Abstract
Introduction:
Cystic echinococcosis (CE) is a zoonotic disease of humans with variable clinical manifestations. Imaging and immunological methods are currently the mainstay of diagnosis of this disease. Although the immunological tests for detection of anti-echinococcal antibodies have several disadvantages, they are widely being used. Antigen is far more superior than antibody detection test as they can provide a specific parasitic diagnosis.
Materials and Methods:
A sandwich enzyme linked immunosorbent assay (ELISA) was designed using antibodies to 24 kDa urinary hydatid antigen for the detection of hydatid antigens in urine, serum and cyst fluid specimens. The performance of this novel test was compared with that of other hydatid antibody detection ELISA and enzyme immune transfer blot (EITB) using radiological and surgical confirmation as the gold standard.
Results:
The antigen detection ELISA showed 100% sensitivity and specificity when tested with cyst fluid. On testing urine and serum, the antigen detection ELISA was found to be more specific than antibody detection ELISA. EITB was found to be the most sensitive and specific test.
Conclusions:
ELISA using polyclonal antibodies against 24 kDa urinary hydatid protein was moderately sensitive to detect hydatid antigen in serum and urine. Hence polyclonal antibodies to 24 kDa urinary hydatid antigen can be used as an alternative source of antibody to detect hydatid antigen in serum, urine and cyst fluid. In the present study, EITB was found to be highly specific test for detection of hydatid antibodiesin serum. 24 kDa protein was found to be specific and of diagnostic value in CE.
KEY WORDS: Cystic echinococcosis, sandwich enzyme linked immunosorbent assay, urinary hydatid antigen
INTRODUCTION
Cystic echinococcosis (CE) caused by the dog tapeworm, Echinococcus granulosus is a chronic zoonotic disease. CE earlier referred to as hydatid disease remains asymptomatic throughout the life in the majority of cases. In symptomatic cases, the clinical manifestations are highly variable.[1]
Clinical diagnosis of CE is frequently difficult, hence always supported by imaging and immunological methods. The immunodiagnostic methods detecting the antibodies have the disadvantages of low specificity and sensitivity and the inability to differentiate between recent and past infections.[2]
Antigen detection tests such as counter current immune electrophoresis (CIEP), co-agglutination (Co-A) and enzyme linked immunosorbent assay (ELISA) are increasingly used now-a-days.[3] In spite of their low sensitivity, the antigen detection tests are superior to serological tests as they can provide a definite parasitic diagnosis.[4] In the present study, a sandwich ELISA using polyclonal antibodies to 24 kDa urinary hydatid antigen was designed and evaluated for its efficacy to detect hydatid antigen in serum, urine and cyst fluid for the diagnosis of CE. Also, the validity of this novel test was compared against that of other serological tests such as ELISA and enzyme immune transfer blot (EITB) for detection of anti-hydatid antibodies.
MATERIALS AND METHODS
Patients
The study was carried out after obtaining clearance from the Institutional Ethical Committee. This 2 years prospective study was systematically conducted at the Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. During the study period, patients surgically or radiologically diagnosed of having CE were followed-up and appropriate samples were collected from these patients after obtaining written consent. A total of 50 patients were included in the study and were divided into the following groups:
Group I included 10 surgically proven and operated cases of CE
Group II included 15 cases of unoperated CE but proved by ultrasonography
Group III included 15 patients with various parasitic diseases other than CE, as control
Group IV included 10 healthy adults (blood donors and students) who had not suffered from CE, as control.
Urinary hydatid antigen preparation
Urinary hydatid antigen obtained from surgically confirmed CE cases was used as the source of hydatid antigen. The method of antigen preparation from urine of surgically confirmed CE cases was as described by Parija et al.[5] Briefly, 50 ml of urine was collected from surgically confirmed cases of CE. Urine was concentrated by trichloro acetic acid (TCA) method. A total volume of 1 ml of urine was mixed with 1 ml of 4M TCA. The mixture was blended in a vortex mixture and centrifuged at 10,000 rpm for 10 min at 4°C. The supernatant was discarded and the pellet was resuspended in 0.1 ml of phosphate buffered saline (PBS) of pH 7.2.[6]
The dialysate was subjected to protein estimation by Lowry et al. method.[7] Approximately, 20 μg of urinary precipitated protein was subjected to sodium dodecyl sulphate – poly acrylamide gel electrophoresis (SDS-PAGE) with 5% stacking gel and 12% separating gel under reducing and denaturing conditions in an electrophoretic cell at 100 v for 1.30 h. The separated urinary protein was stained in Coomasie blue R-250 (0.25% staining solution) and molecular weight of the urinary protein bands was determined by comparing with standard molecular weight marker having a broad range from 175 kDa to 6.5 kDa (New England Biolabs) run alongside, using software provided with the gel documentation system (Vilver Lourmat).
After the SDS-PAGE the concentrated urine revealed a range of protein from 70 kDa to 9 kDa (70 kDa, 41 kDa, 35 kDa, 24 kDa, 14 kDa and 10 kDa). The diagnostically relevant hydatid antigen from the urine of confirmed CE cases was eluted as per the method described by Hager and Burgess.[8] The molecular weight of the eluted hydatid protein from urine of confirmed CE cases was found to be 24 kDa. This fraction of the urinary protein was used as the source of antigen for subsequent tests.
Polyclonal hydatid antibody preparation
Polyclonal hydatidantibodies were raised in rabbits against purified 24 kDa fragment of urinary hydatid antigen of surgically confirmed CE cases. Two rabbits were taken. The rabbits were bled 1 day prior to immunization. This serum was used as the normal rabbit serum control. This method was followed as per previous study by Shariff and Parija.[9] Briefly, the urinary antigen fraction 24 kDa was emulsified with an equal volume of Freund's complete adjuvant. Adult rabbits (3-5 kg) were injected with 0.5 ml of this emulsion to all the four limbs intramuscularly. After 6 weeks, rabbits were injected with 0.5 ml of the same antigen with Freund's incomplete adjuvant. After 10 days, 0.5 ml of blood was collected from the ear vein of the rabbit. The level of antibody raised in rabbit was tested by indirect hemagglutination test using the method employed by Parija et al.[10] When the antibody titer was significant (1:1024 and above) in test bleeding, subsequently blood was collected from rabbits for polyclonal antibodies. These polyclonal antibodies to 24 kDa protein form urine of confirmed CE cases were used to detect antigen in the serum, cyst fluid and urine by ELISA.
Sandwich ELISA procedure
Polyvinyl high binding microtiter ELISA plates were coated with 100 μl of anti-rabbit polyclonal antibodies to 24 kDa fragment urinary hydatid antigen in PBS 7.2 per well and incubated overnight at 4°C. The unabsorbed antibodies were removed by washing the plates with washing buffer, sterile PBS 7.2 containing 0.1% Tween-20 (PBS-T). 50 μl of the analyte was added and incubated for 1.5 h at 37°C. The plates were washed 3 times with PBS-T as before to remove unbound materials from the sample. A total volume of 100 μl volume of rabbit anti-hydatid IgG conjugated to horse radish peroxidase (HRP) was dispensed to all the wells and incubated for 1 h at 37°C in dark. Plates were washed thrice with PBS-T as mentioned before to remove unbound conjugate. A total volume of 100 μl volume of the substrate solution per well (O-phenylene diamine dihydrochloride in 10 ml of PBS 7.2 containing 0.05% Tween-20 and 10 μl hydrogen peroxide) was dispensed to all the wells and incubated for 15-20 min at 37°C in dark for the development of optimum color. The reaction was stopped by adding 50 μl of 2M sulphuric acid per well. The absorbance was recorded at 450 nm using ELISA reader. The cut-off value for sandwich ELISA was determined by the formula, cut-off = mean OD + 2 standard deviation (SD) of healthy normal controls. The cut-off value was calculated as 0.2605 for serum samples, 0.1213 for urine samples and 1.1031 for hydatid cyst fluid samples.
Specimen tested by sandwich ELISA
The newly designed sandwich ELISA was tested with serum, urine and hydatid fluid obtained from various study groups as mentioned earlier. 5 ml of venous blood was collected with aseptic precautions from CE patients and controls and was allowed to clot. The serum was separated and stored at − 20°C until use. 5 ml of urine was collected from each patient in sterile glass vials using aseptic techniques. Specimens were preserved with sodium azide 0.015 mol/L and stored at − 20°C until use.
Urine was concentrated by the TCA precipitation method.[6] In this procedure, 1 ml of urine was mixed with 1 ml of 4M TCA. The mixture was blended in a vortex mixture and centrifuged at 10,000 rpm for 10 min at 4°C. The supernatant was discarded and the pellet was resuspended in 0.1 ml of PBS (pH 7.2).
Human hydatid cyst fluid was obtained from surgically confirmed cases of CE in the liver and was processed according to the method described by Parija et al.[10] The hydatid fluid was aspirated aseptically and checked for the presence of scolices and hooklets. The fluid was filtered using a Seitz filter and checked for sterility aerobically.
Hydatid antibody detection by ELISA
Hydatid antibody was detected in the serum by ELISA. The test was performed by using 24 kDa fragment of urinary hydatid antigen for the detection of hydatid antibodies in the serum samples from the study population. It consisted of the following steps: Polyvinyl high binding microtiter ELISA plates were coated with 100 μl of urinary hydatid antigen in PBS 7.2 per well and incubated overnight at 4°C. The unabsorbed antigen was removed by washing the plates with washing buffer PBS-T. 1:1000 dilution of the patient sera was prepared in PBS. 100 μl of each diluted serum was added and incubated for 1.5 h at 37°C. The plates were washed 3 times with PBS to remove unbound antibodies in serum. A total volume of 100 μl volume of 1:2000 dilution of rabbit anti-human IgG-HRP conjugated secondary antibody was dispensed to all the wells and incubated for half an hour at 37°C in dark. Plates were washed thrice with PBS as before to remove unbound conjugate. 100 μl volume of the substrate solution (O-phenylene diamine dihydrochloride in 10 ml of PBS 7.2 containing 0.05% Tween-20 and 10 μl hydrogen peroxide) was dispensed to all the wells and incubated for 15-20 min at 37°C in dark for the development of optimum color. The reaction was stopped by adding 50 μl of 2M sulfuric acid per well. The absorbance was recorded at 450 nm using ELISA reader. The cut-off value was calculated by the formula: Cut-off = mean OD + 2 SD of healthy normal controls.
Hydatid antibody detection by EITB
EITB was performed to detect anti-hydatid antibodies in the sera of confirmed CE cases (Group 1 and 2) and controls (Group 3 and 4) by using urinary hydatid antigen from surgically confirmed cases. The procedure of EITB for demonstrating reactive antigenic proteins consists of the following steps: The concentrated urine obtained from surgically confirmed CE cases was separated based on the difference in molecular weight by SDS-PAGE as explained earlier. The separated antigens were blotted onto nitro cellulose membrane (NCM) (0.22 μm) by using the blotting apparatus. The transfer was done at constant volt (100 V) for 1 h transfer time. The free reactive sites on the NCM (cut to strips) were blocked by PBS 7.2 containing 2% BSA by incubating for 3 h at 37°C under constant rocking. Membrane strips were washed thrice with PBS-T. 5 ml of 1:100 dilutions of the patient sera was prepared in PBS-T and the membrane strips were incubated for 1.5 h at 37°C under constant rocking. The membrane strips were washed thrice with PBS-T to remove the unbound antibodies. 1:1000 dilution of rabbit anti-human-IgG-HRP conjugated secondary antibody was used with PBS 7.2 containing Tween-20 (0.05%) and the strips are placed in a plastic tray. This was incubated for 0.5 h at 37°C in dark under constant rocking. The membrane strips were washed 3 times with PBS-T to remove the unbound conjugate. 5 ml of substrate solution (6 mg of 3, 3’ diaminobenzidine in 10 ml of PBS 7.2 containing 0.05% Tween-20 and 10 μl H2O2) was dispensed and incubated for 15-20 min at 37°C in dark under constant rocking for the development of color. The reaction was stopped by washing with double distilled water. After blocking with BSA, the membranes containing immobilized proteins were cut into strips and were screened with individual serum from confirmed CE cases and controls. After treating separately with individual serum, strips were subjected to immunostaining to identify the immunodominant fractions.
Statistical analysis
Statistical analysis was performed using Microsoft Excel software 2003 Microsoft Corporation, USA. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of each the diagnostic method were calculated as per the standard method, using the surgical confirmation and ultrasonography as the gold standard.[11]
RESULTS
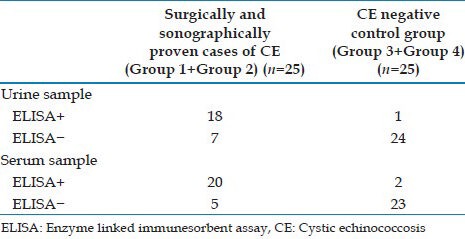
A total number of 25 cases (surgically confirmed CE 10, ultrasound proven CE 15) and 25 controls (controls with other parasitic disease 15, healthy controls 10) were included in the study. The performance of the sandwich ELISA was evaluated using surgical and radiological diagnosis as the gold standard. The sandwich ELISA using polyclonal antibodies demonstrated hydatid antigen in the cyst fluid of all 10 surgically confirmed cases. With urine samples, the sandwich ELISA showed a sensitivity of 72%, specificity of 96%, PPV of 95% and a NPV of 77%. When serum was tested, the sandwich ELISA demonstrated sensitivity, specificity, PPV and NPV of 80%, 92%, 91% and 82% respectively [Table 1].
Table 1.
Performance of the novel sandwich ELISA for urine and serum samples
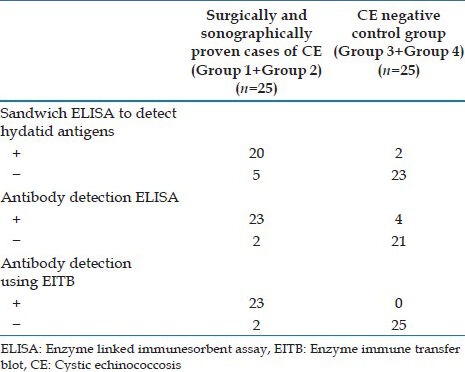
The comparative evaluation of ELISA for detection of hydatid antibody and antigen in serum showed that ELISA for hydatid antibody detection (92%) was more sensitive than that of ELISA for antigen detection (80%). However, the antigen detection ELISA was found to be more specific (92%) than the antibody detection ELISA (84%). Among all the three tests that used serum, EITB for detection of hydatid antibody was found to be the most sensitive (92%) and specific (100%) [Figure 1, Table 2].
Figure 1.
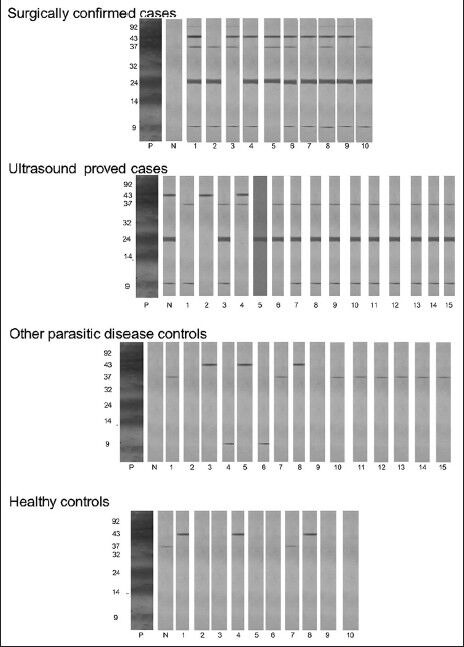
Detection of antibodies in serum of cases and controls by enzyme immune transfer blot using urinary hydatid antigen. P-Standard positive serum, N-Known negative serum, 1-10 and 1-15 represents the number of cases/controls
Table 2.
Performance of the different tests with serum samples
DISCUSSION
CE is a chronic asymptomatic parasitic infection with viable cysts persisting throughout the life of an intermediate hosts.[12] The laboratory methods for the diagnosis of parasitic diseases essentially consist of parasitic, immunodiagnosis and molecular methods. Parasitic diagnosis is the best method to establish the specific diagnosis of the CE, which includes microscopy of the aspirated hydatid fluid to demonstrate brood capsules and protoscolices. Although it confirms the diagnosis of the CE, the greatest disadvantage of diagnostic aspiration is the risk of anaphylactic reaction from leakage of cyst fluid.[13]
Hence, a wide number of serological tests have been developed for the detection of either hydatid antibodies or antigens in sera.[14] However, the antibody-based tests have the disadvantages of low specificity and sensitivity and the inability to differentiate between recent and past infections. The mere demonstration of hydatid antibody doesn’t confirm clinical diagnosis because of non-specific reactions and persistence of anti-hydatid antibodies for several years after surgical removal of hydatid cyst. Hence, diagnostic methods based on the detection of parasite antigens are more useful as it helps in more direct measure of parasite burden, giving an indication of the activity and intensity of infection and also in assessing medical and chemotherapeutic treatment of CE.[15]
In the present study, ELISA was performed using purified 24 kDa urinary hydatid antigen to detect antibodies in the sera of both study and control groups. The test showed a sensitivity of 92% and specificity of 84%. Romia et al.[16] reported a sensitivity of 88.9% and specificity of 96.9% by Dot-ELISA by using antigen B instead. Iacona et al.[17] evaluated ELISA using purified hydatid fluid fraction for demonstration of specific antibodies in human CE. The ELISA showed a sensitivity of 64%. They recommended that ELISA should be considered not as alternative, but as a useful addition to the range of immunodiagnostic tests available for serodiagnosis of CE. In the present study, the ELISA using 24 kDa urinary hydatid antigen showed 16% of false positive reactions. Three out 15 cases of other disease controls (tropical pulmonary eosinophilia, neurocysticercosis and partial seizures) and one out of 10 healthy controls were positive by ELISA. Similar observations have also been documented in a study by Ramzy et al.[18] In their study, with ELISA using camel hydatid cyst fluid observed a false positive reaction of 34.9% with sera from other parasitic diseases.
The EITB assay for the diagnosis of CE has been reported to be more specific than ELISA and Dot-ELISA.[19] The latter two tests although are sensitive but are not highly specific and often show cross reaction with sera from patients with other cestode infections. The EITB combines the high sensitivity and specificity of the immunoenzyme tests with the high resolution of specific proteins in SDS-PAGE. Most of the studies on EITB have used cyst fluid antigen and have observed 8 kDa, 29 kDa and 34 kDa as diagnostically relevant bands and exhibited 91% sensitivity and overall specificity of 97%. The test provided 99% discrimination between seropositive pre-operative CE cases and cross-reactive non-cestode parasitic infections or malignancies.[20] EITB with cyst fluid revealed 44 kDa, 34 kDa, 29 kDa and 8 kDa proteins to be Echinococcus specific and provided 100% sensitivity and specificity in CE.[21]
In the present study, EITB was performed using sera from different groups to identify diagnostically relevant bands by using urinary 24 kDa protein for the first time. In this study, an antibody to 24 kDa fraction was observed to be protein of diagnostically relevance. Similar low molecular weight proteins in other antigen preparations have also been reported in other studies. These are 24 kDa protein of antigen B, 23 kDa protein and 20 kDa of hydatid cyst fluid.[19,22,23] The study showed a sensitivity of 92% and specificity of 100%. The results of the present study suggest that 24 kDa urinary hydatid protein can also serve as an alternate source of antigen. EITB was found to be highly specific test for detection of hydatid antibodies.
Detection of hydatid antigen in the serum or other body fluid is suggested to be more efficacious in the diagnosis of CE than antibody detection.[24] Most antibody assays in CE are neither sensitive nor very specific and there is no correlation between the levels of anti-hyadtid antibodies and the size or location of hydatid cyst. Moreover, anti-hydatid antibodies persist for years after surgical removal of the cyst.[25] The antibody detection assays in CE fails to differentiate the current infection from past due to the residential nature of antibodies in the circulation. These drawbacks could be overcome by the antigen detection tests. Antigen-based tests such as CIEP, Co-A, latex agglutination test and ELISA are increasingly used now-a-days because these tests can detect recent infection.[4,9,26,27] Hydatid antigen is present in serum possibly due to the biological permeability of minute volume of fluid antigen through the hydatid cyst wall, or secreted/excreted metabolic products of the hydatid cyst or due to deformed hydatid cyst induced by chemotherapy.[28] The antigen is present during active infection; detection of serum hydatid antigen always indicates recent infection.[4]
In the present study, the sandwich ELISA using polyclonal antibodies raised against purified 24 kDa urinary hydatid protein showed a sensitivity of 80% and specificity of 92% for diagnosis of CE. The results of the present study are in complete with the study conducted by Kanwar et al., who showed a sensitivity of 85% and 90% respectively for detection of 8 kDa and 116 kDa antigen in the serum for the diagnosis of CE.[29] Employing the double antibody sandwich ELISA using polyclonal antibodies, Gottstien demonstrated circulating antigen in 40% of CE patients.[24] The author suggested that low sensitivity obtained by sandwich ELISA using polyclonal antibodies for the detection of hydatid antigen in the serum is due to the level of passage of parasite proteins from fissuration of the cyst wall. The sandwich ELISA showed a false positive reaction in one case each in other parasitic disease control and healthy controls. The results of the present study suggest that polyclonal antibodies to 24 kDa urinary hydatid antigen can be used to demonstrate hydatid antigen in serum by sandwich ELISA.
Although antigen detection assays in the serum are widely used for most of the parasitic diseases, the collection of blood for serum is an invasive procedure and requires technical expertise and disposable syringes and associated with the risk of acquiring blood-borne infections such as hepatitis B virus and human immunodeficiency virus. Recently, there has been much interest in the collection of body fluids such as urine, saliva and tear drops other than serum for the diagnosis of parasitic infections of these specimens; urine is increasingly used as a specimen alternate to blood for the diagnosis of many parasitic infections.[30] Detection of antigen in urine is a recent approach in diagnosis of many of the parasitic infections including Chaga's disease, leishmaniasis, urinary schistosomiasis, filariasisand malaria.[31,32,33,34] Hydatid antigen detection in the urine for diagnosis of CE was first reported by Parija et al. in 1997 from India by CIEP using hyperimmune antisera rose against crude human hydatid cyst fluid.[5]
In the present study, sandwich ELISA was done to detect hydatid antigen (24 kDa) in the urine of both study group and control group by using polyclonal antibodies against 24 kDa. The assay could detect 7 out of 10 and 11 out of 15 in Group I and Group II respectively. The test had a sensitivity of 72% and specificity of 96%. The sensitivity of sandwich ELISA for detection of hydatid antigen in urine is higher than that of the sensitivities of CIEP (29.6%) and Co-A (47.5%) as reported earlier.[16] However the test failed to detect antigen in the urine of one case whose sera is positive for hydatid antigen by using sandwich ELISA. The sensitivity of sandwich ELISA for detection of hydatid antigen in the urine (72%) is relatively lower compared to that of the sandwich ELISA for detection of hydatid antigen in the serum (80%). This is possibly due to the lower quantity of hydatid antigen excreted in a large volume of urine.[35] The specificity of sandwich ELISA for detection of antigen in urine is slightly higher (96%) than that of the sandwich ELISA for detection of antigen in the serum (92%). In the present study, the test showed a false positive reaction in one case of other parasitic disease control. Based on overall results, showed that sandwich ELISA using the polyclonal antibodies to purified 24 kDa antigen is a moderately sensitive and highly specific test to detect urinary hydatid antigen for the diagnosis of CE. The hydatid cyst fluid is a repository of somatic and functional antigen of parasite origin. It also contains variable amounts of host proteins. The type and concentration of parasite-derived molecules are likely to be different in fertile and non-fertile cysts. It has been demonstrated that the highest concentration of antigenic protein was shown in batches of bovine fertile hydatid cyst fluid than that of bovine non-fertile hydatid cyst fluid.[36] In the present study, the sandwich ELISA test demonstrated hydatid antigen in all 10 surgically confirmed cases (100%). The sensitivity of this method for detection of hydatid antigen in cyst fluid is higher (100%) than hydatid antigen in serum (80%). However, the disadvantage of using cyst fluid as specimen for diagnosis of CE is that cyst fluid is difficult to obtain and any attempts of diagnostic aspiration might lead to anaphylactic shock.[13]
CONCLUSION
In the present study, purified 24 kDa urinary hydatid antigen was used as a source of antigen for the identification of hydatid antibodies in serum and polyclonal antibodies were raised against 24 kDa antigen to detect hydatid antigen in serum, urine and cyst fluid. ELISA using polyclonal antibodies against 24 kDa urinary hydatid protein was moderately sensitive to detect hydatid antigen in serum and urine. Hence, polyclonal antibodies to 24 kDa urinary hydatid antigen can be used as an alternative source of antibody to detect hydatid antigen in serum, urine and cyst fluid. In addition, EITB was found to be highly specific test for detection of hydatid antibodiesin serum. Overall, 24 kDa protein was found to be specific and of diagnostic value in CE.
Footnotes
Source of Support: Institutional Review Board - Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India.
Conflict of Interest: None declared.
REFERENCES
- 1.Williams JF, López Adaros H, Trejos A. Current prevalence and distribution of hydatidosis with special reference to the Americas. Am J Trop Med Hyg. 1971;20:224–36. doi: 10.4269/ajtmh.1971.20.224. [DOI] [PubMed] [Google Scholar]
- 2.Parija SC. 1st ed. New Delhi: AITBS; 1990. Review of Parasitic Zoonoses; p. 463. [Google Scholar]
- 3.Craig PS, Bailey W, Nelson GS. A specific test for the identification of cyst fluid samples from suspected human hydatid infections. Trans R Soc Trop Med Hyg. 1986;80:256–7. doi: 10.1016/0035-9203(86)90029-5. [DOI] [PubMed] [Google Scholar]
- 4.Giri S, Parija SC. A review on diagnostic and preventive aspects of cystic echinococcosis and human cysticercosis. Trop Parasitol. 2012;2:99–108. doi: 10.4103/2229-5070.105174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parija SC, Ravinder PT, Rao KS. Detection of hydatid antigen in urine by countercurrent immunoelectrophoresis. J Clin Microbiol. 1997;35:1571–4. doi: 10.1128/jcm.35.6.1571-1574.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polman K, Diakhate MM, Engels D, Nahimana S, Van Dam GJ, Falcão Ferreira ST, et al. Specificity of circulating antigen detection for Schistosomiasis mansoni in Senegal and Burundi. Trop Med Int Health. 2000;5:534–7. doi: 10.1046/j.1365-3156.2000.00600.x. [DOI] [PubMed] [Google Scholar]
- 7.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 8.Hager DA, Burgess RR. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: Results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980;109:76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- 9.Shariff M, Parija SC. Co-agglutination (Co-A) test for circulating antigen in hydatid disease. J Med Microbiol. 1993;38:391–4. doi: 10.1099/00222615-38-6-391. [DOI] [PubMed] [Google Scholar]
- 10.Parija SC, Mishra SR, Rao RS. Sensitized chick cells in the indirect haemagglutination test for echinococcosis. J Med Microbiol. 1986;22:237–9. doi: 10.1099/00222615-22-3-237. [DOI] [PubMed] [Google Scholar]
- 11.Park K. 17th ed. Jabalpur: M/S Banarsidas Bharot Publishers; 2002. Park's Textbook of Preventive and Social Medicine. [Google Scholar]
- 12.Lightowlers MW. Immunology and molecular biology of Echinococcus infections. Int J Parasitol. 1990;20:471–8. doi: 10.1016/0020-7519(90)90194-r. [DOI] [PubMed] [Google Scholar]
- 13.Parija SC. 4th ed. New Delhi: All India Publishers and Distributers; 2013. Textbook of Medical Parasitology: Protozoology and Helminthology. [Google Scholar]
- 14.Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. 2003;16:18–36. doi: 10.1128/CMR.16.1.18-36.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravinder PT, Parija SC. Countercurrent immuno-electrophoresis test for detection of hydatid antigen in the fluid from hydatid cysts: A preliminary report. Acta Trop. 1997;66:169–73. doi: 10.1016/s0001-706x(97)00036-3. [DOI] [PubMed] [Google Scholar]
- 16.Romia SA, Youssef ME, Handoussa AE, Rizk HM, Sallam SM. Dot-ELISA as a diagnostic test in hydatid disease. J Egypt Soc Parasitol. 1992;22:603–10. [PubMed] [Google Scholar]
- 17.Iacona A, Pini C, Vicari G. Enzyme-linked immunosorbent assay (ELISA) in the serodiagnosis of hydatid disease. Am J Trop Med Hyg. 1980;29:95–102. doi: 10.4269/ajtmh.1980.29.95. [DOI] [PubMed] [Google Scholar]
- 18.Ramzy RM, Helmy H, El Zayyat EA, Rifaat MM, Abdel Hameed DM, Abdel-Baki MH. An enzyme-linked immunosorbent assay for detection of IgG1 antibodies specific to human cystic echinococcosis in Egypt. Trop Med Int Health. 1999;4:616–20. doi: 10.1046/j.1365-3156.1999.00459.x. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd JC, McManus DP. Specific and cross-reactive antigens of Echinococcus granulosus hydatid cyst fluid. Mol Biochem Parasitol. 1987;25:143–54. doi: 10.1016/0166-6851(87)90003-x. [DOI] [PubMed] [Google Scholar]
- 20.Richard-Lenoble D, Smith MD, Loisy M. Human hydatidosis: Evaluation of three serodiagnostic methods, the principal subclass of specific immunoglobulin and the detection of circulating immune complexes. Ann Trop Med Parasitol. 1978;72:553–60. doi: 10.1080/00034983.1978.11719360. [DOI] [PubMed] [Google Scholar]
- 21.Poretti D, Felleisen E, Grimm F, Pfister M, Teuscher F, Zuercher C, et al. Differential immunodiagnosis between cystic hydatid disease and other cross-reactive pathologies. Am J Trop Med Hyg. 1999;60:193–8. doi: 10.4269/ajtmh.1999.60.193. [DOI] [PubMed] [Google Scholar]
- 22.Lightowlers MW, Liu DY, Haralambous A, Rickard MD. Subunit composition and specificity of the major cyst fluid antigens of Echinococcus granulosus. Mol Biochem Parasitol. 1989;37:171–82. doi: 10.1016/0166-6851(89)90149-7. [DOI] [PubMed] [Google Scholar]
- 23.al-Yaman FM, Knobloch J. Isolation and partial characterization of species-specific and cross-reactive antigens of Echinococcus granulosus cyst fluid. Mol Biochem Parasitol. 1989;37:101–7. doi: 10.1016/0166-6851(89)90106-0. [DOI] [PubMed] [Google Scholar]
- 24.Gottstein B. An immunoassay for the detection of circulating antigens in human echinococcosis. Am J Trop Med Hyg. 1984;33:1185–91. doi: 10.4269/ajtmh.1984.33.1185. [DOI] [PubMed] [Google Scholar]
- 25.Rickard MD, Honey RD, Brumley JL, Mitchell GF. Serological diagnosis and post-operative surveillance of human hydatid disease. II. The enzyme-linked immunosorbent assay (ELISA) using various antigens. Pathology. 1984;16:211–5. doi: 10.3109/00313028409059107. [DOI] [PubMed] [Google Scholar]
- 26.Devi CS, Parija SC. A new serum hydatid antigen detection test for diagnosis of cystic echinococcosis. Am J Trop Med Hyg. 2003;69:525–8. [PubMed] [Google Scholar]
- 27.Kanwar JR, Kanwar RK, Grewal AS, Vinayak VK. Significance of detection of immune-complexed 8 kDa hydatid-specific antigen for immunodiagnosis of hydatidosis. FEMS Immunol Med Microbiol. 1994;9:231–6. doi: 10.1111/j.1574-695X.1994.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 28.Craig PS, Rogan MT, Allan JC. Detection, screening and community epidemiology of taeniid cestode zoonoses: Cystic echinococcosis, alveolar echinococcosis and neurocysticercosis. Adv Parasitol. 1996;38:169–250. doi: 10.1016/s0065-308x(08)60035-4. [DOI] [PubMed] [Google Scholar]
- 29.Kanwar JR, Kaushik SP, Sawhney IM, Kamboj MS, Mehta SK, Vinayak VK. Specific antibodies in serum of patients with hydatidosis recognised by immunoblotting. J Med Microbiol. 1992;36:46–51. doi: 10.1099/00222615-36-1-46. [DOI] [PubMed] [Google Scholar]
- 30.Parija SC. Urinary antigen detection for diagnosis of parasitic infections. Parasitol Today. 1998;14:5–6. doi: 10.1016/s0169-4758(97)01148-4. [DOI] [PubMed] [Google Scholar]
- 31.Corral RS, Orn A, Freilij HL, Bergman T, Grinstein S. Purification and characterization of an 80-kilodalton Trypanosoma cruzi urinary antigen. J Clin Microbiol. 1989;27:145–51. doi: 10.1128/jcm.27.1.145-151.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohanteb J, Ardehali SM, Rezai HR. Detection of Leishmania donovani soluble antigen and antibody in the urine of visceral leishmaniasis patients. Trans R Soc Trop Med Hyg. 1987;81:578–80. doi: 10.1016/0035-9203(87)90414-7. [DOI] [PubMed] [Google Scholar]
- 33.Bosompem KM, Arishima T, Yamashita T, Ayi I, Anyan WK, Kojima S. Extraction of Schistosoma haematobium antigens from infected human urine and generation of potential diagnostic monoclonal antibodies to urinary antigens. Acta Trop. 1996;62:91–103. doi: 10.1016/s0001-706x(96)00040-x. [DOI] [PubMed] [Google Scholar]
- 34.Katzin AM, Kimura ES, Alexandre CO, Ramos AM. Detection of antigens in urine of patients with acute falciparum and vivax malaria infections. Am J Trop Med Hyg. 1991;45:453–62. doi: 10.4269/ajtmh.1991.45.453. [DOI] [PubMed] [Google Scholar]
- 35.Hafid J, Tran Manh Sung R, Raberin H, Akono ZY, Pozzetto B, Jana M. Detection of circulating antigens of Toxoplasma gondii in human infection. Am J Trop Med Hyg. 1995;52:336–9. doi: 10.4269/ajtmh.1995.52.336. [DOI] [PubMed] [Google Scholar]
- 36.Irabuena O, Nieto A, Ferreira AM, Battistoni J, Ferragut G. Characterization and optimization of bovine Echinococcus granulosus cyst fluid to be used in immunodiagnosis of hydatid disease by ELISA. Rev Inst Med Trop Sao Paulo. 2000;42:255–62. doi: 10.1590/s0036-46652000000500004. [DOI] [PubMed] [Google Scholar]


