Abstract
BACKGROUND
Previous studies have shown that hormone receptor (HR) and HER2 status influence the outcome of locoregional treatments. However, the interrelationship of these factors with trastuzumab is unclear. We sought to determine the role of HR and HER2 status on the locoregional benefit of trastuzumab treatment in patients with non-metastatic breast cancer.
METHODS
Locoregional outcomes of 5683 women treated in our institution from 2000–2008 for invasive breast cancer were analyzed using Kaplan-Meier and Cox regression methods to compare 6 subgroups: HR+/HER2−, HR−/HER2− (triple-negative), HR+/HER2+ with or without trastuzumab, and HR−/HER2+ with or without trastuzumab.
RESULTS
Overall, LRR was 5% at 5 years among patients with HER2+ disease. Patients with HR+/HER2+ disease treated with trastuzumab had half the rate of LRR as patients that did not receive trastuzumab, whereas patients with HR−/HER2+ disease had similar rates of LRR regardless of trastuzumab treatment. On Cox regression analysis comparing LRR risk to the cohort with HR+/HER2− disease, only the HR+/HER2+ cohort treated with trastuzumab had similar LRR risk (HR 1.24, 95% CI 0.56–2.73, p=0.591). All other subgroups, including the HR+/HER2+ cohort who did not receive trastuzumab, had significantly worse outcomes. LRR risk was highest among patients with triple-negative disease (HR 4.73, 95% CI 3.42–6.54, p<0.001).
CONCLUSION(S)
Among patients with HR+/HER2+ disease, treatment with trastuzumab reduces LRR risk to the more favorable outcome of patients with HR+/HER2− disease. In contrast, the increased LRR risk among patients with HR−/HER2+ disease remains despite treatment with trastuzumab. Additional locoregional strategies are needed in this subgroup of patients.
Keywords: Hormone receptor, locoregional, trastuzumab, non-metastatic, breast cancer
INTRODUCTION
Breast cancer is a heterogeneous disease comprised of biologic subtypes with distinct clinical outcomes. Previous publications have demonstrated survival differences between hormone receptor (HR) positive subtypes versus HER-2/neu (HER2) overexpressing or triple-negative subtypes.1–3 Moreover, differences in locoregional outcomes among molecular classes of breast cancer defined by HR and HER2 status have also been reported4. However, these reports largely predate the trastuzumab era (Herceptin®; F. Hoffmann-La Roche, Basel, Switzerland).
Following several studies5–7 establishing the survival benefit of adjuvant trastuzumab compared to chemotherapy alone, recent reports have examined the systemic efficacy of trastuzumab in relation to hormone receptor status and found trastuzumab to be of equal benefit for HR+/HER2+ and HR−/HER2+ disease7, 8. However, there are no current data available to determine whether the locoregional benefits of trastuzumab for patients with HER2+ disease are dependent on HR status. Indeed, the role of HR status itself varies in the setting of locoregional versus systemic therapy, where HR-negativity is associated with a greater response to chemotherapy and HR-positivity is associated with higher radiosensitivity. Thus, the predictive factors that determine locoregional outcomes may be inherently different than those that determine systemic disease control.
Understanding the relationship between known determinants of locoregional outcome is important, as locoregional disease status directly influences breast cancer-specific survival. In this study, we investigated the inter-relationship of HR and HER2 status with trastuzumab treatment and its effect on locoregional outcomes of patients with non-metastatic breast cancer.
MATERIALS AND METHODS
Women with biopsy-proven, invasive breast cancer and confirmed HER2 and HR status diagnosed between 2000–2008 and treated with definitive locoregional and systemic therapy were included in this analysis. Beginning in 2004, adjuvant trastuzumab was increasingly used in clinical practice at our institution, and the inclusion of patients treated from 2000–2008 ensured an adequate sample size of patients with HER2-positive disease who either received or did not receive trastuzumab as part of their definitive therapy. Patients who developed locoregional recurrence prior to presentation at our institution, or who were found to have locoregional recurrence within 1 month of presentation were excluded (n=414). In total, 5683 women with an index primary breast cancer met inclusion criteria and were included in this analysis. This study was approved by the MD Anderson Institutional Review Board and waiver of consent was obtained.
Patients were divided into receptor subgroups as defined by estrogen receptor (ER), progesterone receptor (PR), and HER2 status and stratified by whether they did or did not receive treatment with trastuzumab. Six patient subgroups were defined in this manner: ER and/or PR-positive, HER2-negative (HR+/HER2−, n=3218), ER and/or PR-positive, HER2-positive not treated with trastuzumab (HR+/HER2+/No Trastuzumab, n=322), ER and/or PR-positive, HER2-positive treated with trastuzumab (HR+/HER2+/Yes Trastuzumab, n=314), ER and PR-negative, HER2-positive not treated with trastuzumab (HR−/HER2+/No Trastuzumab, n=257), ER and PR-negative, HER2-positive treated with trastuzumab (HR-/HER2+/Yes Trastuzumab, n=292), and triple-negative (n=1280). Cases were designated as HER2-positive if they demonstrated gene amplification on fluorescence in-situ hybridization (FISH), or a membranous staining score of 3+ on immunohistochemistry (IHC). HER2 copy number that was not amplified by FISH testing or read as 0–1+ by IHC was designated as HER2-negative. Six cases that were IHC 3+/FISH negative who received trastuzumab were considered to be HER2-positive. Cases that were IHC 2+ without a confirmatory FISH result were excluded from analysis (n=168). For ER and PR status, tumors with 1% or more positive tumor nuclei were considered positive.
Patients were seen in follow-up 4–6 months after completion of their definitive treatment, every 4–6 months during the first 5 years, and annually thereafter with imaging and clinical examination. Follow-up time was calculated from the date of diagnosis to the date of locoregional recurrence, the date when the patient was last known to be free of locoregional recurrence, or death. Any relapse within the intact breast or ipsilateral chest wall, and/or ipsilateral axillary, internal mammary, infraclavicular or supraclavicular nodal basins was considered a locoregional event regardless of systemic disease status. Ten patients were considered to have an event at time zero due to locoregional progression during the course of their curative-intent therapy.
All analyses were performed using SPSS, release 17.0.2 (SPSS Inc., Chicago, IL) and Stata, release 12 (StataCorp LP, College Station, TX). Differences in prognostic factors between patient subgroups were analyzed using the χ2 method. Kaplan-Meier analysis was used to calculate locoregional survival outcomes and compared by the log rank statistic. Cox regression methods were used to estimate locoregional recurrence risk among the 6 receptor subgroups. Covariables included age at diagnosis, menopausal status, stage group, T-stage, N-stage, nuclear grade, lymphovascular space invasion (LVSI), surgery type and receipt of radiation therapy, surgical margin status, and treatment with chemotherapy. The proportional hazards assumption was tested and upheld. All tests were two-sided with a significance level of 0.05.
RESULTS
Baseline Characteristics of Receptor Subgroups
Median follow-up for all patients was 52 months (interquartile range 2 – 121 months). Among the 6 receptor subgroups, the cohort with HR+/HER2− disease had the most favorable characteristics including a higher percentage of Stage I tumors [T1 N0 M0; [T1] tumor 2 cm or less in greatest dimension; [N0] no regional lymph node metastases; [M0] no distant metastases], and fewer tumors of high grade histology. In contrast, the triple-negative cohort had a higher rate of advanced nodal disease and were more likely to receive neoadjuvant chemotherapy.
Baseline characteristics of patients with HR+/HER2+ status stratified according to trastuzumab treatment are listed in Table 1. The vast majority of patients with HR+/HER2+ disease who received trastuzumab were treated after 2004 (p<0.001) and more often presented with N3 disease (p=0.005). They also had higher rates of positive or close surgical margins compared to their counterparts who were not treated with trastuzumab (p=0.006). This was due to a slightly higher number of cases with positive/close surgical margins after 2004 among the HR+/HER2+, trastuzumab-treated cohort undergoing mastectomy (7 of 185 patients) compared to those not treated with trastuzumab (0 of 190 patients). Over time, a slight increase in BCS was seen among patients with HR+/HER2+ disease; however, no corresponding change in the rate of positive/close surgical margins was seen among these patients before and after 2004 whether they were (p=0.504) or were not (p=0.254) treated with trastuzumab. For systemic treatment, HR+/HER2+ patients treated with trastuzumab were more likely to receive both anthracycline- and taxane-based chemotherapy in the neoadjuvant and adjuvant settings (both p<0.001). The majority of patients with HR+ disease were treated with hormone therapy, and there was no significant difference in the frequency of hormone therapy treatment between the HR+/HER2+ trastuzumab-treated cohort compared to those not treated with trastuzumab (p=0.124), although an increasing percentage of postmenopausal women were treated with an aromatase inhibitor versus tamoxifen during the latter part of the study period when trastuzumab was in greater use.
Table 1.
Baseline characteristics among estrogen and/or progesterone receptor positive, HER2-positive subgroups stratified by trastuzumab treatment.
| Characteristic N (%) |
ER/PR+* / HER2+* Without trastuzumab N = 322 |
ER/PR+ / HER2+ With trastuzumab N = 314 |
P-value |
|---|---|---|---|
| Median age (years) | 49 | 48 | 0.873 |
| Menopausal status | |||
| Pre | 163 (51) | 163 (52) | 0.673 |
| Post | 132 (41) | 123 (39) | |
| Unknown | 27 (8) | 28 (9) | |
| T-stage | |||
| T1 | 125 (39) | 123 (39) | 0.768 |
| T2 | 140 (43) | 129 (41) | |
| T3 | 23 (7) | 29 (9) | |
| T4 | 34 (11) | 31 (10) | |
| Unknown | 0 (0) | 2 (1) | |
| N-stage | |||
| N0 | 151 (47) | 155 (49) | 0.012 |
| N1 | 134 (42) | 109 (35) | |
| N2 | 16 (5) | 9 (3) | |
| N3 | 21 (6) | 41 (13) | |
| Unknown | 0 (0) | 0 (0) | |
| Stage Group | |||
| I | 82 (26) | 86 (27) | 0.626 |
| II | 162 (50) | 145 (46) | |
| III | 78 (24) | 81 (26) | |
| Unknown | 0 (0) | 2 (1) | |
| Nuclear grade | |||
| 1 | 4 (1) | 1 (1) | 0.273 |
| 2 | 80 (25) | 90 (28) | |
| 3 | 230 (71) | 220 (70) | |
| Unknown | 8 (3) | 3 (1) | |
| LVSI† | |||
| Negative | 190 (59) | 203 (65) | 0.148 |
| Positive | 125 (39) | 105 (33) | |
| Unknown | 7 (2) | 6 (2) | |
| Surgery/Radiation | |||
| BCT‡ + RT‡ | 118 (37) | 124 (40) | 0.512 |
| Mastectomy + RT | 104 (32) | 112 (36) | |
| Mastectomy no RT | 86 (27) | 74 (23) | |
| Unknown | 14 (4) | 4 (1) | |
| Surgical Margins | |||
| Negative | 298 (93) | 276 (88) | 0.006 |
| Positive/close | 13 (4) | 30 (10) | |
| Unknown | 11 (3) | 8 (2) | |
| Chemotherapy | |||
| Neoadjuvant only | 109 (34) | 71 (23) | <0.001 |
| Adjuvant only | 178 (55) | 161 (51) | |
| Both | 35 (11) | 82 (26) | |
| Chemotherapy Agents | |||
| Anthracycline-based | 86 (27) | 9 (3) | <0.001 |
| Taxane-based | 8 (2) | 41 (13) | |
| Antracycline + Taxane | 215 (67) | 255 (81) | |
| Other/Unknown | 13 (4) | 9 (3) | |
| Era of Treatment | |||
| Before 2004 | 273 (85) | 46 (15) | <0.001 |
| 2004 and after | 49 (15) | 268 (85) | |
ER/PR+ = Estrogen and/or Progesterone Receptor Positive; HER2+ = Human epidermal growth factor receptor 2 positive
LVSI = Lymphovascular space invasion
BCT = Breast conservation therapy; RT = Radiation therapy
Baseline characteristics of patients with HR−/HER2+ disease stratified by trastuzumab treatment are listed in Table 2. Patients with HR−/HER2+ disease treated with trastuzumab were mainly treated after 2004 (p<0.001), and were more likely to have stage III disease due to T4 or N3 disease at presentation (all p<0.010). They were more likely to receive neoadjuvant and adjuvant chemotherapy than their counterparts who were not treated with trastuzumab (all p<0.001).
Table 2.
Baseline characteristics among estrogen and progesterone receptor negative, HER2-positive subgroups stratified by trastuzumab treatment.
| Characteristic N (%) |
ER/PR(−)* / HER2+* Without trastuzumab N = 257 |
ER/PR(−) / HER2+ With trastuzumab N = 292 |
P-value |
|---|---|---|---|
| Median age (years) | 49 | 51 | 0.336 |
| Menopausal status | |||
| Pre | 113 (44) | 122 (42) | 0.307 |
| Post | 110 (43) | 143 (49) | |
| Unknown | 34 (13) | 27 (9) | |
| T-stage | |||
| T1 | 102 (40) | 85 (29) | 0.010 |
| T2 | 102 (40) | 112 (39) | |
| T3 | 19 (7) | 30 (10) | |
| T4 | 33 (12) | 62 (21) | |
| Unknown | 1 (1) | 3 (1) | |
| N-stage | |||
| N0 | 121 (47) | 116 (40) | 0.004 |
| N1 | 95 (37) | 103 (35) | |
| N2 | 18 (7) | 16 (5) | |
| N3 | 21 (8) | 55 (19) | |
| Unknown | 2 (1) | 2 (1) | |
| Stage Group | |||
| I | 70 (27) | 52 (18) | 0.010 |
| II | 114 (44) | 129 (44) | |
| III | 71 (28) | 108 (37) | |
| Unknown | 2 (1) | 3 (1) | |
| Nuclear grade | |||
| 1 | 0 (0) | 0 (0) | 0.837 |
| 2 | 23 (9) | 25 (9) | |
| 3 | 223 (87) | 258 (88) | |
| Unknown | 11 (4) | 9 (3) | |
| LVSI† | |||
| Negative | 154 (60) | 180 (62) | 0.680 |
| Positive | 94 (37) | 102 (35) | |
| Unknown | 9 (3) | 10 (3) | |
| Surgery/Radiation | |||
| BCT‡ + RT‡ | 88 (34) | 88 (30) | 0.081 |
| Mastectomy + RT | 90 (35) | 125 (43) | |
| Mastectomy no RT | 73 (29) | 64 (22) | |
| Unknown | 6 (2) | 15 (5) | |
| Surgical Margins | |||
| Negative | 222 (86) | 260 (89) | 0.278 |
| Positive/close | 23 (9) | 19 (7) | |
| Unknown | 12 (5) | 13 (4) | |
| Chemotherapy | |||
| Neoadjuvant only | 80 (31) | 71 (24) | <0.001 |
| Adjuvant only | 148 (58) | 112 (39) | |
| Both | 29 (11) | 109 (37) | |
| Chemotherapy Agents | |||
| Anthracycline-based | 59 (23) | 10 (3) | <0.001 |
| Taxane-based | 2 (1) | 38 (13) | |
| Antracycline + Taxane | 192 (74) | 240 (82) | |
| Other/Unknown | 4 (2) | 4 (2) | |
| Era of Treatment | |||
| Before 2004 | 213 (83) | 59 (20) | <0.001 |
| 2004 and after | 44 (17) | 233 (80) | |
ER/PR(−) = Estrogen and Progesterone Receptor Negative; HER2+ = Human epidermal growth factor receptor 2 positive
LVSI = Lymphovascular space invasion
BCT = Breast conservation therapy; RT = Radiation therapy
Locoregional Recurrence Outcomes of Receptor Subgroups
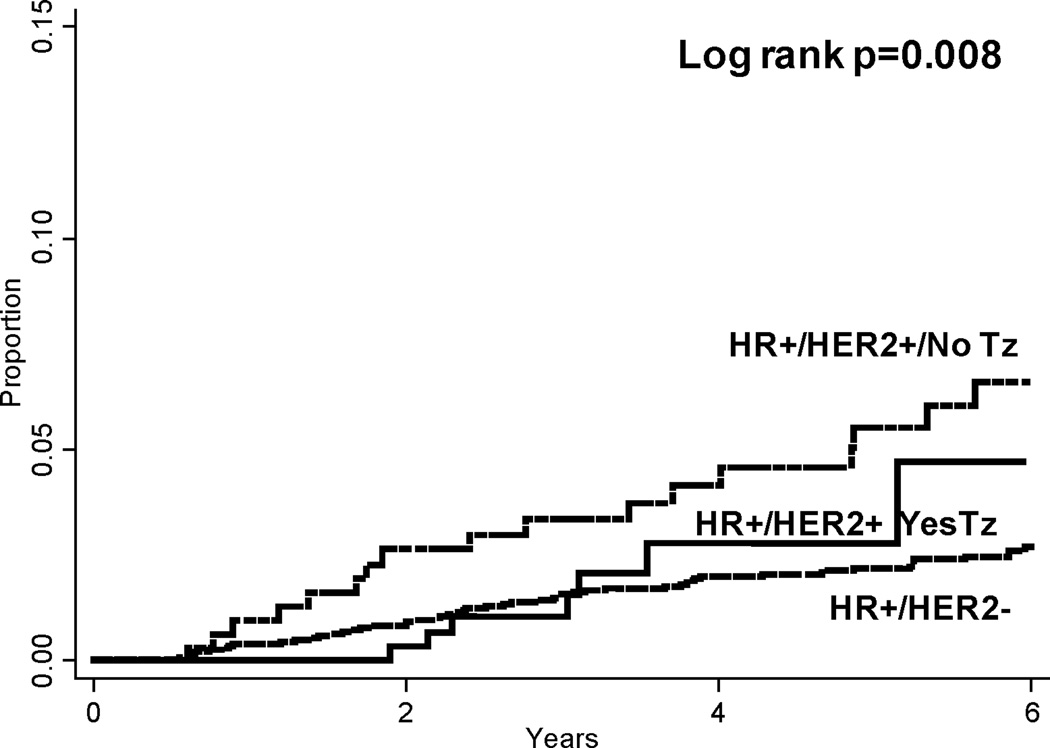
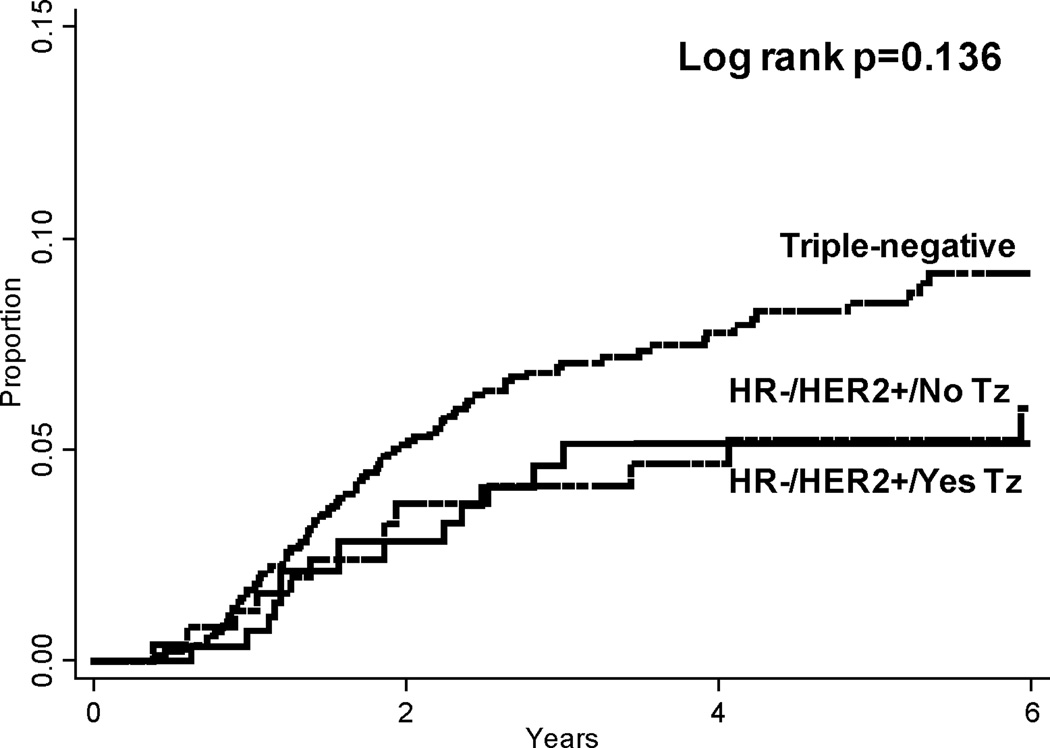
Overall, locoregional recurrence rates were low in the entire cohort. At 5 years, the lowest rate of LRR was seen among patients with HR+/HER2− disease (2% or 72 total locoregional events). Patients with triple-negative disease had the highest LRR rate at 5 years (9%, 102 events). In patients with HER2+ disease, locoregional recurrence rates varied according to hormone receptor status and treatment with trastuzumab. Among patients with HR+/HER2+ disease, 5-year LRR rates were 3% (8 events) among patients treated with trastuzumab versus 6% (18 events) among patients who were not. In contrast, among patients with HR-/HER2+ disease, 5-year LRR rates were similar (6%) whether patients did or did not receive trastuzumab (14 and 17 total locoregional events, respectively). Cumulative LRR rates among all patients with HR-positive disease and HR-negative disease are depicted in Figures 1 and 2, respectively.
Figure 1.
Cumulative locoregional recurrence rates among hormone receptor-positive subgroups. Pairwise comparison HR+/HER2− vs HR+/HER2+/No Tz p=0.002; HR+/HER2− vs HR+/HER2+/Yes Tz p=0.506. HR = Hormone receptor; HER2 = Human epidermal growth factor receptor 2; Tz = Trastuzumab.
Figure 2.
Cumulative locoregional recurrence rates among hormone receptor-negative subgroups. Pairwise comparison triple-negative vs HR−/HER2+/No Tz p=0.187; triple-negative vs HR−/HER2+/Yes Tz p=0.086. HR = Hormone receptor; HER2 = Human epidermal growth factor receptor 2; Tz = Trastuzumab.
On univariate analysis in comparison with the HR+/HER2− cohort, all patient subgroups had higher LRR risk except patients with HR+/HER2+ disease treated with trastuzumab, which did not significantly differ from the referent group. All other intercomparisons between receptor subgroups did not reach statistical significance.
Multivariate Cox Regression Analysis
In comparison with the favorable HR+/HER2− cohort, only patients with HR+/HER2+ disease that were treated with trastuzumab had similar LRR risk (HR 1.24, 95% CI 0.56 – 2.73, p=0.591, Table 3). All other subgroups had a significantly higher risk of LRR, including patients with HR+/HER2+ disease that were not treated with trastuzumab. Patients with HR-/HER2+ disease had greater than twice the risk of LRR regardless of trastuzumab treatment. Patients with triple-negative disease had the highest LRR risk (HR 4.73, 95% CI 3.42 – 6.54, p<0.001). No other intercomparisons between receptor subgroups revealed significant differences in LRR risk, except the triple-negative cohort which consistently demonstrated higher LRR risk than all other subgroups.
Table 3.
Adjusted hazard ratios for locoregional recurrence among all patients.
| Variable | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| Hormone Receptor Subgroup | ||
| ER/PR+* / HER2*-negative | 1.00 | - |
| ER/PR+ / HER2+ / No Trastuzumab | 2.11 (1.25 – 3.58) | 0.005 |
| ER/PR+ / HER2+ / Yes Trastuzumab | 1.24 (0.56 – 2.73) | 0.591 |
| Triple-negative | 4.73 (3.42 – 6.54) | <0.001 |
| ER/PR-negative / HER2+ / No Trastuzumab | 2.32 (1.32 – 4.07) | 0.004 |
| ER/PR-negative / HER2+ / Yes Trastuzumab | 2.01 (1.04 – 3.87) | 0.037 |
| Age at Diagnosis | 0.99 (0.97 – 1.00) | 0.025 |
| Stage Group | ||
| I | 1.00 | - |
| II | 1.68 (1.06 – 2.66) | 0.028 |
| III | 4.28 (2.52 – 7.27) | <0.001 |
| LVSI† | ||
| No | 1.00 | - |
| Yes | 3.06 (2.30 – 4.08) | <0.001 |
| Chemotherapy | ||
| Neoadjuvant | 1.00 | - |
| Adjuvant | 0.64 (0.46 – 0.89) | 0.007 |
| Both | 0.68 (0.43 – 1.06) | 0.091 |
| Surgery/Radiation | ||
| BCT‡ + RT‡ | 1.00 | - |
| Mastectomy + RT | 0.96 (0.67 – 1.38) | 0.836 |
| Mastectomy no RT | 2.01 (1.39 – 2.91) | <0.001 |
ER/PR+ = Estrogen receptor and/or progesterone receptor positive; HER2 = Human epidermal growth factor receptor 2
LVSI = Lymphovascular space invasion
BCT = Breast conservation therapy; RT = Radiation therapy
Addition of an interaction term for receptor subgroup by locoregional treatment to the Cox regression model was not significant (p=0.19), indicating that the LRR risk conferred by the various hormone receptor and HER2 subgroups was independent of surgery type or radiation treatment (data not shown).
DISCUSSION
In this study, we have shown that the inter-relationship of HR and HER2 status on LRR risk is influenced by treatment with trastuzumab, and confirm that receptor subtypes of breast cancer (as defined by HR and HER2 status) affect the risk of LRR after definitive treatment. Our findings suggest that treatment with trastuzumab is associated with reduced LRR risk in patients with HR+/HER2+ disease to a rate similar to that of HR+/HER2− status. In contrast, LRR risk among the HR-negative cohort remains elevated even for the subgroup of patients with HR−/HER2+ disease who receive trastuzumab, compared to patients with HR+ disease.
Among 5 prospective clinical trials evaluating the benefit of adjuvant trastuzumab with standard chemotherapy for high-risk, early stage breast cancer5–7, only one study evaluated the effect of hormone receptor status on the efficacy of trastuzumab. In the combined analysis of the B31/9831 trials, outcomes following adjuvant trastuzumab were similar whether patients had hormone receptor-positive or negative disease, suggesting that the directional benefit of trastuzumab on disease-free survival is independent of ER/PR status7. No locoregional recurrence data stratified according to hormone receptor status has been published from these studies. Moreover, locoregional events comprised fewer than 25% of all first events in the control and experimental arms of both studies, and the impact of LRR on the reported disease-free survival outcomes is significantly less than other classes of events.
Several studies have examined the inter-relationship between HR status and the benefit of trastuzumab in patients treated for metastatic disease. A retrospective analysis of 3 clinical trials evaluating the role of trastuzumab as monotherapy or in combination with chemotherapy as first, second, or third-line treatment in patients with metastatic breast cancer did not reveal a difference in overall response rate or time to progression in relation to hormone receptor status9. In a more recent retrospective study evaluating the role of hormone receptor status and the benefit of trastuzumab among patients with metastatic breast cancer treated at 2 European centers, high expression of ER (≥30% of tumor cells) predicted for reduced response to trastuzumab plus chemotherapy; however, when endocrine therapy was added to the regimen of patients with ER-positive tumors, a significant progression-free survival benefit was seen8. These findings are consistent with two recent randomized trials that examined the benefit of combining trastuzumab and endocrine therapy as first-line treatment in patients with metastatic breast cancer that co-expresses hormone receptor and HER210, 11. In these trials, dual blockade of these signaling pathways led to significant improvement in progression-free survival in the experimental arms.
Recent preclinical work has described the potential interaction between hormone receptor and growth factor receptor signaling. This crosstalk may involve supersensitivity to low residual estrogen concentrations or loss of ER-regulated genes with up-regulation of growth factor signaling, leading to resistance to endocrine therapy as well as ErbB2 tyrosine kinase inhibitors12–14. A recent study demonstrated that following neoadjuvant chemotherapy containing trastuzumab, pathologic response was highest in ER-negative, HER2-positive tumors, and progressively decreased with increasing ER co-expression15. These findings would predict a LRR benefit among patients treated with trastuzumab for ER-negative, HER2+ tumors compared to patients with ER-positive, HER2+ disease. In our study, we did not find a significant difference in LRR risk between these two cohorts, and the hazard ratio for LRR was higher among patients with HR-negative disease. This difference may be attributable to our definition of HR-positivity, which included progesterone receptor expression, as well as the degree of HR receptor positivity which was not quantified in this analysis. Nonetheless, our findings do suggest an interaction between HR and HER2 status and the locoregional response to trastuzumab treatment.
Little clinical data exists on the interaction of hormone receptor and HER2 status on locoregional outcomes in the trastuzumab era. In a recent retrospective study of 582 patients, in which 86% of HER2+ patients received trastuzumab, HER2-positivity was associated with significantly reduced LRR risk 4. This study also examined the LRR risk of the triple-negative subgroup in relation to other receptor subtypes, but did not evaluate the influence of hormone receptor subgroups on the LRR benefit of trastuzumab treatment. Although several reports have now demonstrated the locoregional benefit of trastuzumab treatment in HER2-positive breast cancer4, 5, 16, the locoregional benefit of trastuzumab in relation to hormone receptor status has not been clearly elucidated. To our knowledge, our study is the first to characterize LRR risk among receptor subgroups prior to and since the introduction of trastuzumab. Among patients with HR+/HER2+ disease, we found that treatment with trastuzumab reduced the rate of LRR by 50% compared to patients that were not treated with trastuzumab, whereas patients with HR−/HER2+ disease had similar rates of LRR regardless of trastuzumab treatment. Moreover, we found that all receptor subgroups had higher LRR than the cohort with HR+/HER2− disease, except the HR+/HER2+ cohort treated with trastuzumab whose LRR outcomes did not significantly differ from the favorable HR+/HER2− subgroup. These findings confirm the role of HR-negativity and HER2 overexpression in increasing LRR risk, and help to elucidate the relationship of HR status on locoregional outcomes among patients treated with trastuzumab for HER2+ disease. Moreover, these findings were independent of type of locoregional treatment administered, although the low number of events limited power to identify small differences in LRR risk among receptor subgroups undergoing BCT versus mastectomy.
In addition to the limitations and biases of retrospective studies, the overall low locoregional event rate limited the statistical analysis in this study, despite our sample size of over 5,000 patients. Moreover, the shorter follow-up time of patients who were treated with trastuzumab may overestimate the benefit of treatment. A quantitative analysis of hormone receptor expression was not undertaken in this study, which may have provided additional insight into the interaction between hormone receptor status and locoregional outcomes following treatment with trastuzumab.
This is the first study to examine the predictive efficacy of trastuzumab on locoregional outcomes in relation to hormone receptor and HER2 status. While patients with HR+/HER2+ disease appeared to derive a locoregional control benefit from trastuzumab, we were not able to demonstrate a similar benefit among patients with HR−/HER2+ disease. This novel finding suggests a complex interplay between hormone receptor status, HER2 status, and their corresponding targeted therapies in mediating LRR risk. Future studies evaluating locoregional treatment questions should stratify patients not only based on HR and HER2 status, but also based on treatment with trastuzumab. Such an approach may facilitate more accurate risk assessment among patients with biologically variant disease.
Acknowledgement
This study was supported in part by the MD Anderson Institutional Core Training Grant and Institutional Cancer Center Grant, CA016672 and T32CA77050.
Footnotes
Conflicts of Interest Notification: Shaheenah Dawood, M.D. is a recipient of honoraria from Roche Pharmaceuticals
REFERENCES
- 1.Press MF, Bernstein L, Thomas PA, et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997 Aug;15(8):2894–2904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001 Sep 11;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panoff JE, Hurley J, Takita C, et al. Risk of locoregional recurrence by receptor status in breast cancer patients receiving modern systemic therapy and post-mastectomy radiation. Breast Cancer Res Treat. Aug;128(3):899–906. doi: 10.1007/s10549-011-1495-1. [DOI] [PubMed] [Google Scholar]
- 5.Dahabreh IJ, Linardou H, Siannis F, Fountzilas G, Murray S. Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist. 2008 Jun;13(6):620–630. doi: 10.1634/theoncologist.2008-0001. [DOI] [PubMed] [Google Scholar]
- 6.Gianni L, Dafni U, Gelber RD, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. Mar 12;(3):236–244. doi: 10.1016/S1470-2045(11)70033-X. [DOI] [PubMed] [Google Scholar]
- 7.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005 Oct 20;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 8.Montemurro F, Rossi V, Rocca MC, et al. Hormone-receptor expression and activity of trastuzumab with chemotherapy in HER2-positive advanced breast cancer patients. Cancer. May 19; doi: 10.1002/cncr.26162. [DOI] [PubMed] [Google Scholar]
- 9.Brufsky A, Lembersky B, Schiffman K, Lieberman G, Paton VE. Hormone receptor status does not affect the clinical benefit of trastuzumab therapy for patients with metastatic breast cancer. Clin Breast Cancer. 2005 Aug;6(3):247–252. doi: 10.3816/CBC.2005.n.027. [DOI] [PubMed] [Google Scholar]
- 10.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009 Nov 20;27(33):5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J Clin Oncol. 2009 Nov 20;27(33):5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 12.Xia W, Bacus S, Hegde P, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006 May 16;103(20):7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborne CK, Shou J, Massarweh S, Schiff R. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005 Jan 15;11(2 Pt 2):865s–870s. [PubMed] [Google Scholar]
- 14.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004 Jun 16;96(12):926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 15.Bhargava R, Dabbs DJ, Beriwal S, et al. Semiquantitative hormone receptor level influences response to trastuzumab-containing neoadjuvant chemotherapy in HER2-positive breast cancer. Mod Pathol. Mar 24;(3):367–374. doi: 10.1038/modpathol.2010.209. [DOI] [PubMed] [Google Scholar]
- 16.Kiess AP, McArthur HL, Mahoney K, et al. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer. Sep 1; doi: 10.1002/cncr.26484. [DOI] [PubMed] [Google Scholar]