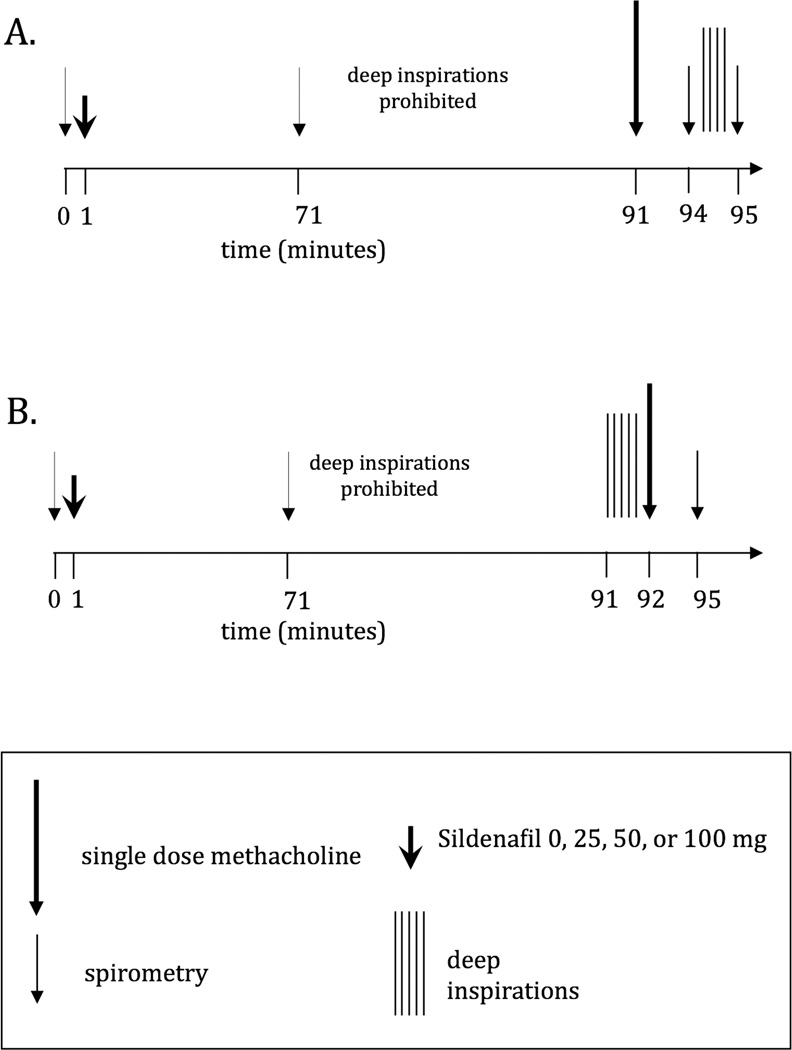
The assessment of DI-induced bronchodilation was performed using multiple modified single dose Mch challenges on separate days, as previously described [31]. At every single dose challenge, after baseline spirometry, study participants were instructed to abstain from taking DIs for 20 minutes. At the end of this period, a single dose Mch (starting at 0.025 mg/ml) was delivered with five tidal inspirations from a deVilbiss 646 nebulizer attached to a model 2A Rosenthal-French dosimeter (Laboratory for Applied Immunology, Inc., Fairfax, VA). Three minutes later, a single full spirometric maneuver was performed and the degree of airways obstruction was calculated by comparing baseline to post Mch FEV1. If the Mch-induced reduction in FEV1 was not greater than 20%, the participant was invited to return on a separate day for another single dose Mch challenge, using the next highest single dose of Mch (e.g. 0.075 mg/ml). This process was continued with additional single dose challenges (0.25, 0.75, 2.5, 7.5 mg/ml) on separate days, until the single dose inducing ≥20% reduction in FEV1 was delivered. At the challenge where 20% or greater reduction in FEV1 was achieved, the participant was instructed to continue the procedure by taking 4 DIs immediately after the single post-Mch spirometry. Another spirometric maneuver was performed immediately after the 4 DIs to calculate the degree to which the participant was able to reverse the Mch-induced airway obstruction (Figure 1A). By measuring the difference between the post- Mch FEV1 and the FEV1 obtained after the 4 DIs, we were able to calculate a measure of bronchodilation induced by the DIs, which we termed bronchodilation (BD) index. This measure, which has been previously described [1], is calculated as follows:
BD index = (1- ((1 - ((FEV1 after Mch and after DI) ÷ (FEV1 baseline))) ÷ (1 - ((FEV1 after Mch) ÷ (FEV1 baseline)))))×100
Figure 1.
A: Schema of protocol. On the study visits that involved the administration of sildenafil, following a brief history and physical examination, spirometry was performed and either nothing, or a single oral dose of sildenafil was administered. Subjects were asked to remain in the laboratory for 70 minutes after sildenafil administration. After 70 minutes, spirometry was repeated. Subsequently, the study participant entered the above-described 20-minute quiet breathing (no deep breaths) period after which the assessment of DI-induced bronchodilation was conducted (see text).
B: Schema of protocol. On the study visits that involved the administration of sildenafil, following a brief history and physical examination, spirometry was performed and either nothing, or a single oral dose of sildenafil was administered. Subjects were asked to remain in the laboratory for 70 minutes after sildenafil administration. After 70 minutes, spirometry was repeated. Subsequently, the study participant entered the above-described 20-minute quiet breathing (no deep breaths) period after which the assessment of DI-induced bronchoprotection was conducted (see text).
To simplify the above equation, the BD index is derived from two components, the reduction in FEV1 from baseline after Mch and after DIs and the reduction in FEV1 from baseline after Mch, but before DIs. Also, a BD index of 100% refers to a DI that reversed all bronchoconstriction to the Mch challenge.
On a separate day, the same single Mch dose used to achieve a 20% reduction in FEV1 was again administered after 20 minutes of quiet breathing. However, prior to the single dose Mch, the subject was instructed to take 5 DIs to prevent the Mch-induced obstruction. By measuring the difference between the Mch-induced reduction in FEV1 on the day no DIs were taken (Figure 1A) versus the day 5 DIs were taken prior to the challenge (Figure 1B), we obtained a measure of bronchoprotection induced by the series of DIs, which we termed bronchoprotection (BP) index. This measure, which has been previously described [1], is calculated as follows:
BP index = (1- ((1 - ((FEV1 after DIs and after MchB) ÷ (FEV1 baselineB))) ÷ (1 - ((FEV1 after MchA)÷(FEV1 baselineA)))))×100
In other words, the BP index is derived from two components, the Mch-induced reduction in FEV1 from baseline on the day 5 DIs preceded the single-dose Mch challenge (B) and the Mch-induced reduction in FEV1 from baseline on the day no DIs were taken prior to the single-dose Mch challenge (A). Also, a BP index of 100% refers to a DI that prevented all bronchoconstriction to the Mch challenge.
Sildenafil dosing
Subjects underwent 8 study visits within a period of 1 to 3 months. After the BP and BD effects of DI were determined, the two study visits described in Figure 1A & 1B were repeated 4 times on separate days, first with no pretreatment and then with sildenafil pretreatment. On the study visits that involved the administration of sildenafil, following a brief history and physical examination, spirometry was performed and a single oral dose of sildenafil was administered beginning from the lowest dose (25 mg). Subjects were asked to remain in the laboratory for 70 minutes after sildenafil administration and vital signs were recorded at 35 and 70 minutes. Caffeinated drinks were prohibited. After 70 minutes, spirometry was repeated. Subsequently, the study participant entered the above-described 20-minute quiet breathing (no deep breaths) period after which the assessment of DI-induced BD and BP was conducted as described above (Figure 1A and B). For safety reasons and since the effects of sildenafil on lung function or AHR were unknown at the time, we chose to start at the lowest dose and administer the next dose in increasing order, on subsequent visits. Consequently, every study participant had 8 study visits (Figure 1A and B) receiving no sildenafil on 2 consecutive visits, 25 mg on 2 consecutive visits, 50 mg on 2 consecutive visits and 100 mg on the last 2 visits.
Data Analysis
Data analysis was performed using JMP 7.0.1 software (SAS Institute Inc.). Because of the limited number of subjects, we took a conservative approach and made no assumptions about the distribution of the data and used nonparametric statistics. We used the Wilcoxon and the Kruskal-Wallis tests to compared the baseline pulmonary function measurements between the subjects with AHR and the healthy subjects, the effect of sildenafil on lung function (change in FEV1 between pre-dosing and 70 minutes post-dosing), the effects of sildenafil on the single-dose Mch-induced decrease in FEV1 from baseline, and the effects of sildenafil on the BP and BD indicies.
To test the effects of dose and the effect of sildenafil on the response to Mch regardless of DI, we performed simple linear regression analyses to evaluate the relationship between the attenuation in the single-dose Mch-induced decrease in FEV1 and the BP and BD indices separately for the group with AHR and the healthy groups.
To examine the effects of the change in baeline FEV1 with increasing sildenafil doses, we performed two-way ANOVA, one with the BP index and the other with the BD index as the dependent variable. The independent variables in both models were the percent change in the response to Mch with increasing sildenafil dose as a continuous independent variable, and the sildenafil dose as a categorical independent variable. Significance was accepted at p≤0.05.
An a priori statistical analysis plan also included an interim analysis. The study was terminated early because not only did we not obtain the expected effect size, in fact we did not even see a trend towards increased deep inspiration-induced BP or BD in the presence of sildenafil. Given that we did not even see a trend, if we were to use the interim data to calculate power for a subsequent study, the number of subjects sufficient to demonstrate a significant result would be approaching infinite. In other words, it was futile to continue the study.
RESULTS
Seven subjects with AHR and 8 healthy individuals were enrolled in the study. On the basis of inclusion criteria, all subjects with AHR had clinical symptoms compatible with asthma and several were using asthma medications. Their demographic data are shown in Table 1a and b. There were no differences in baseline pulmonary function measurements between the subjects with AHR and the healthy subjects with regards to their FEV1 (p=0.76), FVC (p=0.59), or their FEV1/FVC (p=0.87).
Table 1.
| a Characteristics of study participants with AHR | ||||||
|---|---|---|---|---|---|---|
| AHR Subject |
Age | FEV1 % pred |
FVC % pred |
FEV1/ FVC |
PC20 (mg/ml) |
meds |
| 1 | 21 | 89 | 87 | 86 | 7.15 | 1 |
| 2 | 27 | 80 | 94 | 72 | 0.13 | 1, 2, 3, 4 |
| 3 | 21 | 111 | 108 | 85 | 8.9 | - |
| 4 | 27 | 72 | 86 | 68 | 0.24 | 1 |
| 5 | 23 | 91 | 101 | 74 | 1.47 | 1, 3, 5 |
| 6 | 26 | 93 | 89 | 85 | 13.6 | - |
| 7 | 20 | 104 | 106 | 80 | 2.048 | - |
| Mean±SD | 24±3 | 91±13 | 96±9 | 79±7 | ||
| b: Characteristics of healthy study participants | ||||||
|---|---|---|---|---|---|---|
| Healthy Subject |
Age | FEV1 % pred |
FVC % pred |
FEV1/ FVC |
PC20 (mg/ml) |
meds |
| 1 | 22 | 96 | 101 | 81 | >25 | - |
| 2 | 22 | 92 | 97 | 80 | >25 | - |
| 3 | 21 | 121 | 116 | 88 | >25 | - |
| 4 | 19 | 98 | 102 | 81 | >25 | - |
| 5 | 26 | 85 | 106 | 68 | >25 | - |
| 6 | 21 | 86 | 94 | 78 | >25 | - |
| 7 | 39 | 87 | 87 | 83 | >25 | - |
| 8 | 20 | 80 | 87 | 77 | >25 | - |
| Mean±SD | 24±6 | 93±13 | 99±10 | 79±6 | ||
AHR: airways hyperresponsiveness; respiratory medications: 1=inhaled beta-agonist bronchodilators, 2=inhaled corticosteroids, 3=nasal corticosteroids, 4=leukotriene receptor antagonists, 5= antihistamines
The median single dose Mch concentration identified in the screening phase of the study as the dose required to reduce FEV1 by 20% when DIs were withheld was 18.05 mg/ml in the healthy group and 0.75 mg/ml in the AHR group. In the screening phase, the median change in FEV1 after the single dose Mch challenge when DIs were withheld was −33% (−44, −31) (median (25th, 75th percentiles)) and −31% (−44, −25) for the subjects with AHR and the healthy subjects, respectively. In the screening phase of the study, the BP index was 26% (9, 60) and 41% (15, 74) for the subjects with AHR and the healthy subjects, respectively, and the BD index was 28% (11, 34) and 55% (18, 72) for the subjects with AHR and the healthy subjects, respectively.
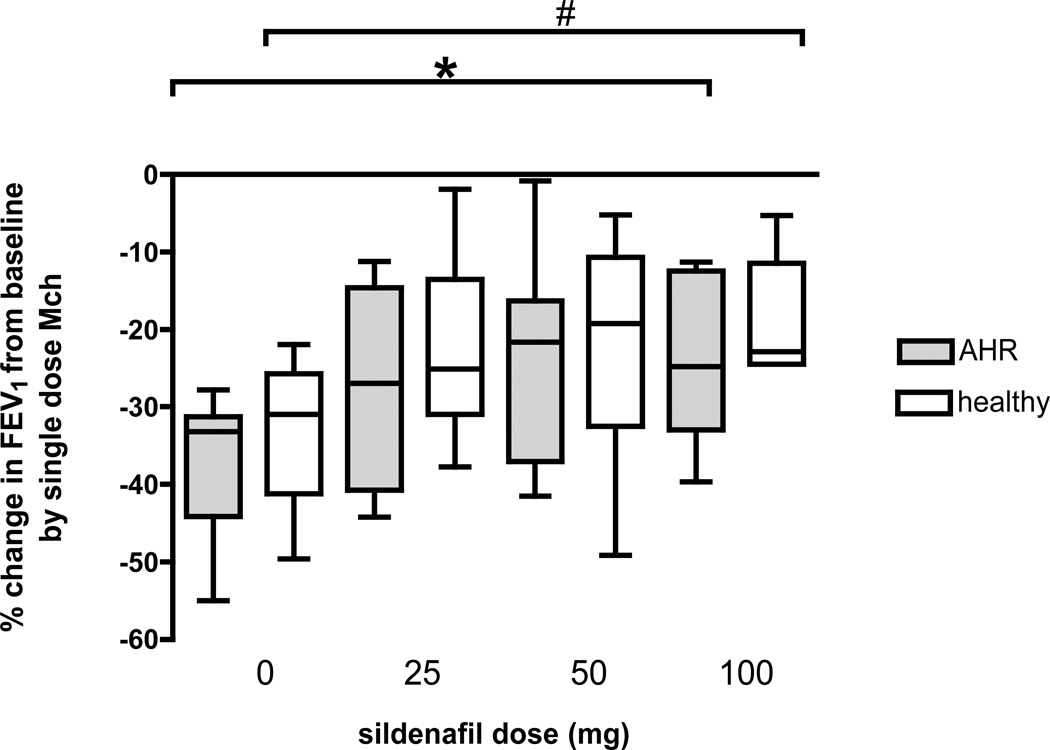
Sildenafil, at any dose, did not influence lung function (data derived from the spirometry that was obtained 70 minutes after dosing) in either group of participants (change in FEV1 between pre-dosing and 70 minutes post-dosing: subjects with AHR, p=0.99 and healthy group, p=0.99). However, the drug caused a dose-dependent attenuation in the single-dose Mch-induced decrease in FEV1 from baseline. Specifically, in subjects with AHR, sildenafil attenuated the Mch-induced decrease in FEV1 by 9% (3, 64), 45% (−12, 61), and 44% (18, 59) for the 25mg, 50mg, and 100mg doses, respectively, compared to Mch challenge without sildenafil pretreatment (p=0.03). In the healthy subjects, sildenafil also attenuated the Mch-induced decrease in FEV1 by 18% (−5,64), 25% (0, 64), and 37% (10, 57) for the 25mg, 50mg, and 100mg doses, respectively, compared to Mch challenge without sildenafil pretreatment (p=0.02). There was no difference in the percent attenuation of the Mch effect between the two participant groups at any dose of sildenafil (for the 25 mg, p=0.91, for the 50 mg, p=0.95, and for the 100 mg dose, p=0.63). The percent changes in FEV1 from baseline by the single dose Mch challenge, at each dose of sildenafil and for each group of participants are presented in Figure 2.
Figure 2.
Box plot graph of the percent fall in FEV1 to the single dose Mch challenge when DIs where withheld for the subjects with AHR (grey bars) and the healthy subjects (white bars) for each dose of sildenafil. There was an increasing attenuation in the fall in FEV1 to the single dose Mch challenge with increasing doses of sildenafil for both the subjects with AHR (*p=0.03) and the healthy subjects (#p=0.02) (Wilcoxon rank sum test).
The primary outcome of our study was the effect of sildenafil on the BP index. We found no significant relationships between the increasing doses of sildenafil and the BP index (p=0.17 and p=0.35 for the subjects with AHR and healthy groups, respectively, Table 2). Similarly, we found no relationship between sildenafil dose and the BD index (p=0.52 and p=0.98 for the AHR and healthy groups, respectively, Table 3). It is interesting to note that a numeric trend towards reduction in BP and BD by sildenafil was observed in subject with AHR, which was practically absent in the healthy group. This resulted in larger differences between the two groups in both the BP and the BD indices: at the 100 mg sildenafil dose, the median BP indices in the AHR and the healthy groups were −27% and 30% (p=0.52), respectively; for the BD index, the median values were 13% and 51% (p=0.08), respectively.
Table 2.
The effect of sildenafil on the bronchoprotection index (median (25th, 75th percentiles))
| Sildenafil dose | AHR participants | Healthy participants |
All participants |
|---|---|---|---|
| 0 mg | 26 (9, 60) | 41 (15, 74) | 36 (13, 69) |
| 25 mg | −13 (−131, 42) | 43 (25, 64) | 28 (−19, 44) |
| 50 mg | 19 (−46, 44) | 59 (19, 80) | 39 (−29, 70) |
| 100 mg | −27 (−59, 33) | 30 (−30, 37) | 26 (−37, 32) |
Table 3.
The effect of sildenafil on the bronchodilation index (median (25th, 75th percentiles))
| Sildenafil dose | AHR participants | Healthy participants |
All participants |
|---|---|---|---|
| 0 mg | 28 (11, 34) | 55 (18, 72) | 33 (13, 63) |
| 25 mg | 13 (−21, 42) | 57 (14, 69) | 28 (−10, 60) |
| 50 mg | 7 (−2, 30) | 49 (11, 86) | 18 (4, 61) |
| 100 mg | 13 (−6, 26) | 51 (20, 69) | 29 (−2, 52) |
AHR: airways hyperresponsiveness
Our data raised the question whether the apparent attenuation of the Mch-induced bronchoconstriction by sildenafil had an impact on the lack of an effect of sildenafil on the DI-induced BP and BD. To address this question, we examined whether there was any relationship between the attenuation in the single-dose Mch-induced decrease in FEV1 and the BP and BD indices. In simple regression analyses, in the subjects with AHR, we found a significant negative relationship between the percent change in the single-dose Mch-induced decrease in FEV1 and the BP (r2=0.57, p<0.0001) or the BD (r2=0.33, p=0.002) indices implying that DI-induced BP and BD were diminished in association with the diminished effects of Mch. These relationships were not significant in the healthy group (for BP index, p=0.08; for BD index, p=0.13).
Since we found a significant correlation between BP/BD indices and the change in the single-dose Mch-induced decrease in FEV1 from baseline in the group with AHR, we examined the effect of the sildenafil dose on BP and BD controlling for the change in the single-dose Mch-induced decrease in FEV1 from baseline. We performed two-way ANOVA, one with the BP index and the other with the BD index as the dependent variable. The independent variables in both models were the percent change in the response to Mch with increasing sildenafil dose as a continuous independent variable, and the sildenafil dose as a categorical independent variable. For the BP index in the group with AHR, the overall model was significant (p=0.01). Controlling for the dose of sildenafil in the subjects with AHR, we found a significant relationship between the percent change in the single-dose Mch-induced decrease in FEV1 and the BP (p=0.02). However, controlling for the percent change in the FEV1 response to Mch with sildenafil, the dose of sildenafil was not significantly related to the change in the BP index (p=0.41). For the BD index, the two-way ANOVA model was not significant in the AHR group (p=0.72).
DISCUSSION
To our knowledge, this is the first study examining the effects of a PDE V inhibitor on the function of DI; as part of the study, we also tested the effect of PDE V inhibition on airway responsiveness measured by Mch challenge. We conducted this study to address the hypothesis that cGMP may play a role in the beneficial effects of deep inspiration. We tried to address this hypothesis in an indirect manner, using a PDE V inhibitor (sildenafil) to reduce the natural degradation of cGMP. The finding that sildenafil caused a dose-dependent protection from the direct bronchoconstrictive effects of Mch regardless of the study participants’ condition confounded our ability to test our hypotheses. However, this represents a novel observation that requires attention.
We and others have previously shown that bronchoconstriction by Mch inhalation can be induced to a similar degree in individuals with AHR and in healthy controls in the absence of DI [32] and that a single dose of Mch, after 20 minutes of quiet breathing, can be used to assess the ability of DI to either protect from or reverse the Mch effects [1,13]. Using this well-established methodology, we achieved an almost identical reduction in FEV1 (> 30%) in individuals with AHR and healthy controls, the required median concentration of the single Mch dose being 18.05 mg/ml in the healthy group and 0.75 mg/ml in the group of study participants with conventionally determined AHR. At the highest dose of sildenafil, we observed an approximately 40% attenuation of the Mch-induced fall in FEV1 compared to the challenge in which no sildenafil was administered. We did not observe a difference in this attenuation between the subjects with AHR and the healthy groups at any dose of sildenafil (Figure 2). The protective effect of sildenafil against Mch cannot be explained by an effect on baseline lung function, which remained unaffected by any sildenafil dose. This study was not designed to explore if sildenafil can improve FEV1 because participants with AHR, although they had a history of respiratory symptoms compatible with asthma were asymptomatic and under good control with average FEV1 96% predicted.
The effect of sildenafil on the response to Mch was not an expected finding and, therefore, the protocol did not include conventional multi-dose Mch provocations that would allow the use of common outcomes such at the PC20. It is, therefore, impossible to interpret the magnitude of the effect we observed with a PC20 outcome in mind. This could be the objective of a follow-up study, where sildenafil would be used to reduce Mch responsiveness; in such study the design should focus on a PC20 outcome obtained through multiple dose Mch provocations.
There are limited and conflicting published works examining the effects of PDE V inhibition on airway reactivity, in animal models. Sousa et al. showed that sildenafil relaxed carbachol contracted and OVA-sensitized rat airways in a concentration-dependent manner [33]. In guinea pigs, Toward et al. showed that sildenafil treatment did not affect histamine-induced bronchoconstriction [34]. Clayton et al, showed that sildenafil had no effect on the levels of TNF-α, IL-4 and IL-5 in the BAL of OVA-challenged mice [35]. These differences may be due to the different agonist or species used. Our data are consistent with those of Sousa et al. who found protection against a cholinergic stimulus.
For our primary outcome, the BP index, as well as for the secondary outcome BD index, we found no effect of sildenafil at any dose (Tables 2 and 3). Both indices appeared to worsen with sildenafil particularly in the subjects with AHR, although these effects did not reach statistical significance. To examine whether this “worsening” of the BP and BD indices with sildenafil was secondary to the drug-induced change in the bronchoconstrictive response to Mch, we constructed multi-variate models, which included the sildenafil dose and the percent change in the Mch-induced effects on FEV1. After controlling for the change in the Mch response, sildenafil had no significant effect on the BP and BD indices. This analysis needs to be interpreted with caution because our small sample size substantially reduces the statistical power to detect independent effects of sildenafil on DI-induced BP or BD. We infer from these results that while sildenafil attenuated the Mch-induced bronchoconstriction when DIs were withheld, it did not change the ability of DIs to either protect from or to reverse Mch-induced bronchoconstriction. Notably, we have previously found that the BP and BD effects of DI become more evident when the Mch-induced bronchoconstriction in the absence of DI is of substantial magnitude [31]. The dependence of the effects of DI on the magnitude of bronchoconstriction can not only explain the trends for worsening of the BP and BD effects by sildenafil in this study, but may have also masked any evidence of improvement in BP and BD. In the absence of any trend, however, the latter possibility is remote.
Sildenafil’s inability to improve the beneficial effects of DI in individuals with AHR raises serious doubts as to whether cGMP dysregulation plays a role in the dysfunction of DI in AHR and asthma. These doubts are further strengthened by the fact that, in the absence of DIs, sildenafil did induce bronchoprotection against Mch, but this effect was of equal magnitude in individuals with AHR and the healthy controls. If the ineffectiveness of DI in AHR and asthma was due to reduced production or rapid degradation of cGMP, one would have expected sildenafil to at least partially restore the DI effects. On the other hand, if the problem lied in airway smooth muscle’s resistance to the relaxation effects of cGMP, sildenafil would have not been as effective in protecting the airways from Mch in the study participants with AHR, compared to the healthy controls.
Even if our results are not supportive of the role of cGMP in the beneficial effects of DI, we cannot eliminate the possibility that endogenous NO is playing a role in these effects. Perez-Zoghbi studied the effects of NO donors on agonist-induced airway contraction and Ca2+ signaling of airway SMCs in lung slices. They found that NO-induced SMC relaxation was mediated by two main mechanisms: (1) a cGMP-dependent mechanism in which NO binds to and activates soluble guanyl cyclase to generate cGMP, or (2) a cGMP-independent mechanism in which a functional alteration of protein (i.e., activation) occurs via nitrosylation of thiol groups [28,36,37]. By administering sildenafil, we assume to have by-passed the NO induction of cGMP, although we did not obtain any measurements of cGMP, to relate changes of this mediator to the inhibitory effects of sildenafil on the Mch-induced bronchoconstriction. We cannot rule out a cGMP-independent NO mechanism.
One limitation of the study was the sample size. The number of subjects in each group was small (n=7 and n=8 for the subjects with AHR and healthy group respectively). This raises the possibility that the study was insufficiently powered to detect a difference in BP or BD if there was a real difference. However, an a priori statistical analysis plan also included an interim analysis. The study was terminated early because not only did we not obtain the expected effect size, but also we did not even see a trend towards increased deep inspiration-induced BP in the presence of sildenafil as depicted in Table 2, and the same observation was made for deep inspiration-induced BD (Table 3). Given that we did not even see a trend, if we were to use the interim data to calculate power for a subsequent study, the number of subjects sufficient to demonstrate a significant result would be approaching infinite. In other words, it was futile to continue the study.
In summary, sildenafil significantly reduced the response to a single dose of Mch in individuals with AHR as well as in healthy participants to a similar extent. However, it did not improve the DI-induced BP or BD effects in the subjects with AHR across a range of doses tested. We infer from these results that the mechanism responsible for the diminution or loss of the ability of DI to protect the airways from Mch-induced bronchoconstriction or to reverse bronchoconstriction in people with AHR (including patients with asthma) is unlikely to be due to dysregulation of cGMP. On the other hand, our data raise the possibility that PDE V inhibition may be of value in the management of asthma as an add-on bronchoprotective agent especially in view of some data indicating that long-acting beta-adrenergic agonists may lose their protective ability against induced bronchoconstriction over time [38], This possibility requires further exploration.
Acknowledgments
(G.P.) Recipient of the George Behrakis Hellenic Fellowship in Respiratory Allergy at the Johns Hopkins Asthma and Allergy Center. This work was supported by the National Institutes of Health, grant numbers RO1HL61277, R01HL62698, RO1HL10342, 1F32HL086179-01 and ES003819
REFERENCES
- 1.Scichilone N, Permutt S, Togias A. The lack of the bronchoprotective and not the bronchodilatory ability of deep inspiration is associated with airway hyperresponsiveness. Am J Respir Crit Care Med. 2001;163:413–419. doi: 10.1164/ajrccm.163.2.2003119. [DOI] [PubMed] [Google Scholar]
- 2.Scichilone N, Pygros G, Kapsali T, Anderlind C, Brown RH, Permutt S, Togias A. Airways hyperresponsiveness and the effects of lung inflation. Int.Arch.Allergy Immunol. 2001;124:262–266. doi: 10.1159/000053728. [DOI] [PubMed] [Google Scholar]
- 3.Scichilone N, Togias A. The role of lung inflation in airway hyperresponsiveness and in asthma. Curr Allergy Asthma Rep. 2004;4:166–174. doi: 10.1007/s11882-004-0063-8. [DOI] [PubMed] [Google Scholar]
- 4.Scichilone N, Permutt S, Bellia V, Togias A. Inhaled corticosteroids and the beneficial effect of deep inspiration in asthma. Am J Respir Crit Care Med. 2005;172:693–699. doi: 10.1164/rccm.200407-955OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fredberg J. Frozen objects: small airways, big breaths, and asthma. J Allergy Clin Immunol. 2000;106:615–624. doi: 10.1067/mai.2000.109429. [DOI] [PubMed] [Google Scholar]
- 6.Gunst S, Wu M. Plasticity of airway smooth muscle stiffness and extensibility: role of length-adaptive mechanisms. J Appl Physiol. 2001;90:741–749. doi: 10.1152/jappl.2001.90.2.741. [DOI] [PubMed] [Google Scholar]
- 7.Pyrgos G, Scichilone N, Togias A, Brown RH. Bronchodilation response to deep inspirations in asthma is dependent on airway distensibility and air trapping. J Appl Physiol. 2011;110:472–479. doi: 10.1152/japplphysiol.00603.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Slats AM, Janssen K, van Schadewijk A, van der Plas DT, Schot R, van den Aardweg JG, de Jongste JC, Hiemstra PS, Mauad T, Rabe KF, Sterk PJ. Bronchial inflammation and airway responses to deep inspiration in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:121–128. doi: 10.1164/rccm.200612-1814OC. [DOI] [PubMed] [Google Scholar]
- 9.Gunst SJ, Shen X, Ramchandani R, Tepper RS. Bronchoprotective and bronchodilatory effects of deep inspiration in rabbits subjected to bronchial challenge. J Appl Physiol. 2001;91:2511–2516. doi: 10.1152/jappl.2001.91.6.2511. [DOI] [PubMed] [Google Scholar]
- 10.Brown RH, Mitzner W. Airway response to deep inspiration: role of nitric oxide. Eur Respir J. 2003;22:57–61. doi: 10.1183/09031936.03.00090403. [DOI] [PubMed] [Google Scholar]
- 11.Brown RH, Mitzner W. Airway response to deep inspiration: role of inflation pressure. J.Appl.Physiol. 2001;91:2574–2578. doi: 10.1152/jappl.2001.91.6.2574. [DOI] [PubMed] [Google Scholar]
- 12.Brown RH, Mitzner W. Duration of deep inspiration and subsequent airway constriction in Vivo. J.Asthma. 2003;40:119–124. doi: 10.1081/jas-120017981. [DOI] [PubMed] [Google Scholar]
- 13.Kapsali T, Permutt S, Laube B, Scichilone N, Togias A. The potent bronchoprotective effect of deep inspiration and its absence in asthma. J Appl Physiol. 2000;89:711–720. doi: 10.1152/jappl.2000.89.2.711. [DOI] [PubMed] [Google Scholar]
- 14.Brown RH, Scichilone N, Mudge B, Diemer F, Permutt S, Togias A. High Resolution Computed Tomographic evaluation of airways distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. Am.J.Respir.Crit.Care Med. 2001;163:994–1001. doi: 10.1164/ajrccm.163.4.2007119. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Bhargava V, Mittal RK. Mechanism of stretch-activated excitatory and inhibitory responses in the lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol. 2009;297:G397–G405. doi: 10.1152/ajpgi.00108.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei B, Chen Z, Zhang X, Feldman M, Dong XZ, Doran R, Zhao BL, Yin WX, Kotlikoff MI, Ji G. Nitric oxide mediates stretch-induced Ca2+ release via activation of phosphatidylinositol 3-kinase-Akt pathway in smooth muscle. PLoS One. 2008;3:e2526. doi: 10.1371/journal.pone.0002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silkoff PE, Sylvester JT, Zamel N, Permutt S. Airway nitric oxide diffusion in asthma: Role in pulmonary function and bronchial responsiveness. Am J Respir Crit Care Med. 2000;161:1218–1228. doi: 10.1164/ajrccm.161.4.9903111. [DOI] [PubMed] [Google Scholar]
- 18.De Sanctis GT, MacLean JA, Hamada K, Mehta S, Scott JA, Jiao A, Yandava CN, Kobzik L, Wolyniec WW, Fabian AJ, Venugopal CS, Grasemann H, Huang PL, Drazen JM. Contribution of nitric oxide synthases 1, 2, and 3 to airway hyperresponsiveness and inflammation in a murine model of asthma. J Exp Med. 1999;189:1621–1630. doi: 10.1084/jem.189.10.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feder LS, Stelts D, Chapman RW, Manfra D, Crawley Y, Jones H, Minnicozzi M, Fernandez X, Paster T, Egan RW, Kreutner W, Kung TT. Role of nitric oxide on eosinophilic lung inflammation in allergic mice. Am J Respir Cell Mol Biol. 1997;17:436–442. doi: 10.1165/ajrcmb.17.4.2845. [DOI] [PubMed] [Google Scholar]
- 20.Samb A, Pretolani M, Dinh-Xuan AT, Ouksel H, Callebert J, Lisdero C, Aubier M, Boczkowski J. Decreased pulmonary and tracheal smooth muscle expression and activity of type 1 nitric oxide synthase (nNOS) after ovalbumin immunization and multiple aerosol challenge in guinea pigs. Am J Respir Crit Care Med. 2001;164:149–154. doi: 10.1164/ajrccm.164.1.2004030. [DOI] [PubMed] [Google Scholar]
- 21.Ten Broeke R, Folkerts G, Leusink-Muis T, Van der Linde HJ, Villain M, Manion MK, De Clerck F, Blalock JE, Nijkamp FP. Calcium sensors as new therapeutic targets for airway hyperresponsiveness and asthma. Faseb J. 2001;15:1831–1833. doi: 10.1096/fj.01-0018fje. [DOI] [PubMed] [Google Scholar]
- 22.Belvisi MG, Stretton CD, Yacoub M, Barnes PJ. Nitric oxide is the endogenous neurotransmitter of bronchodilator nerves in humans. Eur J Pharmacol. 1992;210:221–222. doi: 10.1016/0014-2999(92)90676-u. [DOI] [PubMed] [Google Scholar]
- 23.Ellis JL, Undem BJ. Inhibition by L-NG-nitro-L-arginine of nonadrenergicnoncholinergic- mediated relaxations of human isolated central and peripheral airway. Am Rev Respir Dis. 1992;146:1543–1547. doi: 10.1164/ajrccm/146.6.1543. [DOI] [PubMed] [Google Scholar]
- 24.Fischer A, Hoffmann B. Nitric oxide synthase in neurons and nerve fibers of lower airways and in vagal sensory ganglia of man. Correlation with neuropeptides. Am J Respir Crit Care Med. 1996;154:209–216. doi: 10.1164/ajrccm.154.1.8680682. [DOI] [PubMed] [Google Scholar]
- 25.Raeburn D, Souness JE, Tomkinson A, Karlsson JA. Isozyme-selective cyclic nucleotide phosphodiesterase inhibitors: biochemistry, pharmacology and therapeutic potential in asthma. Prog Drug Res. 1993;40:9–32. doi: 10.1007/978-3-0348-7147-1_3. [DOI] [PubMed] [Google Scholar]
- 26.Nijkamp FP, Folkerts G. Nitric oxide and bronchial hyperresponsiveness. Arch Int Pharmacodyn Ther. 1995;329:81–96. [PubMed] [Google Scholar]
- 27.Pelligrino DA, Wang Q. Cyclic nucleotide crosstalk and the regulation of cerebral vasodilation. Prog Neurobiol. 1998;56:1–18. doi: 10.1016/s0301-0082(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Zoghbi JF, Bai Y, Sanderson MJ. Nitric oxide induces airway smooth muscle cell relaxation by decreasing the frequency of agonist-induced Ca2+ oscillations. J Gen Physiol. 2010;135:247–259. doi: 10.1085/jgp.200910365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scichilone N, Morici G, Marchese R, Bonanno A, Profita M, Togias A, Bonsignore MR. Reduced airway responsiveness in nonelite runners. Med Sci Sports Exerc. 2005;37:2019–2025. doi: 10.1249/01.mss.0000178100.76067.e0. [DOI] [PubMed] [Google Scholar]
- 30.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 31.Scichilone N, Kapsali T, Permutt S, Togias A. Deep inspiration-induced bronchoprotection is stronger than bronchodilation. Am J Respir Crit Care Med. 2000;162:910–916. doi: 10.1164/ajrccm.162.3.9907048. [DOI] [PubMed] [Google Scholar]
- 32.Skloot G, Permutt S, Togias AG. Airway hyperresponsiveness in asthma: a problem of limited smooth muscle relaxation with inspiration. J.Clin.Invest. 1995;96:2393–2403. doi: 10.1172/JCI118296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sousa CT, Brito TS, Lima FJ, Siqueira RJ, Magalhaes PJ, Lima AA, Santos AA, Havt A. Sildenafil decreases rat tracheal hyperresponsiveness to carbachol and changes canonical transient receptor potential gene expression after antigen challenge. Braz J Med Biol Res. 2011;44:562–572. doi: 10.1590/s0100-879x2011007500056. [DOI] [PubMed] [Google Scholar]
- 34.Toward TJ, Smith N, Broadley KJ. Effect of phosphodiesterase-5 inhibitor, sildenafil (Viagra), in animal models of airways disease. Am J Respir Crit Care Med. 2004;169:227–234. doi: 10.1164/rccm.200211-1372OC. [DOI] [PubMed] [Google Scholar]
- 35.Clayton RA, Dick CA, Mackenzie A, Nagasawa M, Galbraith D, Hastings SF, MacKenzie SJ. The effect of selective phosphodiesterase inhibitors, alone and in combination, on a murine model of allergic asthma. Respir Res. 2004;5:4. doi: 10.1186/1465-9921-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaston B, Singel D, Doctor A, Stamler JS. S-nitrosothiol signaling in respiratory biology. Am J Respir Crit Care Med. 2006;173:1186–1193. doi: 10.1164/rccm.200510-1584PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saraiva RM, Hare JM. Nitric oxide signaling in the cardiovascular system: implications for heart failure. Curr Opin Cardiol. 2006;21:221–228. doi: 10.1097/01.hco.0000221584.56372.dc. [DOI] [PubMed] [Google Scholar]
- 38.Bhagat R, Kalra S, Swystun VA, Cockcroft DW. Rapid onset of tolerance to the bronchoprotective effect of salmeterol. Chest. 1995;108:1235–1239. doi: 10.1378/chest.108.5.1235. [DOI] [PubMed] [Google Scholar]