Human cysticercosis – resulting from infection with the larval stage of the pork tapeworm, Taenia solium – is a modern human plague, affecting thousands of people world-wide.[1] In the normal cycle of transmission of this tapeworm, humans harbor the adult parasite in the small intestine as definitive hosts. Thousands of infective eggs are daily detached from the distal end of the adult tapeworm and are passed with feces of Taenia carriers. In places where both improper disposal of human feces and poor husbandry are common, pigs get access to human feces and ingest T. solium eggs. Eggs mature into oncospheres and metacestodes and lodge in the striated muscles and other tissues of pigs, the natural intermediate host; there, metacestodes evolve into larvaes (cysticerci). When humans ingest improperly cooked pork infected with cysticerci, larvae evaginate and adhere to the intestinal mucosa. Then, the rudimentary body begins to grow – forming proglottids – and the life cycle is completed when proglottids become gravid and are passed with human feces. Humans may also become intermediate hosts in the life cycle of T. solium after ingesting its eggs. In these cases, human cysticercosis ensues [Figure 1].
Figure 1.
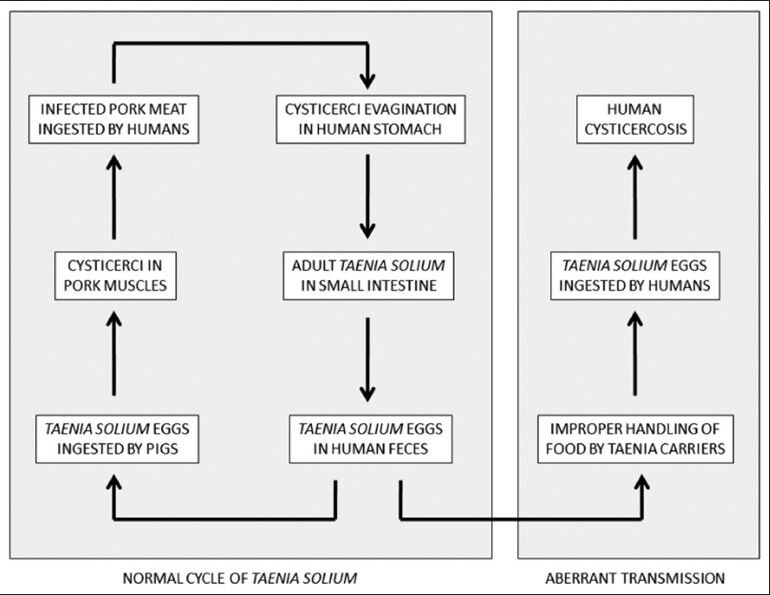
Diagram showing major steps in the normal and aberrant life cycle of Taenia solium (reproduced with permission from Del Brutto. Neurocysticercosis. Continuum (Minneap Minn) 2012;18:1392-1416
Anecdotal data suggested that the most common form of human acquisition of the disease was through the ingestion of vegetables or fruits grown in plantations fertilized with manure containing human feces contaminated with T. solium eggs. However, recent studies showing clusters of cysticercosis patients around Taenia carriers suggested a major role of person-to-person transmission of the disease, changing this traditional, if erroneous, view.[2] Human cysticercosis is mostly transmitted from person-to-person (through non-hygienic handling of food or by direct contact) and the role of infected pigs is to preserve the life cycle of T. solium. This explains the occurrence of autochthonous cases of cysticercosis in non-endemic regions where swine husbandry is adequate or inexistent, as well as the high prevalence of human cysticercosis in countries where most of the population is vegetarian.[3]
Cysticerci may invade almost every organ of the human economy. However, relevant disease is most often related to invasion of the central nervous system, giving rise to a condition called neurocysticercosis. According to conservative figures, neurocysticercosis causes 50,000 deaths/year and is the most common cause of acquired epilepsy worldwide, accounting for up to 30% of the excess fraction of epilepsy seen in the developing world.[4] There are a number of patients where cysticerci lodge in extra neural structures, including subcutaneous tissues, striated muscles and even the heart, liver or other organs. With the exception of some cases of massive muscle infection by hundreds of cysticerci, causing a clinical picture of muscular pseudo-hypertrophy or some sporadic cases of cardiac arrhythmias related to a cardiac cyst, most cases of systemic cysticercosis are clinically irrelevant and their presence is just a useful marker suggesting that a given individual may also have neurocysticercosis.[5,6,7,8,9] In this issue of tropical parasitology, Srikanth and Ananadam as well as Gupta and co-workers (see pages 130-134 and 148-150) described a total of eight patients with symptomatic muscle and subcutaneous cysticercosis, emphasizing on their rarity (even in endemic areas) and their diagnostic considerations.
From the clinical point of view, neurocysticercosis is highly pleomorphic and there is no possibility to define a pathognomonic clinical syndrome. Most of patients develop seizures as the main or sole manifestation of the disease, but this is true only for those with parenchymal brain cysticercosis, since the subarachnoid and ventricular forms of the disease often course with focal neurological deficits or intracranial hypertension. The clinical pleomorphism of neurocysticercosis is directly related to differences in the severity of infection, in the location of lesions within the central nervous system and in the intensity of the reaction of the host immune system against the parasites.
The past few decades witnessed the introduction of modern neuroimaging equipment that, together with the development of reliable immune diagnostic tests, enhanced our diagnostic accuracy for neurocysticercosis. However, both neuroimaging and serologic tests must be interpreted with caution and always in the context of a given patient, to avoid over-and misdiagnosis of neurocysticercosis. Few neuroimaging findings are pathognomonic of neurocysticercosis and most serologic tests may be faced with problems related to poor sensitivity or specificity. With this in mind, we published the first attempt to settle a set of diagnostic criteria for human cysticercosis, based on the objective evaluation of clinical, radiological, immunological and epidemiological data of patients.[10] After some years of experience, the same panel of investigators considered that set to be somewhat complex, as it was developed for both the diagnosis of patients with neurocysticercosis as well as for those with cysticercosis elsewhere in the body. It was then considered that a set of criteria exclusively devoted to the diagnosis of neurocysticercosis would be more practical than the initial ones and our panel agreed upon the elaboration of more accurate and stringent revised criteria exclusively devoted to the diagnosis of neurocysticercosis.[11] As in the initial publication, the revised criteria included four categories of diagnosis (absolute, major, minor and epidemiologic) that were stratified on the basis of their individual strength. Absolute criteria allowed unequivocal diagnosis of neurocysticercosis, major criteria strongly suggested the diagnosis, but could not be used alone to confirm the disease, minor criteria were frequent, but non-specific manifestations of the disease and epidemiologic criteria referred to circumstantial evidence favoring the diagnosis. Interpretation of these criteria allowed two degrees of diagnostic certainty, definitive and probable, according to the likelihood that neurocysticercosis is present in a given patient [Table 1].
Table 1.
Diagnostic criteria for neurocysticercosis

Finally, the introduction of potent cysticidal drugs heralded the start of a new era in the fight against human cysticercosis. Two drugs, praziquantel and albendazole, have proven to be effective and safe and have improved the prognosis of thousands of patients affected by this parasitic disease.[12] There have been, however, a number of problems related to the use of these drugs that have ranged from the occurrence of seizures to the death of the patient soon after therapy.[13] Most of these “adverse effects” of cysticidals have been related to incorrect selection of patients before therapy and to lack of knowledge of the different patterns of disease expression of neurocysticercosis. There are some forms of the disease that do not benefit with specific therapy and more worrisome, some forms that must not be treated as the use of cysticidals may exacerbate the clinical manifestations. Proper knowledge on currently accepted guidelines of therapy for the different forms of neurocysticercosis may help to reduce these adverse reactions [Table 2].
Table 2.
General guidelines for therapy of neurocysticercosis

References
- 1.Del Brutto OH. Neurocysticercosis. Continuum (Minneap Minn) 2013;18:1392–416. doi: 10.1212/01.CON.0000423853.47770.90. [DOI] [PubMed] [Google Scholar]
- 2.Lescano AG, Garcia HH, Gilman RH, Gavidia CM, Tsang VC, Rodriguez S, et al. Taenia solium cysticercosis hotspots surrounding tapeworm carriers: Clustering on human seroprevalence but not on seizures. PLoS Negl Trop Dis. 2009;3:e371. doi: 10.1371/journal.pntd.0000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Brutto OH, García HH. Neurocysticercosis in nonendemic countries: Time for a reappraisal. Neuroepidemiology. 2012;39:145–6. doi: 10.1159/000341693. [DOI] [PubMed] [Google Scholar]
- 4.Garcia HH, Del Brutto OH Cysticercosis Working Group in Peru. Neurocysticercosis: Updated concepts about an old disease. Lancet Neurol. 2005;4:653–61. doi: 10.1016/S1474-4422(05)70194-0. [DOI] [PubMed] [Google Scholar]
- 5.Bothale KA, Mahore SD, Maimoon SA. A rare case of disseminated cysticercosis. Trop Parasitol. 2012;2:138–41. doi: 10.4103/2229-5070.105183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharjee HK, Ramman TR, Agarwal L, Nain M, Thomas S. Isolated cysticercosis of the breast masquerading as a breast tumour: Report of a case and review of literature. Ann Trop Med Parasitol. 2011;105:455–61. doi: 10.1179/1364859411Y.0000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damani M, Mehta VC, Baile RB, Nakwa B. Orbital cysticercosis: A case report. Saudi J Ophthalmol. 2012;26:457–8. doi: 10.1016/j.sjopt.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawhney BB, Chopra JS, Banerji AK, Wahi PL. Pseudohypertrophic myopathy in cysticerosis. Neurology. 1976;26:270–2. doi: 10.1212/wnl.26.3.270. [DOI] [PubMed] [Google Scholar]
- 9.Jakhere SG, Chemburkar VC, Yeragi BS, Bharambay HV. Imaging findings of disseminated cysticercosis with unusual involvement of spleen and pancreas. J Glob Infect Dis. 2011;3:306–8. doi: 10.4103/0974-777X.83547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Brutto OH, Wadia NH, Dumas M, Cruz M, Tsang VC, Schantz PM. Proposal of diagnostic criteria for human cysticercosis and neurocysticercosis. J Neurol Sci. 1996;142:1–6. doi: 10.1016/0022-510x(96)00130-x. [DOI] [PubMed] [Google Scholar]
- 11.Del Brutto OH, Rajshekhar V, White AC, Jr, Tsang VC, Nash TE, Takayanagui OM, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57:177–83. doi: 10.1212/wnl.57.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nash TE, Garcia HH. Diagnosis and treatment of neurocysticercosis. Nat Rev Neurol. 2011;7:584–94. doi: 10.1038/nrneurol.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wadia N, Desai S, Bhatt M. Disseminated cysticercosis. New observations, including CT scan findings and experience with treatment by praziquantel. Brain. 1988;111:597–614. doi: 10.1093/brain/111.3.597. [DOI] [PubMed] [Google Scholar]


