Abstract
Rationale
Research suggests that age and sex are vulnerability factors for drug abuse. However, few studies have systematically examined their interaction.
Objective
The purpose of the present study was to examine male and female, adult and adolescent rats under a procedure that measures responding during periods of signaled availability and nonavailability of iv cocaine and food reinforcers.
Methods
Adolescent and adult rats lever pressed for iv infusions of cocaine or food pellets under a procedure with three components of signaled availability of the reinforcer alternating with two components of signaled nonavailability. Adolescent rats were removed and then later retested under the same conditions as adults, and a group of adult rats was also removed and retested after a similar number of days. A subset of rats earning cocaine infusions under the initial test was later retested with food pellets under the same behavioral task to assess the influence of prior cocaine exposure on subsequent responding for a nondrug reinforcer.
Results
Adolescents (vs. adults) made more responses during periods of signaled iv cocaine availability and nonavailabiltiy, and adult females responded more than adult males during these periods. Responding during periods of signaled nonavailability of iv cocaine and food did not differ between the initial and subsequent retest conditions in adult rats. Further, adult males and females exposed to cocaine during adolescence responded more during periods of food availability compared to cocaine-naïve adults.
Conclusion
These results indicate that sex and age are vulnerability factors in cocaine abuse, and cocaine exposure during critical developmental stages can have long-lasting effects.
Keywords: Adolescence, Sex differences, Cocaine, Rats, Cocaine seeking
Introduction
Individuals who abuse drugs early in life also have an increased likelihood of developing a lifelong problem with drug abuse and of suffering from long-term behavioral and cognitive problems (Brunswick and Messeri 1986; Newcomb and Bentler 1987). Thus, it is imperative to gain an understanding of adolescent drug abuse in order to develop and implement effective treatments that can prevent the progression of this disorder into adulthood. There are several features of adolescence that are thought to increase susceptibility to abuse drugs, such as heightened stress reactivity (Chung and Maisto 2006; Lopez et al. 2005; Rao et al. 1999; Spear 2009), increased proclivity to seek natural rewards (Casey et al. 2008; Spencer et al. 2007), and poor inhibitory control (Casey et al. 2008; Spencer et al. 2007). These factors may converge to enhance drug abuse vulnerability and may explain why adolescents, compared to adults, are more likely to initiate and maintain drug use, are more likely to binge and relapse (Winters and Lee 2008), and are more resistant to treatment (Dennis et al. 2004; Perepletchikova et al. 2008; Winters et al. 2000).
Sex differences also exist in drug abuse. Despite a larger number of men vs. women abusing most drugs, the progression from initial use to abuse (Chen and Kandel 2002), from stable intake to bingeing (Mann et al. 2005; Randall et al. 1999), and from abstinence to relapse is more rapid in women compared to men (Ignjatova and Raleva 2009). There is a trend toward equal prevalence of drug abuse in male and female adolescents (SAMHSA 2010); however, relatively little is known as to whether female and male adolescents differ on various aspects of drug abuse in humans.
Results from animal studies lend cross-species support to these findings with humans and suggest that age and sex may interact to produce additive vulnerability. Similar to findings with humans, adolescent rats self-administered greater amounts of several drugs of abuse (Anker and Carroll 2010a; Chen et al. 2007; Fullgrabe et al. 2007; Levin et al. 2007; Shahbazi et al. 2008; Vetter et al. 2007), showed greater persistence in responding for cocaine (cocaine-seeking behavior), and were more sensitive to the facilitating effects of stress on drug seeking (Anker and Carroll 2010a). In further support of clinical work, results from several animal studies indicate that females outperform males across several phases of the drug abuse process that mark transitions in drug use (Anker and Carroll 2010c; Craft 2008; Lynch 2006), and they suggest there are sex differences in drug abuse vulnerability during adolescence. In two recent studies, females (vs. males) and adolescents (vs. adults) acquired drug self administration faster and responded more for drug infusions under a progressive-ratio schedule for both cocaine (Lynch 2008) and nicotine (Lynch 2009). These results suggest that adolescents and females may have a predisposition for drug abuse, but it has yet to be determined if sex and age interact to produce additive vulnerability. One of the aims of the present study was to examine a potential interaction between age and sex on to cocaine seeking and intake.
One way to measure cocaine seeking in animal models is to measure responding during periods of signaled nonavailability of self-administered iv cocaine (Deroche-Gamonet et al. 2004). In a study by Deroche-Gamonet et al. (2004), responding during periods of signaled nonavailability of self-administered cocaine infusions was highly correlated with other addiction-like behaviors such as continuing to respond for cocaine infusions despite an aversive consequence (i.e., infusions paired with foot shock) and high motivation to take drug as measured by a progressive-ratio schedule. These factors strongly predicted behavior in animal models of relapse and cocaine bingeing (Deroche-Gamonet et al. 2004). However, it has not yet been reported whether responding occurs during the beginning (i.e., extinction responding) or the end (i.e., anticipatory/premature responding) of the period of signaled nonavailability of cocaine. In the present study, we sought to differentiate extinction and anticipatory responding by examining the time course of responses occurring during periods of signaled nonavailability of an iv cocaine reinforcer. We expected that responding during the beginning of these periods would reflect an inability to inhibit a previously reinforced response; hence, a measure of extinction responding, while responding occuring at the end of the signaled nonreward periods, would measure anticipation of the upcoming period of iv cocaine availability.
In a recent study, female (vs. male) rats engaged in more responding during a period of signaled nonavailability of self-administered iv cocaine infusions under a procedure similar to that used by Deroche-Gamonet et al. (2004) (Anker et al. 2008). Previous work also indicates that female rats are more likely to engage in binge-like cocaine intake (Roth and Carroll 2004) and are more sensitive to relapse (Kerstetter et al. 2008; Lynch and Carroll 2000) compared to male rats. Together, these results suggest that increased vulnerability to cocaine abuse in females may be associated, in part, with enhanced cocaine seeking during periods of signaled nonavailability of cocaine. However, it is unknown whether age influences cocaine seeking during these periods or whether sex and age interact to produce an additive effect on this measure. Thus, an additional aim of the present study was to assess cocaine seeking during periods of signaled availability and nonavailability of self-administered iv cocaine in adolescent and adult, male and female rats.
The final aim of the present study was to examine long-term effects of adolescent cocaine exposure on behavior reinforced by a drug (cocaine), a nondrug (food pellets) reward, and a sweet saccharin solution. Relatively little is known of the long-term behavioral consequences of adolescent drug exposure. However, results from animal studies indicate that early exposure to stimulants can have a lasting impact on several behavioral measures. For example, previous studies have shown that exposure to stimulants during adolescence increases stress reactivity, produces a depression-like state characterized by reduced intake of natural rewards such as food and sweet solutions (Bolanos et al. 2003, 2008; Carlezon et al. 2003), and increases sensitivity to stimulants during adulthood (Brandon et al. 2001; Brandon et al. 2003; Crawford et al. 2007).
Materials and methods
Subjects
Eighty Wistar rats served as subjects in the present study. Forty-one rats began testing during adolescence [postnatal day (PND) 23; female 18 and male 23], and 39 rats began testing during adulthood (PND 90; female 21 and male 18). Rats were bred at the University of Minnesota from parents obtained from Harlan Sprague–Dawley, Inc. (Madison, WI, USA) and housed in temperature- (24°C) and humidity-controlled rooms under a constant light/dark cycle (12/12 h with room lights on at 6:00 am) where they had ad libitum access to food and water. Female rats are considered adults on PND 55, and males are considered adults on PND 60 (Ojeda et al. 1980; Spear 2000a, b); thus, adolescence was defined as PND 21 to 55 for females and PND 21 to 60 for males. Estrous cycle was not monitored in the present study, and it was allowed to vary across a range of hormonal conditions. During the experiment, rats had free access to water, and they were fed 16 g (females) or 20 g (males) of ground food (Purina Formula 5001, St. Louis, MO, USA) after cocaine sessions. The amount earned during food sessions was subtracted from these totals. During experimental training, rats lived in their operant conditioning chambers. As soon as stability was achieved on the operant tasks, adolescent rats were removed from the operant conditioning chambers and housed in plastic bins until they were retested on the cocaine- or food-maintained procedures as adults at PND 75 (about 30 days). Other adult rats were also removed and housed in plastic bins for an equivalent length of time as a control for repeated testing. The experimental protocol (1008A87755) was approved by the University of Minnesota Institutional Care and Use Committee, and the experiments were conducted in accordance with the Principles of Laboratory Animal Care (National Research Council 2003). All laboratories were accredited by the American Association for the Accreditation of Laboratory Animal Care.
Apparatus
Cocaine and food self administration
Custom-made operant conditioning chambers were used in the present study as previously described (Carroll et al. 2002). A 35-ml syringe pump (Model PHM-100, Med Associates Inc., St. Albans, VT, USA) located adjacent to the chamber delivered response contingent iv cocaine in the cocaine condition. For the food condition, a food hopper (Coulbourn Instruments, Allentown, NJ, USA), mounted to the outside of the chamber, was used to dispense grain-based 45-mg food pellets (PJA1-0045, Research Diets Inc., New Brunswick, NJ, USA) into a recessed food delivery trough. The experiment was programmed, and data were recorded with Med-PC software (MedAssociates, Inc.) and PC computers.
Drugs
Cocaine HCl was supplied by National Institute of Drug Abuse (Research Triangle Institute, Research Triangle Park, NC), dissolved in 0.9% NaCl at a concentration of 1.6 mg cocaine HCl/1 ml saline and refrigerated. Heparin (1 ml/200 ml saline) was added to the cocaine solution to prevent catheter occlusion from thrombin build-up. The flow rate of each cocaine infusion was 0.025 ml/s, and the duration of pump activation (1 s/100 g of body weight) was adjusted to maintain rats at a dosage of 0.4 mg/kg cocaine. The infusion duration was adjusted approximately every 3 days in order to maintain the 0.4 mg/kg cocaine dose during rapid weight gain in the adolescent groups.
Procedure
Surgical procedure
On PND 23 for adolescents (female and male) and 90 for adults, rats were implanted with a polyurethane intravenous catheter by methods described previously (Carroll and Boe 1982) and fitted with a soft plastic infusion harness (C3236, Plastics One, Roanoke, VA, USA). Following the surgical procedure, rats were allowed a 3-day recovery period during which antibiotic and analgesic medications were administered following guidelines outlined by the University of Minnesota IACUC. After the recovery period, catheters were flushed with saline for adolescents or a heparinized saline solution for adults at 8:00 am daily to prevent catheter blockage, and every 3 days, weights were taken at 3:00 pm. Catheter patency was checked every 7 days by injecting a 0.1-ml solution containing ketamine (60 mg/kg), midazolam (3 mg/kg), and saline. If a loss of the righting reflex was not manifest upon this patency check, a second catheter was implanted in the left jugular vein.
Cocaine-maintained operant procedure
Three days following surgery, 27 adolescent (female 12 and male 15) and 29 adult (female 16 and male 13) rats were trained to self-administer 0.4 mg/kg iv cocaine under a fixed ratio 1 (FR 1) schedule of reinforcement during a procedure modified from Deroche-Gamonet et al. (2004) and previously used in Anker et al. (2008). Each session took place from 9:00 to 11:45 am and consisted of three 45-min periods of signaled availability of self-administered iv infusions of cocaine separated by two 15-min periods of signaled nonavailability of iv cocaine. The cocaine availability periods were signaled by the illumination of the house light, and a response on the active lever produced an infusion of cocaine and illuminated stimulus lights above the lever. Responses during an infusion were recorded but had no consequence. A response on the inactive lever illuminated the lever lights only and was considered a measure of general activity. During the cocaine nonavailability periods, the house light was extinguished, and a green LED light above the active lever was lit. Responses on the active lever and the inactive lever did not activate the infusion pump or illuminate the stimulus lights during this period. Initially, the active lever was baited with a small amount of peanut butter (0.5 g), and each session began with three experimenter-delivered cocaine-priming injections. Once rats began self-administering drug, these measures were discontinued. Stability was defined as three consecutive sessions of over 20 infusions earned per session with no increasing or decreasing trend in infusions and a difference of no more than eight infusions across the 3 days. A subset of adolescent (female 6 and male 7) and adult (female 10 and male 6) rats were retested on a food-maintained operant procedure (described below) to assess the effects of previous cocaine exposure on food-maintained responding.
Food-maintained operant procedure
Fourteen adolescent (female 6 and male 8) and 10 adult (female 5 and male 5) rats were trained to earn food pellets. The experimental procedure was identical to that described for cocaine with the exception that rats did not undergo catheterization surgery and did not receive have their lever baited or receive experimenter-delivered cocaine-priming injections. Stability was achieved when rats earned at least 30 food pellets per session over three consecutive sessions with no increasing or decreasing trend in pellets earned. As with the cocaine-maintained procedure, rats were removed from the operant conditioning chamber following stability of operant responding until being retested on the food-maintained procedure approximately 30 days later.
Saccharin preference test
As another test of the effects of cocaine exposure on subsequent intake of a nondrug substance, adult female and male rats that were either exposed (trained to self-administer cocaine) or naïve to cocaine (trained to self-administer food) during adolescence or adulthood were allowed to drink several concentrations of a saccharin solution. When rats reached stability during the retest condition under either the cocaine- or food-maintained operant procedure, they were again removed from the operant conditioning chambers. Under conditions of food satiation, rats began a series of saccharin preference tests 48 h following the final stable session. In order to obtain a dose–response function of saccharin preference, four saccharin concentrations (0.03%, 0.1%, 0.3%, and 0.6% w/v) were presented concurrently with water. Each concentration was presented one at a time in nonsystematic order for three consecutive days, and intake of saccharin solutions was compared to intake of water alone to calculate a saccharin preference score [(24 h saccharin intake ml–24 h water intake (ml))/(body wt. (grams)×100)] as described previously (Anker et al. 2008; Badia-Elder et al. 1996; Carroll et al. 2008).
Data analysis
The primary dependent measures were reinforced and nonreinforced active lever responses, infusions, food intake (number of pellets), inactive lever responses, and saccharin score. Dependent measures were averaged across 3 days of stable responding, compared between groups, and analyzed using separate two-factor analyses of variance (ANOVAs) with sex and age as the between-subject factors. Mixed-factor ANOVAs with sex as the between-subjects factor and testing condition as the repeated measure (test vs. retest) were used to compare rats that were retested.
Active-lever responses during the first and last 5 min of each of the three periods of signaled availability of iv cocaine or food and the first and last 5 min for the two signaled periods of nonavailabiltiy to each reinforcer were analyzed using separate two-factor repeated-measures ANOVAs with period (e.g., first, second, and last period of cocaine or food availability or first and last period of nonavailabilty of the reinforcer) and 5 min (first or last) as the repeated measures. This was done to determine if there were any within-group differences in responding between different periods of cocaine or food availability and nonavailability and to identify any group trends in responding between the beginning vs. the end of each condition. Saccharin scores were analyzed using a three-factor ANOVA with sex and reinforcer history (food or cocaine) as the between-subjects factors and saccharin concentration as the within-subjects factor. Post hoc tests were conducted using Fisher’s least significant difference protected t tests. All data analyses were conducted using GB Stat (Dynamic Microsystems, Inc., Silver Spring, MD, USA).
Results
Table 1 shows the mean number of days for adolescent and adult rats to reach stability under both reward conditions in addition to the mean age, food intake, and weight during the three stable days during the initial and retest conditions under the behavioral procedures. Results from a mixed-factor ANOVA indicated a significant main effect of sex (F1, 167 =7.00, p <0.01), age (F1, 167=18.28, p <0.01), and condition (food or cocaine) (F1, 167 =36.00, p <0.01), and a significant interaction between age and reinforcer (food or cocaine) condition (F1, 167 =36.00, p<0.01) on the number of days for rats to reach stability. Post hoc analyses indicated that adolescents took significantly fewer days to reach cocaine self-administration than adults (p<0.05) and that adult rats reached the acquisition criteria faster for food than for cocaine (p<0.05). In addition, adult rats acquired the operant task faster during retesting for both cocaine infusions and food pellets (p<0.05), but there was no difference between the test and retest conditions for adolescents. Comparison of food intake between adolescent and adult rats indicated that all adult female and male rats consumed more food than their adolescent counterparts (p< 0.05) with the exception of the adolescent female groups compared to their retest conditions. Weights also differed between groups. All adults weighed more than adolescents, and adult males weighed more than adult females (p< 0.025). There was no significant difference in weight due to reinforcer history for either adolescents or adults.
Table 1.
Mean (±SEM) days to stability, days between retest, age at stability (days), mean weight (grams), and mean intake (grams) for the 3 days of stability for each group and condition
| Cocaine
|
Food
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adolescent → adult
|
Adult → adult
|
Adolescent → adult
|
Adult → adult
|
||||||
| Female | Male | Female | Male | Female | Male | Female | Male | ||
| First test | Days to stability | a16.5 (3.9) | a20.1 (1.8) | 34.0 (8.0) | 28.9 (3.7) | 13.8 (1.4) | 13.3 (1.7) | b14.4 (2.7) | b11.8 (2.4) |
| Age (days) | 50.0 (2.1) | 49.8 (1.9) | 143.2 (14.6) | 123.7 (4.9) | 43.0 (4.2) | 40.1 (1.2) | 98.6 (2.3) | 102.0 (4.3) | |
| Weight (g) | 159.5 (11.2) | 155.4 (12.4) | 295.3 (10.7) | 391.6 (20.4) | 144.8 (17.0) | 146.3 (10.6) | 297.3 (5.0) | 383.8 (6.1) | |
| Food intake (g) | 14.2 (0.8) | 15.3 (0.7) | 15.1 (0.7) | 19.2 (0.5) | 15.8 (0.2) | 20.0 (0) | 16.0 (0) | 19.2 (0.8) | |
| Retest | Days to retest | 28.0 (3.2) | 34.3 (4.1) | 30.0 (1.9) | 28.0 (3.7) | 35.7 (2.1) | 33.0 (3.8) | 23.8 (5.8) | 34.8 (2.6) |
| Days to stability | 15.0 (3.6) | 19.8 (6.2) | c20.8 (3.7) | c14.6 (2.6) | 7.0 (1.8) | 7.1 (0.9) | c5.2 (0.7) | c10.4 (4.2) | |
| Age (days) | 96.5 (3.1) | 102.6 (6.7) | 193.4 (14.1) | 166.0 (3.5) | 84.2 (2.2) | 90.3 (7.2) | 127.0 (6.3) | 144.4 (3.5) | |
| Weight (g) | 278.7 (15.9) | 390.8 (26.8) | 333.2 (15.0) | 471.1 (21.4) | 270.3 (10.0) | 401.8 (31.5) | 334 (13.5) | 495.2 (13.7) | |
| Food intake (g) | 14.2 (0.8) | 18.8 (0.3) | 14.1 (1.4) | 18.4 (0.7) | 16.0 (0.0) | 20.0 (0) | 16.0 (0) | 20.0 (0) | |
Adolescent rats reached stability for cocaine self-administration faster than adults (between- and within-subject comparisons) (p<0.05)
Adult rats took significantly fewer days to reach stability for food than for cocaine (p<0.05)
Adults took significantly fewer days to reach acquisition of lever pressing for iv cocaine and food under the retest vs. the initial testing conditions (p<0.05)
Cocaine condition
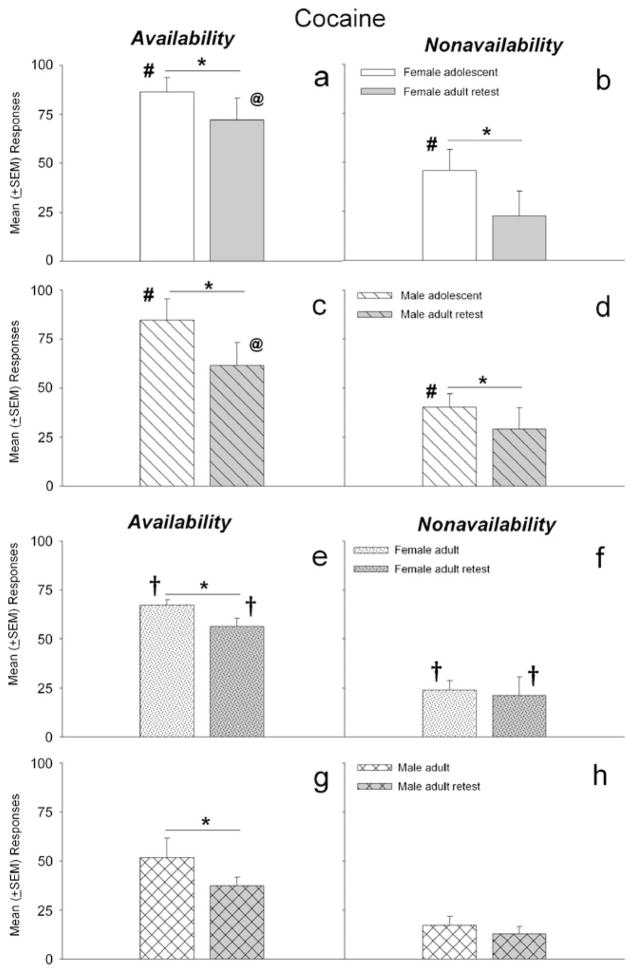
Figure 1 depicts the mean number of responses during periods of signaled availability (left panels) and nonavailability (right panels) of self-administered iv infusions of cocaine. Adolescent female and male rats responded more during periods of cocaine availability than when they were retested under this condition during adulthood (F1, 59=8.32, p<0.01) (Fig. 1a, c), and they made more cocaine-reinforced responses than a separate group of adult rats (F1, 58 =10.67, p<0.01) (Fig. 1a, c vs Fig. 1e, g). In addition, adult rats with previous cocaine testing during adolescence made more reinforced responses than adult rats that received initial cocaine testing as an adult (F1, 27=6.89, p<0.05) (Fig. 1a, c vs Fig. 1e, g). Adult female and male rats also showed diminished reinforced responding during the retest (F1, 57 =14.10, p<0.01) (Fig. 1e, g). There were no sex differences in reinforced responding in the adolescent groups. In contrast, adult females made more responses during periods of signaled availability of iv cocaine than adult male during the initial and subsequent retest (F1, 57 =6.26, p<0.05) (Fig. 1e, g). There were no significant differences in mean cocaine-reinforced responses between adults that were previously tested during adolescence and adults that received their initial testing during adulthood. Thus, adolescents responded more than adults for iv infusions of cocaine and adult (but not adolescent) females responded more than males.
Fig. 1.
Mean (±SEM) responses during signaled availability and nonavailability periods for cocaine-maintained operant sessions. Left panels (a, c, e, and g) depict responding for cocaine under signaled availability, while right panels (b, d, f, and h) depict responses under signaled nonavailability conditions. Panels a–d compare responding maintained cocaine by females and males during adolescence to responses when retested as adults 30 days later. Panels e–h depict responses for adult females and males during initial testing and during a retest 30 days later. Both adolescents and adults responded more during the availability period during the initial test vs. the retest (a, c, e, and g; *p<0.01), and adolescents also responded more during the signaled nonavailability period during the initial test vs. the retest (b and d; *p<0.01). Adolescents responded more during the availability and nonavailability periods than adults during the initial test (a and c vs. e and g and b and d vs f and h; #p<0.01), and among the adults, females responded more than males during both signaled availability and nonavailability periods for both the initial test and retest (e and f vs. g and h; †p<0.05). Adults with adolescent cocaine exposure made more cocaine-reinforced responses than rats that received initial cocaine exposure as adults (a and c vs e and g; @p<0.01)
The right panels of Fig. 1 shows the mean responses during periods of signaled nonavailability of cocaine by female and male adolescent and adult rats. Adolescent female and male rats made significantly more nonreinforced responses as adolescents compared to when they were retested as adults (F1, 59 =7.24, p <0.05) (Fig. 1b, d) and when they were compared to adults in a separate group (F1, 58 =9.38, p <0.01) (Fig. 1b, d vs Fig. 1f, h). In contrast, there were no differences in responding during signaled nonavailability of cocaine by rats that were tested as adults compared to when they were retested 30 days later. As with reinforced responding, adult females made more responses during periods of cocaine nonavailability than adult males (F1, 57=7.41, p <0.05) (Fig. 1f vs h), and this difference was not observed in the adolescent rats (Fig. 1b vs d). There were also no differences between responses during signaled nonavailability of cocaine by adult rats that were tested during adolescence compared to the separate group of adult rats. Thus, adolescents made more nonreinforced responses relative to adults, and adult females made more nonreinforced active-lever responses than adult males. This was not due to a practice effect as adults responded similarly during nonreinforced periods when they were retested.
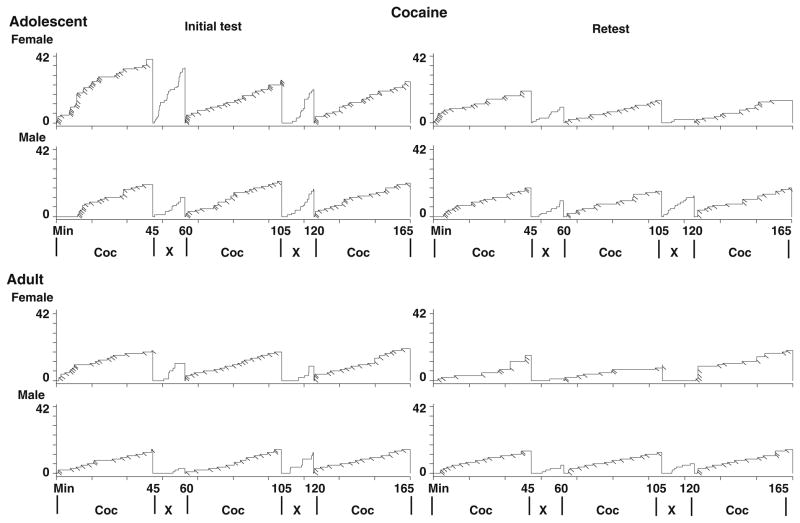
Figure 2 shows representative cumulative records from individual rats during signaled availability and nonavailability of self-administered iv cocaine. Comparison of the first and last 5 min of responding during the first, second, and third periods of cocaine availability indicated that groups responded similarly during each period. However, responding differed between the beginning vs the end of each cocaine availability period with significantly more responses occurring during the first 5 min compared to the last 5 min for all groups (F2, 131=10.06, p<0.01). This indicates that groups loaded on cocaine infusions during the beginning of each period of cocaine availability and subsequently diminished their cocaine intake towards the end of the period.
Fig. 2.
Representative cumulative records of responses (vertical steps) and infusions (downward deflections) during cocaine-maintained operant sessions for adolescents (upper panels) and adults (lower panels) during the initial test and retest
Figure 2 also indicates a trend showing that a majority of responses during periods of signaled nonavailabilty of iv cocaine occurred toward the end of the period compared to the beginning. Analysis of responses during the first and last 5 min for each of these periods indicated that male adolescents and male and female adults responded more during the last 5 min compared to the first 5 min of signaled nonavailability of cocaine (F1, 87=33.44, p <0.01). When retested under the same experimental conditions 30 days later, all groups exhibited a similar trend toward heightened responding during the end of the period of cocaine nonavailability; however, this result was not significant (females, p=0.08; males, p=0.07) (Fig. 2).
Compared to the separate group of adults, adolescents made significantly more inactive lever responses (p<0.05), but they made a comparable number of inactive lever responses when retested as adults (data not shown). Results with cocaine infusions parallel the results with reinforced responses and indicate that adolescents (vs. adults) and adult females (vs. adult males) showed greater cocaine intake.
Food condition
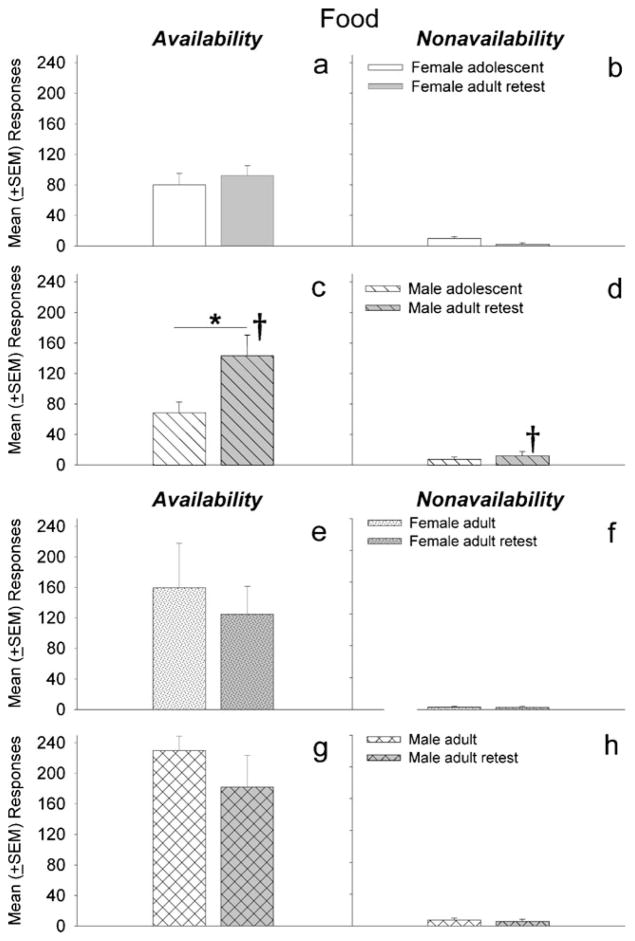
Figure 3 shows the mean number of active lever responses for food pellets during signaled food availability (left panels) and nonavailability (right panels) by female and male adolescent and adult rats. Analysis of reinforced responses during adolescence and later during adulthood indicated a significant effect of age (F1, 27 =13.00, p<0.01) (Fig. 3a, c). There was a significant interaction between sex and age (F1, 27 =6.69, p<0.05), and a subsequent post-hoc test revealed that adult males responded more during periods of food availability than when they were adolescents (p<0.01) and compared to adult females (p<0.05) (Fig. 3a, c). A similar comparison in the adult group that was retested approximately 30 days later indicated no significant sex or retest differences nor an interaction between the two factors (Fig. 3e, g). Between-subject comparisons of adolescents and a separate group of adults indicated that adults responded more than adolescents during periods of food availability (p<0.05), but there were no significant sex differences. Adults that were previously tested during adolescence responded a comparable number of times during the food availability periods as adults that began the study as adults. Results from the food condition suggest that enhanced responding during periods of nonavailability of a reinforcer in the adolescent (vs. adult) and adult female (vs. adult male) groups is specific to the cocaine condition under this procedure.
Fig. 3.
Mean (±SEM) responses during the signaled availability and nonavailability periods for food-maintained operant sessions. Left panels (a, c, e, and g) depict responding for food under signaled availability conditions, while right panels (b, d, f, and h) depict responses under signaled nonavailability conditions. Panels a–d compare responding maintained by food for females and males during adolescence to responses when retested as adults 30 days later. Panels e–h depict responses for adult females and males during initial testing and during a retest 30 days later. Males responded more during the signaled availability period when retested as adults vs. their initial test as adolescents (c; *p<0.05) and also responded more during the signaled availability and nonavailability periods than females retested as adults (c and d vs. a and b; †p<0.01)
For responses (right panels) during periods of signaled nonavailabilty of food, there were no significant main effects, but there was a significant interaction between sex and testing condition/age in adolescent rats that received retesting as adults (F1, 27 =6.20, p <0.05). Post hoc analyses indicated that adult males made more nonreinforced responses on the active lever than adult females (p<0.01) (Fig. 2d). However, there were no differences in non-reinforced responding between the test and retest conditions in adult female and male rats (Fig. 2f, h). Responding during periods of availability and nonavailability of a reinforcer under the iv cocaine and food conditions was compared across subjects, and rats from all groups made more responses during signaled nonavailability of iv cocaine than food (F1, 75=17.41, p <0.01).
Rats earned fewer food pellets during adolescence than when they were retested as adults (F1, 27=13.36, p<0.01) (data not shown); however, there were no sex differences in this measure in adolescents. In adults, there were no differences in the number of pellets earned during the test and retest conditions, but adult male rats earned more food pellets than adult female rats (F1, 19 =5.40, p<0.05). All food pellets were eaten during the food condition. In addition, there were no age/testing or sex differences in inactive lever responding for food pellets (data not shown).
Responding for cocaine and retest with food
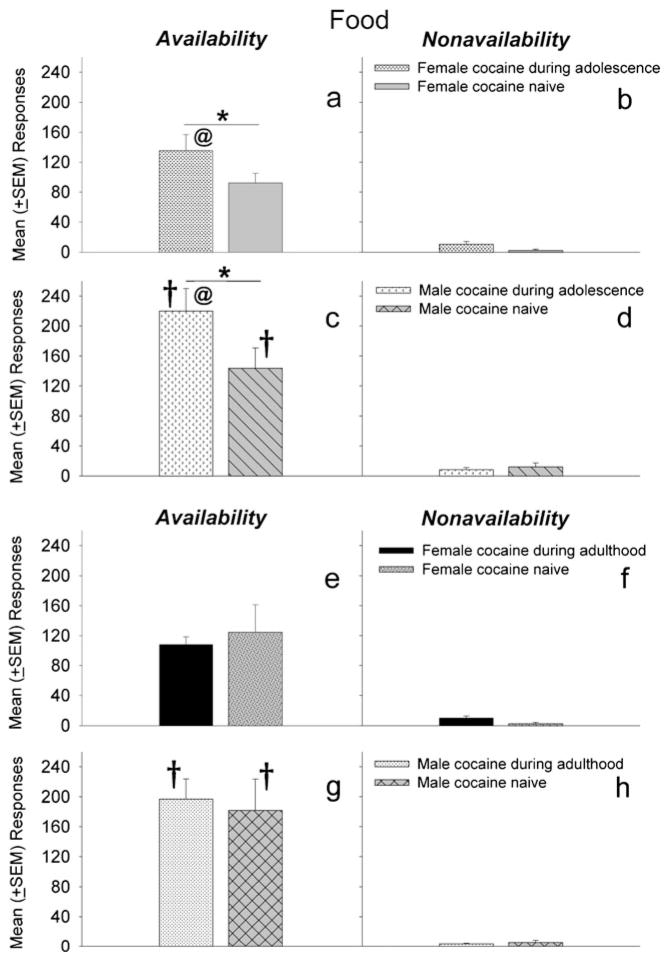
Figure 4 shows the mean number of active lever responses during signaled availability (left panels) and nonavailability (right panels) of food by rats that either responded for food or iv cocaine as adolescents or adults during initial testing. Results indicated that adult female and male rats exposed to cocaine during adolescence responded more during periods of food availability than adults that previously earned food pellets as adolescents (F1, 26 =5.55, p<0.05) (Fig. 4a, c), and males made more reinforced responses than females (F1, 26 =7.23, p <0.05) (Fig. 4c vs a). In contrast, in adult rats, there were no differences in reinforced responses due to reinforcer history (iv cocaine vs food) (Fig. 4e, g); however, males responded more than females during periods of food availability (Fig. 4g vs e) (F1, 25=7.64, p<0.05). There were no significant differences in responding during periods of nonavailability for food between any of the groups (Fig. 4b, d, f, and h). Inactive lever responding was comparable between all groups (data no shown). These data indicate that rats exposed to cocaine during adolescence (but not during adulthood) made more reinforced responses for food pellets during adulthood than their cocaine-naïve food-trained counterparts.
Fig. 4.
Mean (±SEM) responses during the signaled availability and nonavailability periods for food-maintained operant sessions. Left panels (a, c, e, and g) depict responding maintained by food under signaled availability conditions, while right panels (b, d, f, and h) depict responses under signaled nonavailability conditions. Panels a–d compare responding for food pellets between groups of adult rats that earned cocaine or food pellets (cocaine-naïve) during adolescence. Panels e–h compare responding between groups of adult rats that earned cocaine or food pellets (cocaine-naïve) earlier in adulthood. The cocaine-naïve groups are the same as the food retest groups in Fig. 3 and are renamed. Adult females and males exposed to cocaine during adolescence responded more for food during the signaled availability period than cocaine-naïve adult females and males (a and c; *p<0.05). Further, males, whether cocaine-naïve or cocaine-exposed during adolescence or adulthood, responded more than females during the signaled availability period (c and g vs. a and e; †p<0.05). Adult female and male rats with adolescent cocaine exposure make more reinforced responses than females and males that were exposed to cocaine as adults (a and c vs. e and g; @p<0.05)
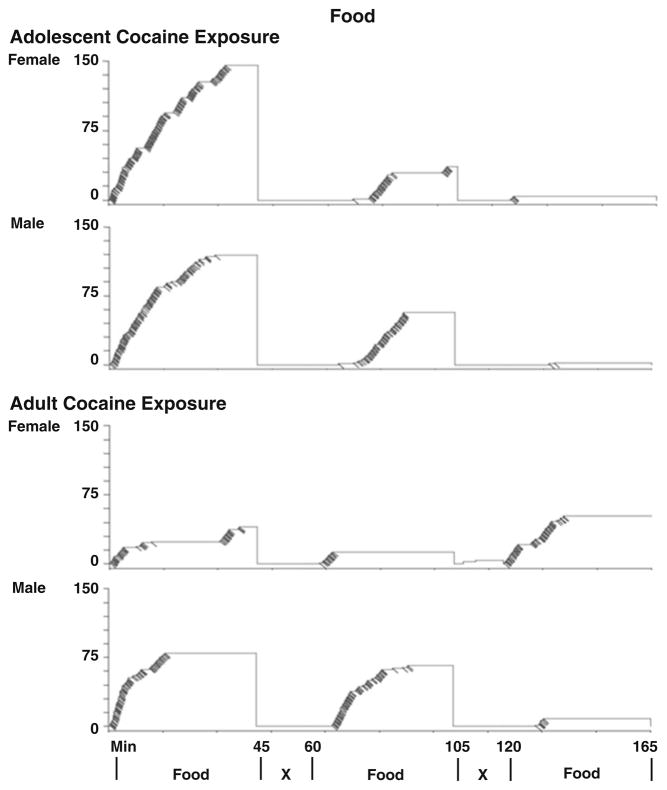
Overall, the topography of responding for food was different than that for cocaine (Fig. 2 vs Fig. 5). The results of the ANOVAs indicated that all groups responded more during the first and second periods of food availability compared to the final signaled food availability period (p< 0.05). In addition, all rats responded more during the first 5 min of the signaled food availability period compared to the last 5 min. Rats responded very little during the signaled food nonavailability periods which may be explained by food satiation during the initial food availability periods. Analysis of responses during the two periods of signaled nonavailability of food indicated no significant differences between the periods.
Fig. 5.
Representative cumulative records of responses (vertical steps) and infusions (downward deflections) during food-maintained operant sessions for adults with previous cocaine exposure during adolescence (upper panels) or adulthood (lower panels)
Saccharin testing
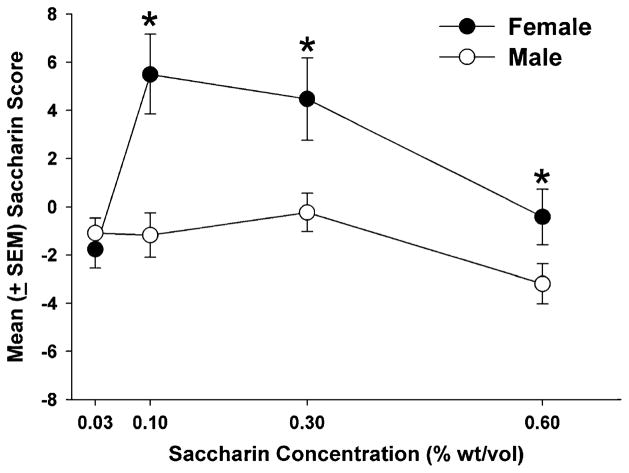
Following the final operant condition, rats were tested for saccharin consumption with four different concentrations of saccharin (0.03%, 0.1%, 0.3%, and 0.6% w/v) to examine how reinforcer history (food vs. cocaine), age of cocaine exposure (adolescent vs. adult), sex, and saccharin concentration influenced saccharin intake. Initial results indicated no significant main effects for reinforcer history or age of cocaine exposure on saccharin intake. However, there was a significant effect of sex (F1, 307 =9.67, p <0.01) and saccharin concentration (F3, 307 =29.77, p <0.01) and a sex X saccharin concentration interaction (F3, 307 =17.63, p < 0.01). Given the lack of an effect of reinforcer history and age, these data were combined and a mixed-factor ANOVA comparing sex and saccharin concentration was conducted. This yielded a significant effect of sex (F1, 307=5.93, p< 0.05), saccharin concentration (F3,307=17.72, p<0.01), and a significant sex X saccharin concentration interaction (F3, 307 =9.19, p<0.01). Post hoc tests indicated that at the three highest saccharin concentrations (0.1%, 0.3%, and 0.6%) females achieved higher saccharin preference scores than males (ps<0.01) (Fig. 6). Also, females consumed less of the highest (0.6%) and lowest (0.03%) concentrations of saccharin compared to the two middle concentrations (0.1% and 0.3%) (p<0.05), while male rats consumed the least amount of the highest concentration of saccharin compared to all lower concentrations (p<0.05). Overall, females and males preferred the 0.1% saccharin concentration to all others (p<0.05).
Fig. 6.
Mean (±SEM) saccharin preference scores at 0.03%, 0.10%, 0.30%, and 0.60% (w/v) saccharin solutions for both females and males. Females had significantly higher saccharin preference scores than males at the 0.10%, 0.30%, and 0.60% saccharin concentrations (*p<0.01)
Discussion
The interaction between age (adolescent vs. adult) and sex was examined on cocaine- and food-seeking behavior under intermittent conditions of signaled availability and non-availability of iv cocaine and food pellets. Adolescents (vs adults) and females (vs males) made more reinforced responses for cocaine, earned more cocaine infusions, and engaged in more bouts of cocaine-seeking behavior during signaled nonavailability of cocaine. However, these differences did not extend to the food condition.
These results suggest that adolescents are more vulnerable to cocaine self-administration than adults. The present finding supports a growing body of animal literature indicating that adolescents are more sensitive to the behavioral effects of cocaine and other stimulants. For example, results from previous studies indicate that adolescent rats were more sensitive to the psychomotor-activating effects of cocaine (Caster et al. 2005; Parylak et al. 2008; Snyder et al. 1998) and showed greater cocaine-induced conditioned place preference (Badanich et al. 2008; Brenhouse and Andersen 2008; Brenhouse et al. 2008; Zakharova et al. 2009) than adults. In addition, adolescent rats were more likely to acquire (Perry et al. 2007) and reinstate (Anker and Carroll 2010a) cocaine-seeking behavior, and they self-administered greater amounts of cocaine (Anker and Carroll 2010a) and amphetamine (Shahbazi et al. 2008) than adult rats. In other self-administration studies that used shorter periods of drug access or larger doses of cocaine, there were no differences in cocaine self-administration between adolescent and adult rats (Kantak et al. 2007; Kerstetter and Kantak 2007; Li and Frantz 2009; Schramm-Sapyta et al. 2009). Previous work indicates that long periods of drug access and smaller doses of cocaine are more likely to reveal individual differences (e.g., phenotype and sex) (Carroll et al. 2008, 2009; Lynch and Carroll 1999; Roth et al. 2004), and this may explain differential results between the adolescent studies.
Adolescents made more active-lever responses during periods of signaled nonavailability of cocaine than adults. This is in line with recent evidence indicating that male adolescent (vs. adult) rats were more resistant to extinction of lever pressing that was measured over several days and was previously reinforced with iv cocaine (Anker and Carroll 2010a). Similarly, under the conditioned place preference paradigm, adolescent (vs. adult) rats required several more sessions to extinguish cocaine-induced conditioned place preference (Brenhouse and Andersen 2008). Others have shown that adolescents (vs. adults) are more resistant to extinction of lever pressing previously maintained by food pellets under a delay-discounting procedure (Adriani and Laviola 2003). These results suggest that adolescents may have an impaired ability to discriminate between conditions of signaled availability and nonavailability of a reinforcer. However, results from the present study indicated no effect of age on responding during signaled nonavailability of a food reinforcer. Others have shown a lack of an age difference in responding for nondrug reinforcers. For example, in a recent study by Li and Frantz (2009), there were no differences between adolescent and adult rats on the reinstatement of cue-induced sucrose responding. Thus, impaired discriminatory control of previously reinforced responding in adolescents may be reinforcer-specific. An additional explanation for increased cocaine seeking in adolescent (vs. adult) rats may be that adolescent rats were more active than adults. However, during the food condition in the present study, there were no age differences in reinforced and non-reinforced responding, suggesting that adolescents were engaged in goal-directed (i.e., cocaine-seeking) behavior.
During adolescence, the frontal cortical system that mediates behavioral inhibition is underdeveloped, as indicated by a slower onset of dendritic pruning in the frontal cortex, and the mesolimbic dopamine system that is involved in reward salience is overdeveloped (Casey et al. 2008). This may account for increased responding during periods of signaled nonavailability of cocaine in adolescent rats. According to Casey et al. (2008), the lag in the maturation of the inhibitory system compared to the reward system results in behavior that is unhibited and largely driven by the salience of the reward.
Responding during periods of signaled nonavailability of a reinforcer is considered a measure of an inability to inhibit a previously reinforced response (Anker et al. 2008). In previous studies, we have described nonreinforced responding following cocaine self administration under a similar procedure in such terms and attributed it to poor discriminatory control of behavior following the presentation of a cue that signals nonavailability of a reinforcer. Thus, in the present study, it was assumed that responses would be higher at the beginning of each period of signaled nonavailability of iv cocaine and would subsequently extinguish. However, a microanalysis of the response topography suggested a more anticipatory-like response during these periods, especially in the adults. Cumulative record data indicated that responding during periods of cocaine nonavailability primarily occurred during the last 5 min of the period compared to the first 5 min. The topography of this responding was similar to the scalloped effect produced by a fixed-interval 15 min period nonavailability of iv cocaine employed in other studies (Arroyo et al. 1998) and suggests that instead of measuring differences in resistance to extinction, this procedure measures anticipatory cocaine-seeking behavior or premature responding. Furthermore, both female and male adult rats engaged in more anticipatory responding at the end (vs. the beginning) of each 15 min period of cocaine nonavailability, while only males from the adolescent group did so. In a recent clinical study, activation of the ventral tegmental area (a structure implicated in assessing reward salience) was higher in anticipation of an upcoming reward under an operant task in adult compared to adolescent humans (Geier et al. 2010). This suggests that adults may be more sensitive than adolescents to time as it relates to the probability of an expected reward.
Chronic exposure to drugs of abuse (Carlezon et al. 2003; Ersche et al. 2008; Guerriero et al. 2006; Wiley et al. 2009) or even a single exposure to a large dose of drug (Caster et al. 2007) can result in long-term changes in behavior maintained by drug and nondrug reinforcers. In the present study, adults with adolescent cocaine exposure made more cocaine-reinforced responses than their counterparts that received initial cocaine exposure as adults, suggesting a sensitization effect. Similar results were seen in adult rats in the food condition. Adult female and male rats with adolescent cocaine exposure made more reinforced responses and earned more food pellets than females and males that were exposed to cocaine as adults. Similar findings were obtained in previous studies showing that exposure to stimulants during adolescence produced long-term sensitization to cocaine (Griggs et al. 2010; Guerriero et al. 2006) and amphetamine (Burton et al. 2010) and altered responding for natural rewards such as food (Bolanos et al. 2003). Together, these results suggest that adolescence marks a period of increased sensitivity to the long-term effects of stimulants on addiction-like behaviors maintained by both drug and nondrug reinforcers.
In the present study, adult females responded more during periods of signaled availability and nonavailability of iv cocaine compared to adult males. Similar results were reported in a recent study in which female rats responded significantly more than male rats during periods of iv cocaine availability and nonavailability under a similar behavioral task (Anker et al. 2008). Together, these findings support other studies reporting enhanced cocaine seeking by female rats during several phases of the drug abuse process, such as acquisition (Anker and Carroll 2010c; Jackson et al. 2006; Lynch 2008; Lynch and Carroll 1999), escalation (Roth and Carroll 2004), extinction (Kerstetter et al. 2008), and reinstatement (Kerstetter et al. 2008; Lynch and Carroll 2000) (for review, see Anker and Carroll 2010c). Several studies indicate that female gonadal hormones mediate cocaine-seeking behavior (for reviews, see Anker and Carroll 2010c; Becker and Hu 2008; Carroll et al. 2009); however, in the present study, the estrous cycle was allowed to vary freely. Future studies may benefit from examining the effects of estrous cycle phase on cocaine seeking during periods of signaled nonavailability of cocaine.
Given that sex, age, and/or previous exposure to stimulants modulate saccharin and/or sucrose consumption (Bolanos et al. 2003; Wiley et al. 2009), an additional aim was to compare males and females with differential reinforcer history (e.g., adults earning cocaine during adolescence vs. adulthood) on saccharin intake. In a previous study, rats that had a history of cocaine self administration consumed less 0.1% saccharin than rats with no history of cocaine self administration (Anker et al. 2008). Long-term decreases in saccharin intake in rats previously exposed to cocaine are thought to be an indicator of anhedonia (Ferguson and Boctor 2010). Results of the present study did not support this finding and indicated no differences between cocaine-exposed and -naïve rats, regardless of whether they were exposed during adolescence or adulthood. However, consistent with an earlier study (Anker et al. 2008), we observed that females preferred higher concentrations of saccharin than males, while both groups equally preferred the lowest concentration of saccharin. Other studies have reported similar sex differences in sweet preference in rats (Carroll et al. 2008; Valenstein et al. 1967).
In summary, the present results indicated that adolescents engaged in more cocaine-seeking (but not food-seeking) behavior during periods of signaled availability and nonavailability of iv cocaine than adults, and females responded more than males during these periods. Early exposure to cocaine during adolescence had long-term consequences on behavior maintained by cocaine and food. Taken together, the results from the present study indicate that age (adolescence) and sex (females) are vulnerability factors in drug abuse, and exposure to cocaine during early development can have long-term effects on behavior maintained by rewards.
Acknowledgments
This research was supported by the National Institute on Drug Abuse grants, R01 DA 003240-27, R01 DA019942-3, K05 015267-08 (MEC), and F31 DA 023301-02 (JJA). The authors would like to thank Thomas Baron, Luke Gliddon, Nathan Holtz, Seth Johnson, Emily Kidd, Brandon Knight, Brianna Lubben, Paul Regier, Amy Saykao, Matthew Starr, Rachael Turner, Troy Velie, and Jeremy Williams for their technical assistance.
References
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with D-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacol Berl. 2010a;208:211–222. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010b;107:264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Gliddon LA, Carroll ME. Impulsivity on a Go/No-go task for intravenous cocaine or food in male and female rats selectively bred for high and low saccharin intake. Behav Pharmacol. 2008;19:615–629. doi: 10.1097/FBP.0b013e32830dc0ae. [DOI] [PubMed] [Google Scholar]
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacol Berl. 1998;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Maldonado AM, Kirstein CL. Early adolescents show enhanced acute cocaine-induced locomotor activity in comparison to late adolescent and adult rats. Dev Psychobiol. 2008;50:127–133. doi: 10.1002/dev.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia-Elder N, Kiefer SW, Dess NK. Taste reactivity in rats selectively bred for high vs. low saccharin consumption. Physiol Behav. 1996;59:749–755. doi: 10.1016/0031-9384(95)02131-0. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolanos CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Willey MD, Maffeo ML, Powers KD, Kinka DW, Grausam KB, Henderson RP. Antidepressant treatment can normalize adult behavioral deficits induced by early-life exposure to methylphenidate. Biol Psychiatry. 2008;63:309–316. doi: 10.1016/j.biopsych.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, White FJ. Adolescent exposure to methylphenidate alters the activity of rat midbrain dopamine neurons. Biol Psychiatry. 2003;54:1338–1344. doi: 10.1016/s0006-3223(03)00787-x. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122:460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunswick AF, Messeri P. Drugs, lifestyle, and health: a longitudinal study of urban black youth. Am J Public Health. 1986;76:52–57. doi: 10.2105/ajph.76.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CL, Nobrega JN, Fletcher PJ. The effects of adolescent methylphenidate self-administration on responding for a conditioned reward, amphetamine-induced locomotor activity, and neuronal activation. Psychopharmacol Berl. 2010;208:455–468. doi: 10.1007/s00213-009-1745-7. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Boe IN. Increased intravenous drug self-administration during deprivation of other reinforcers. Pharmacol Biochem Behav. 1982;17:563–567. doi: 10.1016/0091-3057(82)90319-7. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ. Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav. 2010;58:44–56. doi: 10.1016/j.yhbeh.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacol Berl. 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK. Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol. 2008;19:435–460. doi: 10.1097/FBP.0b013e32830c3632. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anker JJ, Perry JL. Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend. 2009;104(Suppl 1):S70–S78. doi: 10.1016/j.drugalcdep.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. Enhanced behavioral response to repeated-dose cocaine in adolescent rats. Psychopharmacol Berl. 2005;183:218–225. doi: 10.1007/s00213-005-0159-4. [DOI] [PubMed] [Google Scholar]
- Caster JM, Walker QD, Kuhn CM. A single high dose of cocaine induces differential sensitization to specific behaviors across adolescence. Psychopharmacol Berl. 2007;193:247–260. doi: 10.1007/s00213-007-0764-5. [DOI] [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32:700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Chen K, Kandel D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend. 2002;68:65–85. doi: 10.1016/s0376-8716(02)00086-8. [DOI] [PubMed] [Google Scholar]
- Chung T, Maisto SA. Relapse to alcohol and other drug use in treated adolescents: review and reconsideration of relapse as a change point in clinical course. Clin Psychol Rev. 2006;26:149–161. doi: 10.1016/j.cpr.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Exp Clin Psychopharmacol. 2008;16:376–385. doi: 10.1037/a0012931. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Villafranca SW, Cyr MC, Farley CM, Reichel CM, Gheorghe SL, Krall CM, McDougall SA. Effects of early methylphenidate exposure on morphine- and sucrose-reinforced behaviors in adult rats: relationship to dopamine D2 receptors. Brain Res. 2007;1139:245–253. doi: 10.1016/j.brainres.2006.12.079. [DOI] [PubMed] [Google Scholar]
- Dennis M, Godley SH, Diamond G, Tims FM, Babor T, Donaldson J, Liddle H, Titus JC, Kaminer Y, Webb C, Hamilton N, Funk R. The Cannabis Youth Treatment (CYT) study: main findings from two randomized trials. J Subst Abuse Treat. 2004;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacol Berl. 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SA, Boctor SY. Cocaine responsiveness or anhedonia in rats treated with methylphenidate during adolescence. Neurotoxicol Teratol. 2010;32:432–442. doi: 10.1016/j.ntt.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Fullgrabe MW, Vengeliene V, Spanagel R. Influence of age at drinking onset on the alcohol deprivation effect and stress-induced drinking in female rats. Pharmacol Biochem Behav. 2007;86:320–326. doi: 10.1016/j.pbb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs R, Weir C, Wayman W, Koeltzow TE. Intermittent methylphenidate during adolescent development produces locomotor hyperactivity and an enhanced response to cocaine compared to continuous treatment in rats. Pharmacol Biochem Behav. 2010;96:166–174. doi: 10.1016/j.pbb.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Guerriero RM, Hayes MM, Dhaliwal SK, Ren JQ, Kosofsky BE. Preadolescent methylphenidate versus cocaine treatment differ in the expression of cocaine-induced locomotor sensitization during adolescence and adulthood. Biol Psychiatry. 2006;60:1171–1180. doi: 10.1016/j.biopsych.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Ignjatova L, Raleva M. Gender difference in the treatment outcome of patients served in the mixed-gender program. Bratisl Lek Listy. 2009;110:285–289. [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Goodrich CM, Uribe V. Influence of sex, estrous cycle, and drug-onset age on cocaine self-administration in rats (Rattus norvegicus) Exp Clin Psychopharmacol. 2007;15:37–47. doi: 10.1037/1064-1297.15.1.37. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Kantak KM. Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacol Berl. 2007;194:403–411. doi: 10.1007/s00213-007-0852-6. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE. Protracted time-dependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacol Berl. 2008;198:63–75. doi: 10.1007/s00213-008-1089-8. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Frantz KJ. Attenuated incubation of cocaine seeking in male rats trained to self-administer cocaine during periadolescence. Psychopharmacol Berl. 2009;204:725–733. doi: 10.1007/s00213-009-1502-y. [DOI] [PubMed] [Google Scholar]
- Lopez B, Turner RJ, Saavedra LM. Anxiety and risk for substance dependence among late adolescents/young adults. J Anxiety Disord. 2005;19:275–294. doi: 10.1016/j.janxdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacol Berl. 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. 2009;94:43–50. doi: 10.1016/j.pbb.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacol Berl. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacol Berl. 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clin Exp Res. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies; Washington: 2003. p. 209. [PubMed] [Google Scholar]
- Newcomb MD, Bentler PM. The impact of late adolescent substance use on young adult health status and utilization of health services: a structural-equation model over four years. Soc Sci Med. 1987;24:71–82. doi: 10.1016/0277-9536(87)90141-9. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Andrews WW, Advis JP, White SS. Recent advances in the endocrinology of puberty. Endocr Rev. 1980;1:228–257. doi: 10.1210/edrv-1-3-228. [DOI] [PubMed] [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol Biochem Behav. 2008;89:314–323. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perepletchikova F, Krystal JH, Kaufman J. Practitioner review: adolescent alcohol use disorders: assessment and treatment issues. J Child Psychol Psychiatry. 2008;49:1131–1154. doi: 10.1111/j.1469-7610.2008.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Anderson MM, Nelson SE, Carroll ME. Acquisition of i.v. cocaine self-administration in adolescent and adult male rats selectively bred for high and low saccharin intake. Physiol Behav. 2007;91:126–133. doi: 10.1016/j.physbeh.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol. 1999;60:252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- Rao U, Ryan ND, Dahl RE, Birmaher B, Rao R, Williamson DE, Perel JM. Factors associated with the development of substance use disorder in depressed adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38:1109–1117. doi: 10.1097/00004583-199909000-00014. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacol Berl. 2009;206:1–21. doi: 10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M, Moffett AM, Williams BF, Frantz KJ. Age- and sex-dependent amphetamine self-administration in rats. Psychopharmacol Berl. 2008;196:71–81. doi: 10.1007/s00213-007-0933-6. [DOI] [PubMed] [Google Scholar]
- Snyder KJ, Katovic NM, Spear LP. Longevity of the expression of behavioral sensitization to cocaine in preweanling rats. Pharmacol Biochem Behav. 1998;60:909–914. doi: 10.1016/s0091-3057(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Spear L. Modeling adolescent development and alcohol use in animals. Alcohol Res Health. 2000a;24:115–123. [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000b;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Heightened stress responsivity and emotional reactivity during pubertal maturation: implications for psychopathology. Dev Psychopathol. 2009;21:87–97. doi: 10.1017/S0954579409000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Mick E. Attention-deficit/hyperactivity disorder: diagnosis, lifespan, comorbidities, and neurobiology. J Pediatr Psychol. 2007;32:631–642. doi: 10.1093/jpepsy/jsm005. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-38A, HHS Publication No SMA 10-4856 Findings. Office of Applied Studies; Rockville, MD: 2010. Results from the 2009 national survey on drug use and health: volume 1. Summary of National Findings. [Google Scholar]
- Valenstein ES, Kakolewski JW, Cox VC. Sex differences in taste preference for glucose and saccharin solutions. Science. 1967;156:942–943. doi: 10.1126/science.156.3777.942. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley MD, Poveromo LB, Antapasis J, Herrera CM, Bolanos Guzman CA. Kappa-opioid system regulates the long-lasting behavioral adaptations induced by early-life exposure to methylphenidate. Neuropsychopharmacology. 2009;34:1339–1350. doi: 10.1038/npp.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters KC, Lee CY. Likelihood of developing an alcohol and cannabis use disorder during youth: association with recent use and age. Drug Alcohol Depend. 2008;92:239–247. doi: 10.1016/j.drugalcdep.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters KC, Stinchfield RD, Opland E, Weller C, Latimer WW. The effectiveness of the Minnesota Model approach in the treatment of adolescent drug abusers. Addiction. 2000;95:601–612. doi: 10.1046/j.1360-0443.2000.95460111.x. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92:131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]