Abstract
The clinical sequelae from bone metastases, termed skeletal-related events (SREs), are among the most frequent and debilitating complications in patients with advanced cancer. Bone metastases are characterized by pathologically increased osteoclast activity, and accumulating evidence indicates that tumor cells interact within the bone to stimulate the receptor activator of nuclear factor kB (RANK)-RANK ligand (RANKL) pathway. RANKL is an essential mediator of osteoclast formation, function, and survival. Because of the central role of RANKL in cancer-induced bone destruction, the inhibition of RANKL has the potential to result in the reduction of pathologic bone resorption. Denosumab is a fully human monoclonal antibody specific for RANKL that inhibits the formation, activation, and survival of osteoclasts. This in turn decreases bone resorption and reduces cancer-induced bone destruction. In this review, we give an overview of the drug Denosumab with its history, mechanism of action, clinical trial data, adverse effects, and future challenges.
Keywords: Bone metastasis, denosumab, skeletal-related events
Introduction
Bone metastasis
Bone metastases occur in more than 1.5 million patients with cancer worldwide[1] and are most commonly associated with cancers of the prostate, lung, and breast; with incidence rates as high as 75% of patients with metastatic disease.[2,3,4,5,6] Bone metastases can result in incapacitating clinical sequelae including skeletal-related events (SREs).[7] SREs can cause debilitating pain that often requires aggressive management with radiation therapy and narcotic analgesics, pathologic fractures that may impair ambulation, surgery to prevent or treat pathologic fractures or manage pain, and spinal cord compressions that can result in numbness or weakness, urinary or fecal incontinence, and paralysis.
A key objective in managing the skeletal morbidity associated with bone metastases is to inhibit excessive osteolysis and interrupt the vicious cycle of bone destruction, tumor growth, and further bone destruction; thus preventing or delaying the complications from bone metastases (i.e., SREs). The underlying pathophysiology of bone metastases, irrespective of the underlying malignancy and radiographic appearance as osteolytic, mixed, or osteoblastic includes a locally increased pathologic rate of bone remodelling, including increased osteoclast activity.[8,9] Accumulating evidence shows that tumor cells interact within the bone to stimulate the receptor activator of nuclear factor kB (RANK)-RANK ligand (RANKL) system, leading to cancer-induced bone destruction.[8] Additional data suggest that RANKL also may play a role in primary tumorigenesis and metastasis.
RANK and RANKL and role in bone metastasis
The expression of RANKL is controlled by numerous cytokines and hormones, commonly known as regulators of the immune system and calcium homeostasis. Among the proresorptive factors are 1,25(OH) 2 vitamin D3, parathyroid hormone (PTH), and parathyroid hormone-related protein (PTHrP), prostaglandin E2, interleukin-1 and -6, tumor necrosis factor (TNF), prolactin, and corticosteroids. However; estrogens, calcitonin, transforming growth factor-beta, platelet-derived growth factor, and calcium induced osteoprotegerin (OPG) expression, leading to neutralization of RANKL; and thereby inhibition of osteoclastogenesis and resorption.[10]
Recent evidence indicates that osteoblastic metastases form on trabecular bone at sites of previous osteoclast resorption. In fact, such resorption is necessary for subsequent osteoblastic bone formation.[11,12] These findings suggest that prostate cancer cells induce bone production through an overall increase of bone remodelling.[13] In fact, animal models of prostate cancer metastasis have shown that skeletal lesions frequently exhibit increased osteoclast activation and osteolysis, although bone metastases radiologically have an osteoblastic appearance.[14] When neoplastic cells from prostate cancer metastasize to bone, they initially induce osteoclastogenesis and bone resorption. RANK, RANKL, and its soluble decoy receptor OPG play an essential role in the regulation of this process.[15] It has been shown that metastatic prostate cancer cells in bone (and not at other sites) express both RANKL and its “antagonist” OPG. OPG, however, seems to have a primarily nuclear localization in these cells, whereas normally it is expressed in the cytoplasm. This could affect the bioavailability of this protein.[16] Similar findings have been made in in vitro experiments with multiple myeloma cells.[17] Breast cancer cells have been shown not only to express RANK,[18] but also to upregulate RANKL expression by osteoblasts and bone marrow stromal cells.[19] Prostate cancer cells can also upregulate RANKL expression in osteoblasts.[20]
RANKL inhibitors: Development
Numerous experimental models of bone metastasis have shown that RANKL antagonists prevent tumor-associated osteolysis and significantly reduced skeletal tumor burden.[21] Animal models that mimic advanced prostate, breast, or non-small cell lung cancer, representing both osteolytic and osteoblastic skeletal lesions, have demonstrated that the RANKL inhibitors RANK-Fc or OPG-Fc were effective in preventing or delaying of bone metastases and reducing progression of tumors in the skeleton [Figure 1].[22] In addition, preclinical models of multiple myeloma bone disease have shown that inhibition of RANKL reduced osteolysis[23] and tumor burden while increasing survival.[24] Various studies have also evaluated the effect of RANKL inhibition in conjunction with chemotherapeutic agents.
Figure 1.
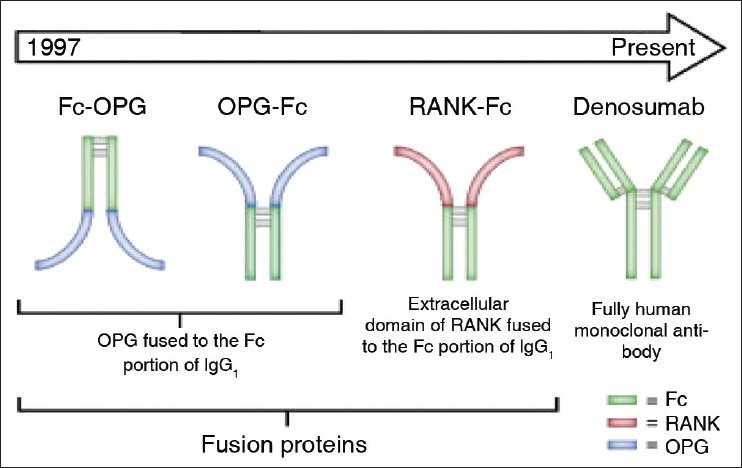
Denosumab-Development
The combination of RANKL inhibition with hormonal therapy or chemotherapy resulted in significantly greater inhibition of skeletal tumor growth than either single agent alone in the breast, prostate, or lung cancer models examined, respectively. In addition to mediating tumor-induced bone destruction, RANKL also seems to be involved in tumorigenesis and metastasis. Treatment of RANK-expressing cancer cell lines with RANKL stimulates production of osteotropic factors and enhances the migration of RANK expressing tumor cells to bone.[25] A model of carcinogen and hormone-induced breast cancer demonstrated that RANKL inhibition with RANK-Fc significantly delayed mammary tumor formation in transgenic mice and almost completely blocked tumor formation in wild-type mice.[26] The reduction and delay in mammary tumor formation was not observed with IV zoledronic acid. This suggests that the effect of RANKL on tumor formation is independent from the effect of RANKL on osteoclastogenesis.
Denosumab: Mechanism of Action
Denosumab is a fully human immunoglobulin G2 monoclonal antibody with high affinity and specificity for human RANKL. By binding to RANKL, denosumab inhibits RANKL from activating its only receptor RANK on the surface of osteoclasts and their precursors. Prevention of RANKL-RANK interaction inhibits osteoclast formation, function, and survival; thereby decreasing bone resorption and interrupting cancer-induced bone destruction.
Denosumab: Drug metabolism and elimination
Denosumab follows nonlinear, dose-dependent pharmacokinetics. The bioavailability of one subcutaneous denosumab injection is 61% and serum concentrations are detected within 1 h. Maximal serum concentrations are achieved in 5-21 days and denosumab may be detectable for 9 months or longer. Based upon monoclonal antibody pharmacokinetics, denosumab is most likely cleared by the reticuloendothelial system with minimal renal filtration and excretion. The elimination half-life of denosumab is 32 days, and the terminal half-life is 5-10 days. Denosumab does not incorporate into bone.
Denosumab: Early phase clinical trials
A phase 1 study compared the safety, pharmacokinetics, and pharmacodynamics of denosumab in patients with multiple myeloma and bone lesions (n = 25) or breast cancer and bone metastases (n = 29) with intravenous (IV) pamidronate.[27] Administration of denosumab resulted in rapid reductions in biochemical markers of bone turnover, in a dose-dependent manner, which lasted up to 13 weeks. Furthermore, safety data suggested that denosumab was well-tolerated by these patients.
Two separate phase 2 trials evaluated the efficacy and safety of multiple dosing regimens in patients with cancer and bone metastases. In patients with breast cancer and bone metastases who were naive to treatment with IV bisphosphonates (n = 255), denosumab suppressed bone turnover similarly to that of IV bisphosphonates.[28,29]
The second phase 2 study evaluated the effect of denosumab treatment in patients with advanced cancer and bone metastases or multiple myeloma with bone disease who had previously been treated with IV bisphosphonates (n = 111) yet still had elevated concentrations of urinary N-telopeptide normalized to urinary creatinine (uNTx/Cr) (≥50 nmol/mmol). A significantly greater proportion of patients who received denosumab had uNTx/Cr levels ≥50 nmol/mmol at week 13 compared with those who continued receiving IV bisphosphonate therapy.[30] These data suggest that denosumab treatment may further suppress markers of bone resorption even in patients who were previously treated with IV bisphosphonates. Although these studies were not powered to detect differences in the incidence of SREs between treatment groups, fewer SREs were experienced by patients in the denosumab than in the IV bisphosphonate groups. Results from both trials also showed that denosumab was well tolerated by this patient population.
These phase 2 trials also compared multiple dosing regimens of denosumab. The 120 mg dose of denosumab administered subcutaneously (SC) once every 4 weeks (Q4W) was the minimal dose that maintained maximal suppression of bone turnover over the entire dosing interval in a high proportion of patients. This dose was selected for use in the registrational trials in patients with advanced cancer and bone metastases.
Denosumab: Pivotal Phase III registrational trials; comparison to zoledronic acid
The first phase III trial to compare directly denosumab to zoledronic acid in patients with metastatic breast cancer had a total of 2,046 biphosphonate naive patients (except treatment with oral bisphosphonates for osteoporosis). Patients received denosumab 120 mg or zoledronic acid 4 mg every 4 weeks. Primary study endpoint was time to first on-study SRE (noninferiority); secondary endpoints consisted of time to first on-study SRE (superiority) and time to first and subsequent on-study SREs.[31] In the denosumab group, a significant delay in time to first on-study SRE was observed (hazard ratio (HR) 0.82; 95% confidence interval (CI) 0.71-0.95; P < 0.001 noninferiority). Median time to first on-study SRE was 26.4 months in patients receiving zoledronic acid, and was 32.4 months in the denosumab group. Furthermore, denosumab reduced the risk of experiencing multiple SREs (analysis of time to first and subsequent on-study SRE) significantly (HR 0.77; 95% CI 0.66-0.89; P = 0.001). A similar time to pain improvement was observed in both treatment arms. Rates of severe and serious adverse events were similar between both treatment groups. Significantly more cases of pyrexia, bone pain, arthralgia, and renal failure were observed in the zoledronic acid group; while hypocalcemia and toothache, not associated with the development of osteonecrosis of the jaw (ONJ), were seen more often in patients receiving denosumab. Importantly, the overall rate of ONJs, was similar in the respective treatment groups [Table 1].[31,32]
Table 1.
Primary end point of three RCT of Denosumab, time to first onset SRE, and results
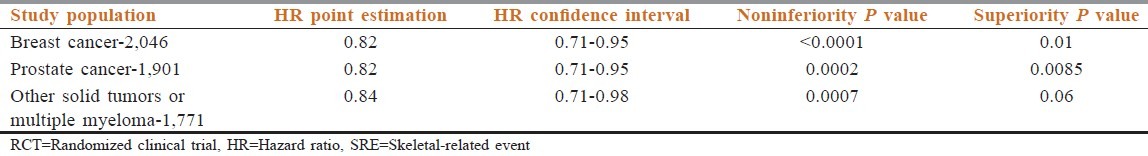
Another phase III trial was conducted in a mixed population of patients with different advanced solid cancers (excluding prostate and breast cancer) and multiple myeloma. Similar to NCT00321464 trial in breast cancer, the primary study endpoint, noninferiority to zoledronic acid, was met (HR 0.84; 95% CI 0.71-0.98; P = 0.0007), although a superiority of denosumab was not established. The trial design and dosage of denosumab and zoledronic acid were identical to the former trial. Overall, 1,776 patients were randomized to receive bone-targeted therapy over a 34-month trial horizon. The investigators reported equivalence in time to first SRE between drugs.[33] The proportion of patients developing any SRE was 31.4 and 36.3% in the denosumab and zoledronic acid groups, respectively (the P value was not significant) (Henry et al., 2011).
In the trial by Fizazi et al., which compared zoledronic acid to denosumab in prostate cancer, 1,901 patients meeting the eligibility criteria were randomized one to one to either denosumab 120 mg subcutaneous injection or to IV injection of zoledronic acid 4 mg adjusted for creatinine clearance.[34] The primary endpoint was time to first SRE. Patients randomized to receive denosumab had a longer time to first SRE (20.7 vs 17.1 months; HR 00.82; P = 0.008). Over the 41-month study duration, the proportion of denosumab and zoledronic acid patients developing at least one SRE was 35.9 and 40.6%, respectively, for an absolute difference of 4.7% [Table 1].
Denosumab was also safer than zoledronic acid in terms of acute drug reactions and renal toxicity. Similar to the results of the other studies the incidence of ONJ and hypocalcemia were higher in the denosumab group
Denosumab: Phase III studies; Prevention of bone loss in cancer patients
NCT00089674, also known as the Hormone Ablation Bone Loss Trial (HALT)-prostate cancer trial, was a randomized double-blind, placebo-controlled phase III trial that accrued 1,468 men with nonmetastatic prostate cancer receiving androgen deprivation therapy (ADT). The purpose was to evaluate denosumab in the prevention of bone loss in this group of patients. The subjects were randomized to either 60 mg of denosumab by subcutaneous injection every 6 months or placebo, together with calcium and vitamin D supplements. The primary endpoint was percent change of bone mineral density (BMD) in the lumbar spine after 24 months of treatment, and fracture rate was a secondary endpoint. The results indicated a significant difference between the two treatment arms, with a 5.6% increase in BMD in the denosumab group and a 1.0% decrease in the placebo group (P = 0.001). There was also a significant difference in vertebral fracture rate at 36 months in favor of denosumab: 1.5 vs 3.9% (P = 0.006). Rates of adverse events were similar between the two groups, and no cases of were reported [Table 2].[35]
Table 2.
Denosumab: Prevention of bone loss studies and the results
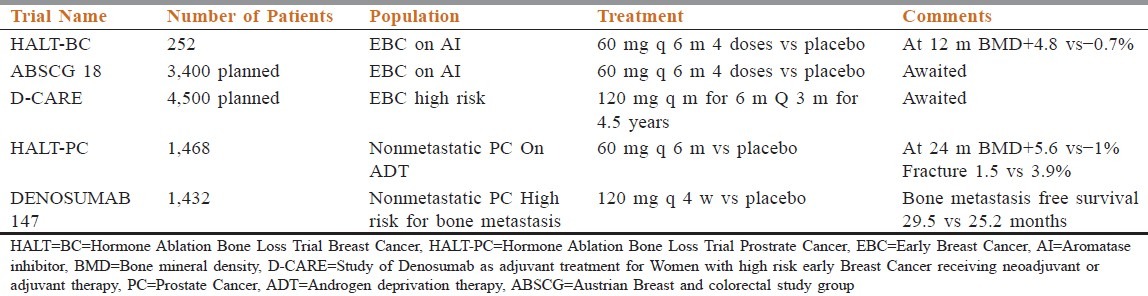
In another phase 3, double-blind, randomized, placebo-controlled study in men with nonmetastatic castration resistant prostate cancer at high risk of bone metastasis received denosumab 120 mg or subcutaneous placebo every 4 weeks. The primary endpoint was bone metastasis-free survival, a composite endpoint determined by time to first occurrence of bone metastasis (symptomatic or asymptomatic) or death from any cause. A total of 1,432 patients were randomly assigned to treatment groups (716 denosumab, 716 placebo). Denosumab significantly increased bone metastasis-free survival by a median of 4.2 months compared with placebo (median 29.5 (95% CI 25.4-33.3) vs 25.2 (22·2-29.5) months; HR 0.85, 95% CI 0.73-0.98, P = 0.028). Denosumab also significantly delayed time to first bone metastasis (33.2 (95% CI 29.5-38.0) vs 29.5 (22.4-33.1) months; HR 0.84, 95% CI 0.71-0·98, P = 0.032). Overall survival did not differ between groups (denosumab 43.9 (95% CI 40.1-not estimable) months vs placebo 44.8 (40.1-not estimable) months; HR 1.01, 95% CI 0.85-1.20, P = 0·91). Rates of adverse events and serious adverse events were similar in both groups, except for ONJ and hypocalcemia. Thirty-three (5%) patients on denosumab developed ONJ vs none on placebo. Hypocalcemia occurred in 12 (2%) patients on denosumab and two (<1%) on placebo.[36]
HALT Breast Cancer (HALT-BC) trial included 252 hormone receptor positive breast cancer patients on aromatase inhibitor therapy in adjuvant clinical setting. They were randomly assigned to receive either 60 mg of subcutaneous denosumab every 6 months (127 patients) or placebo (125 patients). All patients received supplementary vitamin D and calcium. The primary outcome measure was change in BMD. At 12 and 24 months, lumbar spine BMD had increased by 5.5 and 7.6%, respectively, in the denosumab arm vs placebo (P = 0.0001), with decreased bone turnover markers in the denosumab group. No differences in adverse events were found between the two groups.[37]
Denosumab: Safety profile
Denosumab is generally well tolerated. Most significant safety concerns with denosumab are ONJ and infections.
ONJ is a serious adverse event in treatment targeting osteoclasts which include bisphosphonates given intravenously and denosumab. ONJ is a form of avascular necrosis where there is persistence of exposed, necrotic bone in the oral cavity for more than 8 weeks, and where there is no history of local evidence of malignancy or radiation exposure in the affected region.[38] The association between osteoclast-targeted therapy and ONJ was first reported with IV bisphosphonates in the early 2000s. Overall, this relationship has been best described in patients receiving frequent IV bisphosphonate therapy such as pamidronate or zoledronic acid for prevention or management of skeletal-related complications of cancer.[39]
ONJ has also been observed with denosumab therapy, primarily in the cancer patient population with bone metastases where denosumab is given every 4 weeks. An integrated analysis examined the frequency of ONJ in three blinded phase III trials in cancer patients with bone metastases comparing denosumab 120 mg SC vs zoledronic acid 4 mg intravenously given every 4 weeks.[40] In this analysis, the cumulative rate of ONJ was similar for both arms: 1.3 and 1.8% in year 3 for zoledronic acid and denosumab, respectively. The median time of drug exposure before ONJ was 14 months for both treatment groups. Tooth extraction was the main risk factor associated with the development of ONJ.
In trials of patients where denosumab is given every 6 months to prevent bone loss (rather than to treat metastatic disease), ONJ has not been reported. For example, ONJ was not seen in the Future REvascularization Evaluation in patients with Diabetes mellitus: Optimal management of Multivessel disease (FREEDOM) registration trial, which enrolled postmenopausal women between the ages of 60 and 90 years with osteoporosis and randomized 3,902 women to the denosumab arm; denosumab was given at 60 mg SC every 6 months for 3 years.[41] Similarly, in the trials where denosumab was given to prevent bone loss in patients with cancer, ONJ was not observed in the HALT-BC trial[35] or the HALT-PC trial.[37] However, in the denosumab 147 study, where denosumab 120 mg was given every 4 weeks, ONJ occurred in 5% of the denosumab arm over the course of the study. The absence of ONJ observed in the HALT-BC and HALT-PC bone loss prevention trials may be related to the lower intensity of denosumab administration in this patient population, as denosumab was given 60 mg every 6 months vs 120 mg every 4 weeks in the metastatic cancer patient population. Nevertheless, there have been case reports of ONJ where denosumab is given to prevent bone loss. For example, there were two cases of ONJ reported in patients who received denosumab for an additional 2 years after the FREEDOM trial.
Infections
A possible effect on the immune system by denosumab was postulated based on preclinical data where RANKL was found to be a costimulatory cytokine for T-cell activation[42] and lymphocyte development.[43] However, the clinical data with denosumab is conflicting on this issue. A meta-analysis of nine randomized controlled trials where denosumab was used to mitigate bone loss in patients with osteoporosis or early breast cancer (EBC) showed an increased risk for infection with an odds ratio of 4.45 (95% CI 1.15-17.14).[44] On the other hand, in the FREEDOM study (which was analyzed in the above meta-analysis), there was no clear relationship between overall infections and exposure to denosumab.[45] However, skin infections such as cellulitis, including erysipelas, while infrequent, occurred significantly more in the denosumab arm (0.3%) compared to placebo (0.03%).[45] These infections were not related to the injection site.[45] Also related to the skin, eczema occurred more frequently in the denosumab group (3%) compared to the placebo group (1.7%) in this trial.
No dose adjustment is recommended for renal or hepatic impairment with available data for denosumab.
Denosumab: Food and drug administration approved indications and dosage
Prevention of SRE in prevention of bone metastasis from solid tumors; November 2010. Dose 120 mg SC once in 4 weeks
Postmenopausal women with high fracture risk; June 2010. Dose 60 mg SC once in 6 months
For increasing bone mass in patients at high risk of fracture including ADT for nonmetastatic prostate cancer and on aromatase inhibitor (AI) for breast cancer; September 2011. Dose 60 mg SC once in 6 months
Unresectable giant cell tumor of bone in adults and skeletally mature adolescents; June 2013. Dose 120 mg SC once in 4 weeks, additional two doses on day 8 and 15 of 1st month.
Conclusions
Denosumab is an effective agent for minimizing bone loss associated with certain cancer treatments. It has the advantage of convenience with subcutaneous administration and is not associated with acute phase reactions or renal toxicity. Importantly, data are emerging that demonstrates its effect on minimizing disease progression in prostate cancer. It remains to be seen if the use of denosumab to prevent bone loss in the cancer patient population will improve overall survival and how long-term side effects such as ONJ will evolve over time. While the role of denosumab in patients with metastatic bone cancer has been established, denosumab will play an increasing role in the supportive care and treatment of patients with early stage cancer to prevent bone loss.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Jasmin C, Capanna R, Coleman RE, Coia LR, Saillant G, editors. Textbook of bone metastases. Hoboken: John Wiley and Sons; 2005. pp. 105–22. [Google Scholar]
- 2.Buijs JT, van der Pluijm G. Osteotropic cancers: From primary tumor to bone. Cancer Lett. 2009;273:177–93. doi: 10.1016/j.canlet.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 3.Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer. 2000;88:2989–94. doi: 10.1002/1097-0142(20000615)88:12+<2989::aid-cncr14>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–94. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Selvaggi G, Scagliotti GV. Management of bone metastases in cancer: A review. Crit Rev Oncol Hematol. 2005;56:365–78. doi: 10.1016/j.critrevonc.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Viadana E, Cotter R, Pickren JW, Bross ID. An autopsy study of metastatic sites of breast cancer. Cancer Res. 1973;33:179–81. [PubMed] [Google Scholar]
- 7.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243–9s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 8.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 9.Yonou H, Ochiai A, Goya M, Kanomata N, Hokama S, Morozumi M, et al. Intraosseous growth of human prostate cancer in implanted adult human bone: Relationship of prostate cancer cells to osteoclasts in osteoblastic metastatic lesions. Prostate. 2004;58:406–13. doi: 10.1002/pros.10349. [DOI] [PubMed] [Google Scholar]
- 10.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, et al. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001;107:1235–44. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer. 2000;88(suppl 12):2989–94. doi: 10.1002/1097-0142(20000615)88:12+<2989::aid-cncr14>3.3.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Boyce BF, Hughes DE, Wright KR, Xing L, Dai A. Recent advances in bone biology provide insight into the pathogenesis of bone diseases. Lab Invest. 1999;79:83–94. [PubMed] [Google Scholar]
- 14.Yonou H, Kanomata N, Goya M, Kamijo T, Yokose T, Hasebe T, et al. Osteoprotegerin/osteoclastogenesis inhibitory factor decreases human prostate cancer burden in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice. Cancer Res. 2003;63:2096–102. [PubMed] [Google Scholar]
- 15.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANK-L-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Brown JM, Corey E, Lee ZD, True LD, Yun TJ, Tondravi M, et al. Osteoprotegerin and rank ligand expression in prostate cancer. Urology. 2001;57:611–6. doi: 10.1016/s0090-4295(00)01122-5. [DOI] [PubMed] [Google Scholar]
- 17.Farrugia AN, Atkins GJ, To LB, Pan B, Horvath N, Kostakis P, et al. Receptor activator of nuclear factor kappa b ligand expression by human myeloma cells mediates osteoclast formation in vitro and correlates with bone destruction in vivo. Cancer Res. 2003;63:5438–45. [PubMed] [Google Scholar]
- 18.Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–6. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 19.Thomas RJ, Guise TA, Yin JJ, Elliott J, Horwood NJ, Martin TJ, et al. Breast cancer cells interact with osteoblasts to support osteoclast formation. Endocrinology. 1999;140:4451–8. doi: 10.1210/endo.140.10.7037. [DOI] [PubMed] [Google Scholar]
- 20.Fizazi K, Yang J, Peleg S, Sikes CR, Kreimann EL, Daliani D, et al. Prostate cancer cells-osteoblast interaction shifts expression of growth/survival-related genes in prostate cancer and reduces expression of osteoprotegerin in osteoblasts. Clin Cancer Res. 2003;9:2587–97. [PubMed] [Google Scholar]
- 21.Roodman GD, Dougall WC. RANK ligand as a therapeutic target for bone metastases and multiple myeloma. Cancer Treat Rev. 2008;34:92–101. doi: 10.1016/j.ctrv.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Canon JR, Roudier M, Bryant R, Morony S, Stolina M, Kostenuik PJ, et al. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis. 2008;25:119–29. doi: 10.1007/s10585-007-9127-1. [DOI] [PubMed] [Google Scholar]
- 23.Croucher PI, Shipman CM, Lippitt J, Perry M, Asosingh K, Hijzen A, et al. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood. 2001;98:3534–40. doi: 10.1182/blood.v98.13.3534. [DOI] [PubMed] [Google Scholar]
- 24.Vanderkerken K, De Leenheer E, Shipman C, Asosingh K, Willems A, Van Camp B, et al. Recombinant osteoprotegerin decreases tumor burden and increases survival in a murine model of multiple myeloma. Cancer Res. 2003;63:287–9. [PubMed] [Google Scholar]
- 25.Tometsko M, Armstrong A, Miller R, Jones J, Chaisson M, Branstetter D, et al. RANK Ligand directly induces osteoclastogenic, angiogenic, chemoattractive and invasive factors on RANK-expressing human cancer cells MDAMB-231 and PC3. J Bone Miner Res. 2004;19:S25. [Google Scholar]
- 26.Gonzalez-Suarez E, Jacob AP, Jones J, Miller R, Roudier-Meyer MP, Erwert R, et al. RANK ligand mediates progestin-induced mammary epithelial proliferation and carcinogenesis. Nature. 2010;468:103–7. doi: 10.1038/nature09495. [DOI] [PubMed] [Google Scholar]
- 27.Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M, et al. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–8. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- 28.Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25:4431–7. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- 29.Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, et al. Extended efficacy and safety of denosumab in breast cancer patients with bone metastases not receiving prior bisphosphonate therapy. Clin Cancer Res. 2008;14:6690–6. doi: 10.1158/1078-0432.CCR-07-5234. [DOI] [PubMed] [Google Scholar]
- 30.Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–71. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 31.Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, De Boer RH, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J Clin Oncol. 2010;28:5132–9. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 32.Stopeck A, Martin M, Ritchie D, Body JJ, Paterson A, Viniegra M, et al. Effect of denosumab versus zoledronic acid treatment in patients with breast cancer and bone metastases: Results from the extended blinded treatment phase. Cancer Res. 2010:14–21. [Google Scholar]
- 33.Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–32. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 34.Fizazi K, Carducci M, Smith M, Damião R, Brown J, Karsh L, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet. 2011;377:813–22. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith MR, Egerdie B, Hernaxndez Toriz N, Feldman R, Tammela TL, Saad F, et al. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–55. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: Results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Smith J, et al. Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol. 2008;26:4875–82. doi: 10.1200/JCO.2008.16.3832. [DOI] [PubMed] [Google Scholar]
- 38.Task Force. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2007;65:369–76. doi: 10.1016/j.joms.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: Bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–61. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 40.Saad F, Brown JE, Van Poznak C, Ibrahim T, Stemmer SM, Stopeck AT, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: Integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341–7. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 41.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 42.Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, et al. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem. 1997;272:25190–4. doi: 10.1074/jbc.272.40.25190. [DOI] [PubMed] [Google Scholar]
- 43.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteo-clastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 44.Anastasilakis AD, Toulis KA, Goulis DG, Polyzos SA, Delaroudis S, Giomisi A, et al. Efficacy and safety of denosumab in postmenopausal women with osteopenia or osteoporosis: A systematic review and a meta-analysis. Horm Metab Res. 2009;41:721–9. doi: 10.1055/s-0029-1224109. [DOI] [PubMed] [Google Scholar]
- 45.Watts NB, Roux C, Modlin JF, Brown JP, Daniels A, Jackson S, et al. Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: Coinci-dence or causal association? Osteoporos Int. 2012;23:327–37. doi: 10.1007/s00198-011-1755-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


