Abstract
Background
Although osteoporosis affects women of all ages, the impact is most pronounced in frail residents in long term care. Nevertheless, few interventional trials have been performed in this population and few data on therapeutic alternatives are available in this cohort.
Purpose
We describe the challenges and lessons learned in developing and carrying out a trial in frail long term care residents.
Methods
The ZEST (Zoledronic acid in frail Elders to STrengthen bone) study was designed to examine the safety and efficacy of a single-dose therapy for osteoporosis in frail residents in long term care in the Pittsburgh area. Women with osteoporosis who were 65 years of age and older and currently not on therapy, were randomized in a blinded fashion to intravenous zoledronic acid or placebo. Follow-up of each participant was planned for 2 years. All participants received appropriate calcium and vitamin D supplementation.
Results
Seven hundred and thirty-three contacts were made with long term care residents of 9 participating facilities. Of 252 women screened, 181 women were eligible, enrolled, and were randomized. Multiple barriers to research in long term care facilities were encountered but overcome with direct communication, information sessions, in-service trainings and social events. Lessons learned included designing the study in a manner that avoided placing an additional burden on an already overcommitted facility staff, a two-stage consent process to separate screening from randomization, and a flexible examination schedule to accommodate residents while obtaining the necessary outcome measurements. Furthermore, a mobile unit accessible to participants containing state-of-the-art dual x-ray absorptiometry, assessment for vertebral fractures, and phlebotomy equipment allows all assessments to be performed on-site at each facility. Serious adverse events are collected from affiliated hospitals in real time with a novel electronic surveillance system.
Limitations
The major limitation is selection of outcomes that can be assessed at participating facilities and do not require transport of participants to hospitals or clinics.
Conclusions
Clinical research for osteoporosis can be successfully and safely performed with frail residents in long term care facilities. Lessons learned from this study may inform future investigations among frail elderly residents of these facilities.
Keywords: osteoporosis, long term care, frail elderly
Introduction
Although osteoporosis is a devastating condition for individuals of all ages, its impact is most pronounced in the frail elderly. Approximately 85% of women in long-term-care institutions have osteoporosis (1;2), and the incidence of hip or vertebral fractures in this setting is 8 to 9 times that among individuals of similar age and gender in the community (3). Frail elders with hip fractures suffer mortality as high as 24% at one year; survivors never may recover fully (4). Despite these adverse outcomes, most osteoporosis trials have focused on younger postmenopausal women and rarely included residents of long term care facilities. A panel of experts from the March 2000, NIH Consensus Development Conference on Osteoporosis Prevention, Diagnosis and Therapy identified frail nursing home patients as one of the five critical areas of need deserving immediate attention (5).
Background and Rationale for the Clinical Trial
Although there are FDA-approved therapies for prevention and treatment of osteoporosis, approvals were based on studies of younger postmenopausal women living in the community (6–9). Post-hoc subgroup analyses of data from pivotal trials have examined outcomes among healthy older women in these cohorts and reported reduction in the incidence of fractures (10–12). However, to be included in these pivotal trials, women had to be mobile, cognitively intact, healthy and able to go easily to an offsite clinical center for regular evaluations. Even in a placebo-controlled trial that included residents of long-term care facilities, all participants were independent, ambulatory, cognitively intact, and able to self-administer the daily oral bisphosphonate (13).
A challenge for researchers studying long-term-care patients is the negative perception of staff, faculty, physicians and patients regarding clinical research in a “vulnerable population.” The facility administrators and staff are protective of their patients. Family members may perceive that conducting research with their loved one reduces the patient to a “guinea pig”; The physicians may feel threatened and not want their patients, practice, and decision making process observed and judged by outsiders. Physicians also often believe that their patients are too old, too frail or too medicated to be in a research study. Administrators and staff also have concerns that outside research will add a burden and may require additional tasks or procedures, medications or time to manage new medical events brought on as a result of the test intervention or other aspects of study participation by patients.
Because frail, institutionalized elderly women have multiple medical problems and are on multiple medications, many physicians consider the potentially powerful therapies for osteoporosis to have greater risk than benefit and to be unjustified in this population. Moreover, there are concerns regarding reports of severe bone, joint, or muscle pain with oral and intravenous bisphosphonates (14). Furthermore, before a study in this age group in which hip or vertebral fracture is the outcome can be justified, safety, feasibility, maintenance of bone mass and decrease in bone turnover must be demonstrated. This reasoning echoes the Surgeon General’s Report on Bone Health and Osteoporosis, which stated that “Although the most important study outcome is fracture reduction, changes in bone mineral density or bone turnover can be used (in the conduct of clinical studies) as supportive evidence of the effectiveness of treatment” (15).
Therefore, the present clinical trial was designed to set the stage for a definitive fracture reduction study in frail elderly. The design was intended to be inclusive and generalizable by allowing for inclusion of many of the frail, older patients that pivotal trials excluded. If the therapy tested proves to be as safe and effective for bone mass as in non-frail patients, the current lack of enthusiasm for use in the nursing home would be unjustified. In addition, findings from this study will indicate whether a single dose of therapy is beneficial in this cohort. Thus, given the lack of evidence and the enormity of the problem, further investigation was desperately needed.
Our purpose is to describe the barriers, challenges and lessons learned in developing and implementing a trial in frail residents in long term care. Our experience may be useful for designers of other osteoporosis clinical trials as well as other studies that involve patients in the long-term-care setting.
Methods
Design
The ZEST (Zoledronic Acid in Frail Elders to Strengthen Bone) study was a double-blind, placebo-controlled, randomized clinical trial.
Participants
The ZEST study enrolled eligible women resident in long-term-care facilities in the Pittsburgh area. Unless there was a known contraindication for treatment, ambulatory and non ambulatory women were invited to be screened for eligibility; inclusion and exclusion criteria are described in Table 1. Women deemed eligible after screening who consented to randomization were enrolled. In comparison to most pivotal trials for osteoporosis, ZEST had no upper age limit, allowed women with cognitive impairment, immobility, who were on multiple medications (including glucocorticoids and antiseizure medications) and with multiple medical problems.
Table 1.
Inclusion and Exclusion Criteria for Participants in the ZEST Study
| Inclusion Criteria |
|
| Exclusion Criteria |
|
Note Use of glucocorticoids, antiseizure medications, estrogen, raloxifene and calcitonin were permitted. Immobility or cognitive impairment were not exclusion criteria.
Recruitment
We based our sample size justification for the study on published data that suggested that the one-year effect (mean ± standard deviation) of zoledronic acid on BMD is an increase of approximately 4.5±3.6% in the lumbar spine and 3.0 ± 2.9% in the femoral neck (16). We further assumed that treatment effect would be 25% less (i.e. 3.375% and 2.25%, respectively) than published estimates due to the frail elderly nature of our population (17). We conservatively assumed that one-year attrition rate in our study would be 30% (18). This 30% will be effectively imputed to have no change in a last-value-carried-forward analysis based on the intention-to-treat principle. Thus the mean anticipated effect would be reduced by a further 30% after incorporating the imputed zero changes, resulting in conservative estimates of 2.363% and 1.575%, respectively, for the anticipated treatment effect. Further, we also conservatively assumed that the control group would experience no change in BMD during follow-up despite the potential for likely decline over the study period. Therefore, any actual decline in the control group would increase the between group difference in treatment effect and result in greater statistical power than estimated. We also assumed that the control group would experience no change in BMD during follow-up despite the potential for decline over the study period. Thus, a total of 180 women randomized to treatment and placebo in equal proportions was expected to result in 126 completers at the one year, the time for assessment of the primary outcome for efficacy. The 126 completers were estimated to provide 95% statistical power to detect a between group difference of 2.363% in lumbar spine BMD and 86% power to detect a between group difference of 1.575% in femoral neck in two-sided tests conducted at the α=0.05 level.
Recruitment of facilities
Our multidisciplinary study team included geriatricians, endocrinologists, nurses, research coordinators and long term care specialists. To reach our randomization goal of 180 women, we enlisted the participation of nursing homes and assisted living facilities in the Pittsburgh area. Many of these facilities were affiliated with the University of Pittsburgh Medical Center (UPMC); in such cases, the medical director typically was affiliated with the Department of Geriatrics at the University of Pittsburgh. We estimated that we would have a total pool of over 2700 older women from whom to recruit ZEST trial participants.
Recruitment of facilities was a multistep process and began with a meeting of the respective administrators, directors of nursing, clinical directors and staff (Table 2). The staff received material and brochures detailing the purpose, design and timeline of the study. They were informed that the study would not require assistance from the facility staff as study procedures and measurements would be performed by the research staff.
Table 2.
Perceived Barriers to Research Studies Among Elderly Long-Term-Care Patients and Solutions Formulated for the ZEST Trial
| Perceived Barriers | Solutions |
|---|---|
| Complicated medical histories and ongoing issues preven residents from meeting inclusion criteria |
|
| Residents cannot be recruited via the usual means, e.g., physician referral, announcements in the media |
|
| Perception of clinical research as an “experiment” |
|
| Additional burden imposed on staff prone to high rate of turnover |
|
| Patients overwhelmed by a lengthy and detailed consent process for a clinical trial |
|
| Patient reluctance and transportation challenges to travel to a hospital or clinic |
|
| Inability of patients to meet a rigorous follow- up visit schedule |
|
| Unreliability of self-report by residents of adverse events |
|
| Patients cannot expect a direct benefit from study participation within their remaining lifetimes |
|
Following the administrative meeting, letters were sent to physicians affiliated with each facility where personnel indicated a willingness to participate in the study.. The correspondence with the physicians outlined in detail the types of medical testing and assessments of bone mineral density and vitamin D would take place. The study team agreed to provide the physicians, at the request of each participating patient or of the individual with power of attorney, access to all of the baseline information that would be collected. Facility newsletters were sent to family members informing them of the opportunity for women residents to participate. Family members were informed that for any resident deemed cognitively impaired, the family or the designated individual with power of attorney would be involved in the decision making process surrounding participation. Recruitment of long-term-care residents was done in partnership largely with the activity directors and director of nursing at each facility. Social events for recruitment purposes included bingo games, meet and greet events, holiday festivities, social teas, health talks, family nights, etc. The time between first contact with a site and first women enrolled for that site ranged from 3 to 6 months.
Consent Process
For skilled nursing facilities in Pennsylvania (highest level of medical and custodial care), approval was obtained from the Pennsylvania Department of Health in addition to the Institutional Review Board of the University of Pittsburgh before recruitment could begin. A two-stage consent process was utilized whereby the first document described and sought consent for screening procedures only. For each resident who completed screening without being deemed ineligible due to any exclusionary factor or without withdrawing assent to participate in the ZEST trial, a second document was discussed that described randomization and the procedures to be followed throughout the rest of the study. Consent was obtained for all participants; a family member or an individual with power of attorney provided consent for patients with cognitive impairment who assented to participate in the trial.
Intervention
The test intervention, intravenous zoledronic acid 5 mg, was selected because it had been shown to improve bone mass significantly in postmenopausal osteoporotic women in a 1-year clinical trial (16). Because the nursing home residents were difficult to treat and resources were limited, a single dose of intravenous bisphosphonate zoledronic acid was judged to have many advantages. It did not require fasting, administration at a specific time of day, or sitting up post administration, significant practical advantages over a once-weekly dose of oral bisphosphonate. Because the drug was administered as an infusion by study personnel, compliance was not an issue and the long term-care-staff was not burdened with additional responsibilities. All participants received a 45 minute infusion of either zoledronic acid or placebo; the placebo consisted of normal saline similar to the solution for the zoledronic acid. The zoledronic acid and placebo were masked by the research pharmacy so that all the research staff, facility staff, physicians and participants were blinded to the study allocation.
In addition to the infused drug or placebo, all participants received open-label elemental calcium up to 1200 mg per day and 800 units per day of vitamin D in divided doses, depending upon individual dietary calcium intake. At baseline, the dietary calcium intake was calculated with a validated food frequency questionnaire (19); the total daily calcium intake (diet plus supplement) was approximately 1200 mg/day. These daily intake levels of calcium and vitamin D were those recommended by the National Osteoporosis Foundation (20;21) and Institute of Medicine 2010 guidelines (22). The calcium was given in a chewable form, Caltrate plus D which provided 600 mg of elemental calcium and 400 IUs of vitamin D3 per tablet. Residents therefore could chew or suck on the tablets or have them ground and placed in food. The tablets were distributed by the facility staff along with participants’ routine daily medications.
A complex issue was inclusion of a placebo group in a clinical trial for patients with osteoporosis when medications were available that were known to reduce the risk of fracture. A placebo control was utilized for multiple reasons. First, there is genuine uncertainty (“clinical equipoise”) about risk-benefit ratio of current treatments to women eligible for the ZEST trial (23) because the therapy could cause more side effects than benefits. Second, elderly participants may not live long enough to experience any benefit. Third, inclusion of an active comparator would require a larger sample size, which would put more participants at risk of fractures before completion of the trial. Fourth, all participants were treated with the standard of care, i.e., with calcium and vitamin D and allowed to continue with calcitonin, raloxifene or hip protectors whenever these were prescribed by their physicians. Finally, there is uncertainty regarding the benefit of other things considered to be standard of care such as educational modules on osteoporosis evaluation and treatment, academic detailing, and case-based teleconferences on osteoporosis quality improvement (24;25). Therefore, we justified a placebo control arm because it was unknown whether the test intervention was beneficial, a fracture reduction study with an active comparator would result in more fractures (not ethically justifiable), and all participants received the standard of care in long term care.
Schedule
Because frail participants are reluctant to leave the facilities, all study visits and assessments occurred at each participant’s own long-term-care facility. The questionnaires, source evaluations, and consents were obtained in the participant’s room whenever possible. A mobile unit with equipment to assess bone mineral density and to perform ultrasound examinations, EKGs, and phlebotomy was outfitted for the study. After informed consent was obtained, all participants were scheduled for a brief history and physical examination. Baseline safety laboratory assays and 25-hydroxy vitamin D levels were obtained; and residents with vitamin D levels <20 ng/mL were repleated. Follow-up study visits occurred at 6, 12 (the primary outcome time) and 24 months after randomization. The study schedule was flexible so that visits and measurements could be obtained over multiple sessions as needed to allow for changes in clinical status or social events that, for the participant, may take precedence over the study.
Outcome Assessment
The primary outcome variables were changes in bone mineral density of the hip and spine at 12 months. Secondary outcomes included the 12-month changes in 1) markers of bone turnover, 2) safety indicators, including death, other adverse events and abnormal laboratory findings and 3) feasibility and participation. Additional secondary outcomes included 1) bone mineral density at other skeletal sites, 2) functional and cognitive assessments, 3) co-morbidity index and 4) survival for 24 months after enrollment. An exploratory assessment included fragility fractures at 12 and 24 months.
BMD
Outcome measures included bone mineral density of the hip (total hip, femoral neck, trochanter, intertrochanter), spine (posterior-anterior and lateral projections), and forearm (1/3 distal radius) by a mobile DXA unit using a Hologic Discovery A densitometer (Hologic, Inc., Bedford, MA). The precision error of scans on elderly patients using the DXA scanning technique was 1.2% for the total hip, 1.9% for the femoral neck, 1.5% for the PA spine (26), and 1.7% for the lateral spine (27). The DXA unit was easily transported to the long term care facility in the mobile examination unit. Participants had easy access to the DXA unit via an elevator platform that lifts patients in a wheelchair or with a walker along with 1 or 2 assisting staff members onto the mobile unit.
From the study onset, the same two DXA technicians were responsible for obtaining all bone mass measurements. All of these measurements were reviewed by the University of Pittsburgh Osteoporosis Prevention and Treatment Center’s head DXA technician who is certified by the International Society for Clinical Densitometry (ISCD) and American Registry of Radiologic Technologists.
Vertebral Fractures
The presence of vertebral fractures of the thoracic or lumbar spine were determined by Vertebral Fracture Assessment (VFA) performed by DXA, which assesses vertebral fractures from T6 to L4 as the primary classification of vertebral fractures. These evaluations were made using the vertebral fracture software program created by Hologic. Vertebral fractures were assessed at baseline, 6, 12 and 24 months.
Fragility Fractures
From baseline to the end of follow up, all participants were queried every 6 months regarding clinical fragility or non traumatic fractures. A fragility fracture was defined as a fracture following a fall from standing or sitting height. All reported fractures were verified by obtaining, confirmatory radiology report.
Indices of Bone Mineral Metabolism and Turnover
Indices of bone mineral metabolism included 25-hydroxyvitamin D2 and D3 by liquid chromatography/mass spectrometry (LC-MS-MS) (28). To assess bone resorption, serum CTX (Crosslaps One Step ELISA, Osteometer Biotech, Herlev, Denmark; intraassay CV 8%) was measured. The markers of bone formation included P1NP by RIA (UniQ, Orion Diagnostica, Inc., Espoo, Finland; intraassay CV 3.0–4.2%). Additional serum was archived to be assayed at a later date for markers of bone formation, bone-specific alkaline phosphatase (BSAP) by EIA (Alkphase-B kit, Quidel Corp, San Diego, CA; intraassay CV 5.2–5.8%) or osteocalcin by ELISA (Quidel Corp., San Diego, CA).
Safety Monitoring
Safety laboratory evaluations included measurements of hematologic, renal and liver function. Adverse events also were collected. The medical information regarding serious adverse events and hospitalizations are often incomplete because hospital records are not easily accessible by staff of long term care facilities. Given the cognitive impairment of the majority of the participants, self report was unreliable. To ensure improved capture of serious adverse events, we partnered with the clinical research informatics office of the University of Pittsburgh to implement an electronic surveillance system. The study consent form noted that research personnel would have access to medical records of study participants. Once a patient had given consent and enrolled in the study, the patient’s hospital identifiers were added to the study registry file stored within UPMC’s data repository. The data repository receives real-time information from 18 of the UPMC hospitals as well as outpatient clinics and physician offices. Each day, the study registry file was used to search the data repository for any hospital admissions or emergency room visits in the past 24 hours (29). A daily e-mail notification was generated each time a participant was seen in an emergency room or admitted to one of our affiliated hospitals. The electronic surveillance system tracked serious adverse events for approximately 80% of study participants who routinely received care from UPMC related hospitals.
Up to 20% of participants went to hospitals outside of this UPMC network where the electronic surveillance system could not be used. To overcome this obstacle, we placed stickers on each study participant’s chart at the respective long-term-care facility requesting the onsite staff to notify research staff regarding hospitalizations, emergency room visits or adverse events pertaining to that participant. The pertinent details of all serious adverse events then were collected by the study nurses, often at a scheduled follow-up visit, through information supplied by participants, nursing staff or directors of nursing in the conventional manner. For this subset of participants, the information was captured approximately 1 to 6 months after the event, in comparison to real-time acquisition using the electronic surveillance system.
We examined and reported all serious adverse events (hospitalizations, cancer, death) to the Data and Safety Monitoring Board, drug manufacturer and the National Institute of Health program administrator. Unexpected events related to the study intervention were reported to the University of Pittsburgh Institutional Review Board. In addition, we examined all adverse events potentially related to intravenous administration of zoledronic acid (e.g., myalgia, pyrexia, arthralgia, influenza-like illness). We coded all adverse events using the MedDRA coding system and assessed whether the type and frequency of events were similar in the two groups. Following the infusion of zoledronic acid, the ZEST study nurse visited the participant daily for two consecutive days and ascertained any signs or symptoms that appeared or worsened subsequent to the infusion. For participants who could not report events reliably, the nurse reviewed the medical record at each visit for adverse events noted by other staff or reported by family members.
Retention of Participants
To maximize retention, participants received a small stipend (ten-dollar gift card) at each study visit. Birthday cards and holiday cards reminded participants of our gratitude. Patient champions at each site were appointed to encourage fellow residents to participate and remain in the study. Because long-term-care facilities are all encompassing and residents eat and socialize as a group, it was extremely important to ensure that participation was a positive experience for both staff and participating residents as feedback inevitably was shared with potential study participants. A monetary stipend equivalent to 2.5% of the annual salary for a nursing position was provided to each facility on a biannual basis to compensate a staff member or to support an item or venture that would benefit the staff or residents as acknowledgement of any inconvenience resulting from participation in the ZEST trial, regardless of how minimal. For example, these funds went to the purchase of computers, educational materials for residents and in some instances a flat screen television set for lounge areas. These stipends ensured a good working relationship between our study staff and the facility personnel and were a token of gratitude for the willingness to collaborate. The stipend never exceeded $1250 per year.
Results
Current Status
Enrollment
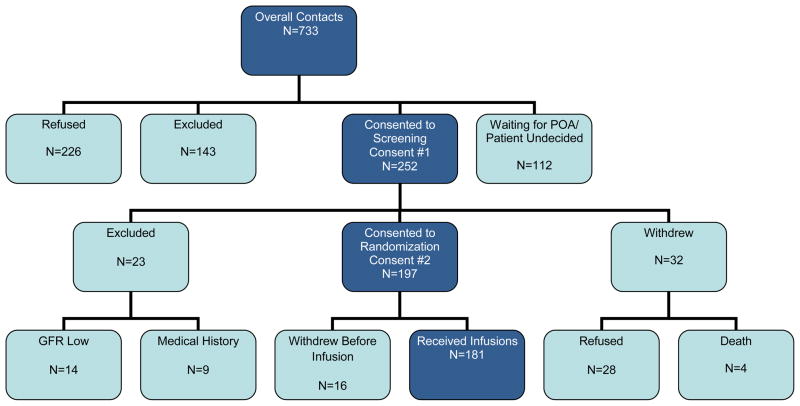
We enrolled participants from nine facilities that ranged in size from 101 residents to 391 residents. The majority of facilities provided both skilled nursing and assisted living at a single center, but one facility had only assisted living residents. To reach our recruitment goal of 180 participants, 733 patients were contacted, 252 consented to screening, 226 refused to participate and 112 participants were undecided or the individuals with power of attorney could not be contacted (Fig 1). The main reason for exclusion of screenees was current bisphosphonate use (n=80, Table 3).
Figure 1.
ZEST Study Design Flow Chart at Completion of Randomization
Table 3.
Number of Screenees for the ZEST Study Who Were Ineligible by Reason for Exclusion
| Reason | Number of Subjects Excluded (N=143) |
Percent of Screened but Excluded (N=143) |
Percent of All Screened (N=733) |
|---|---|---|---|
| On bisphosphonate medication | 80 | 56 | 11 |
| Other osteoporosis medication | 11 | 8 | 2 |
| Allergic to bisphosphonate medications | 1 | <1 | <1 |
| Creatinine clearance <35 mL/min | 6 | 4 | 1 |
| Renal failure or on dialysis | 5 | 3 | 1 |
| In Hospice care | 12 | 8 | 2 |
| Died during screening | 5 | 3 | 1 |
| Too young (age < 65 years) | 3 | 2 | <1 |
| Men | 11 | 8 | 2 |
| Moved out of participating long-term-care facility | 3 | 2 | <1 |
| Other | 6 | 4 | 1 |
Participant Characteristics
The mean age of participants at baseline was 85.3 ± 5.1 (mean ± SD) years with a range from 70.4 to 98.9 years. Most participants were Caucasian/White (97.2%); and the remaining participants (2.8%) were African-Americans. Approximately 29% of participating residents said they had been told by a physician that they had osteoporosis and 63.5% reported a fracture as an adult. The most commonly used medications at baseline were antidepressants (39.2%), thyroid hormone (30.9%), sedative hypnotics (16.0%), glucocorticoids (9.9%), antacids (6.1%), and. Of the supplements consumed at baseline, those used by 64.6% of participants contained calcium and those used by 67.4% of participants contained vitamin D.
Limitations and Advantages
The ZEST trial has several limitations. The measurement outcomes were limited to assessments that could be performed in the long-term-care facility or in the mobile unit. Although we did not have ready access for x-rays, we measured bone mineral density using state-of-the-art methods and assessed vertebral fractures by DXA equipment in the mobile van; otherwise vertebral fractures would be difficult or impossible to measure in a long-term-care population. Patients were recruited in the facilities by informational gatherings, social events, flyers and word of mouth. Those who consented to participate may be different than those who did not, as generally is true for most clinical trials. We believe this study was more generalizable than most since we allowed patients with cognitive impairment, poor mobility, and/or on multiple medications which cause bone loss to participate. A third limitation was our primary outcome of bone mineral density rather than fracture. However, prior to a larger study with fractures as the primary outcome, data demonstrating maintenance or improvement of bone density with zoledronic acid in a frail cohort should be demonstrated. A large fracture reduction study, requiring thousands of patients who experience hundreds of fractures, would be unjustified until it has been shown the zoledronic acid (or some other medication) is safe and efficacious. Estimates of fracture incidence among the ZEST trial participants will be useful when designing trials with fractures as the primary outcome.
This study also has many advantages. It was one of a handful of clinical trials of treatment for osteoporosis among frail long-term-care residents. We developed a real-time system for assessment of serious adverse events. The use of a single infusion therapy eliminated issues of adherence and compliance in long-term-care residents. We utilized a mobile unit so all primary and secondary outcomes could be assessed on-site. Finally our team was a multidisciplinary group of geriatricians, endocrinologists, nurses and long-term-care specialists.
Conclusions and Summary of Lessons Learned
The barriers to research in nursing homes can be overcome with open lines of communication utilizing meetings, information sessions and in-service training. Administrators of participating facilities, along with staff members and the families of the residents, must be comfortable with the study design and requirements. Their cooperation and support can be achieved through meetings, socials and holiday parties and directed letters. It is imperative that the study not impose additional burden on the already overcommitted staff of participating facilities and that all assessments be performed by study staff. It is necessary to continue to provide feedback on the importance of the study and the appreciation of the research team for their participation to the staff, residents of the facilities and their families.
Adequate time should be built into the study plan for each assessment that takes account of the setting and health of target participants compared to younger, healthier, or more mobile outpatients. The trial design must allow for the measurements to be performed on a flexible schedule to account for the comfort and needs of each participant. Study activities and measurements should fit into the routine schedules of the nursing homes so the procedures are not disruptive. Onsite study examinations and measurements eliminates the need for participants to leave the facility for assessments. A two-staged consent process for screening and randomization is helpful.
Serious adverse events should be collected in a timely manner; an electronic surveillance system can facilitate their ascertainment. Adverse events and serious adverse events collected should be organized by systems, using a schema such as MedDRA, to permit identification of serious adverse events in the frail elderly that have not been reported in younger populations.
In conclusion very few studies have examined osteoporosis prevention or treatment among residents in long-term-care facilities who are fragile, cognitively impaired or immobilized. However, these patients have the highest risk of fractures and the highest morbidity and mortality resulting from fractures. Evidence from well-designed clinical trials is required to document the safety and efficacy of treatments in such patients. The ZEST trial demonstrated that these studies can be performed safely in frail elderly patients. Lessons learned from our ZEST experience may be useful to designers of future clinical trials among long-term-care residents.
Acknowledgments
Funding for this project was provided by a generous donation from the Holleran family and NIH awards R01 AG028068, University of Pittsburgh Clinical Translational Research Center RFA-RM-06-002, Clinical Translational Science Institute Ul1 RR024153 NIH/NCRR, Pittsburgh Older American’s Independence Center P30 AG024827 and 2K24DK062895. Study medication and matching placebo were provided by Novartis (Novartis Pharmaceuticals, East Hanover, NJ, USA).
References
- 1.Zimmerman SI, Girman CJ, Buie VC, Chandler J, Hawkes W, Martin A, et al. The prevalence of osteoporosis in nursing home residents. Osteoporos Int. 1999;9:151–7. doi: 10.1007/s001980050129. [DOI] [PubMed] [Google Scholar]
- 2.Komar L, Nieves J, Cosman F, Rubin A, Shen V, Lindsay R. Calcium homeostasis of an elderly population upon admission to a nursing home. J Am Geriatr Soc. 1993;41:1057–64. doi: 10.1111/j.1532-5415.1993.tb06452.x. [DOI] [PubMed] [Google Scholar]
- 3.Simonen O, Mikkola T. Senile osteoporosis and femoral neck fractures in long-stay institutions. Calcif Tissue Int. 1991;49(Suppl):S78–S79. doi: 10.1007/BF02555097. [DOI] [PubMed] [Google Scholar]
- 4.Fisher ES, Baron JA, Malenka DJ, Barrett JA, Kniffin WD, Whaley FS, et al. Hip fracture incidence and mortality in New England. Epidemiology. 1991;2:116–22. doi: 10.1097/00001648-199103000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Anonymous. NIH consensus declares osteoporosis a major public health issue. Osteoporosis Report. 2000;16(1):1. [Google Scholar]
- 6.Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 7.Chesnut CH, III, Silverman S, Andriano K, Genant H, Gimona A, Harris S, et al. A randomized trial of nasal salmon calcitonin in postmenopausal women with established osteoporosis: the Prevent Recurrence of Osteoporotic Fractures Study. Am J Med. 2000;109:267–76. doi: 10.1016/s0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- 8.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA. 1999;282:637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 10.Boonen S, Klemes AB, Zhou X, Lindsay R. Assessment of the relationship between age and the effect of risedronate treatment in women with postmenopausal osteoporosis: a pooled analysis of four studies. JAGS. 2010;58:658–63. doi: 10.1111/j.1532-5415.2010.02763.x. [DOI] [PubMed] [Google Scholar]
- 11.Boonen S, Black DM, Colon-Emeric CS, Eastell R, Magiasis B, Magaziner JS, et al. Efficacy and safety of a once-yearly intravenous zoledronic acid 5mg for fracture prevention in elderly postmenopausal women with osteoporosis aged 75 and older. J Am Geriatr Soc. 2010;58:292–9. doi: 10.1111/j.1532-5415.2009.02673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boonen S, Marin F, Mellstrom D, Xie L, Desaiah D, Krege JH, et al. Safety and efficay of teraparatide in elderly women with established osteoporosis: bone anabolic therapy from a geriatric perspective. J Am Geriatr Soc. 2006;54:782–9. doi: 10.1111/j.1532-5415.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 13.Greenspan SL, Schneider DL, McClung MR, Miller PD, Schnitzer TJ, Bonin R, et al. Alendronate improves bone mineral density in elderly women with osteoporosis residing in long-term care facilities: A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;136:742–6. doi: 10.7326/0003-4819-136-10-200205210-00009. [DOI] [PubMed] [Google Scholar]
- 14.Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med. 2005;165:346–7. doi: 10.1001/archinte.165.3.346-b. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 2004. [Google Scholar]
- 16.Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346:653–61. doi: 10.1056/NEJMoa011807. [DOI] [PubMed] [Google Scholar]
- 17.Trevisan C, Bigoni M, Cherubini R, Steiger P, Randelli G, Ortolani S. Dual x-ray absorptiometry for the evaluation of bone density from the proximal femur after total hip arthroplasty: analysis protocols and reproducibility. Calcif Tissue Int. 1993;53:158–61. doi: 10.1007/BF01321831. [DOI] [PubMed] [Google Scholar]
- 18.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 19.Dawson-Hughes B, Jacques P, Shipp C. Dietary calcium intake and bone loss from the spine in healthy postmenopausal women. Am J Clin Nutr. 1987;46:685–7. doi: 10.1093/ajcn/46.4.685. [DOI] [PubMed] [Google Scholar]
- 20.National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Belle Mead, NJ: Excerpta Medica; 2003. [Google Scholar]
- 21.Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, D.C: National Academy Press; 1997. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. [Google Scholar]
- 22.Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium Food and Nutrition Board. [PubMed] [Google Scholar]
- 23.Rosen CJ, Khosla S. Placebo-controlled trials in osteoporosis-proceeding with caution. N Engl J Med. 2010;363(14):1365–70. doi: 10.1056/NEJMsb1002227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colon-Emeric CS, Lyles KW, House P, et al. Randomized trial to improve fracture prevention in nursing home residents. Am J Med. 2007;120:886–92. doi: 10.1016/j.amjmed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crilly RG, Hillier LM, Mason M, Gutmanis I, Cox L. Prevention of hip fractures in long-term care: relevance of community-derived data. J Am Geriatr Soc. 2010;58:738–45. doi: 10.1111/j.1532-5415.2010.02766.x. [DOI] [PubMed] [Google Scholar]
- 26.Varney LF, Parker RA, Vincelette A, Greenspan SL. Classification of osteoporosis and osteopenia in postmenopausal women is dependent on site-specific analysis. J Clin Densitom. 1999;2:275–83. doi: 10.1385/jcd:2:3:275. [DOI] [PubMed] [Google Scholar]
- 27.Greenspan SL, Maitland-Ramsey L, Myers E. Classification of osteoporosis in the elderly is dependent on site-specific analysis. Calcif Tissue Int. 1996;58:409–14. doi: 10.1007/BF02509439. [DOI] [PubMed] [Google Scholar]
- 28.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005 doi: 10.1210/jc.2004-2364. In press. [DOI] [PubMed] [Google Scholar]
- 29.Yount RJ, Vries JK, Councill CD. The medical archival system: An information retrieval system based on distributed parallel processing. Information Processing and Management. 1991;27(4):379–89. [Google Scholar]