Abstract
Background:
Although Elizabethkingia meningoseptica (Chryseobacterium meningosepticum) infections in immunocompromised hosts have been recognised, clinical data detailing these infections remain limited, especially from India. Antimicrobial susceptibility data on E. meningoseptica remain very limited, with no established breakpoints by Clinical and Laboratory Standards Institute (CLSI). The organism is usually multidrug resistant to antibiotics usually prescribed for treating Gram-negative bacterial infections, a serious challenge to the patient and the treating clinicians.
Materials and Methods:
The analysis was done in a tertiary care oncology and stem cell transplant center. Susceptibility testing and identification of E. meningoseptica was done using Vitek auto analyzer. Records of immunocompromised patients with E. meningoseptica bacteremia were analysed from January 2009 to March 2012.
Results:
A total of 29 E. meningoseptica bacteremia cases were documented between 2009 and 2012. Eleven patients were immunocompromised. Three were post stem cell transplant and one was post cord blood transplant. The mean age of the patients was 48.4 years. Mean Charlson’s comorbidity index was 5.7. Four had solid organ malignancies, five had hematological malignancies, and two had lymphoreticular malignancy. Eight patients had received chemotherapy. Mean Apache II score was 18. Mean Pitts score for bacteremia was 4.7. Two were neutropenic (one post SCT, one MDS post chemo) with a mean white blood cell (WBC) count of 450/mm3 . Ten had a line at the time of bacteremia. Mean duration of the line prior to bacteremia was 8 days. Eight had line-related bacteremia. Three had pneumonia with secondary bacteremia. All received combination therapy with two or more antibiotics which included cotrimoxazole, rifampicin, piperacillin–tazobactam, tigecycline, or cefepime–tazobactam. All the isolates showed in vitro resistance to ciprofloxacin. Five patients died, but a multivariate analysis was not done to calculate the attributable mortality.
Conclusion:
In our study, central line was the commonest risk factor for E. meningosepticum bacteremia, although a multivariate analysis was not done. There has not been much of a change in the susceptibility pattern of these organisms over 3 years, with good susceptibility to piperacillin–tazobactam and cotrimoxazole. Even though uncommon, E. meningoseptica is an important pathogen, especially in immunocompromised hosts with indwelling devices.
Keywords: Bacteremia, Chryseobacterium meningosepticum, Elizabethkingia meningoseptica, immunocompromised host
Introduction
Elizabethkingia meningoseptica (Chryseobacterium meningosepticum) is a ubiquitous Gram-negative bacillus described by Elizabeth O. King in 1959.[1] Although E. meningoseptica infections in immunocompromised hosts are a well-known entity,[2] limited clinical data are available from the Indian subcontinent. This organism is usually resistant to most antibiotics prescribed for treating Gram-negative bacterial infections, including extended-spectrum beta-lactam agents and aminoglycosides, a serious challenge to the patient and the treating clinicians.[3]
Antimicrobial susceptibility data on E. meningoseptica also remains very limited, with no established breakpoints by Clinical and Laboratory Standards Institute (CLSI).[4]
Materials and Methods
The analysis was done in a tertiary care oncology and stem cell transplant center. Bacterial identification was done by using mini API strips – Rapid ID32E and ID32GN (bioMerieux) from January 2011 to May 2011 and thereafter by using VITEK2 compact system. Susceptibility testing was performed by a standardised disk diffusion method according to CLSI guidelines on Muller Hinton agar from January 2009 to May 2011.[5,6] From June 2011 to March 2012, susceptibility testing was performed by using the instrument VITEK2 compact. As there is no established breakpoint for Chryseobacterium by CLSI, the interpretive breakpoints for Pseudomonas were used. For tigecycline, the breakpoint for Enterobacteriaceae was used.[7]
The institutional ethics committee approval was obtained prior to analysis and publication.
The isolates were tested against piperacillin-tazobactam 100/10 μg, gentamicin 10 μg, amikacin 30 μg, netilmycin 30 μg, ceftazidime 30 μg, cefoperazone-sulbactam 75/30 μg, cefepime 30 μg, cefepime/tazobactam 30/10 μg, imipenem 10 μg, meropenem 10 μg, ciprofloxacin 5 μg, trimethoprim/sulfamethoxazole 1.25/23.75 μg, and tigecycline 15 μg. While clear-cut CLSI guidelines are available for Enterobacteriaceae and Pseudomonas, the breakpoint of the most of the antibiotics, the guidelines for antibiotics such as cefoperazone–sulbactam and cefepime/tazobactam are not elucidated in the current CLSI guidelines. Hence, the breakpoints of cefoperazone and cefepime were applied for cefoperazone/sulbactam and cefepime/tazobactam, respectively. Antibiotic disks were obtained from BD BBL (USA), Oxoid (UK), and HiMedia Lab (India).
Only the records of patients who had a solid organ, hematological, and lymphoreticular malignancy with E. meningoseptica bacteremia were analysed from January 2009 to March 2012. Patient details like age, sex, underlying immunocompromising condition with comorbidities, chemotherapeutic agents used, ICU stay, and central line (both central line and peripheral line samples grew Chryseobacterium with no other identifiable source) were looked into. Pitt’s bacteremia score and Charlson comorbidity index were calculated for all patients. Outcome of the patients (28-day mortality) was also analysed; however, the attributable mortality was not calculated.
Results
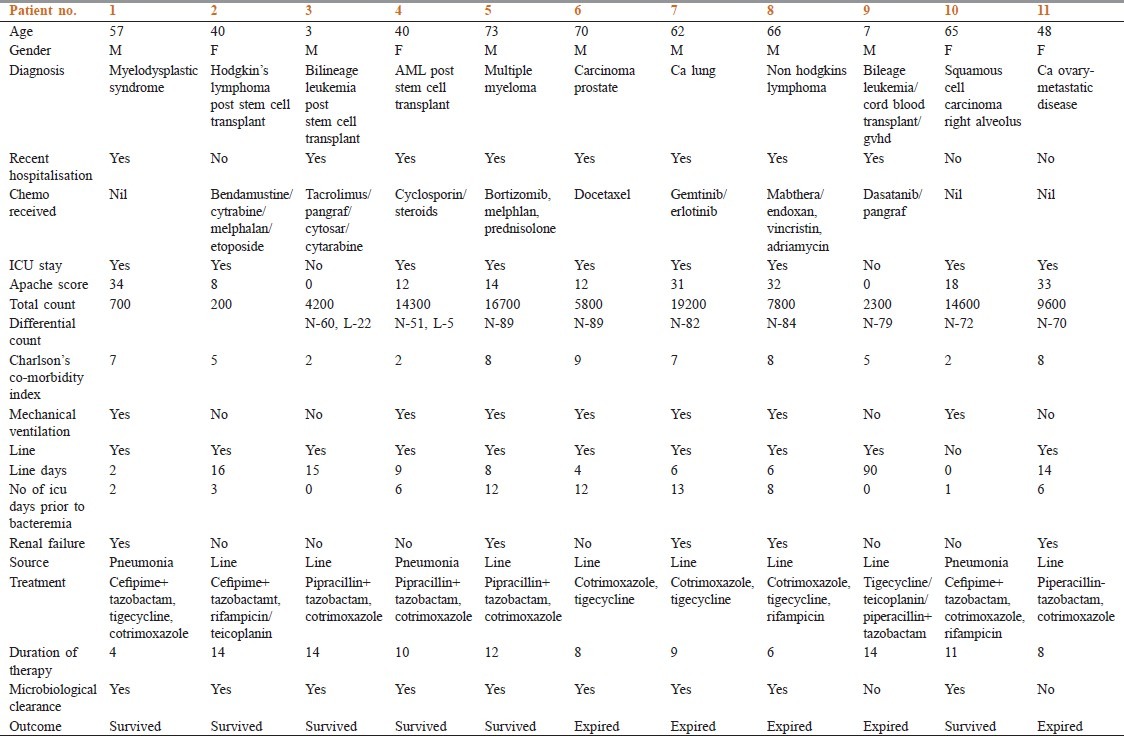
A total of 29 cases with E. meningoseptica bacteremia were documented between 2009 and 2012. Eleven patients were immunocompromised [Figure 1]. The mean age of the patients was 48.4 years. Seven were males and four were females. Mean Charlsons comorbidity index was 5.7. Four of them had solid organ malignancies, five had hematological malignancies, and two had lymphoreticular malignancy. Eight patients had received chemotherapy. Three patients were post stem cell transplant and one patient was post cord blood transplant. Eight patients had history of a recent hospitalization. At the time of bacteremia, eight patients were in the ICU. Mean Apache II score was 18. Mean Pitt score for bacteremia was 4.7. Out of the 11 patients, 2 were neutropenic (one post Stem Cell transplant, one Myelodysplastic Syndrome post chemo) with a mean white blood cell (WBC) count of 450/mm3 . Mean WBC among non-neutropenics was 10,833/mm3 (range 2300-19,200) and the mean neutrophil count was 75% (range 2500-15,754; mean 7441). Six patients required mechanical ventilation. Ten patients had a line at the time of bacteremia. Mean duration of the line prior to bacteremia was 8 days. Eight out of the 11 patients had line-related bacteremia. Three patients had pneumonia with secondary bacteremia. All the patients received combination therapy with two or more antibiotics, which included cotrimoxazole, rifampicin, piperacillin–tazobactam, tigecycline, or cefepime–tazobactam. All the isolates showed in vitro resistance to ciprofloxacin. Ten isolates were susceptible to piperacillin-tazobactam and cotrimoxazole. Six isolates were sensitive to tigecycline. Mean duration of therapy was 10 days. Five out of the eight isolates tested were sensitive to cefepime–tazobactam. Five out of 11 patients died, but a multivariate analysis was not done to calculate the attributable mortality. Clinical and laboratory details of the patients are elaborated in Table 1a,b,c.
Figure 1.
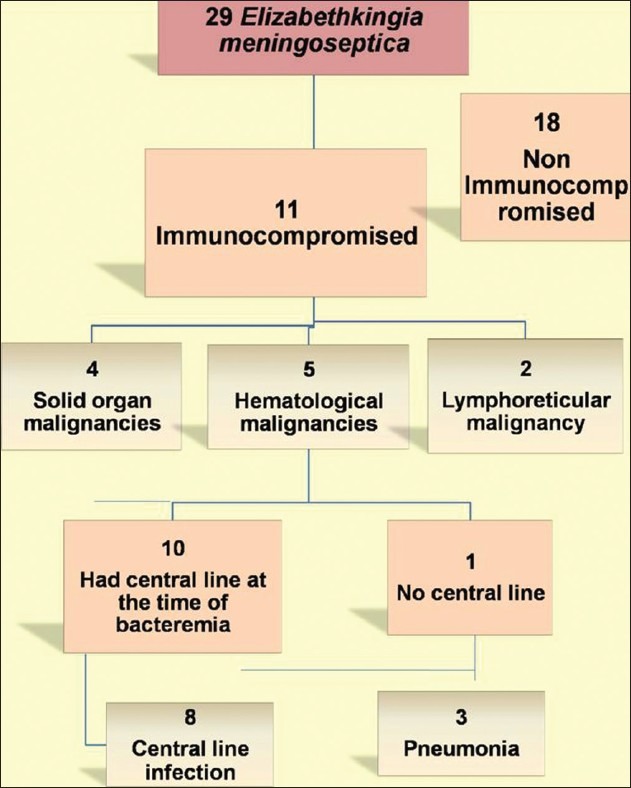
Analysis of patients with Chryseobacteremia
Table 1.
Clinical and laboratory details of the patients
Discussion
E. meningoseptica is associated primarily with meningitis in neonates and a variety of infections in immunocompromised patients. Clinical data detailing these infections remain limited. Infections with E. meningoseptica are not very common; however, they are clinically important as the organism is intrinsically resistant to multiple antibiotics which are routinely used to treat a patient with suspected sepsis.[2] Infections that have been reported with C. meningoseptica are pneumonia, meningitis, and catheter-related blood stream infections. However, there have been cases of biliary sepsis, osteomyelitis, and keratitis in the literature as well.
Environmental studies have shown Chryseobacteria can survive in chlorine-treated water. They often are found to colonise sink basins and taps and in ventilator tubing. They can also colonise patients via contaminated medical equipments involving fluids, for example, respirators, intubation tubes, humidifiers, incubators for newborns, ice chests, and syringes.[8,9,10] Surgically implanted devices such as intravascular catheters and prosthetic valves can also be contaminated with this organism.[8,9,10]
Infections with C. meningoseptica have been associated with prolonged hospitalisation, prior exposure to multiple antibiotics, and immunocompromised host.[11] Our series analysed bacteremic isolates only and the main source for bacteremia was the central line (8 out of 11) followed by pneumonia. C. meningosepticum was the second most common cause of Gram-negative infection in a dialysis unit as per a Greek study.[12] The same study also showed that Pseudomonas aeruginosa was the most prevalent isolate in all types of water samples, followed by C. meningosepticum in tap and treated water and by Escherichia coli in dialysate.[12]
A study from Taiwan on the analysis of adult patients with E. meningoseptica bacteremia showed that 86% of the patients had nosocomial infections and 60% had acquired the infection in the ICUs, and the most common underlying diseases were malignancy (36%) and diabetes mellitus (25%). This study showed a 14-day mortality of 23.4%.[13]
In our study, 8 out of the 11 patients were in the ICU at the time of development of bacteremia. All patients in our series, being oncology patients, were immunocompromised. In our study, 5 out of 11 patients died. However, the attributable mortality was not calculated.
Multivariate analysis from studies have shown that E. meningoseptica bacteremia acquired in an ICU and presence of effective antibiotic treatment after the availability of culture results were independent predictors of 14-day mortality.[7] E. meningoseptica infection is very challenging as the organism is inherently multidrug resistant and only a limited range of antibiotic classes are available for treatment. Studies have shown that susceptibility of the isolates was relatively high (>50%) only to piperacillin, piperacillin-tazobactam, trimethoprim-sulfamethoxazole, and ciprofloxacin. More than 80% of the isolates tested were susceptible to trimethoprim-sulfamethoxozole, moxifloxacin, and levofloxacin.[7] Our study showed similar susceptibilities except for fluoroquinalones where all the 11 isolates showed in vitro resistance to ciprofloxacin. Ten isolates were susceptible to piperacillin-tazobactam and cotrimoxazole. Six isolates were sensitive to tigecycline. Five out of the eight isolates tested were sensitive to cefepime-tazobactam. Rifampicin and cotrimoxazole may be used in the treating regimen.[14,15] Rifampin was active against the majority of strains in an in vitro study. Hence, rifampicin can be used in combination to treat severe invasive infections.[16] Many studies have shown that vancomycin has marginal in vitro activity against Chryseobacterium spp. isolates. There are reports of successful usage of vancomycin to treat Chryseobacterium infections.[16,17,18] However, when the isolates were tested for vancomycin susceptibility, a majority of the isolates showed an intermediate susceptibility.[10]
C. meningosepticum is a biofilm-forming organism. Various studies have shown that mortality is associated with the use of central venous catheters, initial inappropriate antimicrobial therapy, and higher biofilm production by the organism. The outcome of patients with biofilm-forming C. meningosepticum infection was adversely affected by the choice of inappropriate antimicrobial therapy and the use of long-term indwelling intravascular catheters.[19] Presence of effective antibiotic treatment after the availability of culture results was an independent predictor of 14-day mortality. The 14-day mortality was higher among patients receiving carbapenems than fluoroquinolones or other antimicrobial agents.[7]
Conclusions
In our series of oncology patients, central line was the commonest risk factor for E. meningosepticum bacteremia, although a multivariate analysis was not done. There has not been much of a change in susceptibility pattern of this organism over 3 years, with good susceptibility observed to piperacillin-tazobactam and cotrimoxazole. In contrary to other studies, all our isolates were ciprofloxacin resistant. Even though uncommon, E. meningoseptica is an important pathogen, especially in immunocompromised hosts with indwelling devices.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.King EO. Studies on a group of previously unclassified bacteria associated with meningitis in infants. Am J Clin Pathol. 1959;31:241–7. doi: 10.1093/ajcp/31.3.241. [DOI] [PubMed] [Google Scholar]
- 2.Ceyhan M, Celik M. Elizabethkingia meningosepticum (Chryseobacterium meningosepticum) Infections in Children. Int J Pediatr. 2011:215–37. doi: 10.1155/2011/215237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin PY, Chu C, Su LH, Huang CT, Chang WY, Chiu CH. Microbiological Analysis of Bloodstream Infections Caused by Chryseobacterium meningosepticum in Nonneonatal Patients. J Clin Microbiol. 2004;42:3353–5. doi: 10.1128/JCM.42.7.3353-3355.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirby JT, Sader HS, Walsh TR, Jones RN. Antimicrobial Susceptibility and Epidemiology of a Worldwide Collection of Chryseobacterium spp.: Report from the SENTRY Antimicrobial Surveillance Program (1997–2001) J Clin Microbiol. 2004;42:445–8. doi: 10.1128/JCM.42.1.445-448.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wayne PA. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. Clinical and Laboratory Standards Institute. 2009;29:M100–S19. [Google Scholar]
- 6.Mouton JW, Melchers R, Mil AV. Poster session presented at ICAAC. Boston, USA: 2010. In Vitro Activity of Cefepime alone and in combination with Tazobactam against ESBL producers; p. A-2251. [Google Scholar]
- 7.Hsu MS, Liao CH, Huang YT, Liu CY, Yang CJ, Kao KL, et al. Clinical features, antimicrobial susceptibilities, and outcomes of Elizabethkingia meningoseptica (Chryseobacterium meningosepticum) bacteremia at a medical center in Taiwan, 1999-2006. Eur J Clin Microbiol Infect Dis. 2011;30:1271–8. doi: 10.1007/s10096-011-1223-0. [DOI] [PubMed] [Google Scholar]
- 8.Du Moulin GC. Airway colonization by Flavobacterium in an intensive care unit. J Clin Microbiol. 1979;10:155–60. doi: 10.1128/jcm.10.2.155-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoque SN, Graham J, Kaufmann ME, Tabaqchali S. Chryseobacterium (Flavobacterium) meningosepticum outbreak associated with colonization of water taps in a neonatal intensive care unit. J Hosp Infect. 2001;47:188–92. doi: 10.1053/jhin.2000.0908. [DOI] [PubMed] [Google Scholar]
- 10.Kirby JT, Sader HS, Walsh TR, Jones RN. Antimicrobial susceptibility and epidemiology of a worldwide collection of Chryseobacterium spp: Report from the SENTRY antimicrobial surveillance program (1997-2001) J Clin Microbiol. 2004;42:445–8. doi: 10.1128/JCM.42.1.445-448.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bloch KC, Nadarajah R, Jacobs R. Chryseobacterium meningosepticum: An emerging pathogen among immunocompromised adults. Report of 6 cases and literature review. Medicine (Baltimore) 1997;76:30–41. doi: 10.1097/00005792-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Arvanitidou M, Vayona A, Spanakis N, Tsakris A. Occurrence and antimicrobial resistance of Gram-negative bacteria isolated in haemodialysis water and dialysate of renal units: Results of a Greek multicentre study. J Appl Microbiol. 2003;95:180–5. doi: 10.1046/j.1365-2672.2003.01966.x. [DOI] [PubMed] [Google Scholar]
- 13.Lin YT, Chiu CH, Chan YJ, Lin ML, Yu KW, Wang FD, et al. Clinical and microbiological analysis of Elizabethkingia meningoseptica bacteremia in adult patients in Taiwan. Scand J Infect Dis. 2009;41:628–34. doi: 10.1080/00365540903089476. [DOI] [PubMed] [Google Scholar]
- 14.Isaac MI, Neetoo Y. An outbreak of Elizabethkingia meningoseptica neonatal meningitis in Mauritius. J Infect Dev Ctries. 2011;5:834–9. doi: 10.3855/jidc.1885. [DOI] [PubMed] [Google Scholar]
- 15.Hirsh BE, Wong B, Kiehn TE, Gee T, Armstrong D. Flavobacterium meningosepticum bacteremia in an adult with acute leukemia. Use of rifampin to clear persistent infection. Diagn Microbiol Infect Dis. 1986;4:65–9. doi: 10.1016/0732-8893(86)90058-1. [DOI] [PubMed] [Google Scholar]
- 16.Di Pentima MC, Mason EO, Jr, Kaplan SL. In vitro antibiotic synergy against Flavobacterium meningosepticum: Implications for therapeutic options. Clin Infect Dis. 1998;26:1169–76. doi: 10.1086/520309. [DOI] [PubMed] [Google Scholar]
- 17.Du Moulin GC. Airway colonization by Flavobacterium in an intensive care unit. J Clin Microbiol. 1979;10:155–60. doi: 10.1128/jcm.10.2.155-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser SL, Jorgensen JH. Reappraisal of the antimicrobial susceptibilities of Chryseobacterium and Flavobacterium species and methods for reliable susceptibility testing. Antimicrob Agents Chemother. 1997;41:2738–41. doi: 10.1128/aac.41.12.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin PY, Chen HL, Huang CT, Su LH, Chiu CH. Biofilm production, use of intravascular indwelling catheters and inappropriate antimicrobial therapy as predictors of fatality in Chryseobacterium meningosepticum bacteremia. Int J Antimicrob Agents. 2010;36:436–40. doi: 10.1016/j.ijantimicag.2010.06.033. [DOI] [PubMed] [Google Scholar]


