Abstract
The interaction between neurons, astrocytes and endothelial cells plays a central role coupling energy supply with changes in neuronal activity. For a long time it was believed that glucose was the only source of energy for neurons. However, a growing body of experimental evidence indicates that lactic acid, generated by aerobic glycolysis in perivascular astrocytes, is also a source of energy for neuronal activity, particularly when the supply of glucose from the intravascular space is interrupted. Adenosine monophosphate-activated protein kinase (AMPK) is an evolutionary conserved kinase that couples cellular activity with energy consumption via induction of the uptake of glucose and activation of the glycolytic pathway. The uptake of glucose by the blood-brain barrier (BBB) is mediated by the transporter GLUT1, which is abundantly expressed in endothelial cells and astrocytic end-feet processes. Tissue-type plasminogen activator (tPA) is a serine proteinase that is found in endothelial cells, astrocytes and neurons. Genetic overexpression of neuronal tPA or treatment with recombinant tPA (rtPA) protects neurons from the deleterious effects of metabolic stress or excitotoxicity, via a mechanism independent of tPA’s ability to cleave plasminogen into plasmin. The work presented here shows that exposure to metabolic stress induces the rapid release of tPA from neurons but not from astrocytes. This tPA induces AMPK activation, membrane recruitment of GLUT1, and GLUT1-mediated glucose uptake in astrocytes and endothelial cells. Our data indicate that this is followed by the synthesis and release of lactic acid from astrocytes, and that the uptake of this lactic acid via the monocarboxylate transporter-2 (MCT-2) promotes survival in neurons exposed to metabolic stress.
Keywords: Tissue-type plasminogen activator (tPA), Glucose metabolism, Neuroprotection, Adenosine monophosphate-activated protein kinase (AMPK), Monocarboxylate transporter-2 (MCT-2)
1.0. Introduction
Astrocytes constitute a functional and anatomical link between neurons and endothelial cells. Indeed, almost 95% of the vascular surface of the BBB is ensheathed by perivascular astrocytes and the opposite pole of each astrocyte enters in contact with approximately 30 000 synapses (Bushong et al., 2002). Therefore, astrocytes are uniquely positioned to couple the uptake of nutrients from the intravascular space with changes in neuronal demands.
Glucose was considered for a long time to be the only source of energy for neurons (Simpson et al., 2007). However, a growing body of experimental evidence shows that lactate generated by astrocytes, via activation of the glycolytic pathway, is also a fuel for neuronal activity (Fox et al., 1988). These and other observations led to postulate the existence of an astrocyte-neuron lactate shuttle (ANLS) in which astrocytes metabolize glucose to lactate which is uptaken by neurons as an energy source (Pellerin and Magistretti, 1994) via the monocarboxylate transporter-2 (MCT-2), that is abundantly expressed in neurons (Simpson et al., 2007). Although lactate is able to sustain basal neuronal metabolism, glucose is a required substrate for synaptic activity (Bak et al., 2006), suggesting that while glucose is the main fuel for neuronal activity under physiological conditions, lactate synthesized and released by astrocytes is the principal source of energy when the supply of glucose is interrupted.
Adenosine monophosphate-activated protein kinase (AMPK) is an evolutionary conserved kinase that acts as an energy sensor, promotes cellular adaptation to metabolic stress, and couples cellular activity with energy consumption (Amato and Man, 2011). AMPK phosphorylation at Thr172 induces the uptake of glucose and turns on catabolic pathways that generate ATP such as glycogenolysis and glycolysis (Hardie, 2007). The uptake of glucose by endothelial cells and astrocytes in mediated by GLUT1, a member of the SLC2 family of transport proteins (Joost and Thorens, 2001, Joost et al., 2002) abundantly expressed in the luminal and abluminal surface of endothelial cells and in astrocytic end-feet processes. In the brain GLUT1 is detected in two different molecular weights: a 55 kDa form expressed only in the endothelial cells of the BBB, and a 45 kDa isoform found in perivascular astrocytes (Birnbaum et al., 1986). Variations in the concentration of glucose in the intravascular space induce changes in the expression of GLUT1 (Vannucci et al., 1997) and its redistribution from an intracellular pool to the luminal and abluminal membranes of endothelial cells, and to the end-feed processes of perivascular astrocytes (Simpson et al., 2007). Remarkably, astrocytes not only uptake glucose but also are the only cells in the brain able to store it as glycogen.
Tissue-type plasminogen activator (tPA) is a serine proteinase found in neurons, astrocytes and endothelial cells (Yepes et al., 2009). Our early work indicates that genetic overexpression of neuronal tPA or treatment with rtPA protects neurons from the deleterious effects of ischemia and deprivation of oxygen and glucose, via a mechanism independent of tPA’s ability to cleave plasminogen into plasmin (Echeverry et al., 2010, Wu et al., 2012, Wu et al., 2013). The results presented here show that oxygen and glucose deprivation (OGD) induces the rapid release of tPA from cerebral cortical neurons but not from astrocytes. This tPA activates AMPK in astrocytes and endothelial cells, promotes the recruitment of GLUT1 to their plasma membrane, and induces GLUT1-mediated uptake of glucose, followed by synthesis and release of lactic acid from astrocytes. Remarkably, we found that MCT2-mediated uptake of lactic acid promotes neuronal survival following exposure to OGD conditions. In summary, here we report that neuronal tPA mediates the metabolic coupling between neurons, astrocytes and endothelial cells. Based on our data, we propose a model where in response to an increase in metabolic demands tPA is released from neurons. This tPA activates a cell signaling pathway in perivascular astrocytes and endothelial cells that promotes the uptake of glucose and the activation of the glycolytic pathway with the synthesis and release of lactic acid that is used by neurons as a metabolic fuel.
2.0. Experimental Procedures
2.1. Reagents
Recombinant murine tPA and an ELISA kit that detects active tPA were acquired from Molecular Innovations (Novi, MI). Other reagents were 2-N (7-nitrobenz-2-oxa-1,3-diazol-4-yl-amino)-2-deoxyglucose (2-NBDG), goat alexa conjugated secondary antibodies and 4'-6-Diamidino-2-phenylindole (DAPI; Invitrogen; Grand Island, NY), antibodies against glial fibrillary acidic protein (GFAP), AMPK phosphorylated at Thr172 (Cell Signaling Technology; Denvers, MA) and the glucose transporter GLUT1, lactic acid, and L-Lactate acid assay kit (abcam, Cambridge, MA), advasep-7, phloretin and Cyano-4-hydroxycinnamic acid and 1,4-Dideoxy-1,4-imino-D-arabinitol hydrochloride (Sigma; St Louis, MO), the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide ((MTT) assay. ATCC; Manassas, VA), the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific Inc., Waltham, MA), 5-Aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (Cell Signaling Technology; Denvers, MA), and rat brain microvascular endothelial cells and attachment factor solution (Cell Applications, INC., San Diego, CA).
2.2. Cell cultures and determination of cell survival
Rat brain microvascular endothelial cells (RBMVEC) were plated on T75 flasks coated with Attachment Factor solution and maintained in an humidified incubator at 37°C and 5% CO2 until they reached 80% confuency. Cells were used at passages 2 – 8. Astrocytes and cerebral cortical neurons were cultured from 1-day-old and E16–18 wild-type mice, respectively, as described elsewhere (Polavarapu et al., 2007, Echeverry et al., 2010). Briefly, the cerebral cortex was dissected, transferred into Hanks' balanced salt solution containing 100 units/ml penicillin, 100 µg/ml streptomycin, and 10 mm HEPES, and incubated in trypsin containing 0.02% DNase at 37°C for 15 min. Tissue was then triturated, and the supernatant was re-suspended in B27-supplemented neurobasal medium containing 2 mM l-glutamine and plated onto 0.1 mg/ml poly-l-lysine-coated wells. To study the effect of lactic acid on neuronal survival cerebral cortical neurons were maintained during 55 minutes in medium with no glucose and exposed in an anaerobic chamber to < 0.1% oxygen (Hypoxygen; Frederick, MD) in the presence of either vehicle (control), or 5 mM of lactic acid alone or in combination with 0.5 mM of cyano-4-hydroxycinnamic acid. Twenty-four hours later cell survival was quantified with the MTT assay following manufacturer’s instructions and as described elsewhere (Echeverry et al., 2010). Results are given as a percentage of cell survival compared to cultures maintained under physiological conditions. Each experiment was performed in cultures from three different animals and each observation was repeated 12 times.
2.3. TPA activity assay
The culture media of wild-type neurons, astrocytes and brain microvascular endothelial cells was sampled after 0, 1, 5, 15, 30 and 60 minutes of exposure to oxygen and glucose deprivation (OGD) conditions. The concentration of tPA was quantified with an ELISA kit following manufacturer’s instructions. Results were normalized to protein concentration in each well. As controls, tPA concentration was quantified at identical time points in sister cultures maintained under normal oxygen and glucose concentrations. Each experiment was performed with cultures from three different animals and each observation was repeated 10 times.
2.4. Western blot analysis
Extracts prepared from astrocytes and brain microvascular endothelial cells and incubated 0 – 60 minutes with 5 nM of tPA were homogenized. Protein concentration was quantified using the BCA assay and 15 µg of protein were loaded per sample, separated by 4–20% precast linear gradient polyacrylamide gel (Bio-Rad, Hercules, CA), transferred to a PVDF membrane by semi-dry transfer system, blocked with 5% non-fat dry milk in Tris-buffered saline pH 8.0 with 0.1% Tween 20 buffer, and immunoblotted with antibodies against pAMPK and GLUT1. Each observation was repeated 4 times.
2.5. Quantification of glucose uptake
Astrocytes and endothelial cells were treated 0 – 60 min with 5 nM of tPA, or with vehicle (control), or with a combination of 5 nM of tPA and 100 µM of phloretin. A sub-set of cells were incubated during 30 minutes with 2.5 mM of AICAR either alone or in combination with 5 nM of tPA. Cells were washed twice with PBS, incubated 30 minutes with 300 µM of 2-NBDG in the presence of 0.5 mM of glucose to minimize competition with dye uptake, and washed again with PBS. Fluorescence was measured in a spectrofluorophotometer at 528/485 nm. Results were normalized to protein concentration in each well. Each observation was repeated 12 times in cultures from 3 different animals.
2.6. Immunohistochemistry
Astrocytes and brain microvascular endothelial cells were kept in neurobasal medium with no glucose during 30 minutes before adding 5 nM of tPA. Following 5 minutes of incubation cells were fixed, rinsed in 10% goat serum/0.25% casein, incubated overnight with antibodies against GLUT1 (1:50) or pAMPK (Thr172; 1:100), co-stained with DAPI and antibodies against mouse GFAP (astrocytes) or with phalloidin (endothelial cells), and double- labeled with Alexa 594-conjugated goat anti mouse antibodies (1:2500), and visualized with an Olympus BX51 fluorescent microscope using an Olympus DP70 digital camera controlled by Olympus DP software.
2.6. Lactic acid measurement
Astrocytes, neurons and endothelial cells were treated 0 – 60 minutes with 5 nM of tPA, alone or in combination with either 60 µM of 1,4-Dideoxy-1,4-imino-D-arabinitol hydrochloride or 100 µM of phloretin. Cells incubated in medium without tPA for similar periods of time were used as controls. At the end of each time-point lactic acid was measured in the medium using a colorimetric lactate assay kit, following manufacturer’s instructions. Briefly, at the end of each incubation period, 50 µl of the conditioned media were added to 50 µl of a reaction mixture that contained Lactate Assay Buffer, Lactate Substrate Mix and Lactate Enzyme Mix at volume ratios of 46:2:2 µl respectively. The medium-reagent mixtures were then incubated at room temperature for 30 minutes and optical density was measured at OD450nm. Results were normalized to protein concentration for each experiment. Each experiment was repeated 12 times from cultures from 3 different animals.
2.7. Statistical analysis
Values are expressed as percentage or mean ± SD when appropriate. Student's t-test was used for normally distributed data and Wilcoxon's signed rank test was used for data that was not normally distributed. 2-way ANOVA was used for comparisons between groups. P values of less than 0.05 were considered significant.
3.0. Results
3.1. Neurons are the main source of tPA in the CNS during metabolic stress
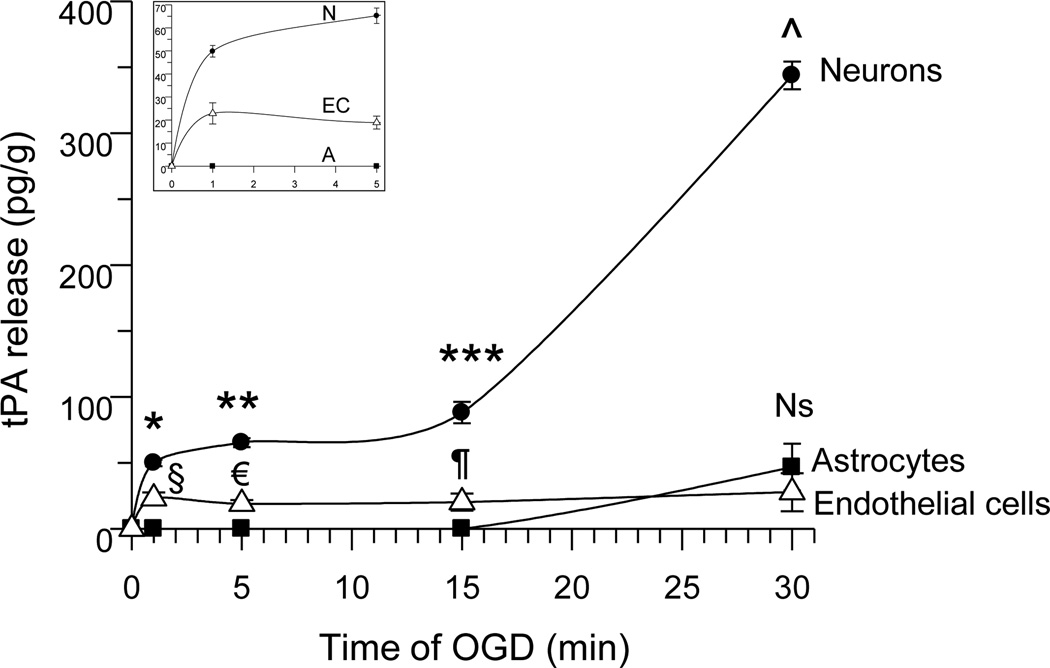
First we quantified the release of tPA from cerebral cortical neurons, astrocytes and brain microvascular endothelial cells exposed 0 – 30 minutes to oxygen and glucose deprivation (OGD). We found that 1 and 5 minutes of OGD induce the release of tPA from neurons (49.83 +/− 2.5 pg/g and 65.26 +/− 3.33 pg/g, respectively) and to lesser extent from brain microvascular endothelial cells (22.9 +/− 4.6 pg/g and 18.9 +/− 2.8 pg/g). In contrast, astrocytes released tPA only after 30 minutes of OGD (46.7 +/− 17.8 pg/g), simultaneously with an additional increase in the release of neuronal tPA (46.7 +/− 17.8 pg/g; Fig 1; n = 10; p < 0.05). Together, our data indicate that under conditions of metabolic stress, neurons release tPA at a greater rate than astrocytes or microvascular endothelial cells.
Figure 1. Neurons are the main source of tPA in the brain under metabolic stress.
Mean concentration of tPA in the culture media of neurons (black circles), astrocytes (black squares) and endothelial cells (white triangles) following 0, 1, 5, 15 or 30 minutes of exposure to oxygen and glucose deprivation (OGD) conditions. The inset depicts a magnification of the release of tPA during the first 5 minutes of OGD. §, € and ¶: p < 0.05 compared to sister cultures maintained under physiological conditions or with astrocytes kept under OGD conditions for similar periods of time. Ns: non-significant compared to endothelial cells. *, **, ***, and ^ : p < 0.05 compared to tPA released from sister cultures maintained under physiological conditions, or from astrocytes or endothelial cells following 1, 5, 15 or 30 minutes of OGD. Lines denote mean +/− SD; n = 10 per time-point in each experimental group.
3.2. Neuronal TPA induces AMPK activation in astrocytes and endothelial cells
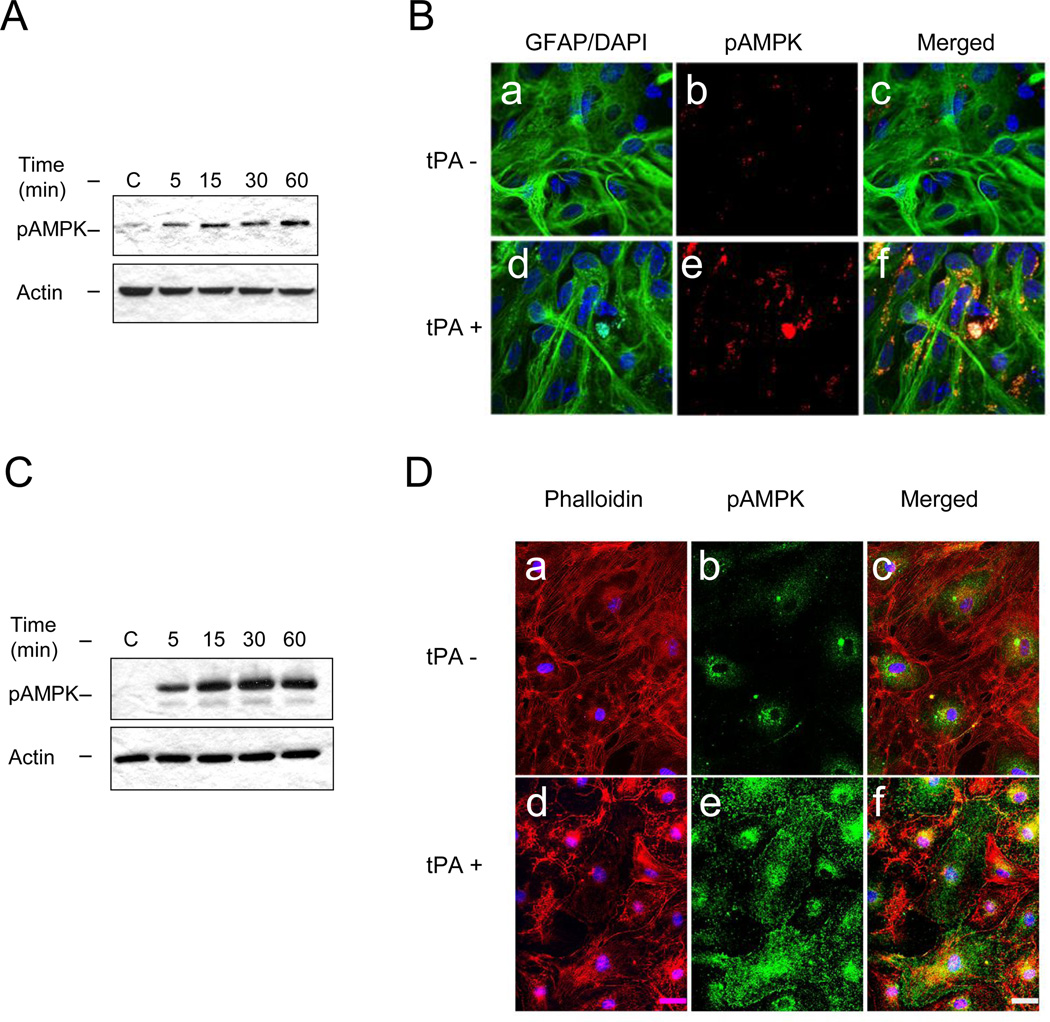
Based on our observations we postulated that the rapid release of neuronal tPA is the first step of a sequence of events aimed at obtaining energy substrates needed to sustain neuronal activity during OGD. Because AMPK promotes cellular adaptation during metabolic stress (Hardie, 2007, Weisova et al., 2009), we studied the expression of AMPK phosphorylated at Thr172 (pAMPK) in astrocytes and endothelial cells following 0 – 60 minutes of incubation with 5 nM of tPA. Our results indicate that tPA induces AMPK activation in astrocytes and endothelial cells (Fig 2), and that this effect is observed as early as after 5 minutes of treatment, a time point when tPA is released from neurons but not from astrocytes (Fig 1).
Figure 2. TPA induces AMPK activation in astrocytes and endothelial cells.
A & C. Representative Western blot analysis of AMPK phosphorylated at Thr172 (pAMPK) in astrocytes (A) and brain microvascular endothelial cells (C) incubated 0 – 60 minutes with 5 nM of tPA. B & D. Representative micrograph of pAMPK expression in astrocytes (B) and brain microvascular endothelial cells (D) incubated 5 minutes with vehicle (tPA−, panels a – c) or with 5 nM of tPA (tPA+; panels d – f). Green: GFAP in B and pAMPK in D; blue: DAPI in B and D; red: pAMPK in B and phalloidin in D. Magnification 60 ×.
3.3. TPA induces membrane recruitment of GLUT1 and GLUT1-mediated uptake of glucose by astrocytes and endothelial cells
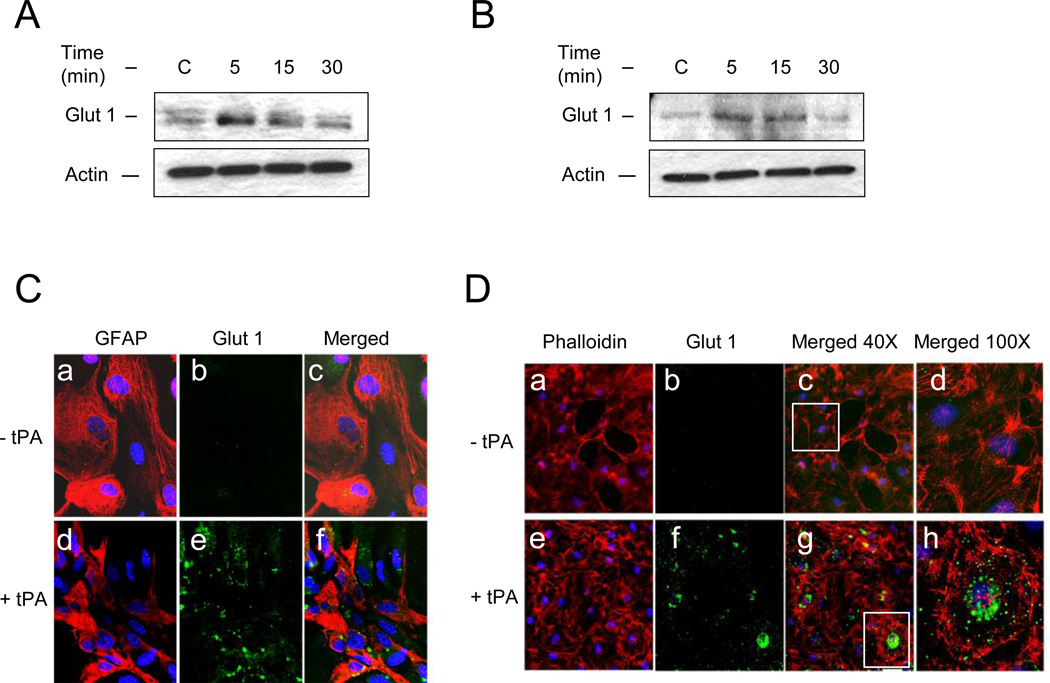
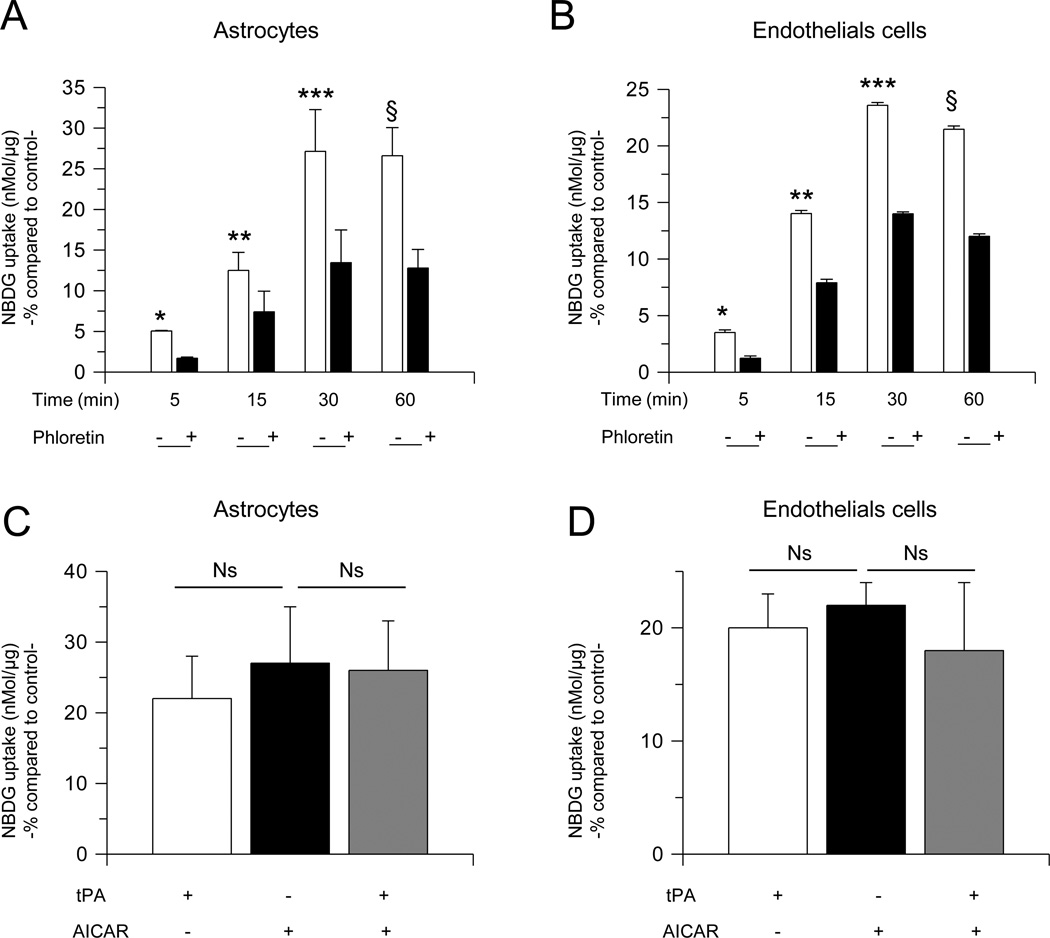
AMPK activation induces the uptake of glucose, which in the BBB is mediated by the glucose transporter GLUT1 (Weisova et al., 2009). Thus we decided to study the expression of GLUT1 in the cell membrane of astrocytes and endothelial cells following 0 – 30 minutes of incubation with 5 nM of tPA or a comparable volume of vehicle (control). We found that tPA induces a rapid and transient recruitment of GLUT1 to the plasma membrane of both, endothelial cells and astrocytes (Fig 3). To investigate whether tPA-induced membrane recruitment of GLUT1 has an effect on the uptake of glucose, we quantified the uptake of a fluorescent deoxyglucose analog (2-NDBG) by astrocytes and brain microvascular endothelial cells incubated 0 – 60 minutes with 5 nM of tPA, alone or in the presence of 100 µM of phloretin, a non-competitive GLUT1 antagonist. We found that compared to cells treated with vehicle (control) for similar periods of time, incubation with tPA during 5, 15, 30 and 60 minutes increases the uptake of glucose in astrocytes by 5 +/− 0.07 %, 12.50 +/− 2.22 %, 27.14 +/− 5.13 %, and 26.61 +/− 3.44 %, and in endothelial cells by 3.5 +/− 0.2 %, 14 +/− 0.28 %, 23.59 +/− 0.24, and 21.47 +/− 0.30, respectively, and that this effect is attenuated at each time point by GLUT1 antagonism (Fig 4; n= 26 per experimental condition; p< 0.05). To further determine whether the effect of tPA on glucose uptake was mediated by AMPK activation, we studied the uptake of 2-NDBG in astrocytes incubated with 2.5 mM of the AMPK activator AICAR, either alone or in combination with 5 nM of tPA, or with 5 nM of tPA alone. Our results indicate that AICAR mimics the effect of tPA, and that the combination of tPA and AICAR has a similar effect than either tPA or AICAR alone (Fig. 4 C & D; n = 8 per experimental condition, p : non-significant).
Figure 3. TPA induces membrane recruitment of GLUT1 in astrocytes and endothelial cells.
A & B. Representative Western blot analysis of GLUT1 expression in membrane extracts from astrocytes (A) and endothelial cells (B) following 0 – 30 minutes of incubation with 5 nM of tPA. C & D. Representative micrograph of GLUT1 expression in astrocytes (C) and endothelial cells (D) following 5 minutes of incubation with vehicle (− tPA), or 5 nM of tPA (+ tPA). Red in C is GFAP and in D is phalloidin; blue and green in C & D is DAPI and GLUT1, respectively. Magnification 60× in C, 40× in D panels a – c and e – g. Panels d & h in D correspond to magnification of insets depicted in c & g.
Figure 4. TPA induces GLUT1-mediated glucose uptake in astrocytes and endothelial cells.
A & B. Mean increase in the uptake of a fluorescent deoxyglucose analog (2-NBDG) in astrocytes (A) and endothelial cells (B) incubated during the indicated periods of time with 5 nM of tPA, alone (−) or in combination with the GLUT1 antagonist phloretin (+). Lines denote SD. n = 26 per experimental condition; *, *, ***, and ξ in A & B: p < 0.05 compared to astrocytes (A) or endothelial cells (B), respectively, incubated with vehicle (control) or with tPA in the presence of phloretin. C & D. Mean increase in the uptake of a fluorescent deoxyglucose analog (2-NBDG) in astrocytes (C) and endothelial cells (D) incubated during 30 minutes with either 5 nM of tPA, or 2.5 mM of AICAR, or with a combination of tPA and AICAR. n = 8 per experimental condition. Lines denote SD. Ns: non-significant Results in A– D are given as a percent increase compared to 2-NBDG uptake (nMol/g) by cells treated during a similar period of time with vehicle (control).
3.4. TPA induces the synthesis and release of lactic acid from astrocytes via activation of the glycolytic pathway
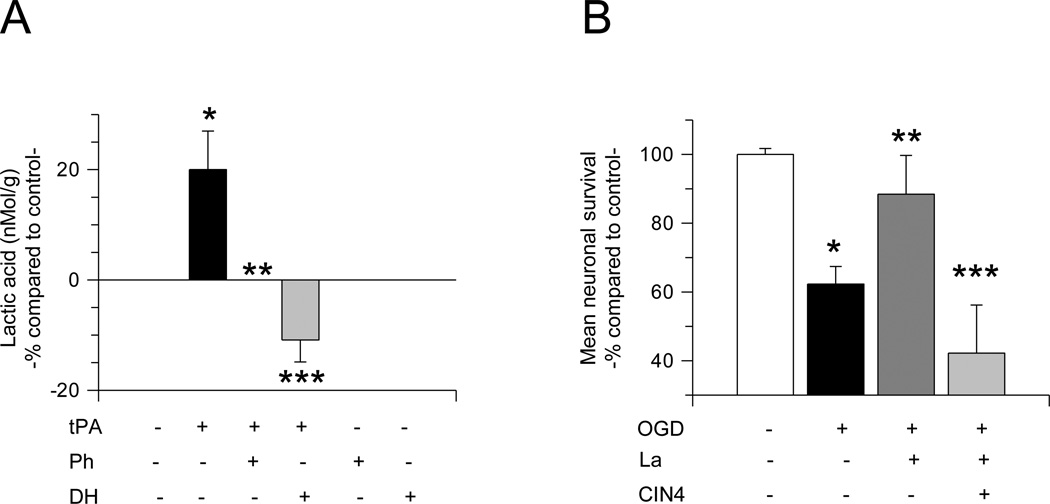
To investigate whether tPA-induced GLUT1-mediated glucose uptake has an effect on the synthesis and release of lactic acid from astrocytes, we quantified the concentration of lactic acid in the culture media of astrocytes following 30 minutes of incubation with 5 nM of tPA, alone or in combination with either 100 µM of phloretin, or 60 µM of the glycogen phosphorylase inhibitor 1,4-Dideoxy-1,4-imino-Darabinitol hydrochloride (DH). Our data indicate that compared to cells treated with vehicle (control), tPA induces a 20 +/− 7% increase in the release of lactic acid from astrocytes and that this effect is abrogated by either GLUT1 antagonism with phloretin (0 %) or inhibition of the glycolytic pathway with DH (−10.88 +/− 4%; n = 12; p < 0.05; Fig 5A). Importantly, we did not detect lactic acid synthesis in neurons or endothelial cells treated with tPA (data not shown).
Figure 5. TPA induces the synthesis and release of lactic acid from astrocytes.
A. Mean concentration of lactic acid in the culture media of astrocytes incubated 30 minutes with 5 nM of tPA alone or in combination with 100 µM the GLUT1 antagonist phloretin or 60 µM of the glycolysis inhibitor 1,4-Dideoxy-1,4-imino-D-arabinitol hydrochloride (DH). Lines denote SD. n = 12 per experimental condition. * p < 0.05 compared to lactic acid concentration in the culture media of astrocytes not incubated with tPA. ** and ***: p < 0.05 compared to lactic acid concentration in the culture media of astrocytes treated with tPA alone. B. Mean cell survival in cerebral cortical neurons maintained under physiological conditions (white bar), or exposed to 55 minutes of oxygen and glucose deprivation (OGD) conditions alone (black bar), or in the presence of either 5 mM of lactic acid (La; dark gray bar), or 0.5 mM of the MCT-2 antagonist cyano-4-hydroxycinnamic acid (CIN4; light gray). * p < 0.05 compared to cells maintained under physiological conditions. ** p < 0.05 compared to cells exposed to OGD conditions in the ansence of lactic acid. *** p < 0.05 compared to cells exposed to OGD conditions in the presence of lactic acid alone. Each observation was repeated 12 times from cultures from three different animals.
3.5. The uptake of lactic acid protects neurons from OGD-induced cell death
To investigate whether tPA-induced synthesis and release of lactic acid from astrocytes has an effect on neuronal survival, we quantified cell survival in cerebral cortical neurons exposed to 55 minutes of OGD conditions in the presence of 5 mM of lactic acid, alone or in combination with 0.5 mM of α-Cyano-4-hydroxycinnamic acid (CIN4), an inhibitor of the monocarboxylate transporter 2 (MCT2), which mediates the uptake of lactic acid by neurons. We found that cell survival decreases from 100 +/− 1.7 % in neurons maintained under physiological conditions, to 62.3 +/− 5.1 % in neurons exposed to OGD conditions. Remarkably, survival in neurons exposed to OGD conditions in the presence of lactic acid was 88.4 +/− 11.30 %, and this protective effect was abrogated by MCT2 inhibition (Fig 5B; n = 12; p < 0.05).
4.0. Discussion
The functional unit assembled by neurons, astrocytes and endothelial cells accounts for over 90% of glucose metabolism in the cerebral cortex (Attwell and Laughlin, 2001). Owning to their simultaneous interaction with endothelial cells and neurons, astrocytes play a fundamental role in this unit, pairing neuronal activity with the uptake and supply of energy substrates. This process has a pivotal importance not only to maintain neuronal activity under physiological conditions but also to promote neuronal survival under metabolic stress.
Oxygen and glucose deprivation causes a rapid increase in tPA activity in the abluminal side of the BBB (Yepes et al., 2003). The data presented herein show that neurons and not astrocytes are the main source of this tPA, and more importantly, that tPA released from neurons activates a cell signaling pathway in astrocytes and endothelial cells that increase the supply of nutrients and promotes neuronal survival under conditions of metabolic stress. More specifically, our data suggest that tPA may pair neuronal demands with either the extraction of glucose from the intravascular space or the degradation of glycogen stored in perivascular astrocytes.
AMPK is an evolutionarily conserved sensor of cellular stress and unmet metabolic demands in multiple organs including the brain (Hardie, 2007). The activation of AMPK inhibits ATP-consuming processes such as protein synthesis, and induces glucose uptake and glycolysis in an attempt to restore ATP production. More importantly, AMPK activation promotes neuronal survival under conditions of metabolic stress (Weisova et al., 2009). The transport protein GLUT1 mediates the uptake of glucose by endothelial cells and perivascular astrocytes in the BBB (Barros et al., 2007). One of the main effects of AMPK activation is the induction of glucose uptake via recruitment of GLUT1 to the cell membrane. Our data indicate that tPA induces AMPK activation, membrane recruitment of GLUT1, and GLUT1-mediated glucose uptake in astrocytes and endothelial cells.
A growing body of evidence indicates that regional stimulation of glycolysis and glycogenolysis are the main sources of energy during neuronal activation. Hence, neurons and astrocytes form a functional energy supply unit where glucose is uptaken by endothelial cells from the intravascular space and stored as glycogen by astrocytes where it is processed via the glycolytic pathway to lactate than then is exported to neurons as an energy source. This process, also known as lactate shuttle, is particularly important during periods of increased energy demand (Tsacopoulos and Magistretti, 1996). In agreement with these observations, it has been shown that lactate is a very efficient oxidative substrate for neurons (Pellerin and Magistretti, 2003), and that under glutamatergic stimulation lactate is the preferred source of neuronal energy (Porras et al., 2004). However, under physiological conditions glucose is used by neurons to assure vesicular filling and neurotransmitter homeostasis (Bak et al., 2006).
We found that tPA induces the release of lactic acid from astrocytes but not from neurons. This effect requires either the uptake of glucose from the extracellular space, or degradation of glycogen stores, which in the brain are found only in astrocytes. Together our results suggest a model where increased neuronal activity under physiological conditions induces the release of tPA from neurons, which then activates a cell signaling pathway in astrocytes and endothelial cells that promotes the uptake of glucose from the intravascular space. Alternatively, in conditions where the supply of glucose from the intravascular space is interrupted tPA released from neurons induces the degradation of glycogen stores in perivascular astrocytes. Either the uptake of glucose from the intravascular space, or the degradation of glycogen stores in perivascular astrocytes, is mediated by tPA-induced AMPK activation and leads to the synthesis and release of lactic acid from astrocytes.
The monocarboxylate transporter-2 (MCT2) mediates the uptake of lactic acid by neurons. MCT2 is abundantly found in post-synaptic densities (Pierre et al., 2002), its expression increases during synaptogenesis, and MCT2-mediated lactate uptake sustains action potential propagation and promotes survival in neurons exposed to metabolic stress (Gladden, 2004). Our data show not only that tPA induces lactic acid synthesis and release from astrocytes via GLUT1-mediated glucose uptake and activation of the glycolytic pathway, but that MCT2-mediated lactic acid uptake prevents cell death in neurons exposed to oxygen and glucose deprivation. In summary, our data indicate that neuronal tPA plays a central role in neuroglial coupling and suggest a model where an increase in neuronal metabolic demands either by augmentation in neuronal activity, or interruption in the supply of oxygen and glucose, induces the release of tPA from neurons which activates a cell signaling pathway in astrocytes that promotes the synthesis of lactic acid either from glucose taken from the intravascular space or from degradation of glycogen stores. This lactic acid is then used by neurons as an energy source promoting cell survival under conditions of metabolic stress.
Highlights.
Metabolic stress induces the rapid release of tissue-type plasminogen activator (tPA) from cerebrocortical neurons.
Neuronal tPA activates a signaling pathway in astrocytes that induces the uptake of glucose and the release of lactic acid.
The uptake of lactic acid by neurons promotes survival in cells exposed to oxygen and glucose deprivation conditions.
Acknowledgements
This work was supported in part by National Institutes of Health Grants NS-079331 (to MY), NS-062073 (to M.Y) and VA MERIT award BX000474 (to MY).
Glossary
- tPA
Tissue-type plasminogen activator
- GLUT1
glucose transporter -1
- MCT2
monocarboxylate transporter-2
- AMPK
Adenosine monophosphate-activated protein kinase
- OGD
Oxygen and glucose deprivation
- MCT-2
Monocarboxylate transporter-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amato S, Man HY. Bioenergy sensing in the brain: the role of AMP-activated protein kinase in neuronal metabolism, development and neurological diseases. Cell Cycle. 2011;10:3452–3460. doi: 10.4161/cc.10.20.17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Bak LK, Schousboe A, Sonnewald U, Waagepetersen HS. Glucose is necessary to maintain neurotransmitter homeostasis during synaptic activity in cultured glutamatergic neurons. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:1285–1297. doi: 10.1038/sj.jcbfm.9600281. [DOI] [PubMed] [Google Scholar]
- Barros LF, Bittner CX, Loaiza A, Porras OH. A quantitative overview of glucose dynamics in the gliovascular unit. Glia. 2007;55:1222–1237. doi: 10.1002/glia.20375. [DOI] [PubMed] [Google Scholar]
- Birnbaum MJ, Haspel HC, Rosen OM. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverry R, Wu J, Haile WB, Guzman J, Yepes M. Tissue-type plasminogen activator is a neuroprotectant in the mouse hippocampus. J Clin Invest. 2010;120:2194–2205. doi: 10.1172/JCI41722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Gladden LB. Lactate metabolism: a new paradigm for the third millennium. The Journal of physiology. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nature reviews Molecular cell biology. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Joost HG, Bell GI, Best JD, Birnbaum MJ, Charron MJ, Chen YT, Doege H, James DE, Lodish HF, Moley KH, Moley JF, Mueckler M, Rogers S, Schurmann A, Seino S, Thorens B. Nomenclature of the GLUT/SLC2A family of sugar/polyol transport facilitators. American journal of physiology Endocrinology and metabolism. 2002;282:E974–E976. doi: 10.1152/ajpendo.00407.2001. [DOI] [PubMed] [Google Scholar]
- Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review) Molecular membrane biology. 2001;18:247–256. doi: 10.1080/09687680110090456. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Food for thought: challenging the dogmas. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2003;23:1282–1286. doi: 10.1097/01.WCB.0000096064.12129.3D. [DOI] [PubMed] [Google Scholar]
- Pierre K, Magistretti PJ, Pellerin L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2002;22:586–595. doi: 10.1097/00004647-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Polavarapu R, Gongora MC, Yi H, Ranganthan S, Lawrence DA, Strickland D, Yepes M. Tissue-type plasminogen activator-mediated shedding of astrocytic lowdensity lipoprotein receptor-related protein increases the permeability of the neurovascular unit. Blood. 2007;109:3270–3278. doi: 10.1182/blood-2006-08-043125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras OH, Loaiza A, Barros LF. Glutamate mediates acute glucose transport inhibition in hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:9669–9673. doi: 10.1523/JNEUROSCI.1882-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Weisova P, Concannon CG, Devocelle M, Prehn JH, Ward MW. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:2997–3008. doi: 10.1523/JNEUROSCI.0354-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Echeverry R, Wu J, An J, Haile WB, Cooper DS, Catano M, Yepes M. Tissue-type plasminogen activator protects neurons from excitotoxin-induced cell death via activation of the ERK1/2-CREB-ATF3 signaling pathway. Molecular and cellular neurosciences. 2013;52:9–19. doi: 10.1016/j.mcn.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Wu J, Nicholson AD, Echeverry R, Haile WB, Catano M, An J, Lee AK, Duong D, Dammer EB, Seyfried NT, Tong FC, Votaw JR, Medcalf RL, Yepes M. Tissue-type plasminogen activator regulates the neuronal uptake of glucose in the ischemic brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:9848–9858. doi: 10.1523/JNEUROSCI.1241-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci. 2009;32:48–55. doi: 10.1016/j.tins.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]