Abstract
Background:
This single center retrospective analysis was undertaken to identify the incidence, clinical impact, and prognostic factors for mortality associated with fungal blood stream infections (BSI) in cancer patients.
Materials and Methods:
One hundred and twenty four patients had 169 episodes of fungal BSI. Incidence has not changed over a 10 year period but non albicans candida species are the predominant fungal isolates. Mortality with fungal BSI was significantly higher than that with other microbial agents. Risk of mortality was associated with renal dysfunction and Candida albicans as the isolate. Type of chemotherapy, patient characteristics, and neutrophil count did not influence the mortality following fungal BSI.
Conclusion:
Fungal BSI is rare and the incidence has not changed in this hospital. Mortality associated with fungal BSI is high. Risk score at the time of developing fungal BSI has prognostic potential to identify patients with higher risk of mortality associated with fungal BSI.
Keywords: Fungal blood stream infections, oncology, hematology
Introduction
Blood stream infections (BSI) are a significant cause of morbidity and mortality in hematology and oncology patients.[1,2] There are many studies evaluating the risk factors for bacteremia and poor outcomes in patients with cancer. Some of the well-established risk factors include use of central venous access device (CVD),[3] prolonged antibiotic use,[4] nature of underlying malignancy,[5] intensity of treatment,[6] duration of neutropenia, intensity of neutropenia,[7,8] comorbidities, breakage of mucosal barriers,[9,10] and use of corticosteroids.[11] Various sources have reported information about BSI with bacterial agents in cancer patients of all ages,[12] and increasing incidence of resistant organisms has been noted.[13] Fungal BSI is not a common event, but is known to be associated with high risk of mortality in oncology patients and also in intensive care units.[14,15] Even though similar increase in BSI with Candida species has been seen,[16,17,18] there is very little data about the incidence and risk factors associated with fungal BSI in cancer patients. Last decade has seen significant developments in the field of oncology with availability of newer classes of drugs, the use of intensive treatment regimens and offering more intensive treatments to patients with advancing age. It is well-established that the use of azoles as prophylaxis during anticancer therapy reduces the risk of Candida infections,[19,20] but the impact of other fungal BSI on mortality has not been extensively reported. This single center analysis was undertaken to evaluate the spectrum of pathogens isolated, changes in temporal patterns, antibiotic sensitivities, and impact on mortality within 30 days in patients with hematological and nonhematological malignancies.
Materials and Methods
The Christie National Health Service (NHS) Foundation Trust is a stand-alone Cancer Center located in the North-West region of England. This retrospective analysis was carried out at The Christie NHS Foundation between the time period from April 2002 to April 2012 as a part of the ongoing infection control program. The data collection and analysis was approved by Hospital Audit Committee. As this was a part of the ongoing infection control program and the analysis was retrospective not involving patient particulars, informed consent was not obtained specifically from individual patients for this project. A total of 54,788 blood cultures were requested in 3,750 patients with cancer at this hospital. Of these, 9,628 (17.6%) yielded positive results. Amongst them, 124 patients had positive fungal isolate on 169 occasions and formed the sample population for this study. Patient characteristics of individual episodes are shown in Table 1. Details of full blood investigations at the detection of fungal BSI are shown in Table 2. As patients with all types of malignancies were included in the analysis, treatments included surgery, radiotherapy, or chemotherapy. Chemotherapy was classified as low intensity if the anticipated duration of neutropenia was likely to be less than 2 weeks and high intensity if it was likely to be more than 2 weeks. The study group also included patients who received radiotherapy, but were not neutropenic and post-operative cases. Exact details of treatment regimen were not available in many cases as they were treated in satellite peripheral centers attached to The Christie Hospital.
Table 1.
Patient characteristics

Table 2.
FBC and biochemical parameters at the detection of fungal BSI

Microbiological investigations
All patients with oncology diagnosis who developed fever had standard investigations in the form of full blood count (FBC) and differential, renal function and liver function assessment, chest X-ray, C-reactive protein estimation, and blood cultures from central venous access (if in situ) and peripheral blood. Patients were treated with antimicrobials according to hospital policy that uses first-line choice of beta-lactamase resistant penicillin and gentamicin. Subsequent modifications were done according to results of microbiology, clinical conditions, or additional investigations including high resolution computed tomography (CT), scan of chest, and CT of paranasal sinuses.
For the purpose of analysis, events in which the same organism was isolated from the central venous device and peripheral blood on the same day, the same organism was isolated from different lumens on the same day or the same organism isolated on two different days, but from the same sample was considered as a single episode.
Antifungal prophylaxis
Prophylaxis policy for individuals was according to the institutional disease specific guidelines. Hematology patients undergoing chemotherapy or stem cell transplant received either fluconazole or itraconazole as antifungal prophylaxis, except patients with acute lymphoblastic leukemia who received liposomal amphotericin 3 days a week as prophylaxis. Neutropenic patients also received quinolone prophylaxis and patients at risk of pneumocystis infections (myeloma, autograft and allograft) received prophylaxis with either cotrimoxazole or inhalational pentamidine.
Statistical analysis
The data were analyzed in June 2013. STATA v 10 (STATA corp, Texas USA)[21] was used for all the statistics. Incidence of and mortality with fungal BSI was estimated using Kaplan-Meir[22] method. For temporal trend analysis, patients were divided into two groups, that is, between 2002-2007 and 2007-2012. As the aim of analysis was to ascertain the impact of fungal BSI on survival, entry time was the date of positive fungal BSI till date of death within 30 days or last follow-up. Univariate analysis was used to evaluate the effect of demographic factors, disease parameters, and biochemical parameters on the incidence of fungal BSI and comparisons made using log-rank method. Multivariate analysis using cox regression analysis[23] was used to identify factors independently influencing the risk of mortality. Factors identified in multivariate analysis were utilized to design the risk score predictive of mortality.
Results
Out of 3,750 patients with cancer who had blood cultures sent, 124 (3.3%) were positive for fungal isolates. As many patients had polymicrobial BSI, analysis was done for each episode and out of 9,628 BSI, 169 (1.8%) were due to fungal pathogens. Common underlying diagnosis was acute leukemia in hematological cancers and gastrointestinal malignancy in nonhematological cases. There was no difference in the incidence of fungal BSI between the years 2002-2007 and 2007-2012 (87/4522:1.9% vs 82/3838:2.1%, P = 0.49). The incidence was not related to intensity of preceding chemotherapy, but surprisingly there was a lower chance of fungal BSI in post-hematopoetic stem cell transplant (HSCT) cases (8 vs 23%). Interestingly, 42 episodes developed in patients who did not receive any chemotherapy leading up to development of BSI. Source of blood sample was CVD in 51% cases and peripheral blood in 47% cases. Biochemical and blood parameters are shown in Table 2. Surprisingly, only 19% patients had neutropenia at the time of event. Renal impairment as determined by raised serum creatinine or raised blood urea nitrogen was seen in 29% cases, while raised bilirubin was present in 36% cases. Fungal isolate was Candida albicans (n = 36), Candida parapsilosis (n = 51), Candida glabrata (n = 15), Candida guilliermondii (n = 14), other non-albicans Candida (n = 38), Rhizobium (n = 10), Rhodotorula (n = 2), and one each had Nocardia, Saccharomyces, and Fusarium.
Mortality and predictors of mortality
Mortality within 30 days of fungal BSI was used as a marker to assess the clinical impact. Twenty-eight of 169 episodes resulted in mortality at or before 30 days (30 day probability 17.4%, standard error: +2.7, [Figure 1]. In the entire population of 9,768 positive BSI, mortality was significantly higher with fungal isolates compared to other organisms (17.4 vs 11%, P = 0.019, [Figure 2]. In univariate analysis, there was no effect of gender (female: 13/81 vs male: 15/88; P = 0.99), type of malignancy (hematology: 6/44 vs 22/125, P = 0.5), source of culture (CVD: 9/87 vs peripheral: 16/71 vs not specified: 3/11, P = 0.07) or neutropenia (absolute neutrophil count (ANC) < 0.5: 5/30 vs ANC > 0.5 23/139, P = 0.95). There was significantly higher risk of mortality with blood urea nitrogen (BUN) above 10 (20/51 vs 8/118, P < 0.0001), creatinine above 120 (12/43 vs 16/126, P = 0.009), bilirubin more than 50 (15/60 vs 13/109, P = 0.02), alkaline phosphatase above 100 (17/68 vs 11/101, P = 0.01), AST above 50 (14/55 vs 14/114, P = 0.018), and gamma glutamyl transpeptidase (GGT) above 150 (21/95 vs 7/74, P = 0.025). In multivariate cox analysis [Table 3], only BUN above 10 (hazard ratio (HR): 5.2, 95% confidence interval (CI): 2.6-10.4, P = 0.001) [Figure 3] creatinine above 120 (HR: 3.03, 95% CI: 1.3-7.04, P = 0.01) [Figure 4] and Candida albicans isolate (HR: 2.35, 95% CI: 1.12-4.9, P = 0.023) [Figure 5] were independently associated with an increased risk of mortality. These three risk factors were combined to divide the patients into two groups, those with one or less risk factor and those with more than one risk factor and the mortality was significantly higher in the latter group (20/54 vs 8/115, P < 0.0001) [Figure 6].
Figure 1.

Probability of 30 day mortality with fungal blood stream infections
Figure 2.

Comparison of 30 day mortality between fungal BSI and BSI with other microbes (P = 0.019)
Table 3.
Multivariate cox analysis

Figure 3.

Effect of raised urea on 30 day mortality with fungal blood stream infections (P > 0.001)
Figure 4.
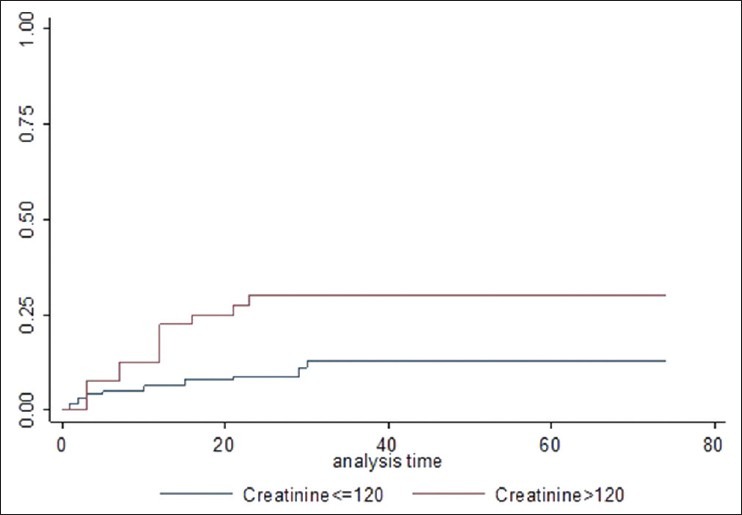
Effect of raised creatinine on 30 day mortality with fungal blood stream infections (P = 0.01)
Figure 5.

Effect of Candida sub-species on 30 day mortality (P = 0.023)
Figure 6.
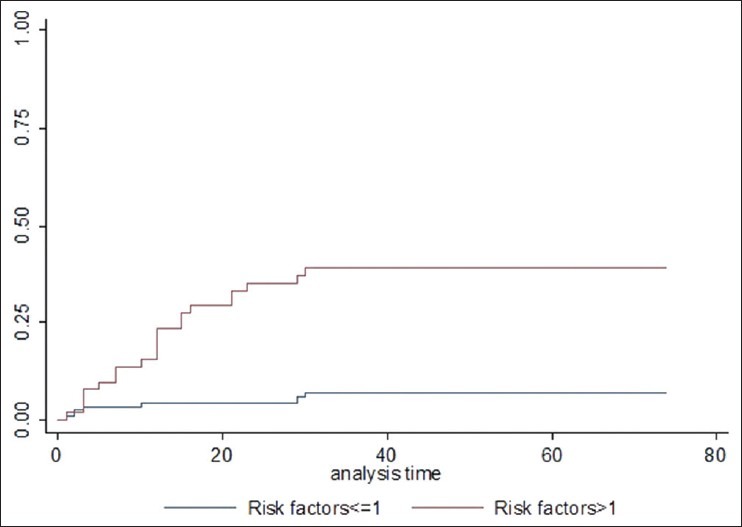
Effect of risk factors on 30 day mortality with fungal blood stream infections (P > 0.0001)
Discussion
The importance of Candida species as a cause of BSI has been highlighted in many studies in the past few years. In a study analyzing nosocomial BSI in the United States (Surveillance and Control of Pathogens of Epidemiological Importance (SCOPE)), Candida species was found to be the fourth most common cause of BSI.[17] Another study reported 14.5% increase in mortality associated with Candida BSI.[18] The risk factors for the development of Candida septicemia are well-described, but BSI due to other fungal agents is a rare event. Therefore, very little is known about these cases. The aim of this look back study was to evaluate if the fungal BSI are an increasing problem in our practice. In view of the recent developments in the anticancer therapeutics it is important to know the impact on the infective complications and the associated agents that may compromise the overall outlook for the patient. There is limited data on the temporal changes of fungal isolates in cancer patients. It was reassuring to know that the incidence of fungal BSI is low and has not increased over time. This is in contrast to the reports from American and European Registries[17,18] and the reasons are unclear. One of the possibilities is the low number of cases of fungal BSI in our study. Routine use of azoles as prophylaxis in neutropenic patients as has been described to reduce the incidence of fungal infections[19] may be a factor, but this is unlikely to be the sole factor, as 81% of our patients were not neutropenic and hence would not have been on prophylactic azoles. In this study we did not isolate any mould BSI and whether this due to patient selection, use of high-efficiency particulate air or over representation of bacterial pathogens remains to be evaluated.
It was interesting to find that neutrophil counts do not correlate with fungal BSI, as prolonged neutropenia is supposed to be the most important risk factor for invasive fungal infections.[24,25] Whether this relates to different behavior of organ specific fungal infections and fungal BSI remains to be determined. This probably reflects in the fact that a significant majority of fungal BSI developed in patients who were not in the post-cancer therapy period. It is difficult to speculate if this could be related to the underlying immune dysfunction arising from previous therapy like in post HSCT cases. The small number of cases did not permit us to address this specific question.
Although the percentage of Candida species has not changed over two time periods it was noteworthy that non-albicans Candida are the predominant isolates. It has been well-established in animal experiments that Candida albicans is more likely to pass across mucosal barriers, is more likely to be associated with septicemia and has worse prognosis.[26,27] Our study confirms the prognostic importance of Candida albicans. As we do not undertake routine susceptibility testing it is not possible to comment upon the drug resistance patterns in our sample group.
Patients who develop infective complications need antimicrobial agents that are known to cause organ toxicity especially to kidneys and liver. Hence, events after the development of infections and the need for antimicrobial agents cannot be manipulated to avoid the outcome. For this reason, we chose parameters at the time of fungal BSI to assess if it was possible to predict the outcome in this group. The risk score identified in this study not only helps to predict the outcome in patients with fungal BSI, but also confirms that it is the organ function and not only the organism that decides the outcome of the infective event. To our knowledge, this is the first study to demonstrate the prognostic and predictive significance of objective organ function parameters on the mortality associated with fungal BSI.
In this analysis we have focused on the events after development of BSI and as such it does not answer the question if it is possible to predict who is likely to develop fungal BSI, but this may serve as a baseline to assess the interventions that may be needed in high risk patients. It is recommended that routine surveillance, aseptic techniques and avoidance of unscrupulous use of antimicrobials be undertaken in patients with hematological and nonhematological malignancies.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Rodríguez L, Ethier MC, Phillips B, Lehrnbecher T, Doyle J, Sung L. Utility of peripheral blood cultures in patients with cancer and suspected blood stream infections: A systematic review. Support Care Cancer. 2012;20:3261–7. doi: 10.1007/s00520-012-1471-2. [DOI] [PubMed] [Google Scholar]
- 2.Sancho S, Artero A, Zaragoza R, Camarena JJ, González R, Nogueira JM. Impact of nosocomial polymicrobial bloodstream infections on the outcome in critically ill patients. Eur J Clin Microbiol Infect Dis. 2012;31:1791–6. doi: 10.1007/s10096-011-1503-8. [DOI] [PubMed] [Google Scholar]
- 3.Mollee P, Jones M, Stackelroth J, van Kuilenburg R, Joubert W, Faoagali J, et al. Catheter-associated bloodstream infection incidence and risk factors in adults with cancer: A prospective cohort study. J Hosp Infect. 2011;78:26–30. doi: 10.1016/j.jhin.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: A persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syrjälä H, Ohtonen P, Kinnunen U, Räty R, Elonen E, Nousiainen T, et al. Finnish Leukemia Group. Blood stream infections during chemotherapy-induced neutropenia in adult patients with acute myeloid leukemia: Treatment cycle matters. Eur J Clin Microbiol Infect Dis. 2010;29:1211–8. doi: 10.1007/s10096-010-0984-1. [DOI] [PubMed] [Google Scholar]
- 6.Orasch C, Weisser M, Mertz D, Conen A, Heim D, Christen S, et al. Comparison of infectious complications during induction/consolidation chemotherapy versus allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:521–6. doi: 10.1038/bmt.2009.187. [DOI] [PubMed] [Google Scholar]
- 7.Samonis G, Vardakas KZ, Maraki S, Tansarli GS, Dimopoulou D, Kofteridis DP, et al. A prospective study of characteristics and outcomes of bacteremia in patients with solid organ or hematologic malignancies. Support Care Cancer. 2013;21:2521–6. doi: 10.1007/s00520-013-1816-5. [DOI] [PubMed] [Google Scholar]
- 8.Kontoyiannis DP, Vaziri I, Hanna HA, Boktour M, Thornby J, Hachem R, et al. Risk factors for Candida tropicalis fungemia in patients with cancer. Clin Infect Dis. 2001;33:1676–81. doi: 10.1086/323812. [DOI] [PubMed] [Google Scholar]
- 9.Gottfredsson M, Vredenburgh JJ, Xu J, Schell WA, Perfect JR. Candidemia in women with breast carcinoma treated with high-dose chemotherapy and autologous bone marrow transplantation. Cancer. 2003;98:24–30. doi: 10.1002/cncr.11470. [DOI] [PubMed] [Google Scholar]
- 10.Viscoli C, Girmenia C, Marinus A, Collette L, Martino P, Vandercam B, et al. Candidemia in cancer patients: A prospective multicenter surveillance study by the Invasive Fungal Infection Group. Clin Infect Dis. 1999;28:1071–9. doi: 10.1086/514731. [DOI] [PubMed] [Google Scholar]
- 11.Dix D, Cellot S, Price V, Gillmeister B, Ethier MC, Johnston DL, et al. Association between corticosteroids and infection, sepsis, and infectious death in pediatric acute myeloid leukemia (AML): Results from the Canadian infections in AML research group. Clin Infect Dis. 2012;55:1608–14. doi: 10.1093/cid/cis774. [DOI] [PubMed] [Google Scholar]
- 12.Liu CY, Liao CH, Chen YC, Chang SC. Changing epidemiology of nosocomial bloodstream infections in 11 teaching hospitals in Taiwan between 1993 and 2006. J Microbiol Immunol Infect. 2010;43:416–29. doi: 10.1016/S1684-1182(10)60065-5. [DOI] [PubMed] [Google Scholar]
- 13.Liss BJ, Vehreschild JJ, Cornely OA, Hallek M, Fätkenheuer G, Wisplinghoff H, et al. Intestinal colonisation and blood stream infections due to vancomycin-resistant enterococci (VRE) and extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBLE) in patients with haematological and oncological malignancies. Infection. 2012;40:613–9. doi: 10.1007/s15010-012-0269-y. [DOI] [PubMed] [Google Scholar]
- 14.Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: Data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48:1695–703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 15.Leroy O, Gangneux JP, Montravers P, Mira JP, Gouin F, Sollet JP, et al. AmarCand Study Group. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: A multicenter, prospective, observational study in France (2005-2006) Crit Care Med. 2009;37:1612–8. doi: 10.1097/CCM.0b013e31819efac0. [DOI] [PubMed] [Google Scholar]
- 16.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: A persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wisplinhoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 18.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: A propensity analysis. Clin Infect Dis. 2005;41:1232–39. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 19.Marr KA, Seidel K, Slavin MA, Bowden RA, Schoch HG, Flowers ME, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: Long-term follow-up of a randomized, placebo-controlled trial. Blood. 2000;96:2055–61. [PubMed] [Google Scholar]
- 20.Marr KA, Seidel K, White TC, Bowden R. Candidemia in allogenic blood and marrow transplant recipients: Evolution of risk factors after the adoption of prophylactic fluconazole. J Infect Dis. 2000;181:309–16. doi: 10.1086/315193. [DOI] [PubMed] [Google Scholar]
- 21.Stata Statistical Software: Release 10. College Station, TX: StataCorp LP; 2007. StataCorp. [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–81. [Google Scholar]
- 23.Cox DR. Regression Models and Life Tables (with Discussion)”. J Royal Statistical Society, Series B. 1972;34:187–220. [Google Scholar]
- 24.Nesher L, Rolston KV. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection. 2013 doi: 10.1007/s15010-013-0525-9. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim KY, Pierrotti LC, Freire MP, Gutierrez PP, Duarte LD, Bellesso M, et al. Health care-associated infections in hematology-oncology patients with neutropenia: A method of surveillance. Am J Infect Control. 2013 doi: 10.1016/j.ajic.2013.03.299. [DOI] [PubMed] [Google Scholar]
- 26.Mellado E, Cuenca-Estrella M, Regadera J, Gonzalez M, Diaz-Guerra TM, Rodriguez-Tudela JL. Sustained gastrointestinal colonization and systemic dissemination by Candida albicans, Candida tropicalis and Candida parapsilosis in adult mice. Diagn Microbiol Infect Dis. 2000;38:21–8. doi: 10.1016/s0732-8893(00)00165-6. [DOI] [PubMed] [Google Scholar]
- 27.Anaissie E, Hachem R, K-Tin-U C, Stephens LC, Bodey GP. Experimental hematogenous candidiasis caused by Candida krusei and Candida albicans: Species differences in pathogenicity. Infect Immun. 1993;61:1268–71. doi: 10.1128/iai.61.4.1268-1271.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


